Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.104024
Revised: February 25, 2025
Accepted: April 22, 2025
Published online: June 15, 2025
Processing time: 181 Days and 6.5 Hours
Glycated hemoglobin (HbA1c), the gold standard for assessing glycemic control, has limited ability to reflect the risks of hypoglycemia and glycemic variability, raising great concerns, especially in patients with type 1 diabetes (T1D). The glycemia risk index (GRI), a composite metric derived from continuous glucose monitoring (CGM), has emerged as a potential solution by systematically in
To evaluate whether the GRI addresses HbA1c limitations.
We analyzed 328 patients with T1D using 681 CGM and clinical data points. Linear mixed-effects models were used to address the relationship between the GRI and HbA1c within repeated-measures data. Correlation and cluster analyses were used to assess the comprehensive GRI reflection of seven key ambulatory glucose profile parameters.
The GRI exhibited linear correlations with HbA1c (r = 0.53), time in range (r =
The GRI offers a comprehensive view of glycemic control in T1D. Combining HbA1c with the GRI enables accurate assessment for managing glycemic control in patients with T1D.
Core Tip: This study highlights the glycemia risk index (GRI) as a comprehensive metric that captures the multidimensional aspects of glycemic control, effectively addressing the limitations of glycated hemoglobin (HbA1c) in type 1 diabetes (T1D). Analyzing 328 patients with T1D, GRI showed stronger correlations with time below range and coefficient of variation than HbA1c, providing a more nuanced reflection of glycemic risks. Clustering of continuous glucose monitoring data identified distinct glycemic control subgroups, demonstrating GRI’s ability to differentiate varying risks of hypoglycemia, hyperglycemia, and glycemic variability. Integrating HbA1c with the GRI enables a more accurate and holistic assessment, optimizing glycemic management in patients with T1D.
- Citation: He BB, Liu ZZ, Xu RY, Fan L, Guo R, Deng C, Xie YT, Zhou ZG, Li X. Glycated hemoglobin is not enough: The role of glycemia risk index for glycemic control assessment in type 1 diabetes. World J Diabetes 2025; 16(6): 104024
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/104024.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.104024
Assessing glycemic control is crucial for optimizing patient outcomes and reducing the risk of complications during diabetes management. Type 1 diabetes (T1D) presents unique challenges because of absolute insulin deficiency, resulting in pronounced glucose fluctuations and susceptibility to hypoglycemia and hyperglycemia. Despite treatment ad
Glycated hemoglobin (HbA1c) is internationally recognized as the gold standard for assessing diabetes prognosis and predicting the risk of diabetes-related complications[4-7]. Its standardized testing methods, ease of measurement, and cost-effectiveness make it indispensable for glucose management in most healthcare systems[8]. However, HbA1c does not provide information on hypoglycemic events or glycemic variability (GV)[9,10], and its results can be influenced by various factors[11,12]. Therefore, additional glycemic control metrics are needed to complement HbA1c levels for more effective glucose management.
The development of continuous glucose monitoring (CGM) technology has provided more detailed and comprehensive glucose data[13]. The American Diabetes Association (ADA) recommends using the ambulatory glucose profile (AGP) to summarize CGM data, which includes standard metrics such as time in range (TIR), percentage times in very-low-glucose hypoglycemia (VLow), low-glucose hypoglycemia (Low), high-glucose hyperglycemia (High), and very-high-glucose hyperglycemia (VHigh), mean glucose (MG) level or glucose management indicator (GMI), and coefficient of variation (CV)[14]. However, these seven metrics are highly interdependent, making treatment optimization challenging and unpredictable[15]. Improving one metric, such as TIR, a widely recognized CGM metric recommended in recent guidelines for assessing glycemic control in patients with diabetes, may improve or worsen others. Novel metrics such as the glycemia risk index (GRI), which provides a more holistic evaluation of glycemic control in individuals with diabetes, are desirable[16].
The GRI is a composite score developed to assess the quality of glycemic control by weighing the risks of long-term complications from hypoglycemia and hyperglycemia[16]. It was established by 330 international diabetes experts based on clinical risk from the CGM data of 225 patients with insulin-treated diabetes. The GRI was developed to address the need for comprehensive blood glucose management and combines multiple metrics derived from past CGM data to make optimal treatment decisions. As a single index, it reflects the risks of hypoglycemia and hyperglycemia, emphasizing clinically high-risk hypoglycemia[17,18] and showing a strong correlation with TIR. This index is expected to effectively reflect the status of multiple metrics and guide glucose adjustment.
Research on the GRI in patients with T1D is limited, primarily focusing on its correlation with other CGM parameters[19,20] or its role as an assessment tool for evaluating diabetes treatment strategies[21-23]. The specific application value of the GRI in assessing glycemic control quality in patients with T1D remains insufficiently understood. This study aimed to investigate the specific role of the GRI in discerning multidimensional glycemic control states and determine whether it can address the limitations of HbA1c levels, providing a more precise assessment tool for glycemic control in patients with T1D.
This cross-sectional study aimed to investigate the clinical value of the GRI in patients with T1D using follow-up data. Participants were recruited from a follow-up cohort at the Second Xiangya Hospital, Central South University, Changsha, Hunan Province, China. Information on this cohort can be found in previously published data[24-26]. The enrollment period spanned from December 2018 to March 2023. All participants wore a flash glucose monitoring (FGM) system (Freestyle Libre, Abbott Diabetes Care, Witney, United Kingdom) for 14 days before their visit, and CGM data were obtained from their daily lives.
A total of 328 patients with T1D using the FGM system were included. The inclusion criteria were as follows: (1) Meeting the 1999 World Health Organization diagnostic criteria for diabetes; (2) Insulin-dependent treatment since diagnosis; (3) Age over 4 years (as the FGM system is specifically indicated for this age group and above); and (4) Duration of T1D lasting over 3 months and follow-up intervals of at least 3 months to ensure accurate HbA1c reflection of glycemic control. The exclusion criteria were as follows: (1) Use of other CGM equipment; (2) Presence of anemia and other blood disorders; (3) Recent complications, including ketoacidosis, acute and chronic infection, surgery, trauma, and other stress states; (4) Long-term use of glucocorticoids or immunomodulators; (5) Unwillingness to wear FGMs equipment or allergies to the equipment; (6) Acute and chronic hepatic and renal insufficiency; and (7) Pregnancy or preparing for pregnancy or breastfeeding. Before commencing the study, the source cohort was registered at ClinicalTrials.gov (No. 146 NCT03610984).
At each visit, we collected demographic data, including age, sex, diabetes duration, height, weight, body mass index, blood pressure, and insulin use. Venous blood samples were obtained to measure HbA1c, fasting C-peptide (FCP), and 2-hour C-peptide (2hCP) levels after a mixed-meal tolerance test (MMTT). The standard MMTT (57% carbohydrates, 24% fat, and 19% protein) was performed after fasting for 8-10 hours. Patients maintained their nighttime long-acting insulin regimen before the test and omitted their normal morning insulin levels. Insulin pump users maintained a background basal rate but refrained from taking the morning bolus. HbA1c levels were measured using automated high-performance liquid chromatography (VARIANT II Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA, United States). Serum C-peptide levels were detected using chemiluminescence with an Adivia Centaur XP immunoassay system (Siemens, Munich, Germany).
CGM data were collected using a Freestyle Libre sensor applied to the patient’s upper arm, recording glucose levels at 15-minute intervals for 14 days. Patients received adequate training and were informed of necessary precautions before wearing the FGM. TIR, time above range (TAR), and time below range (TBR) were calculated based on the target glycemic range defined as 3.9 mmol/L and 10.0 mmol/L. TBR and TAR were subclassified into two levels based on blood glucose values. Level 1 TBR (Low) ranges from 3.0 mmol/L to < 3.9 mmol/L, and level 2 TBR (VLow) represents values < 3.0 mmol/L. Level 1 TAR (High) ranges from > 10.0 mmol/L to 13.9 mmol/L, and level 2 TAR (VHigh) represents values > 13.9 mmol/L. Other CGM-derived metrics include MG, GMI, and CV, standard deviation of glucose. CV and standard deviation are both metrics of GV.
