Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.108262
Revised: April 26, 2025
Accepted: May 29, 2025
Published online: July 15, 2025
Processing time: 90 Days and 23.3 Hours
Type 2 diabetic nephropathy (T2DN) is a severe complication of diabetes mellitus, and identifying biomarkers for its prognosis remains a critical challenge. Previous studies have suggested potential roles of microRNAs (e.g., miR-495-3p), adiponectin (ADPN), and cardiometabolic index (CMI) in metabolic and renal pathologies. However, their combined predictive value for T2DN prognosis is not well understood.
To explore serum miR-495-3p, ADPN, and CMI levels in T2DN and their value in predicting prognosis.
A total of 98 T2DN patients (study group) and 49 type 2 diabetic patients with normal renal function (control group) were enrolled from February 2020 to February 2022. Serum levels of miR-495-3p, ADPN, and CMI were measured in both groups. Patients were followed up for 6 months to assess prognosis. Dif
The study group exhibited significantly lower miR-495-3p levels and higher ADPN and CMI levels compared to the control group (P < 0.05). Poor prognosis patients had even lower miR-495-3p and higher ADPN and CMI levels than those with good prognosis (P < 0.05). Multivariate analysis identified alanine amino
Low miR-495-3p and high ADPN and CMI levels are linked to T2DN and poor prognosis, highlighting their potential for risk prediction and clinical management.
Core Tip: This study identifies serum microRNA-495-3p (miR-495-3p), adiponectin (ADPN), and cardiometabolic index (CMI) as novel biomarkers for predicting poor prognosis in type 2 diabetic nephropathy (T2DN). Lower miR-495-3p and higher ADPN/CMI levels correlate with worse renal outcomes, offering clinical utility for risk stratification. Multivariate analysis confirms their independent predictive value alongside liver/kidney function markers. These findings highlight miR-495-3p’s potential as a key prognostic indicator, advancing personalized management strategies for T2DN.
- Citation: Xu XC, Fang HJ, Huang HY. Prognostic value of microRNA-495-3p, adiponectin, and cardiometabolic index in type 2 diabetic nephropathy. World J Diabetes 2025; 16(7): 108262
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/108262.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.108262
Type 2 diabetes mellitus is a disease with increased blood glucose as the main clinical manifestation, which is prone to be complicated by diabetic nephropathy[1]. Diabetic nephropathy and end-stage renal disease are serious kidney diseases. One of the main causes of diabetic nephropathy and end-stage renal disease is long-term uncontrolled diabetes. When diabetes is not effectively managed, high blood glucose levels can damage the small blood vessels and glomeruli of the kidney, leading to progressive impairment of renal function. This process is often accompanied by the development of hypertension, as diabetes and hypertension influence each other and together increase the burden on the kidneys.
As the disease progresses, patients with diabetic nephropathy and end-stage renal disease often require renal replacement therapy such as renal dialysis or kidney transplantation, which adds to the complexity of treatment. A subset of patients with these nephropathies have a poor quality of prognosis because impaired kidney function may lead to other health problems, such as cardiovascular disease and anemia[2]. Previous studies[3] have shown that type 2 diabetic patients with nephropathy have a worse prognosis than type 2 diabetic patients without nephropathy. Therefore, it is urgent to find the factors that affect the prognosis of patients with diabetic nephropathy. In the study of type 2 diabetic nephropathy (T2DN) and its prognosis, the roles of microRNA-495-3p (miR-495-3p), adiponectin (ADPN) and cardiometabolic index (CMI) are particularly important[4]. miR-495-3p, as a microRNA, plays a key role in regulating gene expression and cellular function, and may affect the development of diabetes and its complications. ADPN, a hormone secreted by adipocytes, plays a central role in the regulation of insulin sensitivity and energy metabolism, and its changes in patients with type 2 diabetes have important effects on the disease process and prognosis. The CMI measures cardiac function and metabolic health, which are critical in patients with diabetic kidney disease because of the cardiovascular complications associated with diabetes. Considering the roles and changes of these three together could provide deeper insights into the understanding of the pathogenesis of T2DN and its prognosis, thereby contributing to the development of more effective treatment and management strategies.
