Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.101173
Revised: March 20, 2025
Accepted: April 8, 2025
Published online: June 15, 2025
Processing time: 278 Days and 16 Hours
In this editorial, we discuss the recent article by Regassa et al, published in the World Journal of Diabetes, which highlights the potential role of platelet indices (PI) in predicting poor glucoregulation in patients with type 2 diabetes mellitus (T2DM). Given the high morbidity and mortality associated with T2DM, there is a constant need to find new and accessible methods for predicting and treating individuals with this condition. The pathophysiology of T2DM involves systemic inflammation, metabolic dysfunction, and an increased risk of vascular injury, which are commonly associated with the development of microvascular and macrovascular complications, such as cardiovascular diseases and neuropathies. The link between these complications and T2DM requires further elucidation but may be explained by prolonged exposure to high glycemic levels and increased advanced glycation end products. PI might play an important role in determining whether some individuals are prone to poor glucoregulation. Recent evidence encourages the scientific efforts to demonstrate the consistency of this role and its applicability in monitoring glucoregulation, underscoring the importance of the study by Regassa et al.
Core Tip: This editorial discusses the role of platelet indices (PI) such as platelet count, mean platelet volume, plateletcrit, platelet large cell ratio, and platelet distribution width as potential predictors of poor glucoregulation in type 2 diabetes mellitus (T2DM). Emerging evidence shows that elevated PI levels correlate with inadequate glucoregulation and microvascular complications. Incorporating PI into routine assessments may offer a cost-effective tool for predicting glycemic outcomes in patients with T2DM, especially in resource-limited settings.
- Citation: Medeiros GA, de Santana JBF, Zajdenverg L, Negrato CA. Is there a role for platelet indices in predicting poor glucoregulation in type 2 diabetes mellitus? World J Diabetes 2025; 16(6): 101173
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/101173.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.101173