The GRI comprises two key components: The hypoglycemic component (GRI-lo) and the hyperglycemic component (GRI-hi). GRI-lo and GRI-hi were calculated using the formulas “VLow + (0.8 × Low)” and “VHigh + (0.5 × High),” respectively. The GRI is computed as the weighted sum of these two indices, GRI = 3.0 × GRI-lo + 1.6 × GRI-hi, with a maximum permissible value of 100. The GRI ranges from 0 to 100, where 0 indicates minimal risk and 100 represents maximum risk. For enhanced risk classification, the GRI was divided into five zones (labeled A to E), each covering a range of 20. Zone A (GRI ≤ 20) signifies the lowest risk, while zone E (GRI: 81-100) represents the highest risk[16]. A two-dimensional GRI grid graph was plotted to reflect the composition of hypoglycemia and hyperglycemia, with GRI-lo on the horizontal axis and GRI-hi on the vertical axis.
All participants used the same CGM device, with data obtained from their daily lives. The patients were recruited from a single study cohort and received intensive insulin therapy while continuously monitored by a team of investigators and specialists. Data from all participants were collected and analyzed using identical methods and tools. To minimize the risk of selection bias, we employed a consecutive recruitment strategy to include eligible patients with T1D.
The primary analysis focused on the relationship between the GRI and key AGP parameters. We first established the correlation between the GRI and each AGP key parameter through correlation analysis and then clarified how the GRI reflects these parameters using cluster analysis. Secondary analysis was used to examine the relationship between the GRI and HbA1c levels and their influencing factors. We compared the correlation coefficients of GRI with TBR and CV with those of HbA1c with TBR and CV. Using a linear mixed-effects model, we determined the impact of CGM-related metrics, particularly TBR and CV, on the relationship between GRI and HbA1c to clarify how GRI complements HbA1c in reflecting glycemic control aspects, such as hypoglycemia and GV.
All data were analyzed using the SPSS or R software. Normally distributed variables (assessed by the Kolmogorov-Smirnov test) are presented as mean ± SD, skewed variables as median interquartile range 25-75 percentile P25-75, and categorical data as numbers and percentages. The two groups were compared using the unpaired student’s t-test and Mann-Whitney U test. One-way analysis of variance or Kruskal-Wallis rank sum tests were performed to determine the differences between groups. Categorical variables were assessed using the χ2 or Fisher’s exact test, as appropriate. Pearson and Spearman correlations were calculated between the GRI, HbA1c, and CGM metrics. A linear mixed-effects model was used to characterize the relationship between the GRI and TIR or HbA1c, accounting for multiple measurements from the same patient. Unsupervised hierarchical clustering was performed on standardized CGM data using the R stats package to group participants based on their CGM metrics, with k-means as the similarity measure. Linear discriminant analysis (LDA) was used for group discrimination analysis between the GRI-lo and GRI-hi derived from the CGM. P < 0.05 was considered statistically significant.
The analysis encompassed a dataset of 681 instances from 328 cases of T1D, with 171 patients undergoing one to seven follow-up visits (Supplementary Figure 1). Of the 328 patients, 194 (59.1%) were female, and 236 (72.0%) experienced onset during childhood or adolescence, with a median age of onset of 10 (6-18) years (Supplementary Table 1). Analysis of these 681 medical records revealed a median duration of 28 (13-49) months. The patients’ ages ranged from 4 to 71 years, comprising 210 (30.5%) adults and 471 (69.5%) children or adolescents. Most participants (67.0%) received multiple daily injections as the treatment method. The levels of HbA1c, TIR, and CV were 7.20% (6.60%-8.00%), 65% (52%-77%), and 39% (34%-44%), respectively. Less than half of patients with T1D achieved the blood glucose target: 273 (40.1%) had HbA1c < 7%, 257 (37.7%) had TIR > 70%, and 241 (35.4%) had CV < 36% (Table 1).
| Characteristic | Total (n = 6811) | GRI zone | P value | ||||
| Zone A (0-20), (n = 731) | Zone B (21-40), (n = 1851) | Zone C (41-60), (n = 1881) | Zone D (61-80), (n = 1331) | Zone E (81-100), (n = 1021) | |||
| Age, years (IQR) | 13 (9-21) | 15 (11-23) | 13 (8-21) | 12 (8-25) | 13 (9-17) | 12 (9-16) | 0.150 |
| Age subgroup, children and adolescents | 471 (69.5) | 42 (57.5) | 126 (68.5) | 125 (66.8) | 100 (75.8) | 78 (76.5) | 0.034 |
| Sex, female | 378 (55.5) | 35 (47.9) | 111 (60.0) | 104 (55.3) | 73 (54.9) | 55 (53.9) | 0.504 |
| Duration, months (IQR) | 28 (13-49) | 13 (8-34) | 23 (11-42) | 29 (14-47) | 35 (18-54) | 35 (20-59) | < 0.001 |
| SBP, mmHg (IQR) | 106 (99-117) | 107 (101-118) | 105 (98-113) | 106 (97-118) | 107 (100-115) | 109 (100-120) | 0.087 |
| DBP, mmHg (IQR) | 64 (58-71) | 67 (60-73) | 64 (57-70) | 63 (58-71) | 65 (57-70) | 66 (60-71) | 0.136 |