Lipid metabolism disorders and obesity are risk factors for diabetic nephropathy. CMI, a novel indicator combining triglyceride and waist-to-height ratio, has been shown to be a useful predictor of cardiometabolic risk in previous studies[4-6]. ADPN, a protective cytokine secreted by adipocytes, is located on chromosome 3q27, which contains susceptibility genes for type 2 diabetes and metabolic syndrome[7,8]. According to studies, miR-495-3p has been confirmed to affect the pathological process of podocyte injury in patients with diabetic nephropathy by regulating nucleoporin 160[9]. The role of miR-495-3p in the prognosis of diabetic nephropathy needs to be further clarified. This study aims to explore the correlation of miR-495-3p, ADPN and CMI with T2DN and its prognosis.
A total of 147 patients were enrolled in this study from February 2020 to February 2022 at Jinhua Municipal Central Hospital. Among them, 98 patients who met the diagnostic criteria for T2DN according to the Guidelines for the Prevention and Treatment of Diabetic Kidney Disease in China[10] were included in the study group. Meanwhile, 49 patients with type 2 diabetes mellitus and normal renal function, treated during the same period, were randomly selected as the control group at a ratio of 2:1 (study group:control group).
Inclusion criteria: (1) All enrolled patients met the World Health Organization diagnostic criteria for type 2 diabetes mellitus; (2) Patients in the study group additionally met the diagnostic criteria for diabetic nephropathy, defined as a glomerular filtration rate < 60 mL/minute/1.73 m2 or a urinary albumin-to-creatinine ratio > 30 mg/g for more than 3 months; (3) Age ≥ 18 years; and (4) Complete clinical data, good compliance, able to complete treatment and follow-up.
Exclusion criteria: (1) Patients with primary renal disease; (2) Patients with urinary tract infection, glomerulonephritis or other renal diseases; (3) Pregnant or lactating women; (4) Type 1 diabetes or other special type; and (5) Presence of thyroid disease.
This study was approved by the Ethics Committee of Jinhua Municipal Central Hospital. Signed written informed consents were obtained from the patients and/or guardians.
The general data of the patients were collected, including age, gender, body mass index (BMI), and disease duration. On admission, 24 hours systolic blood pressure, 24 hours diastolic blood pressure and heart rate were measured by J760 automatic electronic sphygmomanometer (Omron, Japan). 2 mL of venous blood was collected, and the glycated hemoglobin level was measured with a glycated hemoglobin meter (NycoCard Reader I, Norway). The levels of fasting blood glucose, triglyceride, total cholesterol, low density lipoprotein, high density lipoprotein, aspartate aminotransferase, alanine aminotransferase, urea nitrogen and fasting insulin were detected by automatic biochemical analyzer (IDEXX, Catalyst One). The kits were purchased from Shanghai Yanmeng Biotechnology Co., LTD (Shanghai, China). Waist-to-height ratio = waist circumference (cm)/height (cm). CMI = triglyceride/high-density lipoprotein × waist-height ratio.
Five ml of fasting venous blood was collected and the supernatant was removed after centrifugation (3000 r/minute, 10 minutes). The level of ADPN was detected by enzyme-linked immunosorbent assay.
Venous blood was similarly drawn from the patients, and serum was obtained as described above. The level of miR-495-3p was detected by real-time fluorescence quantitative polymerase chain reaction. The total RNA of the sample was extracted according to the instructions of the kit, and then reverse transcription was performed after the purity was qualified. A 20 μL reaction system was prepared and 40 cycles were carried out according to the following reaction conditions: 95 °C for 5 minutes; 95 °C for 30 seconds; 60 °C for 30 seconds; 72 °C for 30 seconds. The expression level of miR-495-3p was calculated by the 2-∆∆Ct method. Primers: Upstream 5’-CGTCCTGACATGATGCATGACC-3’, downstream 5’-TCAGGCTCGCACTAAGCGTCG-3’; The primers were designed and synthesized by Shanghai Kemin Biotechnology Co., LTD. U6 was used as an internal control.