Type 2 diabetes mellitus (T2DM) is a chronic metabolic condition that affects millions of people globally and is associated with several long-term complications, including cardiovascular diseases. Inadequate glucoregulation is a critical factor contributing to these complications and is associated with high morbidity and mortality[1]. Attention has turned to lower-cost and technically easier alternatives that can predict the effectiveness of glucoregulation in people living with T2DM. Among these, platelet indices (PI) have emerged as potential indicators due to their relationship with inflammatory and metabolic processes[2].
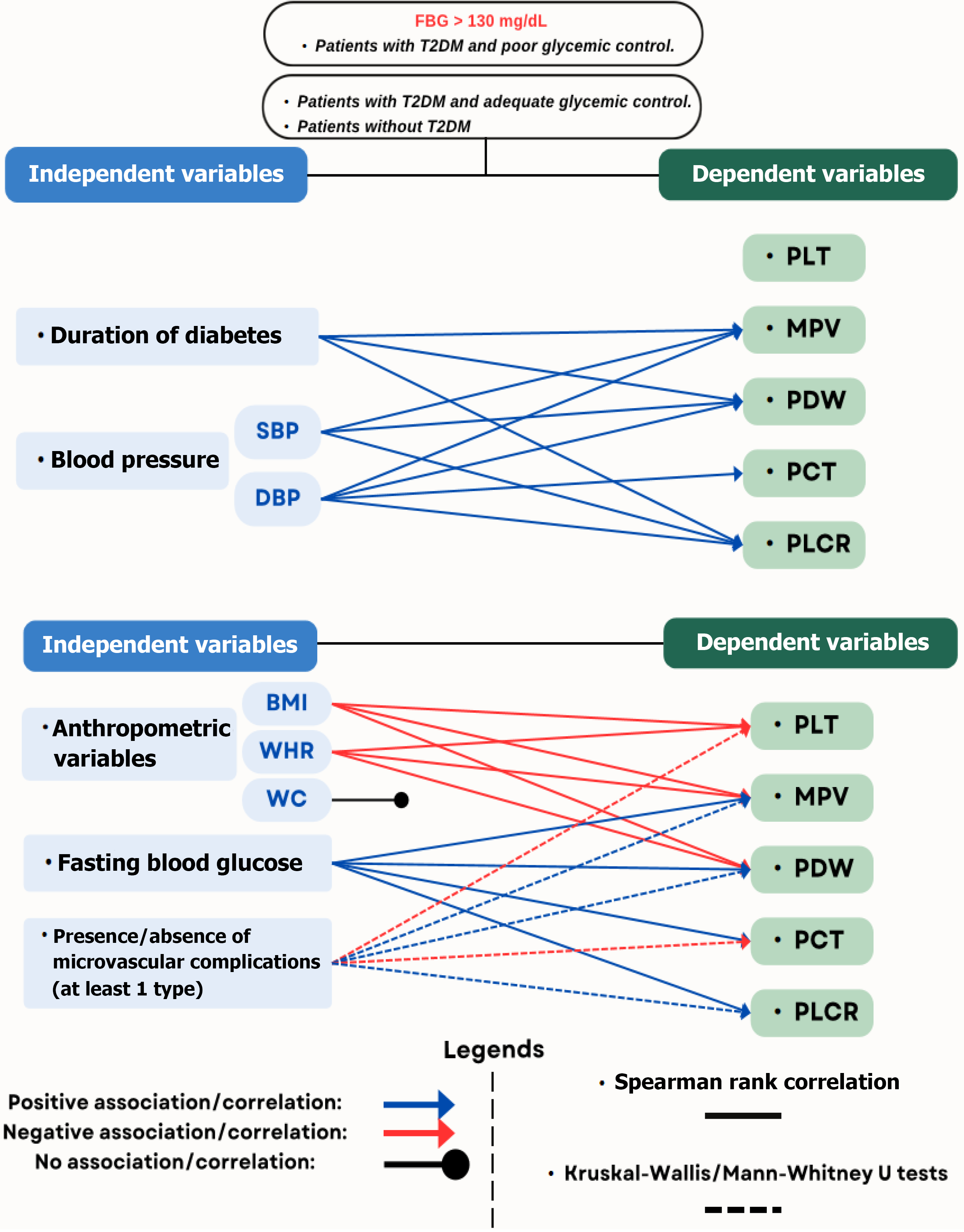
The applicability of this concept was assessed in the study published by Regassa et al[3] in the World Journal of Diabetes, which examined the use of PI as prognostic predictors of suboptimal glucoregulation among adult subjects with T2DM. It revealed that increased PI are significantly elevated in individuals with poor glucoregulation in T2DM, indicated by fasting blood glucose (FBG) levels exceeding 130 mg/dL, when comparing those with adequate glucoregulation and healthy controls.
Moreover, it was highlighted that these indices could also correlate positively with anthropometric variables, including body mass index (BMI), waist circumference (WC), and waist-to-hip ratio. Additional correlated variables include systolic blood pressure, diastolic blood pressure, FBG, duration of T2DM, and the occurrence of microvascular complications. This editorial briefly discusses the role of PI in predicting poor glucoregulation and the prognosis of subjects with T2DM[3].
PI, such as platelet count, mean platelet volume (MPV), platelet large cell ratio, plateletcrit (PCT), and amplitude of platelet distribution, also known as platelet distribution width (PDW), have been investigated as predictors of glucoregulation in T2DM. Studies suggest that high PI levels are associated with poorer glucoregulation, indicated by high glycated hemoglobin values (HbA1c), and microvascular and macrovascular complications. For example, Citirik et al[4] evaluated the potential of MPV, PDW, and PCT as parameters for detecting subclinical platelet activation in diabetic retinopathy. Demirtas et al[5] demonstrated that increased MPV is significantly associated with elevated HbA1c levels, reinforcing the hypothesis that chronic inflammation, commonly found in T2DM, can alter platelet function and quantity.
