Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.97336
Revised: November 11, 2024
Accepted: December 25, 2024
Published online: March 15, 2025
Processing time: 238 Days and 1.4 Hours
Small blood vessels in the eyes are more susceptible to injury, which can lead to complications. However, since diabetic retinopathy is often a serious clinical condition, most of this study focuses on the vascular system of the choroid. As part of this study, we looked at how gymnemic acid (from Gymnema sylvestre) and glabridin (from Glycyrrhiza glabra, or licorice) might help diabetic rats’ choroid structural change and blood vessels.
To explore the effects of glabridin and gymnemic acid on the structural changes of the choroidal layer and choriocapillaris as well as the expression of vascular endothelial growth factor (VEGF) and cluster of differentiation (CD) 31 in diabetic rat’s eye.
The male Wistar rats were separated into five groups: The control group (control), the diabetic group (DM), the diabetic rats treated with glabridin 40 mg/kg body weight (DM + GB), the diabetic rats treated with gymnemic acid 400 mg/kg body weight (DM + GM), and the diabetic rats treated with glyburide 4 mg/kg body weight (DM + GR).
There was an increase in the thickness of both the choroid layer and the wall of the arteries in the DM. A decrease in vascularity and choroidal impairment was found in DM rats. After eight weeks of experimentation, the choroidal thickness increased, and the walls of choroid arteries. The choroidal thickness in the DM + GB was 15.69 ± 1.54 μm, DM + GM was 14.84 ± 1.31, and DM + GR groups was 16.45 ± 1.15 when compared with DM group (27.22 ± 2.05), the walls thickness of choroid arteries in the DM + GB was 10.23 ± 1.11, DM + GM was 10.41 ± 1.44, and DM + GR was 9.80 ± 1.78 when compared with DM group (16.35 ± 5.01), The expression of VEGF and CD31 was lower compared to the DM group.
In diabetic choroidopathy, hyperglycemia and inflammation cause damage to the neurovascular unit and blood-retinal barrier. Anti-VEGF treatments can slow or reverse the progression of the disease. According to current research findings, glabridin and gymnemic acid can reduce damage to the choroid, which is a factor that can sometimes result in vision loss.
Core Tip: To investigate the effects of glabridin and gymnemic acid on the choroidal layer and choriocapillaris in diabetic rats’ eyes, as well as the expression of vascular endothelial growth factor and cluster of differentiation 31. Five groups were created out of the Wistar rats. In the diabetic group, there was an increase in the thickness of both the choroid layer and the wall of the arteries as well as choroidal impairment. The choroidal thickness, and the walls of the choroid arteries in diabetic rats treated with glabridin 40 mg/kg body weight, the diabetic rats treated with gymnemic acid 400 mg/kg body weight, and the diabetic rats treated with glyburide 4 mg/kg body weight groups all rose. When compared to the group with diabetes, the expression of vascular endothelial growth factor and cluster of differentiation 31 was shown to be lower.
- Citation: Matsathit U, Komolkriengkrai M, Khimmaktong W. Glabridin and gymnemic acid alleviates choroid structural change and choriocapillaris impairment in diabetic rat’s eyes. World J Diabetes 2025; 16(3): 97336
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/97336.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.97336
Diabetes issues can eventually result in blindness and give rise to the serious condition known as diabetic retinopathy (DR). High blood sugar makes the vascular wall less effective because it kills intramuscular pericytes and makes the basement membrane thicker. This happens when microvascular retinal changes occur. The small blood vessels in the eyes are particularly vulnerable to poor blood glucose control, which can cause damage. Studies on humans and animals have mostly examined the retinal vasculature rather than the choroid vasculature since DR is typically a serious clinical issue. In DR, persistent hyperglycemia causes oxidative stress, inflammation, and metabolic imbalances in retinal cells, notably in retinal ganglion cells, photoreceptors, and retinal pigment epithelial (RPE) cells. This is especially true in retinal cells that are responsible for eye vision. The following factors set off a chain reaction of molecular changes that ultimately result in apoptosis[1-3]. Pathophysiology of DR is characterized by the presence of Müller cell destruction and abnor
Even though DR is well understood and frequently used as a diagnostic tool to track the advancement of the illness, a routine clinical ophthalmology evaluation of the choroid is quite uncommon[8]. According to new research, the diabetic choroid may experience comparable phenomena[9]. Also, DR has been seen in alloxan and streptozotocin (STZ) diabetes models[10]. The rats and monkeys that develop diabetes on their own show choroidal vascular leakage and capillary dropout and display evidence of choroidal neovascularization. The thickening of the basement membrane coined the term diabetic choroidopathy[11]. It is possible that choroidal vasculopathy that results from diabetes plays a significant role in the development of DR. Diabetic choroidopathy may also be the cause of the inexplicable loss of visual function that can happen to diabetic subjects who do not have retinopathy[9].