The serum levels of miR-495-3p, ADPN and CMI in the study group and the control group were detected, and the differences between the groups were compared. The patients were followed up for 6 months, and the treatment effect of the patients in the study group was evaluated after 6 months. The clinical symptoms of the patients in the study group were significantly improved or basically disappeared, and the serum creatinine level was reduced by more than 30% or maintained at the normal level. According to the prognosis after treatment, the study group was further divided into two subgroups: The good prognosis group (n = 61), including patients with effective treatment, and the poor prognosis group (n = 37), including patients whose clinical symptoms did not significantly improve and/or whose serum creatinine level did not decrease by more than 30% or remained above the normal level. The differences in serum miR-495-3p, ADPN and CMI levels between the two groups were compared, and the factors affecting the prognosis of the patients were studied. The value of miR-495-3p, ADPN and CMI levels in predicting poor prognosis of patients was analyzed.
SPSS 23.0 software (IBM, Armonk, NY, United States) was used for data processing. Enumeration data such as gender, smoking history, drinking history were expressed as (n/%), and χ2 test was performed. Measurement data such as miR-495-3p, ADPN and CMI levels, and blood lipids were expressed as (mean ± SD), and independent sample t test was performed between groups. Multivariate logistic regression analysis was used to explore the influencing factors affecting the prognosis of patients. Receiver operating characteristic (ROC) curve was used to explore the value of the above indicators in predicting poor prognosis of patients. α = 0.05 was considered as the test level.
A total of 147 patients with T2DN treated in our hospital from February 2020 to February 2022 were enrolled. There were 98 cases in the study group and 49 cases in the control group. The baseline data of the two groups are summarized in Table 1. The general data between the two groups were compared, and there were no significant differences in other data between the two groups except serum creatinine, 24 hours urinary protein quantitative, and urinary microalbumin-to-creatinine ratio (P > 0.05).
| Variables | Control (n = 49) | Study (n = 98) | χ2/t value | P value | |
| Age (years) | 46.01 ± 6.18 | 45.89 ± 4.13 | -0.140 | 0.889 | |
| Gender | Male | 20 (40.82) | 42 (42.86) | 0.056 | 0.813 |
| Female | 29 (59.18) | 56 (57.14) | |||
| BMI (kg/m2) | 25.11 ± 2.15 | 25.41 ± 2.18 | 0.790 | 0.431 | |
| Smoking | Yes | 12 (24.49) | 23 (23.47) | 0.019 | 0.891 |
| No | 37 (75.51) | 75 (76.53) | |||
| Duration of disease (years) | 5.23 ± 1.16 | 5.11 ± 1.03 | -0.638 | 0.524 | |
| Drinking | Yes | 16 (32.65) | 30 (30.61) | 0.063 | 0.801 |
| No | 33 (67.35) | 68 (69.39) | |||
| Serum creatinine (μmol/L) | 65.15 ± 7.15 | 115.26 ± 30.16 | -11.452 | < 0.001 | |
| 24-hour urinary protein quantification (mg/24 hours) | 226.21 ± 21.05 | 521.15 ± 45.26 | -43.280 | < 0.001 | |
| Urinary albumin-to-creatinine ratio (mg/g) | 21.02 ± 2.65 | 44.26 ± 5.12 | -29.805 | < 0.001 | |
The level of serum miR-495-3p in the study group was lower than that in the control group, and the levels of ADPN and CMI were higher than those in the control group (P < 0.05). Refer to Table 2.
| Group | miR-495-3p | ADPN (μg/mL) | CMI |
| Control (n = 49) | 0.75 ± 0.13 | 8.36 ± 1.02 | 0.51 ± 0.16 |
| Study (n = 98) | 0.59 ± 0.21 | 12.66 ± 1.23 | 0.92 ± 0.14 |
| t value | 4.881 | -21.101 | -15.950 |
| P value | < 0.001 | < 0.001 | < 0.001 |
When the poor prognosis group was compared with the good prognosis group, it was found that the level of serum miR-495-3p in the poor prognosis group was lower than that in the latter, while the levels of ADPN and CMI were higher (P < 0.05). Refer to Table 3.