Increasing evidence suggests that chronic inflammation and insulin resistance play a crucial role in modulating platelet function in T2DM[6,7]. Elevated levels of pro-inflammatory cytokines, such as TNF-α and IL-6, contribute to platelet activation by enhancing NF-κB signaling and upregulating adhesion molecules like P-selectin, thereby fostering a pro-thrombotic state. Additionally, insulin resistance disrupts the PI3K/Akt signaling pathway, which is essential for both glucose metabolism and platelet regulation. Under normal conditions, insulin promotes nitric oxide production through this pathway, reducing platelet reactivity; however, in the presence of insulin resistance, PI3K/Akt dysfunction shifts signaling toward the MAPK pathway, thereby exacerbating platelet hyperactivity and damaging the glycocalyx, which leads to endothelial dysfunction[8]. This process is further aggravated by the production of reactive oxygen species (ROS) and by impaired IRS-1/2 signaling. ROS directly degrades the endothelial glycocalyx, promoting vascular inflammation and endothelial dysfunction[8]. These effects indirectly perpetuate an inflammatory and thrombotic cycle, potentially contributing to platelet activation and exacerbating vascular injury[8,9]. As a result, PI, such as MPV and PDW, are increasingly recognized as potential biomarkers of metabolic imbalance in T2DM, reflecting the heightened thrombotic risk associated with this disease[6,7].
Novel studies also associate the gut microbiome with T2DM, and PI might serve as a marker of this interaction by reflecting metabolic dysregulation and systemic inflammation. Changes in gut microbial composition can influence platelet activation through microbial metabolites, such as short-chain fatty acids and lipopolysaccharides, which modulate immune responses and insulin sensitivity. These mechanisms may explain the pro-thrombotic state observed in T2DM-linking gut dysbiosis, PI, and disease progression[10]; however, much remains to be explored.
PI are simple, low-cost, and widely available in routine blood tests. Growing evidence suggests that increased MPV and changes in PDW are associated with an exacerbated inflammatory response, which may negatively influence glucoregulation in patients with T2DM. MPV is an independent risk factor for macrovascular complications such as acute myocardial infarction, coronary artery disease[11,12], peripheral artery disease[13], and cerebral ischemia[14,15]. It has also been widely studied as an independent marker of endothelial dysfunction and altered microcirculation, which are central mechanisms in the development of microvascular complications, including erectile dysfunction[16,17].
Studies across different populations have provided valuable data on how PI can predict glucoregulation in T2DM, with the most recent evidence emerging from India. Chawla et al[18] evaluated MPV, PDW, and PCT as predictive biomarkers of microvascular complications in Indian patients with T2DM. The results indicated that HbA1c levels were significantly correlated with elevated MPV, PDW, and PCT levels. Thus, PI may serve as predictive biomarkers of microvascular complications in T2DM, such as neuropathy and retinopathy. Khanna et al[19] reported similar findings, stating that MPV, PDW, and P-LCR were significantly higher in patients with T2DM who had microvascular complications when compared with patients without complications in India. Nimmala et al[20] observed that MPV was significantly higher in subjects with T2DM compared to controls. MPV was associated with microvascular complications and positively correlated with age and T2DM duration.
The use of HbA1c as a predictive biomarker of poor glucoregulation is widely accepted[21,22]. Schoos et al[23] investigated the impact of HbA1c levels on residual platelet reactivity and outcomes following the insertion of coronary drug-eluting stents in subjects with T2DM. They found that those with poor glucoregulation, indicated by HbA1c > 8.5%, had a higher risk of stent thrombosis and cardiac death after percutaneous coronary intervention. A positive association was also found between HbA1c and high platelet reactivity, suggesting a potential area of research regarding pro-inflammatory interventions and PI in subjects with T2DM and poor glucoregulation.