| BMI for children and adolescents, kg/m2 (IQR) | 0.14 (-0.56 to 0.84) | 0.03 (-0.55 to 0.77) | 0.06 (-0.63 to 0.60) | 0.15 (-0.56 to 0.87) | 0.39 (-0.52 to 1.03) | 0.27 (-0.38 to 0.85) | 0.2942 |
| BMI for adult, kg/m2 (IQR) | 21.33 (19.55-23.19) | 21.48 (19.26-22.15) | 21.63 (19.97-22.75) | 20.43 (19.49-23.52) | 21.60 (20.17-23.45) | 21.03 (19.77-23.45) | 0.489 |
| Treatment method | 0.366 | ||||||
| CSII | 225 (33.0) | 19 (26.0) | 67 (36.2) | 66 (35.1) | 45 (33.8) | 28 (27.5) | |
| MDI | 456 (67.0) | 54 (74.0) | 118 (63.8) | 122 (64.9) | 88 (66.2) | 74 (72.5) | |
| FCP, pmol/L (IQR) | 33 (17-121) | 153 (56-209) | 58 (17-137) | 24 (17-76) | 20 (17-77) | 17 (17-77) | < 0.001 |
| 2hCP, pmol/L (IQR) | 53 (17-203) | 340 (178-582) | 109 (17-266) | 35 (17-162) | 22 (17-134) | 19 (17-115) | < 0.001 |
| HbA1c, % (IQR) | 7.20 (6.60-8.00) | 6.45 (6.05-6.70) | 6.90 (6.56-7.40) | 7.30 (6.81-7.90) | 7.80 (7.00-8.29) | 8.36 (7.23-9.00) | < 0.001 |
| HbA1c < 7% | 273 (40.1) | 65 (89.0) | 98 (53.0) | 61 (32.4) | 31 (23.3) | 18 (17.6) | < 0.001 |
| MG, mmol/L (IQR) | 8.10 (7.01-9.41) | 7.01 (6.47-7.30) | 7.65 (6.84-8.31) | 8.39 (7.35-9.37) | 9.18 (7.36-10.37) | 10.98 (7.95-12.54) | < 0.001 |
| GMI (%) | 6.93 ± 0.87 | 6.33 (6.10-6.45) | 6.61 (6.26-6.89) | 6.92 (6.48-7.35) | 7.26 (6.48-7.77) | 8.04 (6.73-8.71) | < 0.001 |
| GMI < 7% | 417 (61.2) | 73 (100) | 158 (85.4) | 105 (55.9) | 51 (38.3) | 30 (29.4) | < 0.001 |
| CV, % (IQR) | 39 (34-44) | 29 (27-31) | 35 (33-38) | 40 (38-42) | 44 (40-48) | 47 (40-52) | < 0.001 |
| CV < 36% | 241 (35.4) | 73 (100.0) | 105 (56.8) | 33 (17.6) | 13 (9.8) | 17 (16.7) | < 0.001 |
| VLow, % (IQR) | 1.3 (0.2-3.6) | 0.1 (0.0-0.3) | 0.8 (0.3-1.7) | 1.9 (0.5-3.6) | 3.5 (1.1-7.2) | 4.2 (0.8-11.5) | < 0.001 |
| Low, % (IQR) | 4.5 (2.3-7.1) | 2.4 (1.2-3.3) | 4.3 (2.3-6.3) | 5.3 (2.8-7.5) | 5.5 (3.1-9.2) | 4.7 (2.3-9.8) | < 0.001 |
| TBR, % (IQR) | 6 (3-11) | 2 (1-4) | 5 (3-8) | 7 (4-12) | 9 (5-16) | 9 (4-22) | < 0.001 |
| TBR < 4% | 238 (34.9) | 58 (79.5) | 71 (38.4) | 54 (28.7) | 29 (21.8) | 26 (25.5) | < 0.001 |
| TAR, % (IQR) | 26 (13-41) | 9 (5-12) | 19 (11-26) | 30 (19-39) | 40 (25-51) | 54 (31-67) | < 0.001 |
| TAR < 25% | 329 (48.3) | 73 (100.0) | 132 (71.4) | 67 (35.6) | 34 (25.6) | 23 (22.5) | < 0.001 |
| High, % (IQR) | 19 (11-25) | 8 (5-11) | 16 (10-22) | 22 (16-27) | 24 (18-31) | 23 (15-27) | < 0.001 |
| VHigh, % (IQR) | 5 (1-13) | 0 (0-1) | 2 (1-5) | 8 (3-11) | 13 (4-21) | 30 (12-37) | < 0.001 |
| TIR, % (IQR) | 65 (52-77) | 89 (86-91) | 76 (70-80) | 63 (57-69) | 50 (45-60) | 37 (29-47) | < 0.001 |
| TIR > 70% | 257 (37.7) | 73 (100.0) | 140 (75.7) | 42 (22.3) | 2 (1.5) | 0 (0.0) | < 0.001 |
| GRI (IQR) | 47 (33-69) | 15 (11-18) | 32 (27-36) | 49 (45-54) | 69 (64-74) | 92 (85-100) | < 0.001 |
| GRI-lo (IQR) | 5 (2-10) | 2 (1-3) | 4 (2-6) | 6 (3-10) | 8 (4-14) | 8 (3-20) | < 0.001 |
| GRI-hi (IQR) | 15 (7-27) | 5 (2-6) | 11 (6-15) | 19 (11-25) | 27 (14-36) | 42 (22-51) | < 0.001 |
| Number of hypoglycemia at night (IQR) | 3.0 (1.0-5.0) | 1.0 (0.0-3.0) | 3.0 (1.0, 5.0) | 4.0 (2.0-6.0) | 4.0 (2.0-6.0) | 2.5 (1.0-6.0) | < 0.001 |
| Number of severe hypoglycemia at night (IQR) | 1.00 (0.00-2.00) | 0.00 (0.00-0.00) | 1.00 (0.00-1.00) | 1.00 (0.00-2.00) | 2.00 (1.00-4.00) | 2.00 (0.00-5.00) | < 0.001 |
The overall GRI level was 47 (33-69), with a higher proportion of patients observed in zones B (27.2%) and C (27.6%). Significant differences in glycemic controls and clinical indicators were found across the different GRI zones. With increasing GRI zones, the duration of diabetes, HbA1c, MG, GMI, CV, TBR, TAR, GRI-lo, and GRI-hi, and the frequency of nocturnal hypoglycemia gradually increased; however, TIR, FCP, and 2hCP experienced a corresponding gradual decrease. The proportion of patients with HbA1c < 7% and TIR > 70% decreased as GRI zone levels increased. The proportion of patients with HbA1c < 7% decreased from 89.0% in GRI zone A to 17.6% in GRI zone E, and the proportion of patients with TIR > 70% decreased from 100.0% in GRI zone A to 0 in GRI zone E. Variations were observed in the compliance rates of different metrics in each GRI zones. For instance, in GRI zone E, characterized by notably high GRI levels, none of the patients achieved a TIR > 70%, whereas the proportions of patients achieving HbA1c < 7%, CV < 36%, TBR < 4%, and TAR < 25% remained 17.6%, 16.7%, 15.5%, and 22.5%, respectively (Table 1).
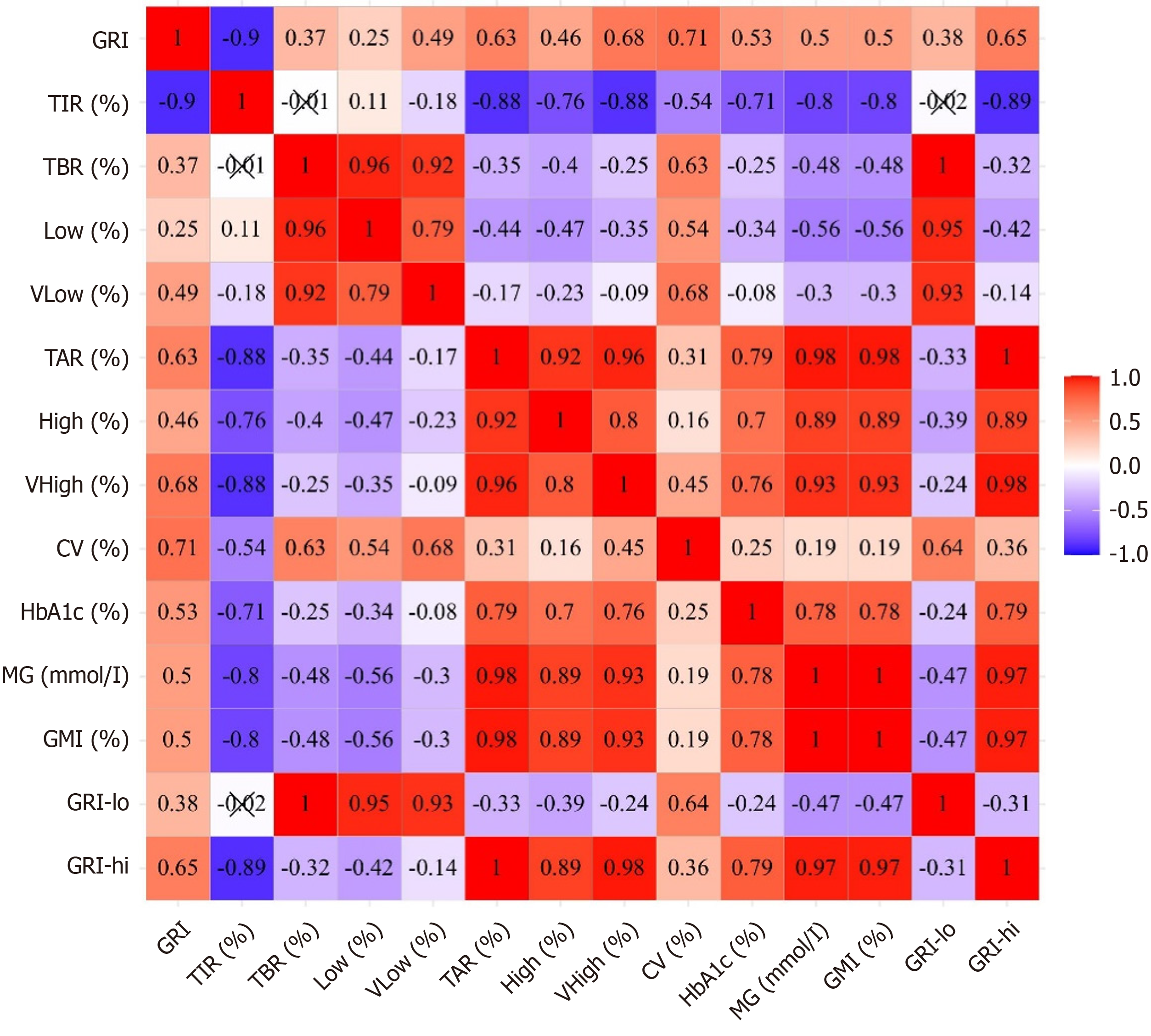
HbA1c negatively correlated with TIR, TBR, Low, and VLow, and positively correlated with TAR, High, VHigh, MG, and CV. The correlations between HbA1c and TAR, MG, TIR, TBR, and CV showed a gradual decrease, with weaker correlations observed for TBR and CV. GRI was negatively correlated with TIR and positively correlated with TAR, High, VHigh, TBR, Low, VLow, MG, and CV (Figure 1). The correlation between GRI and TIR (r = -0.90) was the strongest. However, TBR, TAR, CV, and MG significantly contributed to deviations in GRI from the estimated GRI-TIR mean trend (Supplementary Table 2).