In experimental models like type 1 diabetes, STZ is a commonly used drug to generate insulin-dependent diabetic mellitus. STZ causes potentially dangerous systemic microvascular changes and consequences. It also has toxic effects on islet beta cells. The vascular corrosion casting technique combined with scanning electron microscopy (SEM) has become an important basic tool for learning about how organ microvasculation works. This will help future physiological and pathological studies. The three-dimensional architecture of the vascular bed and network can be identified using this conventional method[12]. This technique produces a readily comprehensible, clear, and identifiable three-dimensional picture of the entire choroid as well as any enlarged localized lesions, all while revealing the vascular architecture.
Glibenclamide or glyburide is the most frequently employed standard drug in the treatment of STZ-induced diabetes. It is a drug that effectively stimulates the secretion of insulin from β cells. The mechanism of action of glibenclamide in hyperglycemic conditions is to reduce blood glucose by stimulating insulin production from the preexisting β cells of the pancreas in STZ diabetic rodents[13]. In addition to this direct action, it also demonstrates pancreatic effects. The sulfonylurea receptor 1 in the pancreatic β cells is the site of binding for this drug[14]. This inhibition results in the depolarization of the cell membrane and the activation of voltage-dependent calcium channels[15]. It is one of the most effective treatments for diabetes, as it can elevate the intracellular calcium concentration in β cells, which in turn induces the release of insulin.
At present, modern methods of treating diabetic eye disease have many effects on patients. In addition, as the cost of treatment is quite expensive and can result in various side effects, there a currently much research on herbs that have the power to cure diabetes. Gymnemic acid is an important substance in Gymnema sylvestre, which is an herb used to treat diabetes[16]. It has the effect of increasing the efficiency of β-cell function in the pancreas and slowing down the absorption of glucose in the small intestine. This results in an increase in the amount of insulin in the body and can reduce blood sugar levels in diabetic rats[17]. Currently, there are research studies on diabetes that affect various organs in the body. In this paper, we studied the action of gymnemic acid as an alternative option for treating diabetic conditions. Within the scope of this research project, the effects of gymnemic acid derived from Gymnema sylvestre on microvascularity and structural issues within the choroids of rats that had been given STZ to induce diabetes were investigated.
Glabridin is a polyphenolic flavonoid that is also recognized as an active component of the Glycyrrhiza glabra, which is also known as the licorice plant. STZ produced diabetic rats have shown that it lowers blood glucose levels[18]. Addition-ally, it has demonstrated evidence of having an antioxidant effect in the kidneys of diabetic’s mice through an increase in superoxide dismutase (SOD) and a decrease in malondialdehyde (MDA) content[19]. The use of glabridin in diabetic rats resulted in a reduction in serum glucose as well as hepatic collagen type I, which caused the livers of the diabetic rats to return to their normal structures[18]. Because of this, glabridin’s ability to lower blood sugar and fight inflammation and scar tissue may make it an important component of future drug development plans for diabetes. For that reason, in this study, we used a microscope to examine how gymnemic acid and glabridin affect the blood vessels in the choroidal layer of diabetic rats’ eyes and compare the levels of vascular endothelial growth factor (VEGF) and cluster of differentiation (CD) 31 proteins after gymnemic acid treatment. These two proteins cause angiogenesis, a process that can result in eye damage and DR. Therefore, it is expected that this research project will yield multiple benefits and be a guideline for using herbs to prevent diabetes related eye complications from causing vision loss.
In this investigation, we used male Wistar rats that were eight weeks old and weighed between 200 g and 250 g. At the Southern Laboratory Animal Facility at Prince of Songkla University, a total of fifty rats were housed in an environmentally controlled laboratory environment. The conditions included a humidity level of 50% ± 10%, a temperature of
One week after becoming accustomed to their new environment, the rats were divided at random into five groups (each with ten members). In the control group, the rats received a standard rat diet. The standard diet was given to the rats in the diabetic group, which was denoted by the acronym STZ. To induce hyperglycemia, a single dosage of STZ, i.e., 60 mg/kg (Sigma-Aldrich; Merk KgA, Germany), that was dissolved in 0.1 mol/L in citrate buffer was administered intraperitoneally to all rats, except for those in the control group of rats. Citric buffer was the only substance that was administered to the control rats[20]. The diabetic rats in the diabetic rats treated with glabridin 40 mg/kg body weight group (DM + GB) were given a standard diet and were also given glabridin from licorice 4 mg/kg body weight (purified > 98% via high performance liquid chromatography analysis performed by Shaanxi Langrun Biotechnology Co., Ltd., Xi’an, Shaanxi Province, China) in 0.5 mL of 0.5% tween 80 solution as a supplement. The diabetic rats in the diabetic rats treated with gymnemic acid 400 mg/kg body weight (DM + GM) group were given a standard diet and were also given gymnemic acid from Gymnema sylvestre 400 mg/kg body weight (purified > 75% via high performance liquid chromatography analysis) in 0.5 mL of 0.5% tween 80 solution as a supplement. The diabetic rats in the diabetic rats treated with glyburide 4 mg/kg body weight (DM + GR) group were given a standard diet and were also given glyburide (Sigma-Aldrich; Merk KGaA) at 4 mg/kg[20,21] in 0.5 mL of 0.5% tween 80 solution as a supplement. Blood glucose levels were tested using a glucometer manufactured by Roche Diagnostics GmbH in Mannheim, Germany, 3 days after an injection of STZ. The rats that had a blood glucose level higher than 250 mg/dL were categorized as diabetic rats. The level of glucose in the blood was measured once a week. Eight weeks after receiving therapy, the rats were sacrificed under anesthesia using an excessive dose of sodium pentobarbital (200 mg/kg; intraperitoneal injection), and a blood sample was taken from their hearts using a cardiac puncture. After dissection, the eyes were placed in 10% formalin buffer for preservation.