Despite its widespread use, HbA1c has several limitations, including cost and the requirement for specialized laboratory equipment capable of performing high-performance liquid chromatography or immunoassay-based methods for accurate measurement[4]. In contrast, PI are derived from routine blood tests through an automated blood count, making them a more accessible and cost-effective option for monitoring glucoregulation in these settings[4]. This ad
Therefore, PI are emerging as promising additional tools for monitoring and managing T2DM, and as preliminary screening measures to identify individuals at risk for diabetes-related complications, thereby prompting early in
The study by Regassa et al[3] exhibits significant heterogeneity due to variability in population characteristics, clinical parameters, laboratory measurements, and treatment regimens. Differences in disease duration, BMI, WC, and the presence of microvascular complications contribute to inconsistencies, while variations in laboratory techniques and the use of different analyzers (CobasC311 and Sysmex XN550) may affect PI results. Furthermore, the inclusion of patients on different antidiabetic treatments, without controlling for antiplatelet medication use, further complicates the in
Integrating the assessment of PI into clinical practice may offer a practical and cost-effective method for identifying individuals with poor glucoregulation. However, it is essential that further studies, particularly those of a long-term, longitudinal, and multicenter nature, should be conducted to confirm these findings and validate the use of PI as reliable predictors of glucoregulation in diverse T2DM populations. Exploring their application is strongly recommended to enhance clinical diagnostic accuracy and prognostic assessment across various pathological states, particularly in inflammatory and thrombotic conditions.
Relevant research questions, such as “What is the longitudinal relationship between PIs and glucoregulation in T2DM?” and “How do PIs compare with traditional biomarkers of glucoregulation in terms of sensitivity and spe
For example, metabolomic studies, such as the one conducted by Jin and Ma[25], offered new insights into the mechanisms underlying glucoregulation in kidney and cardiovascular disease among individuals with T2DM. Similar studies could complement the interpretation of PI in T2DM, including comparisons with HbA1c levels.
The flowchart in Figure 1 illustrates the interaction between the dependent and independent variables of the study by Regassa et al[3].
In the study published in this issue, Regassa et al[3] found an association between PI measurements and glucoregulation in T2DM, suggesting the potential utility of these markers in T2DM follow-up. However, the association between PI and more accurate indicators of glucoregulation, such as HbA1c, remains limited. Although promising, these indices should be interpreted with caution, and their association with other markers and clinical outcomes should be clarified. Advances in the understanding of PI in T2DM may yield new tools to improve disease management and potentially reduce both costs and the risk of diabetes-related complications.
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 5829] [Article Influence: 1457.3] [Reference Citation Analysis (37)] |
| 2. | Pogorzelska K, Krętowska A, Krawczuk-Rybak M, Sawicka-Żukowska M. Characteristics of platelet indices and their prognostic significance in selected medical condition - a systematic review. Adv Med Sci. 2020;65:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Regassa DA, Berihun GA, Habtu BF, Haile WB, Nagaash RS, Kiya GT. Platelet indices as predictors of poor glucoregulation in type 2 diabetes mellitus adults at Bishoftu General Hospital, Ethiopia. World J Diabetes. 2024;15:1889-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (3)] |
| 4. | Citirik M, Beyazyildiz E, Simsek M, Beyazyildiz O, Haznedaroglu IC. MPV may reflect subcinical platelet activation in diabetic patients with and without diabetic retinopathy. Eye (Lond). 2015;29:376-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Demirtas L, Degirmenci H, Akbas EM, Ozcicek A, Timuroglu A, Gurel A, Ozcicek F. Association of hematological indicies with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med. 2015;8:11420-11427. [PubMed] |