To clarify whether the GRI has an advantage over HbA1c and TIR in reflecting the risk of hypoglycemia and GV, we compared their respective correlation coefficients with TBR and CV. GRI exhibited a moderate positive correlation with TBR (r = 0.37), whereas the correlation between HbA1c and TBR was weak (r = -0.25), and no correlation was observed between TIR and TBR. The correlation coefficients of the GRI, TIR, and HbA1c with CV showed a gradually decreasing trend (Figure 1). To confirm the stability of the findings, we performed a subgroup analysis in patients with at least two clinical visits. The results were consistent with the overall cohort, supporting the robustness of the observed associations between GRI, HbA1c, and TBR, CV (Supplementary Figure 2).
HbA1c was positively correlated with the GRI (r = 0.53). TBR, CV, TIR, and MG significantly contributed to deviations in GRI from the estimated GRI-HbA1c mean trend. Specifically, for a given HbA1c level, each 1% increase in TBR was associated with a 1.87% increase in GRI [95% confidence interval (CI): 1.72-2.01; P < 0.001], each 1% increase in CV was associated with a 1.94% increase in GRI (95%CI: 1.80-2.10; P < 0.001), and each 1% increase in TIR was associated with a 1.44 decrease in GRI (95%CI: 1.38-1.50; P < 0.001) (Supplementary Table 3). Conversely, for a given GRI level, each 1% increase in TBR was associated with a 0.079 decrease in HbA1c level (95%CI: 0.072-0.086; P < 0.001), each 1% increase in CV was associated with a 0.035 reduction in HbA1c level (95%CI: 0.023-0.047; P < 0.001), and each 1% increase in TIR was associated with a 0.071 decrease in HbA1c level (95%CI: 0.064-0.077; P < 0.001) (Supplementary Table 4).
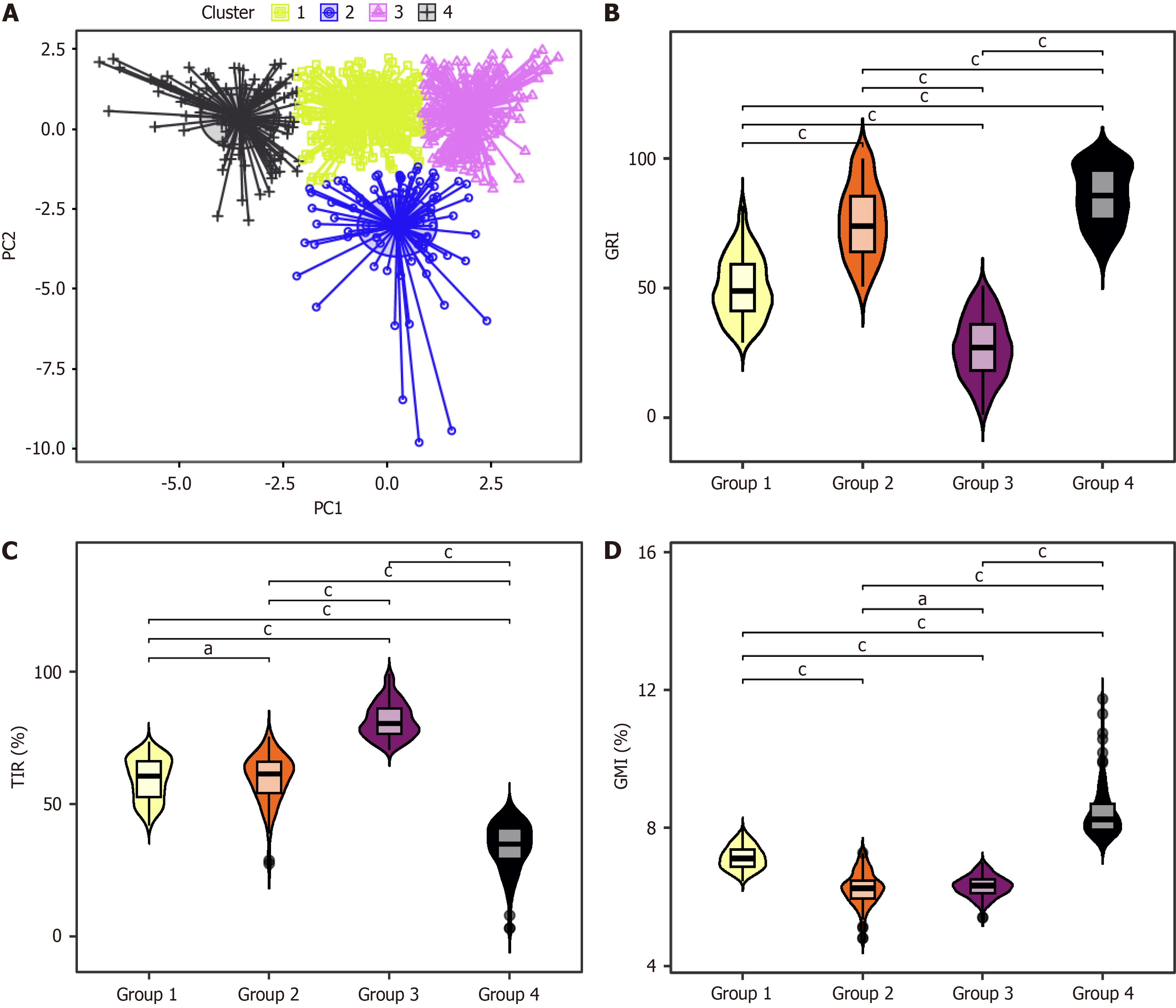
We conducted a clustering analysis to elucidate how the single-parameter GRI reflects the specific situation of the multiple parameters in the AGP. Through integrating GRI and seven parameters from AGP, including TIR, VLow, Low, High, VHigh, GMI, and CV, our study revealed distinct glycemic profiles among patients, classifying them into four groups (Figure 2A). Group 1 was the moderate-risk group, characterized by moderate TIR levels, with 77% (197/256) of patients having a TIR between 50% and 70%. The GMI of all patients falls between 6.5% and 8%, with moderate risks of CV, hypoglycemia, and hyperglycemia, primarily dominated by mild hyperglycemia. Group 2 was the high-risk group for hypoglycemia, with 76.9% (70/91) of patients having TIR between 50% and 70% and 94.5% having GMI < 7%. This group faced a heightened risk of hypoglycemia, a comparatively lower risk of hyperglycemia (mainly mild), and the greatest GV. Group 3 was the optimal glycemic control group, with all patients achieving TIR levels exceeding 70% and 99.6% of patients with a GMI < 7%. The risks of hyperglycemia, hypoglycemia, and GV were relatively low in this group, and C-peptide levels were the highest. Group 4 was the high-risk group for hyperglycemia and was characterized by the poorest TIR control, as all patients had TIR levels below 50%. While the risk of hypoglycemia is minimal, the primary concern is hyperglycemia, particularly VHigh, accompanied by moderate GV (Table 2). Notably, the GRI levels across these groups exhibited statistically significant differences; however, no differences were found in TIR and GMI. TIR was similar between groups 1 and 2, and GMI was comparable between groups 2 and 3 (Figure 2B-D). These findings underscore the distinctiveness of the GRI in discerning multidimensional glycemic control states.