| Group | miR-495-3p | ADPN (μg/mL) | CMI |
| Good outcome (n = 61) | 0.64 ± 0.11 | 11.98 ± 1.03 | 0.88 ± 0.10 |
| Poor outcome (n = 37) | 0.51 ± 0.16 | 13.78 ± 0.92 | 0.99 ± 0.14 |
| t value | 4.762 | -8.724 | -4.527 |
| P value | < 0.001 | < 0.001 | < 0.001 |
Univariate analysis showed that there were no significant differences in age, gender, course of disease, BMI, fasting blood glucose, fasting insulin, glycosylated hemoglobin, heart rate, and 24 hours diastolic blood pressure between the good prognosis group and the poor prognosis group (P > 0.05). The levels of low density lipoprotein, triglyceride, total cholesterol, alanine aminotransferase, aspartate aminotransferase, urea nitrogen, 24 hours systolic blood pressure, and serum creatinine in the poor prognosis group were higher than those in the good prognosis group, and the level of high density lipoprotein was lower than that in the good prognosis group (P < 0.05). Refer to Table 4.
| Group | Good outcome (n = 61) | Poor outcome (n = 37) | t value | P value |
| Age (years) | 45.98 ± 5.78 | 45.74 ± 6.23 | 0.193 | 0.847 |
| Gender (male/female) | 26/35 | 16/21 | 0.004 | 0.952 |
| Duration (years) | 5.01 ± 1.03 | 5.27 ± 1.23 | -1.125 | 0.263 |
| BMI (kg/m2) | 25.31 ± 1.39 | 25.57 ± 1.46 | -1.220 | 0.226 |
| Fasting insulin (mIU/L) | 14.26 ± 1.56 | 14.55 ± 1.68 | -0.867 | 0.388 |
| FBG (mmol/L) | 8.99 ± 1.56 | 8.91 ± 1.61 | 0.243 | 0.808 |
| Glycosylated hemoglobin (%) | 9.95 ± 1.22 | 9.89 ± 0.81 | 0.265 | 0.791 |
| Low density lipoprotein (mmol/L) | 1.97 ± 0.61 | 3.77 ± 0.52 | -14.948 | < 0.001 |
| High density lipoprotein (mmol/L) | 1.36 ± 0.45 | 0.89 ± 0.21 | 5.963 | < 0.001 |
| Triglyceride (mmol/L) | 1.39 ± 0.34 | 3.23 ± 0.56 | -20.266 | < 0.001 |
| Total cholesterol (mmol/L) | 4.46 ± 1.78 | 8.78 ± 1.26 | -12.918 | < 0.001 |
| Heart rate (beats/minute) | 70.28 ± 8.13 | 71.55 ± 8.46 | -0.738 | 0.462 |
| 24-hour systolic blood pressure (mmHg) | 122.46 ± 21.05 | 152.12 ± 21.02 | -8.061 | 0.000 |
| 24-hour diastolic blood pressure (mmHg) | 78.16 ± 3.79 | 78.68 ± 4.11 | -0.638 | 0.525 |
| Alanine aminotransferase (U/L) | 30.55 ± 9.05 | 85.11 ± 8.46 | -29.642 | < 0.001 |
| Aspartate aminotransferase (U/L) | 32.11 ± 8.12 | 76.15 ± 7.48 | 149.069 | < 0.001 |
| Urea nitrogen (mmol/L) | 8.44 ± 1.78 | 16.66 ± 1.22 | -32.857 | < 0.001 |
| Serum creatinine (μmol/L) | 87.26 ± 12.02 | 126.26 ± 9.64 | -16.730 | < 0.001 |
With the patient’s prognosis as the dependent variable (good prognosis = 1, poor prognosis = 0), Multivariate analysis was performed on the indicators with differences in the above single factor analysis (low density lipoprotein, high density lipoprotein, triglyceride, total cholesterol, 24 hours systolic blood pressure, alanine aminotransferase, aspartate aminotransferase, urea nitrogen, serum creatinine, miR-495-3p, ADPN, and CMI were included in the measured values), and the results are shown in Table 5. Alanine aminotransferase, aspartate aminotransferase, urea nitrogen, creatinine, miR-495-3p, ADPN and CMI all affected the prognosis of patients (P < 0.05). Refer to Table 5.