As a part of the preparation for the histological investigation, the eye tissues of all the groups were dissected and were then promptly fixed in 10% formalin. This was carried out to assess the histological alterations and measure the lumen diameter of the choroidal arteries. These were subjected to a graded sequence of ethanol that progressed through 70%, 80%, 90%, 95%, and 100% for one hour each, with two changes in between. Before filtering, three changes of xylene lasting thirty minutes each were employed as a cleaning reagent. The tissue was then fixed in paraffin, cut into sections that were five micrometers thick, and stained with hematoxylin eosin staining (HE) and Masson’s trichrome (Sigma-Aldrich; Merck KGaA). An Olympus light microscope, model BX-50, manufactured in Japan by Olympus, was used to inspect, and photograph each segment. The Olympus cellSens software was used to obtain a measurement of the thickness of the choroid and the wall thickness of the arteries.
An immunohistochemical approach was used to examine the levels of VEGF and CD31 expression in the choroid layer of the eye. Briefly, each slide was deparaffinized in xylene, hydrated in gradient ethanol, permeabilized in phosphate buffer saline (PBS) buffer, and then blocked with serum in PBS. Finally, the slides were washed in PBS. At a temperature of 4 °C for one night, the slides were treated with rabbit anti-VEGF (1:200, ab 9570; Abcam, Cambridge, MA, United States) and mouse anti-CD31 antibodies (mouse mAb 3528, Cell Signaling Technology), diluted at 1:200 in blocking serum. The sections were washed three times with PBS before being placed in blocking solution with a fluorescein horse anti-mouse IgG containing 2 heavy chains (H) and 2 light chains (L) (H + L) antibody and a Texas red goat anti-rabbit IgG (H + L) antibody (1:200; Vector Laboratories, Inc.). They were left there for two hours at room temperature to find VEGF and CD31. A fluorescent microscope (model BX-50; manufacturer: Olympus, Japan) was used to evaluate the images. Within each specimen, five photos at 600-fold magnification in standard fields of 810965 μm2 were chosen at random for examination. Image J software from the National Institutes of Health was used to quantify the chemiluminescence intensity. The optical density of each sample was standardized based on the photo at 600-fold area. The photos were selected after being gray scaled with 8 bits and then transformed. To obtain accurate measurements of the area, integrated density, and background intensity, the optical density calculation followed Fu et al’s instructions, as shown in the choroidal layer[22].
The left ventricle of each group of rats was perfused with a 0.9% sodium chloride solution to flush the blood out of the blood vessels. After that, a combination of plastic called PU4ii casting resin, which is based on polyurethane (vasQtec), was injected into the blood circulation of rats. To ensure that the plastic was properly polymerized, the animal that had plastic injected into it was first left at room temperature for 30 minutes, and then it was submerged in hot water for 3 hours. After the polymerization process was complete, the eye was extracted, and the tissue was then corroded in a 10% potassium hydroxide solution at room temperature for 30-40 days. The vascular cast for the eye was given a rinsing under a tap with a gentle stream of water and then washed in multiple changes of distilled water to remove any leftover tissues. After that, it was allowed to air dry at ambient temperature, and then it was mounted to a metal stub using double glue tape and carbon paint before it was coated with gold using a sputtering apparatus. Finally, an SEM (JEOL JSM-5400) with a 10 KV accelerating voltage was used examine the choroid area of the eye cast. A piece of software called SemAfore was used to determine the diameter of the replica vascular bed in the choroid.
The findings were summarized using the mean value together with the standard error of the mean (SEM). The study of statistics was carried out with the use of analysis of variance, and then the Bonferroni posttest was used. It was determined that statistical significance was reached when the value of P was lower than 0.05.
The blood sugar level was measured every week of the experiment for 8 weeks and glycated hemoglobin (HbA1c) was measured after 8 weeks experiment. It was found that the blood sugar level (Table 1) and HbA1c (Table 2) of the rats in the diabetes group increased significantly. Statistically significant (P < 0.001 and P < 0.0001, respectively) when compared to the rats in the control group. In the 8th week of the experiment, the rats in the diabetes group had blood sugar levels equal to 353.83 ± 35.16 mg/dL, while the control group had a blood sugar level of 74.67 ± 3.31 mg/dL (Table 1).