| Characteristic | Cluster | P value | |||
| Group 1 (n = 256) | Group 2 (n = 91) | Group 3 (n = 232) | Group 4 (n = 102) | ||
| TIR (IQR) | 61 (53-66) | 61 (54-66) | 80 (76-86) | 35 (29-41) | < 0.001 |
| TIR subgroup | < 0.001 | ||||
| < 50% | 42 (16.4) | 13 (14.3) | 0 (0.0) | 102 (100.0) | |
| 50%-70% | 197 (77.0) | 70 (76.9) | 0 (0.0) | 0 (0.0) | |
| > 70% | 17 (6.6) | 8 (8.8) | 232 (100.0) | 0 (0.0) | |
| TAR (IQR) | 34 (28-41) | 17 (11-24) | 11 (7-16) | 60 (53-68) | < 0.001 |
| TAR < 25% | 29 (11.3) | 70 (76.9) | 230 (99.1) | 0 (0) | < 0.001 |
| TBR (IQR) | 5 (3-8) | 20 (17-26) | 6 (3-10) | 4 (1-7) | < 0.001 |
| TBR < 4% | 103 (40.2) | 0 (0) | 85 (36.6) | 50 (49.0) | < 0.001 |
| CV (IQR) | 40 (37-43) | 48 (44-52) | 34 (30-37) | 42 (36-49) | < 0.001 |
| CV < 36% | 53 (20.7) | 3 (3.3) | 158 (68.1) | 27 (26.5) | < 0.001 |
| GRI (IQR) | 49 (41-59) | 74 (64-85) | 27 (18-36) | 86 (77-95) | < 0.001 |
| MG, mmol/L (IQR) | 8.84 (8.27-9.45) | 6.81 (6.13-7.36) | 7.01 (6.51-7.43) | 11.48 (10.80-12.54) | < 0.001 |
| HbA1c, % (IQR) | 7.59 (7.10-8.09) | 6.80 (6.40-7.25) | 6.60 (6.30-6.94) | 8.66 (8.10-9.19) | < 0.001 |
| HbA1c < 7% | 45 (17.6) | 52 (57.1) | 175 (75.4) | 1 (1) | < 0.001 |
| GMI, % (IQR) | 7.12 (6.87-7.38) | 6.24 (5.95-6.48) | 6.33 (6.12-6.51) | 8.25 (7.96-8.71) | < 0.001 |
| GMI < 7% | 100 (39.1) | 86 (94.5) | 231 (99.6) | 0 (0) | < 0.001 |
| High, % (IQR) | 24 (22-28) | 14 (9-17) | 10 (7-14) | 28 (23-33) | < 0.001 |
| VHigh, % (IQR) | 9 (6-13) | 3 (1-6) | 1 (0-2) | 31 (26-37) | < 0.001 |
| VLow, % (IQR) | 1.0 (0.3-2.6) | 9.6 (7.1-13.3) | 0.8 (0.1-2.1) | 0.9 (0.1-2.7) | < 0.001 |
| Low, % (IQR) | 3.9 (2.0-5.6) | 10.8 (9.2-12.5) | 4.9 (2.6-7.2) | 2.6 (0.8-4.3) | < 0.001 |
| FCP, pmol/L (IQR) | 27 (17-106) | 17 (17-51) | 76 (17-161) | 30 (17-97) | < 0.001 |
| 2hCP, pmol/L (IQR) | 43 (17-169) | 17 (17-125) | 159 (17-357) | 45 (17-132) | < 0.001 |
| GRI-lo (IQR) | 4 (2-7) | 18 (15-23) | 5 (2-8) | 4 (1-6) | < 0.001 |
| GRI-hi (IQR) | 21 (17-27) | 10 (6-14) | 6 (4-9) | 45 (39-51) | < 0.001 |
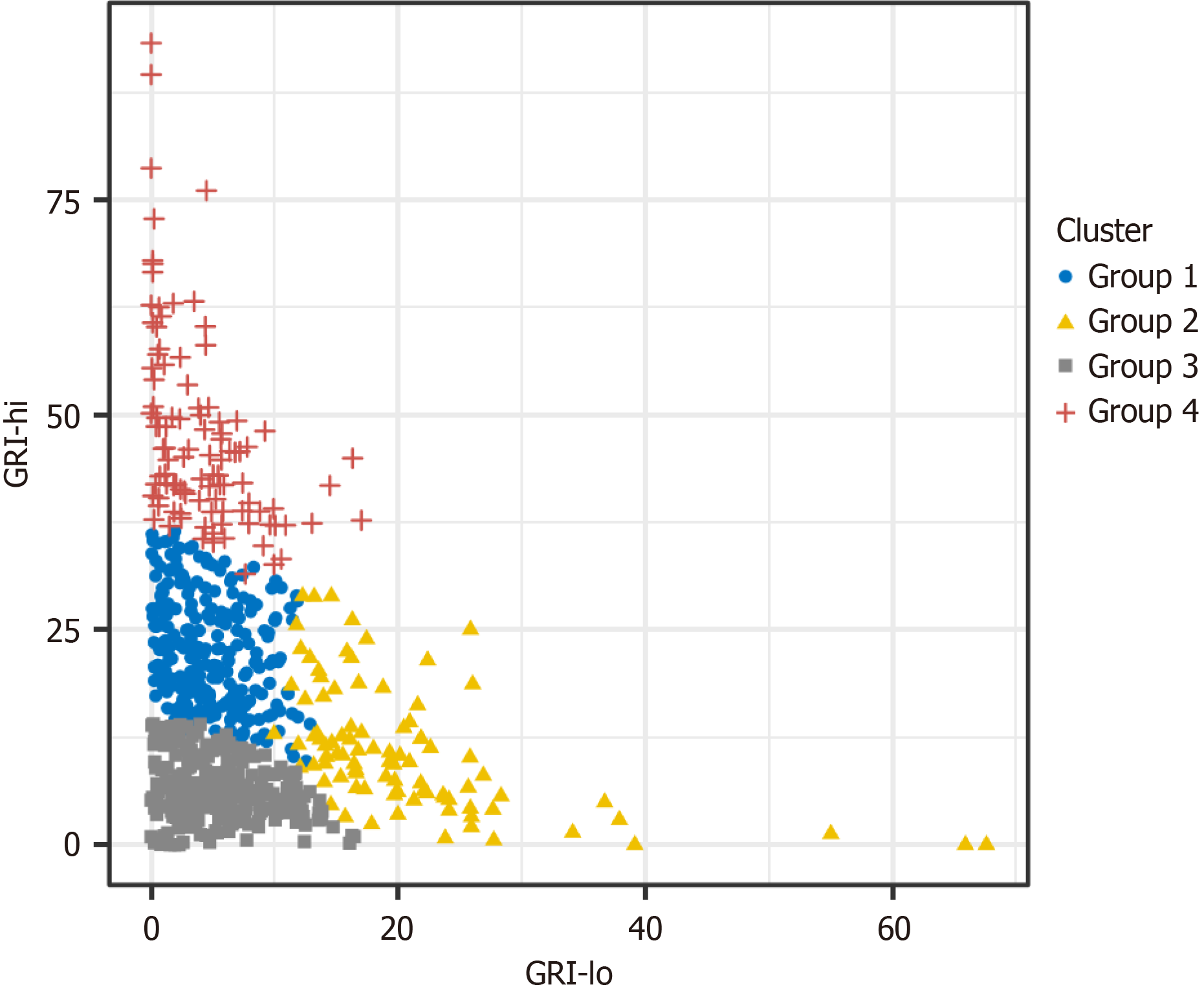
The GRI values of the four groups showed overlaps, making it challenging to infer the specific conditions of the seven AGP parameters solely based on the GRI values. Enhancing the GRI grid two-dimensional plot can facilitate a clear differentiation of these four patient groups (Figure 3). The LDA revealed that using GRI-lo and GRI-hi achieved a grouping discrimination accuracy of 94% in the current dataset, whereas using the eight parameters from the cluster analysis for grouping discrimination achieved 94.9% accuracy. The similarity between these results suggests that utilizing the GRI-lo and GRI-hi can distinguish the four groups.