| Variables | B | SE | Wald | P value | OR | 95%CI | |
| Lower limit | Upper limit | ||||||
| Alanine aminotransferase | 5.377 | 1.330 | 16.341 | 0.000 | 216.393 | 15.959 | 234.134 |
| Aspartate aminotransferase | 10.403 | 4.175 | 6.208 | 0.013 | 42.088 | 9.201 | 179.584 |
| Urea nitrogen | 1.753 | 0.414 | 17.959 | 0.000 | 5.774 | 2.566 | 12.991 |
| miR-495-3p | -7.256 | 1.934 | 14.071 | 0.000 | 0.001 | 0.000 | 0.031 |
| ADPN | 1.754 | 0.342 | 26.379 | 0.000 | 5.778 | 2.958 | 11.284 |
| CMI | 8.821 | 2.265 | 15.173 | 0.000 | 375.821 | 80.045 | 575.438 |
| Creatinine | 0.388 | 0.188 | 4.259 | 0.048 | 1.474 | 1.020 | 2.131 |
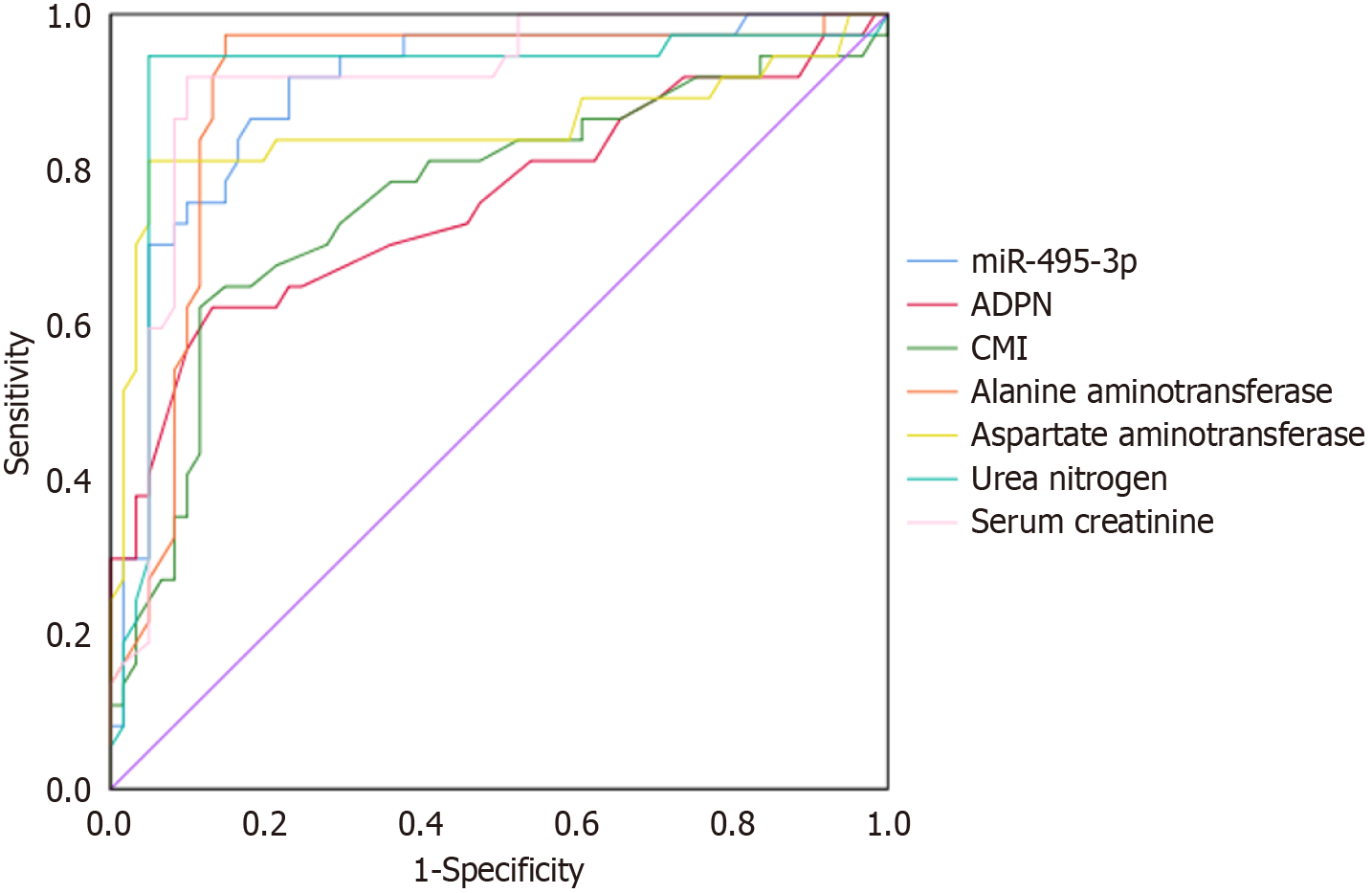
ROC curve analysis showed that serum miR-495-3p, ADPN, CMI, alanine aminotransferase, aspartate aminotransferase, urea nitrogen and serum creatinine had certain predictive value for poor prognosis of patients. The area under the curve values were 0.762, 0.902, 0.757, 0.899, 0.852, 0.916, 0.910, respectively (P < 0.05). Refer to Table 6 and Figure 1.