| Week | C (mg/dL) | DM (mg/dL) | DM + GB (mg/dL) | DM + GM (mg/dL) | DM + GR (mg/dL) |
| 1 | 70.5 ± 4.92 | 360.50 ± 32.331 | 314.83 ± 30.711 | 362.89 ± 49.291 | 297.67 ± 20.69 |
| 2 | 72.00 ± 5.49 | 290.50 ± 34.441 | 311.67 ± 49.731 | 269.22 ± 35.601 | 241.00 ± 36.433 |
| 3 | 79.17 ± 3.25 | 351.17 ± 43.251 | 305.33 ± 52.591 | 188.00 ± 41.511 | 176.50 ± 38.653 |
| 4 | 73.33 ± 3.57 | 253.67 ± 30.751 | 170.67 ± 41.11 | 240.89 ± 48.701 | 187.50 ± 45.61 |
| 5 | 72.00 ± 3.98 | 301.50 ± 55.411 | 137.00 ± 40.872 | 289.78 ± 50.062 | 192.50 ± 54.94 |
| 6 | 73.58 ± 3.55 | 351.00 ± 34.821 | 69.50 ± 13.693 | 249.78 ± 49.682 | 149.00 ± 47.483 |
| 7 | 73.00 ± 4.25 | 457.00 ± 20.991 | 158.33 ± 37.643 | 259.00 ± 45.532 | 165.83 ± 49.043 |
| 8 | 74.67 ± 3.31 | 353.83 ± 35.161 | 174.50 ± 30.993 | 213.44 ± 40.732 | 160.67 ± 52.463 |
After the rats induced with diabetes were treated with glabridin (DM + GB), gymnemic acid (DM + GM), and glibenclamide (DM + GR), it was found that the animals in all three groups of these groups displayed a statistically significant decrease in blood sugar levels (P < 0.001, P < 0.01, P < 0.001, respectively) when compared to the rats in the diabetes group, with the mean sugar levels of the rats being equal to 174.50 ± 30.99, 213.44 ± 40.73, and 160.67 ± 52.46 mg/dL, respectively (Table 3).
| Week | C (g) | DM (g) | DM + GB (g) | DM + GM (g) | DM + GR (g) |
| 1 | 256.66 ± 5.25 | 178.83 ± 3.565 | 173.33 ± 4.715 | 240.83 ± 4.241 | 197.66 ± 8.245 |
| 2 | 286.50 ± 6.17 | 187.33 ± 5.525 | 181.16 ± 5.935 | 216.38 ± 10.033 | 221.33 ± 14.332 |
| 3 | 305.66 ± 8.55 | 178.83 ± 7.605 | 181.00 ± 8.095 | 232.33 ± 8.133 | 215.50 ± 19.721 |
| 4 | 325.50 ± 8.17 | 167.50 ± 10.455 | 179.16 ± 9.155 | 231.66 ± 9.194 | 211.33 ± 11.814 |
| 5 | 342.66 ± 9.02 | 171.83 ± 12.389 | 166.50 ± 13.52 | 263.33 ± 15.158 | 212.16 ± 10.286 |
| 6 | 352.50 ± 8.01 | 173.50 ± 11.019 | 179.16 ± 13.28 | 267.50 ± 15.318 | 209.33 ± 7.856 |
| 7 | 370.50 ± 8.66 | 159.50 ± 13.529 | 175.50 ± 13.04 | 275.83 ± 16.868 | 199.00 ± 8.036 |
| 8 | 371.00 ± 9.27 | 182.00 ± 13.829 | 191.00 ± 14.61 | 286.66 ± 18.287 | 222.83 ± 6.256 |
In the study of the body weight of laboratory rats, the rats were weighed every week of the experiment for 8 weeks. It was found that in the 8th week of the experiment, the body weight of the rats in the diabetes group decreased significantly (P < 0.001) when compared to the rats in the control group. In the 8th week of the experiment, the rats in the diabetes group had an average body weight of 182.00 ± 13.82 grams, while the control group had an average body weight of 371.00 ± 9.27 grams. After being treated with glabridin (DM + GB), gymnemic acid (DM + GM), and glibenclamide (DM + GR) in rats induced with diabetes, it was found that in all three groups of rats there was an increase in mean body weight compared to the rats in the diabetes group, with the mean body weight of the rats being 191.00 ± 14.61, 286.66 ± 18.28, and 222.83 ± 6.25 grams (Table 3).