The following formulas can be used to specify the exact grouping: Group 1 is g1 (X) = -9.115 + 0.479 × GRI-lo + 0.587 × GRI-hi; Group 2 is g2 (X) = -15.386 + 1.09 × GRI-lo + 0.498 × GRI-hi; Group 3 is g3 (X) = -3.014 + 0.332 × GRI-lo + 0.218 × GRI-hi; and Group 4 is g4 (X) = -30.873 + 0.73 × GRI-lo +1.177 × GRI-hi. The group to which a patient belongs can be determined by calculating the formula that yields the highest value.
When HbA1c was < 7%, a higher GRI was accompanied by increased TBR, GRI-lo, and CV, all of which decreased as GRI declined. When HbA1c was ≥ 7%, a higher GRI was accompanied by increased TAR, GRI-hi, and SD, all of which decreased as GRI declined, resulting in improved TIR (Table 3).
| Characteristic | HbA1c < 7% | P value | HbA1c ≥ 7% | P value | ||
| Baseline (n = 31) | First follow-up | Baseline (n = 47) | First follow-up | |||
| Age, years (IQR) | 11 (7-22) | 12 (8-22) | 0.612 | 11 (7-20) | 12 (8-21) | 0.490 |
| Duration, month (IQR) | 24 (8-43) | 31 (17-55) | 0.137 | 19 (11-34) | 28 (18-40) | 0.022 |
| Treatment method | 0.799 | 0.472 | ||||
| CSII | 15 (48.4) | 14 (45.2) | 34 (72.3) | 37 (78.7) | ||
| MDI | 16 (51.6) | 17 (54.8) | 13 (27.7) | 10 (21.3) | ||
| HbA1c, % (IQR) | 6.4 (6.1-6.8) | 6.6 (6.3-6.9) | 0.079 | 8.0 (7.5-8.4) | 7.5 (7.1-8.2) | 0.044 |
| FCP, pmol/L (IQR) | 27 (17-110) | 17 (17-101) | 0.404 | 26 (17-98) | 23 (17-105) | 0.724 |
| 2hCP, pmol/L (IQR) | 50 (17-271) | 17 (17-226) | 0.581 | 69 (17-157) | 35 (17-151) | 0.957 |
| CV, % (IQR) | 39 ± 8 | 34 ± 7 | 0.025 | 38 (33-43) | 38 (34-42) | 0.958 |
| SD | 2.7 ± 0.8 | 2.6 ± 0.7 | 0.492 | 3.6 (3.2-4.2) | 3.3 (2.7-3.9) | 0.022 |
| GMI, % (IQR) | 6.3 ± 0.5 | 6.5 ± 0.5 | 0.086 | 7.4 (7.0-8.1) | 6.9 (6.6-7.7) | 0.004 |
| VLow, % (IQR) | 3.9 (1.1-5.2) | 0.9 (0.1-2.3) | 0.002 | 0.5 (0.04-2.2) | 1.1 (0.3-2.5) | 0.228 |
| Low, % (IQR) | 7.9 (5.4-10.4) | 3.6 (2.2-7.3) | 0.003 | 3.0 (1.0-5.1) | 4.0 (2.1-6.0) | 0.137 |
| TBR, % (IQR) | 12 (6-17) | 5 (2-9) | < 0.001 | 3.3 (1.1-7.4) | 5.4 (2.4-8.5) | 0.100 |
| TIR, % (IQR) | 73 (64-80) | 78 (65-84) | 0.217 | 50 ± 16 | 62 ± 18 | 0.001 |
| TAR, % (IQR) | 13 (8-21) | 12 (8-28) | 0.502 | 45 ± 18 | 33 ± 18 | 0.002 |
| High, % (IQR) | 11 (7-17) | 11 (7-22) | 0.458 | 27 ± 7 | 22 ± 10 | 0.003 |
| VHigh, % (IQR) | 1.3 (0.2-2.9) | 1.9 (0.3-5.1) | 0.476 | 14 (6-27) | 7 (2-16) | 0.006 |
| GRI-lo (IQR) | 10 (5-14) | 4 (2-8) | 0.001 | 2.7 (0.9-6.6) | 4.4 (2.0-7.2) | 0.096 |
| GRI-hi (IQR) | 7 (4-12) | 7 (4-17) | 0.502 | 27 (20-42) | 16 (11-34) | 0.002 |
| GRI (IQR) | 48 ± 23 | 33 ± 18 | 0.007 | 56 (46-85) | 42 (34-66) | 0.004 |
Overall, a simultaneous decrease in HbA1c and GRI was associated with reduced TAR and SD, accompanied by an increased in TIR. If HbA1c remained stable or increased while GRI declined, TBR and CV decreased. Conversely, if HbA1c remained stable or decreased while GRI increased, TBR and CV increased. Concurrent increases in both HbA1c and GRI was associated with elevated TAR and CV, accompanied by a marked decline in TIR (Table 3 and Supplementary Table 5).
In this observational study, we found that the novel CGM-derived metric, GRI, exhibited significant linear correlations with the seven parameters of AGP. By considering the numerical values of the GRI and its hypoglycemia and hyperglycemia components, we could distinguish different glycemic control situations reflected by the seven key AGP indicators. The GRI showed stronger correlations with TBR and CV than the traditional glucose control metric, HbA1c, highlighting its potential to reflect the risks of hypoglycemia and GV. The above characteristics make GRI an ideal metric to supplement the limitations of HbA1c.
Managing blood glucose in T1D has long been a clinical challenge. Our study reaffirms the arduous nature of achieving glycemic control in T1D, as many patients did not meet the recommended HbA1c and TIR targets despite using CGM and continuous subcutaneous insulin infusion. This finding indicates that the current glycemic management approaches have shortcomings. Effective blood glucose monitoring is crucial for achieving satisfactory glycemic control. The in
The GRI is a novel composite metric derived from CGM data that considers hypoglycemia and hyperglycemia. Our analysis of patients with T1D across all age groups revealed a gradual increase in blood glucose levels, heightened risk of hyperglycemia and hypoglycemia, increased GV, and a gradual decrease in glycemic target attainment rates with increasing GRI zones. Additionally, the GRI was significantly correlated with TIR, TBR, TAR, GMI, and CV, aligning with findings of previous studies on adult patients with T1D[9,10]. Cluster analysis revealed that the GRI could distinguish four groups with different glycemic control profiles, each exhibiting unique glycemic patterns. Group 1 was characterized by moderate glycemic control. Group 2 had the highest risk of developing hypoglycemia. Group 3 showed the best glycemic control, with 100% of patients having a TIR of > 70%. Group 4 had the highest risk of hyperglycemia. Using the GRI-lo and GRI-hi parameters, we effectively identified the multidimensional glycemic control status of patients without relying on multiple AGP parameters. The above indicates that the GRI, as a singular metric, can provide a multidimensional representation of glycemic control, including target range achievement, hyperglycemia, hypoglycemia, and GV.