| Variable | AUC | Cut-off value | 95%CI | Sensitivity | Specificity | P value | |
| Lower limit | Upper limit | ||||||
| ADPN | 0.902 | 12.675 | 0.837 | 0.967 | 0.919 | 0.770 | < 0.001 |
| CMI | 0.757 | 0.965 | 0.650 | 0.863 | 0.622 | 0.869 | < 0.001 |
| miR-495-3p | 0.762 | 0.525 | 0.657 | 0.867 | 0.622 | 0.885 | < 0.001 |
| Alanine aminotransferase | 0.899 | 45.320 | 0.828 | 0.970 | 0.973 | 0.852 | < 0.001 |
| Aspartate aminotransferase | 0.852 | 54.150 | 0.755 | 0.949 | 0.811 | 0.951 | < 0.001 |
| Urea nitrogen | 0.916 | 13.990 | 0.842 | 0.990 | 0.946 | 0.951 | < 0.001 |
| Serum creatinine | 0.910 | 45.380 | 0.847 | 0.973 | 0.919 | 0.902 | < 0.001 |
Diabetic nephropathy is the main cause of end-stage renal disease. If the disease cannot be effectively controlled, it can threaten the life of patients.
The results of this study showed that serum miR-495-3p levels were significantly lower in the study group compared to the control group, whereas ADPN and CMI levels were significantly higher. Among patients with poor prognosis, serum miR-495-3p levels were lower, while ADPN and CMI levels were higher than in those with good prognosis. Multivariate analysis further indicated that miR-495-3p, ADPN, and CMI each had predictive value for poor prognosis in patients. Chen et al[11] have shown that insufficient levels of miR-495-3p can affect the expression of CRBN or TRAF6, which together promote the damage of renal cortical proximal tubular epithelial cells and affect renal function. The finding in this study that insufficient levels of miR-495-3p can adversely affect patient prognosis is consistent with previous reports. Low expression of miR-495-3p has been shown to impair the viability of glomerular cells and promote their apoptosis, thereby contributing to the development and progression of diabetic nephropathy. Fu et al[12] have shown that the more serious the kidney injury, the worse the prognosis of diabetic patients. A greater degree of glomerular interstitial injury increases intraglomerular pressure during filtration, leading to further damage to glomerular epithelial cells. Lower expression levels of miR-495-3p are associated with more severe damage, greater disease progression, and reduced potential for disease reversal. Therefore, miR-495-3p expression may serve as a reliable prognostic indicator in clinical diabetic nephropathy. ADPN has several beneficial effects, including the correction of glucose and lipid disorders, anti-inflammatory properties, and the enhancement of insulin sensitivity. According to the study by Speedtsberg and Tepel[13], the level of ADPN was significantly increased in patients with diabetic nephropathy, which was analyzed to be caused by the decreased ability of the kidney to clear it. The increased level of this factor is also a predictor of renal dysfunction, and it has certain value in the prognosis evaluation of patients with diabetic nephropathy.