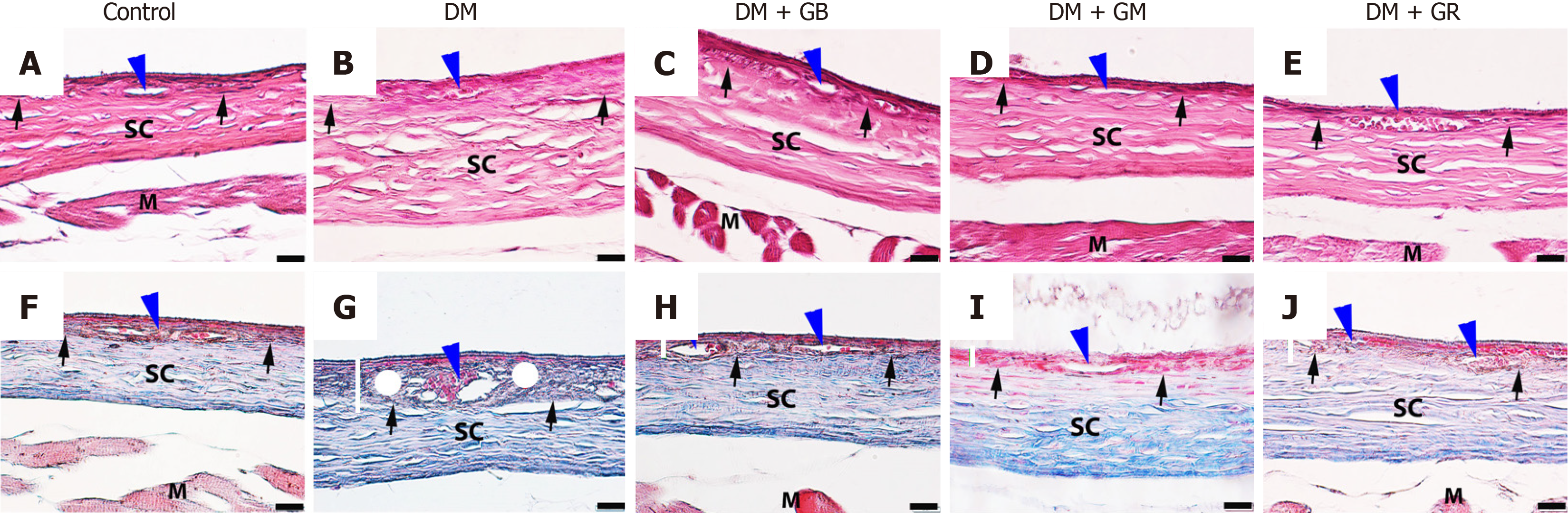
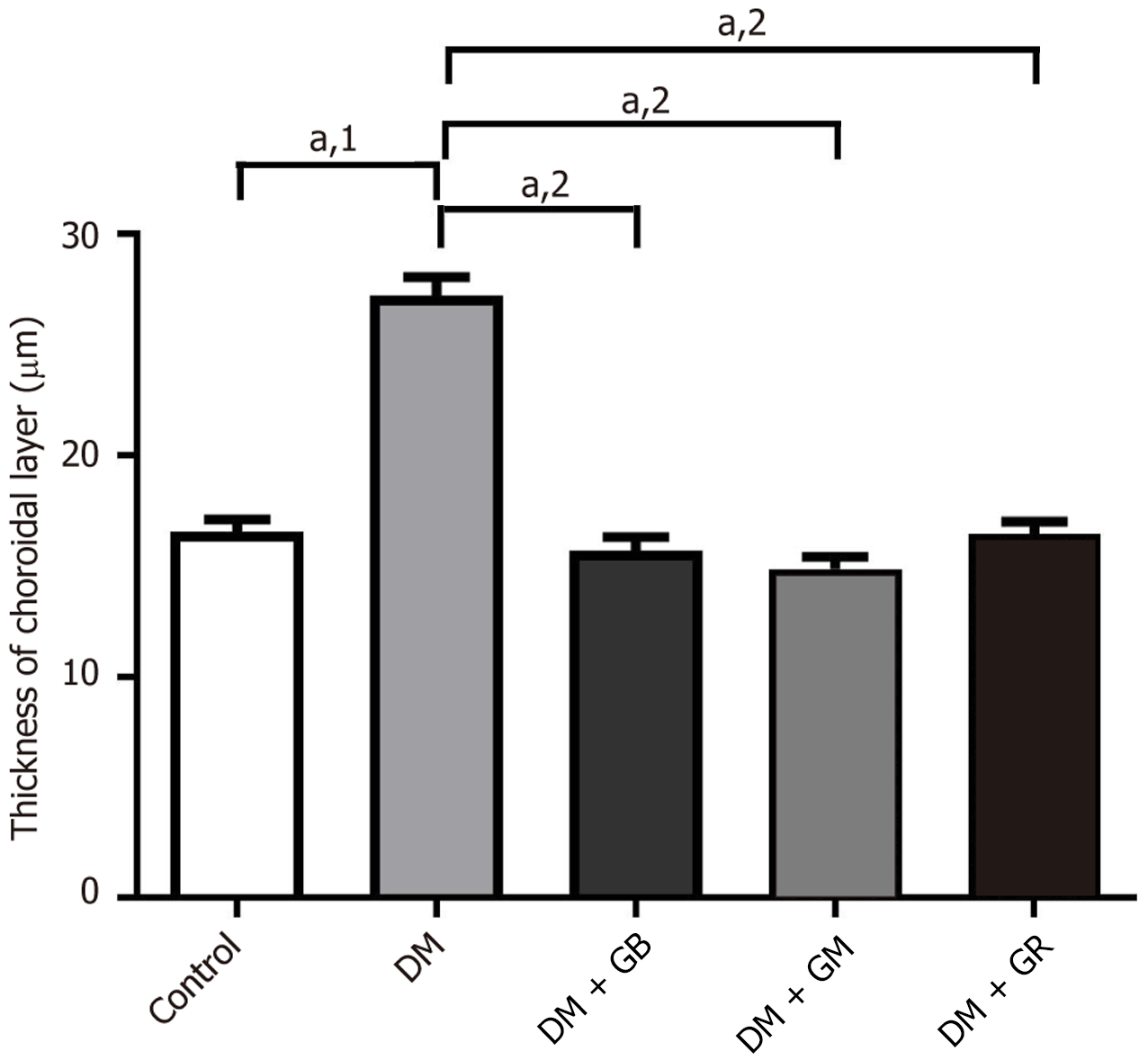
The choroid layer is characterized by loose connective tissue and a high number of blood vessels that serve as its source of nourishment. Within this layer, an intricate network of blood vessels may be found. At the end of the 8-week experiment, eye tissues were stained with Masson’s trichrome (Figure 1). It was found that there was no pathology in the choroidal tissue or blood vessels in the control group (Figure 1). The average thickness of the choroid layer in the control group was 16.56 μm ± 0.56 μm (Figure 2). It was determined through the process of measuring the dimensions of the choroid artery that the average thickness of the arterial wall was 9.48 μm ± 1.13 μm, while the lumen width was measured as being 50.47 μm ± 11.37 μm. High blood sugar levels caused diabetes in the diabetes group of rats, which had the pathological characteristics of the choroidal tissue and blood vessels (Figure 1). The walls of the blood vessels showed that collagen fibers were building up all over the choroidal and scleral tissues. The thickness of the choroid layer and wall of choroidal arteries increased, exhibiting an increased thickness of collagen fibers which caused the lumen diameter to narrow. The average thickness of the choroid layer in diabetes rats was 27.22 μm ± 0.87 μm (Figure 2). The mean choroidal artery wall thickness increased by 16.35 μm ± 5.01 μm compared to the control group, as shown in Table 4.