The four groups with different glycemic profiles require distinct management strategies, and their clinical outcomes warrant further investigation. Group 1 patients exhibited moderate GRI levels and corresponding risks of hyperglycemia, hypoglycemia, and GV, indicating a need for comprehensive glycemic management to meet treatment goals. The median HbA1c of these patients was 7.59%, close to the T1D guideline recommendation of maintaining HbA1c levels below 7.5% for children and adolescents at risk of hypoglycemia[27]. A cohort study of the Swedish population with T1D found that severe complications primarily occurred when HbA1c levels exceeded 8.6%, and mild complications were more common when HbA1c exceeded 7%[28]. Further investigation is warranted to determine whether mild complications are predominant in this group. Group 2 patients, with higher GRI scores, require particular attention because of their very high risk of hypoglycemia and GV. Frequent hypoglycemic events can lead to severe complications and mortality. Additionally, the risk of complications significantly rises with increased GV[29]. Efforts should focus on reducing these risks in this group. Group 3 had the lowest GRI score, indicating good glycemic control. Group 4 patients had the highest GRI levels and primarily needed to reduce the risk of hyperglycemia, particularly extreme hyperglycemia. This group warrants particular attention regarding the risk of complications. Although research on the association between GRI and diabetes complications remains limited, studies in patients with T2D have shown a correlation between elevated GRI and increased risks of diabetic retinopathy[30] and diabetic nephropathy[31]. Therefore, the GRI reflects multidimensional glycemic control and can be used clinically to assess and guide individual patient treatments. In the future, the GRI may assist in developing automated insulin delivery algorithms and predicting long-term diabetes complications.
As a cornerstone of diabetes management, HbA1c remains indispensable, yet its limitations warrant careful consideration[9-11,32,33]. Our study found that HbA1c alone failed to differentiate distinct glycemic profile groups, aligning with previous findings that individuals with the same HbA1c levels may exhibit vastly different glucose patterns[34]. Therefore, HbA1c should be interpreted in the context of individual patient characteristics and complemented by additional monitoring methods, particularly the multidimensional insights offered by CGM[35]. Our study found that, compared to the classic glycemic control metric HbA1c, GRI showed a stronger correlation with TBR and CV. Conversely, HbA1c showed weak or no correlation with these parameters, consistent with previous studies[9,10,36]. This finding suggests that the GRI better reflects the risk of hypoglycemia and GV than HbA1c. To further validate the effectiveness of the GRI in supplementing HbA1c to assess these risks, our study delves into multiple perspectives.
First, when the GRI remains stable, a decrease in HbA1c level may coincide with an increase in TIR, TBR, and CV. This result implies that even if HbA1c and TIR indicate improved glycemic control, patients may still face heightened risks of hypoglycemia and GV, which the GRI can more accurately reflect. Even when patients with T1D meet the traditional optimal glycemic control standard (HbA1c ≤ 7%), 39% still exhibit poor glycemic control according to GRI assessments[37]. An improvement in TIR may be accompanied by a deterioration in TBR and CV, manifesting as elevated GRI levels[16]. Researchers have recommended combining TIR and TBR for glycemic assessment[38], and the introduction of GRI provides a single metric that can offer clearer risk assessment in these complex situations and guide treatment ad
In clinical practice, GRI serves as a valuable complement to HbA1c in assessing glycemic risk and guiding treatment decisions. A high GRI in patients with HbA1c < 7% indicates an increased risk of hypoglycemia and GV, suggesting the need for insulin dose reduction, while a high GRI in patients with HbA1c ≥ 7% reflects a greater risk of hyperglycemia and GV, necessitating insulin dose escalation. During follow-up, the combined assessment of HbA1c and GRI offers a more comprehensive evaluation of glycemic status. A simultaneous decline in HbA1c and GRI signifies improved multidimensional glycemic control, whereas a concurrent rise in both parameters signals worsening control with a higher risk of hyperglycemia and GV. If HbA1c remains stable or rises while GRI decreases, the risk of hypoglycemia and GV is reduced, whereas if HbA1c remains stable or declines while GRI increases, the risk is elevated. These findings underscore the limitations of relying solely on HbA1c and highlight the importance of integrating GRI into routine clinical assessments. Monitoring and maintaining GRI within an optimal range alongside HbA1c standards can facilitate a more precise evaluation of glycemic control, guide individualized treatment adjustments, and ultimately improve diabetes management strategies.
Although our study provides valuable insights, it has certain limitations. First, the CGM data were collected over 14 days, which does not fully align with the 90-day timeframe of HbA1c and may have affected their correlation. However, prior studies have shown a strong agreement between 14-day and 90-day GRI values (R2 = 0.88)[40], supporting the use of 14-day CGM data for estimating GRI. Second, the relatively small sample size, geographic concentration of participants, and non-normal distribution of some glucose parameters limit the generalizability of our findings. Future studies should prioritize recruiting larger, more diverse cohorts that include individuals from multiple regions, ethnicities, and tr
GRI is an effective metric for assessing glycemic control in patients with T1D, offering a concise yet comprehensive reflection of multiple dimensions of glycemic control. It addresses the limitations of HbA1c in identifying hypoglycemia risk and GV, providing a comprehensive view of glycemic control for individuals in whom HbA1c interpretation may be confounded. Combining HbA1c with the GRI will provide a more thorough and accurate assessment for managing glycemic control in patients with T1D. Future research should investigate the association between GRI and complication risks, and develop machine learning-based GRI treatment response models to achieve personalized therapy and advance precision diabetes management.
The authors thank all of the patients, nurses, doctors and technicians involved at National Clinical Research Center for Metabolic Diseases for their efforts in data and sample collection. We would like to express gratitude to Professor Yang XL (Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China) for his assistance with statistical analysis. We also thank Jiang XF, Gong YQ and Xu YL for their kind support.