Chen et al[14] have found that the degree of visceral obesity has a certain relationship with diabetic nephropathy, and the value of using evaluation indicators of visceral obesity to predict diabetic nephropathy has been clinically confirmed. The CMI has the advantages of ease of calculation and high safety. Currently, there are few studies examining whether CMI can predict the prognosis of diabetic nephropathy. Based on the results of this study, a higher CMI reflects a greater degree of visceral obesity and more severe insulin resistance, both of which contribute to endothelial dysfunction, promote the development of microangiopathy, and negatively impact patient prognosis[15]. Furthermore, elevated CMI is associated with increased release of free fatty acids from visceral adipose tissue, indirectly indicating heightened secretion of pro-inflammatory factors and abnormal glomerular endothelial function[16]. These mechanisms may help explain the association between CMI and the development and prognosis of diabetic nephropathy. Therefore, greater attention should be paid to screening for diabetic nephropathy in patients with type 2 diabetes who present with visceral obesity.
Univariate analysis revealed that levels of low-density lipoprotein, triglycerides, total cholesterol, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and serum creatinine were significantly higher in the poor prognosis group than in the good prognosis group, while high-density lipoprotein levels were lower (P < 0.05). Further analysis confirmed that alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine, miR-495-3p, ADPN, and CMI are risk factors affecting the prognosis of diabetic nephropathy. These findings indicate that prognosis is closely related to glucose and lipid metabolism, as well as hepatic and renal function. In cases of hyperlipidemia, lipids gradually deposit on arterial walls, leading to thickening, hardening, and atherosclerosis. Renal artery atherosclerosis reduces renal perfusion, aggravates kidney injury, and ultimately impairs prognosis[17,18]. Elevated aspartate aminotransferase and alanine aminotransferase suggest impaired glycogen synthesis in the liver, which can worsen diabetes, induce microvascular renal abnormalities, and contribute to poorer outcomes[19]. Additionally, patients with high levels of blood urea nitrogen and serum creatinine present with marked renal dysfunction, which is typically difficult to reverse with medical therapy and is associated with a worse prognosis[20]. This study has several limitations. First, it was a single-center study with a relatively small sample size, which may limit the generalizability of the findings. Second, the follow-up period was only six months, so long-term prognostic value of the biomarkers could not be assessed. Third, although several clinical variables were analyzed, other potential confounding factors such as medication use and lifestyle were not fully controlled. Finally, the study was observational and did not explore the underlying molecular mechanisms. Future multi-center studies with larger cohorts, longer follow-up, and mechanistic investigations are needed to validate and extend these findings.
In summary, this study demonstrates that decreased serum miR-495-3p levels and increased ADPN and CMI levels are significantly associated with T2DN and poor prognosis. Multivariate analysis confirmed that miR-495-3p, ADPN, and CMI, along with key liver and kidney function markers, serve as independent predictors of adverse outcomes in T2DN patients. These findings suggest that the combined assessment of miR-495-3p, ADPN, and CMI may provide valuable clinical utility for early risk stratification and personalized management of T2DN. Further large-scale, multi-center studies with longer follow-up are warranted to validate these biomarkers and to elucidate their underlying mechanisms in the progression of diabetic nephropathy.
| 1. | Obaid AA, Mujalli A, Farrash WF, Tayeb RH, Bougeis RJ, Aljehani AA, Alshehri BA, Sharaf SE, Alqurashi SF. Relationship of Vitamin-D Deficiency with Kidney Disease in Patients with Type-2 Diabetes Mellitus (T2DM) in the Makkah Region: A Cross-Sectional Study. Diabetes Metab Syndr Obes. 2024;17:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Yan J, Li B, Chen Y, Gu C, Dai G, Zhang Q, Zheng Z, Luo D, Zhao S, Zhou C. Prevalence and predictors of developing vision-threatening diabetic retinopathy within the first three years of type 2 diabetes. Front Endocrinol (Lausanne). 