On the other hand, when glabridin, gymnemic acid, and glyburide were given to the DM + GB group, DM + GM group, and DM + GR group (Figure 1), these rats displayed thinner choroidal layers than the diabetes rats (Figure 1). It was found that blood vessels were reduced in choroid artery wall pathology and restored to a better condition close to normal. The average thicknesses of the choroid layer in diabetes rats were 15.69 μm ± 0.63 μm, 14.87 μm ± 0.53 μm, and 16.44 μm ± 0.57 μm, respectively (Figure 2). The mean wall thicknesses of choroidal arteries in the DM + GB group, DM + GM group, and DM + GR group were 10.23 μm ± 1.11 μm, 10.41 μm ± 1.44 μm, and 9.80 μm ± 1.78 μm, respectively. The mean diameters of the lumen were 52.52 μm ± 11.10 μm, 53.71 μm ± 7.12 μm, and 51.58 μm ± 8.11 μm, respectively, as shown in Table 4.
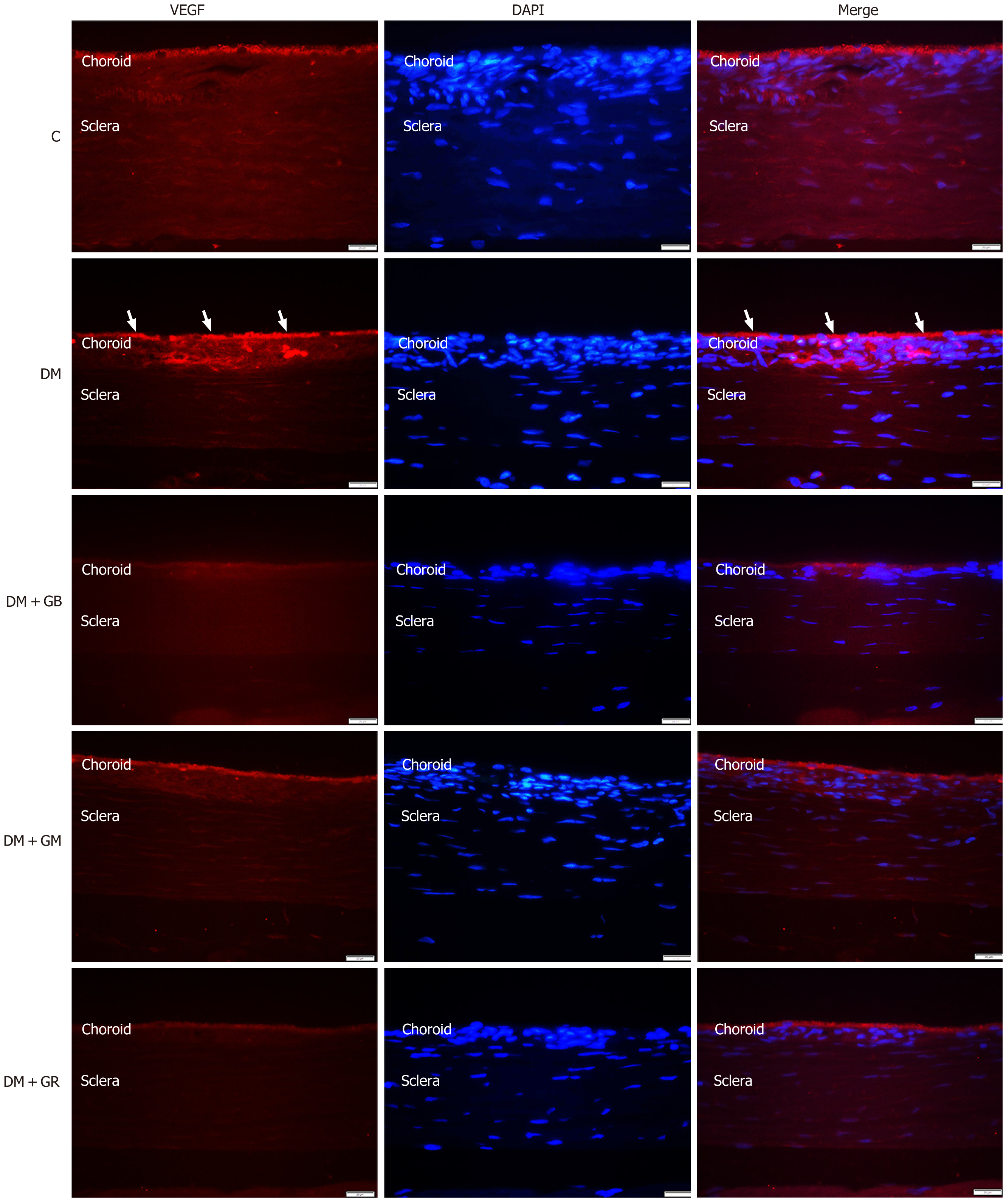
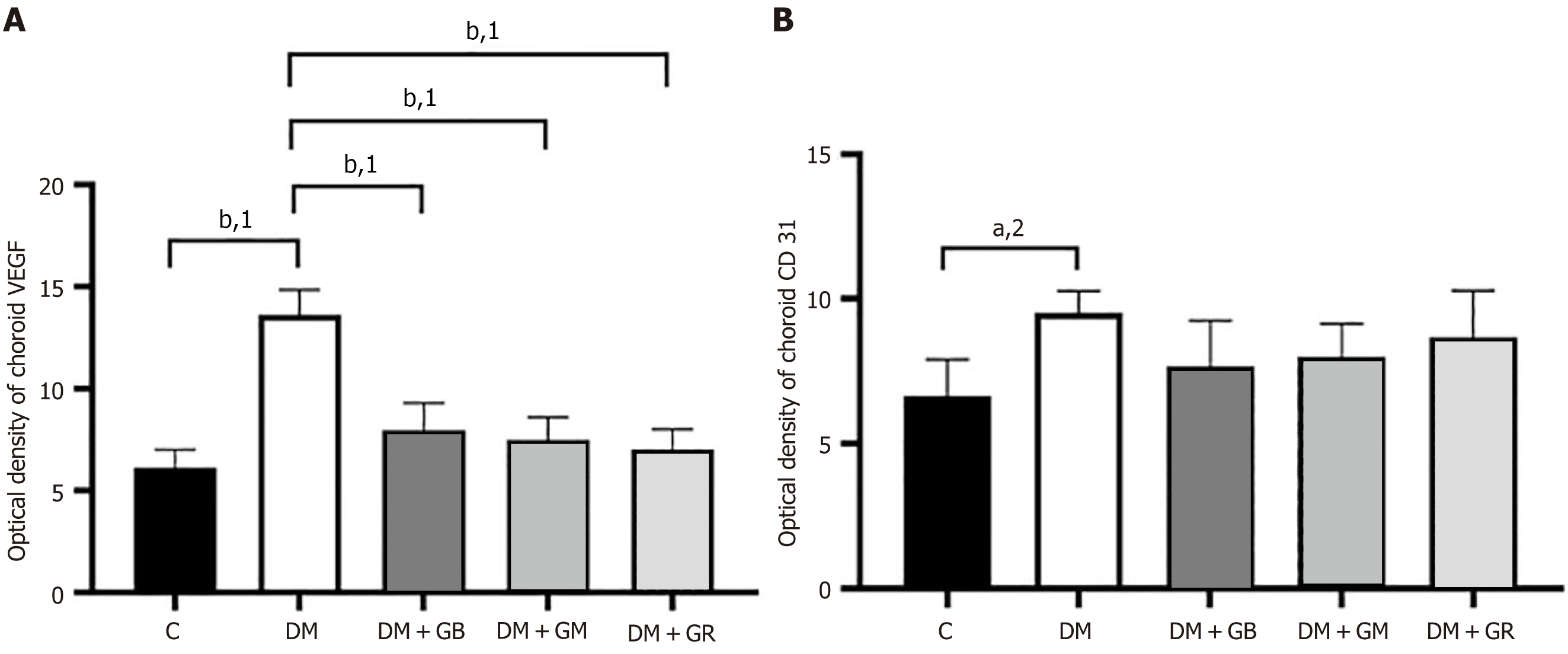
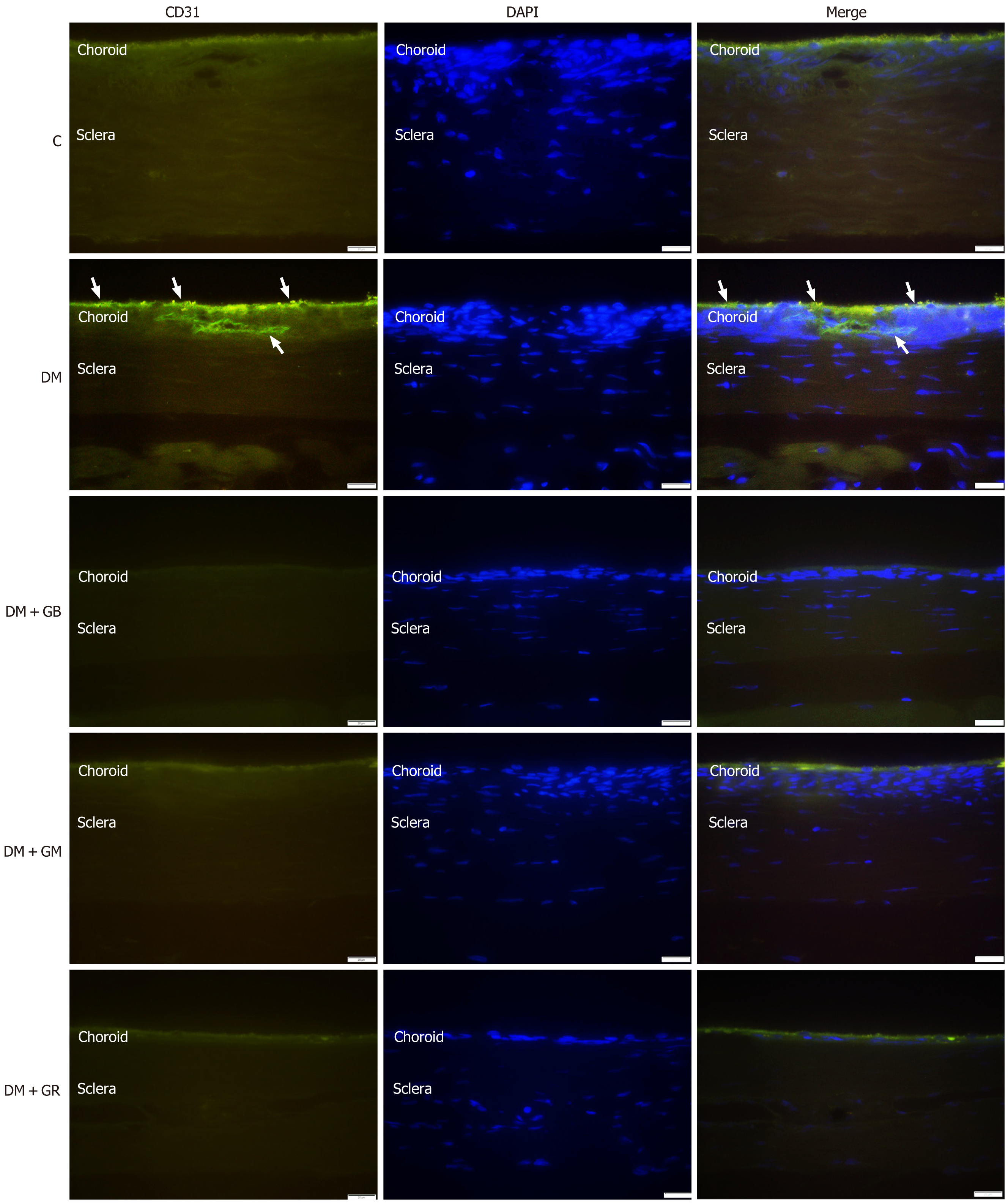