| 1. | Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1501] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 2. | Malik FS, Sauder KA, Isom S, Reboussin BA, Dabelea D, Lawrence JM, Roberts A, Mayer-Davis EJ, Marcovina S, Dolan L, Igudesman D, Pihoker C; SEARCH for Diabetes in Youth Study. Trends in Glycemic Control Among Youth and Young Adults With Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2022;45:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Sandy JL, Tittel SR, Rompicherla S, Karges B, James S, Rioles N, Zimmerman AG, Fröhlich-Reiterer E, Maahs DM, Lanzinger S, Craig ME, Ebekozien O; Australasian Diabetes Data Network (ADDN); T1D Exchanged Quality Improvement Collaborative (T1DX-QI); Prospective Diabetes Follow-Up Registry Initiative (DPV). Demographic, Clinical, Management, and Outcome Characteristics of 8,004 Young Children With Type 1 Diabetes. Diabetes Care. 2024;47:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 4. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16441] [Article Influence: 498.2] [Reference Citation Analysis (4)] |
| 5. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care. 2016;39:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 6. | Hainsworth DP, Bebu I, Aiello LP, Sivitz W, Gubitosi-Klug R, Malone J, White NH, Danis R, Wallia A, Gao X, Barkmeier AJ, Das A, Patel S, Gardner TW, Lachin JM; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Risk Factors for Retinopathy in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care. 2019;42:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 7. | Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 675] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 8. | Selvin E. The Glucose Management Indicator: Time to Change Course? Diabetes Care. 2024;47:906-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Haynes A, Hermann JM, Miller KM, Hofer SE, Jones TW, Beck RW, Maahs DM, Davis EA, Holl RW; T1D Exchange, WACDD and DPV registries. Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes. 2017;18:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Piona C, Marigliano M, Mozzillo E, Rosanio F, Zanfardino A, Iafusco D, Maltoni G, Zucchini S, Piccinno E, Delvecchio M, Maffeis C. Relationships between HbA1c and continuous glucose monitoring metrics of glycaemic control and glucose variability in a large cohort of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2021;177:108933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284-4291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci Transl Med. 2016;8:359ra130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 13. | Battelino T, Alexander CM, Amiel SA, Arreaza-Rubin G, Beck RW, Bergenstal RM, Buckingham BA, Carroll J, Ceriello A, Chow E, Choudhary P, Close K, Danne T, Dutta S, Gabbay R, Garg S, Heverly J, Hirsch IB, Kader T, Kenney J, Kovatchev B, Laffel L, Maahs D, Mathieu C, Mauricio D, Nimri R, Nishimura R, Scharf M, Del Prato S, Renard E, Rosenstock J, Saboo B, Ueki K, Umpierrez GE, Weinzimer SA, Phillip M. Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 2023;11:42-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 473] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 14. | American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S111-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 274] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 15. | Kompala T, Wong J, Neinstein A. Diabetes Specialists Value Continuous Glucose Monitoring Despite Challenges in Prescribing and Data Review Process. J Diabetes Sci Technol. 2023;17:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Klonoff DC, Wang J, Rodbard D, Kohn MA, Li C, Liepmann D, Kerr D, Ahn D, Peters AL, Umpierrez GE, Seley JJ, Xu NY, Nguyen KT, Simonson G, Agus MSD, Al-Sofiani ME, Armaiz-Pena G, Bailey TS, Basu A, Battelino T, Bekele SY, Benhamou PY, Bequette BW, Blevins T, Breton MD, Castle JR, Chase JG, Chen KY, Choudhary P, Clements MA, Close KL, Cook CB, Danne T, Doyle FJ 3rd, Drincic A, Dungan KM, Edelman SV, Ejskjaer N, Espinoza JC, Fleming GA, Forlenza GP, Freckmann G, Galindo RJ, Gomez AM, Gutow HA, Heinemann L, Hirsch IB, Hoang TD, Hovorka R, Jendle JH, Ji L, Joshi SR, Joubert M, Koliwad SK, Lal RA, Lansang MC, Lee WA, Leelarathna L, Leiter LA, Lind M, Litchman ML, Mader JK, Mahoney KM, Mankovsky B, Masharani U, Mathioudakis NN, Mayorov A, Messler J, Miller JD, Mohan V, Nichols JH, Nørgaard K, O'Neal DN, Pasquel FJ, Philis-Tsimikas A, Pieber T, Phillip M, Polonsky WH, Pop-Busui R, Rayman G, Rhee EJ, Russell SJ, Shah VN, Sherr JL, Sode K, Spanakis EK, Wake DJ, Waki K, Wallia A, Weinberg ME, Wolpert H, Wright EE, Zilbermint M, Kovatchev B. A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings. J Diabetes Sci Technol. 2023;17:1226-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 175] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 17. | Muneer M. Hypoglycaemia. Adv Exp Med Biol. 2021;1307:43-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (1)] |
| 19. | Karakus KE, Shah VN, Klonoff D, Akturk HK. Changes in the glycaemia risk index and its association with other continuous glucose monitoring metrics after initiation of an automated insulin delivery system in adults with type 1 diabetes. Diabetes Obes Metab. 2023;25:3144-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Kim JY, Yoo JH, Kim JH. Comparison of Glycemia Risk Index with Time in Range for Assessing Glycemic Quality. Diabetes Technol Ther. 2023;25:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Piona C, Marigliano M, Roncarà C, Mozzillo E, Di Candia F, Zanfardino A, Iafusco D, Maltoni G, Zucchini S, Piccinno E, Delvecchio M, Passanisi S, Lombardo F, Bonfanti R, Maffeis C. Glycemia Risk Index as a Novel Metric to Evaluate the Safety of Glycemic Control in Children and Adolescents with Type 1 Diabetes: An Observational, Multicenter, Real-Life Cohort Study. Diabetes Technol Ther. 2023;25:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 22. | Eviz E, Yesiltepe Mutlu G, Karakus KE, Can E, Gokce T, Muradoglu S, Hatun S. The Advanced Hybrid Closed Loop Improves Glycemia Risk Index, Continuous Glucose Monitoring Index, and Time in Range in Children with Type 1 Diabetes: Real-World Data from a Single Center Study. Diabetes Technol Ther. 2023;25:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Santova A, Plachy L, Neuman V, Pavlikova M, Petruzelkova L, Konecna P, Venhacova P, Skvor J, Pomahacova R, Neumann D, Vosahlo J, Strnadel J, Kocourkova K, Obermannova B, Pruhova S, Cinek O, Sumnik Z. Are all HCL systems the same? long term outcomes of three HCL systems in children with type 1 diabetes: real-life registry-based study. Front Endocrinol (Lausanne). 2023;14:1283181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 24. | Xie Y, Shi M, Ji X, Huang F, Fan L, Li X, Zhou Z. Insulin resistance is more frequent in type 1 diabetes patients with long disease duration. Diabetes Metab Res Rev. 2023;39:e3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Wang Q, Xie Z, Huang G, Fan L, Li X, Zhou Z. The impact of family history of type 2 diabetes on clinical heterogeneity in idiopathic type 1 diabetes. Diabetes Obes Metab. 2023;25:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Wang Q, Chen Y, Xie Y, Xia Y, Xie Z, Huang G, Fan L, Zhou Z, Li X. Type 2 Diabetes Family History as a Significant Index on the Clinical Heterogeneity Differentiation in Type 1 Diabetes. J Clin Endocrinol Metab. 2023;108:e1633-e1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S180-S199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 28. | Lind M, Pivodic A, Svensson AM, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA(1c) level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ. 2019;366:l4894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2021;20:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Lu J, Ni J, Wang M, Shen Y, Lu W, Zhu W, Bao Y, Rodbard D, Vigersky RA, Jia W, Zhou J. Association between glycaemia risk index (GRI) and diabetic retinopathy in type 2 diabetes: A cohort study. Diabetes Obes Metab. 2023;25:2457-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 31. | Yoo JH, Kim JY, Kim JH. Association Between Continuous Glucose Monitoring-Derived Glycemia Risk Index and Albuminuria in Type 2 Diabetes. Diabetes Technol Ther. 2023;25:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1-S115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 751] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 33. | Nielsen LR, Ekbom P, Damm P, Glümer C, Frandsen MM, Jensen DM, Mathiesen ER. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 34. | Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2022;10:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care. 2023;46:e151-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 36. | Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, Rodbard D. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J Diabetes Sci Technol. 2019;13:614-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 37. | Oriot P, Hermans MP, Vandelaer A, Philips JC, Prévost G. Glycemic Risk Index (GRI) variability in type 1 diabetes adults with HbA1c ≤ 7%: Insights for clinical evaluation and intervention. Diabetes Metab. 2024;50:101520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Sterner Isaksson S, Imberg H, Hirsch IB, Schwarcz E, Hellman J, Wijkman M, Bolinder J, Nyström T, Holmer H, Hallström S, Ólafsdóttir AF, Pekkari S, Polonsky W, Lind M. Discordance between mean glucose and time in range in relation to HbA(1c) in individuals with type 1 diabetes: results from the GOLD and SILVER trials. Diabetologia. 2024;67:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 39. | He B, Fan L, Deng C, Liu F, Xie Y, Zhou Z, Li X. Implications of glycemic risk index across different levels of glycated hemoglobin (HbA1c) in type 1 diabetes. Chin Med J (Engl). 2024;137:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Shah VN, Sakamoto C, Pyle L. Optimal Sampling Duration for Continuous Glucose Monitoring for the Estimation of Glycemia Risk Index. Diabetes Technol Ther. 2023;25:140-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 41. | Freckmann G, Pleus S, Schauer S, Link M, Jendrike N, Waldenmaier D, Haug C, Stuhr A. Choice of Continuous Glucose Monitoring Systems May Affect Metrics: Clinically Relevant Differences in Times in Ranges. Exp Clin Endocrinol Diabetes. 2022;130:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/