2023;14:1305378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Zhao P, Lv X, Zhou Z, Yang X, Huang Y, Liu J. Indexes of ferroptosis and iron metabolism were associated with the severity of diabetic nephropathy in patients with type 2 diabetes mellitus: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1297166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Lazzer S, D'Alleva M, Isola M, De Martino M, Caroli D, Bondesan A, Marra A, Sartorio A. Cardiometabolic Index (CMI) and Visceral Adiposity Index (VAI) Highlight a Higher Risk of Metabolic Syndrome in Women with Severe Obesity. J Clin Med. 2023;12:3055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 5. | Fernández-Veledo S, Marsal-Beltran A, Vendrell J. Type 2 diabetes and succinate: unmasking an age-old molecule. Diabetologia. 2024;67:430-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Wang X, Liu M, Li X, Zhang M, Xu F, Liu H, Wu H. Utilizing molecular docking and cell validation to explore the potential mechanisms of lupenone attenuating the inflammatory response via NF-κB pathway. Sci Rep. 2024;14:625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Piran N, Farhadian M, Soltanian AR, Borzouei S. Diabetic foot ulcers risk prediction in patients with type 2 diabetes using classifier based on associations rule mining. Sci Rep. 2024;14:635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Karakasis P, Popovic DS, Patoulias D, Koufakis T, Papanas N, Fragakis N, Rizzo M. The Effect of Sodium-Glucose Cotransporter Inhibitors on Renal Function as Adjunctive to Insulin in Adults with Type 1 Diabetes: An Updated Multilevel Meta-analysis of Randomized Controlled Trials. Diabetes Ther. 2024;15:521-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Guo X, Huang M, Yang D, Luo Z. Expression and Clinical Significance of Plasma miR-223 in Patients with Diabetic Nephropathy. Int J Endocrinol. 2023;2023:9663320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Cheng Y, Liu Y, Lin L, Li D, Peng L, Zheng K, Tao J, Li M. The effects of Tripterygium wilfordii Hook F on renal outcomes in type 2 diabetic kidney disease patients with severe proteinuria: a single-center cohort study. Ren Fail. 2024;46:2295425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Chen J, Li W, Cao J, Lu Y, Wang C, Lu J. Risk factors for carotid plaque formation in type 2 diabetes mellitus. J Transl Med. 2024;22:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 12. | Fu Z, Geng X, Liu C, Shen W, Dong Z, Sun G, Cai G, Chen X, Hong Q. Identification of common and specific fibrosis-related genes in three common chronic kidney diseases. Ren Fail. 2024;46:2295431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Speedtsberg ES, Tepel M. Narrative review investigating the nephroprotective mechanisms of sodium glucose cotransporter type 2 inhibitors in diabetic and nondiabetic patients with chronic kidney disease. Front Endocrinol (Lausanne). 2023;14:1281107. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Chen W, Zheng L, Wang J, Lin Y, Zhou T. Overview of the safety, efficiency, and potential mechanisms of finerenone for diabetic kidney diseases. Front Endocrinol (Lausanne). 2023;14:1320603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhang YY, Chen BX, Chen Z, Wan Q. Correlation study of renal function indices with diabetic peripheral neuropathy and diabetic retinopathy in T2DM patients with normal renal function. Front Public Health. 2023;11:1302615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Karimi MA, Vaezi A, Ansari A, Archin I, Dadgar K, Rasouli A, Ghannadikhosh P, Alishiri G, Tizro N, Gharei F, Imanparvar S, Salehi S, Mazhari SA, Etemadi MH, Alipour M, Deravi N, Naziri M. Lipid variability and risk of microvascular complications in patients with diabetes: a systematic review and meta-analysis. BMC Endocr Disord. 2024;24:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Zhao JM, Su ZH, Han QY, Wang M, Liu X, Li J, Huang SY, Chen J, Li XW, Chen XY, Guo ZL, Jiang S, Pan J, Li T, Xue W, Zhou T. Deficiency of Trex1 leads to spontaneous development of type 1 diabetes. Nutr Metab (Lond). 2024;21:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | He H, Wang H, Chen X, Zhong Y, Huang XR, Ma RC, Wang C, Lan HY. Treatment for type 2 diabetes and diabetic nephropathy by targeting Smad3 signaling. Int J Biol Sci. 2024;20:200-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Jairoun AA, Ping CC, Ibrahim B. Pharmacotherapeutic considerations and treatment patterns of antihyperglycemic agents for diabetic nephropathy: a review of the literature. Eur Rev Med Pharmacol Sci. 2023;27:12058-12069. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Guo J, Tian X, Liu H, Liu WJ. Editorial: Autophagy and hypoxia-inducible factor in diabetes. Front Endocrinol (Lausanne). 2023;14:1349432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/