| 6. | Maravilla Domínguez MA, Zermeño González ML, Zavaleta Muñiz ER, Montes Varela VA, Irecta Nájera CA, Fajardo Robledo NS, Zavaleta Muñiz SA. Inflammation and atherogenic markers in patients with type 2 diabetes mellitus. Clin Investig Arterioscler. 2022;34:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kucuk I, Tural E, Doğantekin B, Kaplan AT, Kucuk E, Onde ME. Evaluation of platelet indices and pro-inflammatory cytokines in type 2 diabetic patients with retinopathy. Rev Assoc Med Bras (1992). 2022;68:1537-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Milusev A, Rieben R, Sorvillo N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front Cardiovasc Med. 2022;9:897087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Henein MY, Vancheri S, Longo G, Vancheri F. The Role of Inflammation in Cardiovascular Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 445] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 10. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 5014] [Article Influence: 358.1] [Reference Citation Analysis (1)] |
| 11. | Ding L, Sun L, Wang F, Zhu L, Zhang T, Hua F. Clinical Significance of Platelet Volume and Other Platelet Parameters in Acute Myocardial Infarction and Stable Coronary Artery Disease. Arq Bras Cardiol. 2019;112:715-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tavil Y, Sen N, Yazici H, Turfan M, Hizal F, Cengel A, Abaci A. Coronary heart disease is associated with mean platelet volume in type 2 diabetic patients. Platelets. 2010;21:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Berger JS, Eraso LH, Xie D, Sha D, Mohler ER 3rd. Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999-2004. Atherosclerosis. 2010;213:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Rajakumar I, Vidya TA, Ramachandran K, Hussain A, Aarthi J, Poovitha M, Madhavan K, Kumar JS. Platelet indices as prognostic markers of ischemic stroke and their correlation with lipid profile. Clin Neurol Neurosurg. 2024;237:108119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Li Y, Xiang W, Xue H, Meng T, Zhang T, Zhang J, Wang J, Zhao J, Wang B. The impact of platelet indices on ischemic stroke: a Mendelian randomization study and mediation analysis. Front Neurol. 2023;14:1302008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Peng J, An J, Chen Y, Zhou J, Xiang B. The associations among platelet count, mean platelet volume, and erectile dysfunction: an observational and Mendelian randomization study. Sex Med. 2024;12:qfae093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Huang YY, Ye N, Peng DW, Li GY, Zhang XS. Peripheral platelet count is a diagnostic marker for predicting the risk of rapid ejaculation: findings from a pilot study in rats. Asian J Androl. 2025;27:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Chawla R, Sahu J, Punyani H, Jaggi S. Evaluation of platelet volume indices as predictive biomarkers of microvascular complications in patients with type 2 diabetes. Int J Diabetes Dev Ctries. 2021;41:89-93. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Khanna P, Salwan SK, Sharma A. Correlation of Platelet Indices in Patients With Type 2 Diabetes Mellitus and Associated Microvascular Complications: A Hospital-Based, Prospective, Case-Control Study. Cureus. 2024;16:e55959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Nimmala SG, Gokhale VS, Yadav P, Mangudkar S, Malik S. A Study of the Mean Platelet Volume and Plasma Fibrinogen in Type Two Diabetes Mellitus Patients Versus Healthy Controls and Their Role as Early Markers of Diabetic Microvascular Complications. Cureus. 2024;16:e65458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Geta ET, Terefa DR, Hailu WB, Olani W, Merdassa E, Dessalegn M, Gelchu M, Diriba DC. Effectiveness of shared decision-making for glycaemic control among type 2 diabetes mellitus adult patients: A systematic review and meta-analysis. PLoS One. 2024;19:e0306296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Falk EM, Staab EM, Deckard AN, Uranga SI, Thomas NC, Wan W, Karter AJ, Huang ES, Peek ME, Laiteerapong N. Effectiveness of Multilevel and Multidomain Interventions to Improve Glycemic Control in U.S. Racial and Ethnic Minority Populations: A Systematic Review and Meta-analysis. Diabetes Care. 2024;47:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Schoos MM, Dangas GD, Mehran R, Kirtane AJ, Yu J, Litherland C, Clemmensen P, Stuckey TD, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Brodie BR, Mazzaferri EL Jr, Maehara A, Stone GW. Impact of Hemoglobin A1c Levels on Residual Platelet Reactivity and Outcomes After Insertion of Coronary Drug-Eluting Stents (from the ADAPT-DES Study). Am J Cardiol. 2016;117:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, Singh S. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Jin Q, Ma RCW. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/