During the research that was conducted on the expression of VEGF protein in the choroidal layer of the eye, it was discovered that the expression of VEGF protein in the choroid of the diabetic group rose in comparison with the control group, particularly in the choriocapillaris and the RPE (Figure 3 and Figure 4). It was found that the level of VEGF protein in the choroid layer of diabetic rats was statistically significantly higher than that of control rats (Figure 3). The mean fluorescence intensity of VEGF protein in the choroid of diabetic rats was 13.62 ± 0.71, while in the control group, the mean VEGF protein fluorescence intensity was only 6.12 ± 0.51. When studying the expression of VEGF protein in the choroid layer of rats in the DM + GB, DM + GM, and DM + GR groups (Figure 4A), it was found that the expression of VEGF protein was significantly decreased compared to rats in the diabetic group, with the mean fluorescence intensity of VEGF protein being 7.96 ± 0.77, 7.47 ± 0.65, and 7.015 ± 0.57, respectively.
An examination of the expression of CD31 protein in the choroid layer of diabetic rats revealed a statistically significant increase in the expression of CD31 protein (Figure 4 and Figure 5) in comparison to the expression of CD31 protein in control rats, particularly in the region of the RPE, choriocapillaris, and the wall of blood vessels. It was found that the mean CD31 protein intensity in the choroid layer of diabetic rats was 9.53 ± 0.44, whereas the mean CD31 protein intensity in the control group was 6.64 ± 0.73 (Figure 4B). It was also found that the DM + GB, DM + GM, and DM + GR groups had lower levels of CD31 protein expression in the choroid layer compared to the diabetic group. The CD31 protein had a mean fluorescence intensity of 7.67 ± 0.91, 7.99 ± 0.65, and 8.66 ± 0.94, respectively, from the three different samples (Figure 4B).
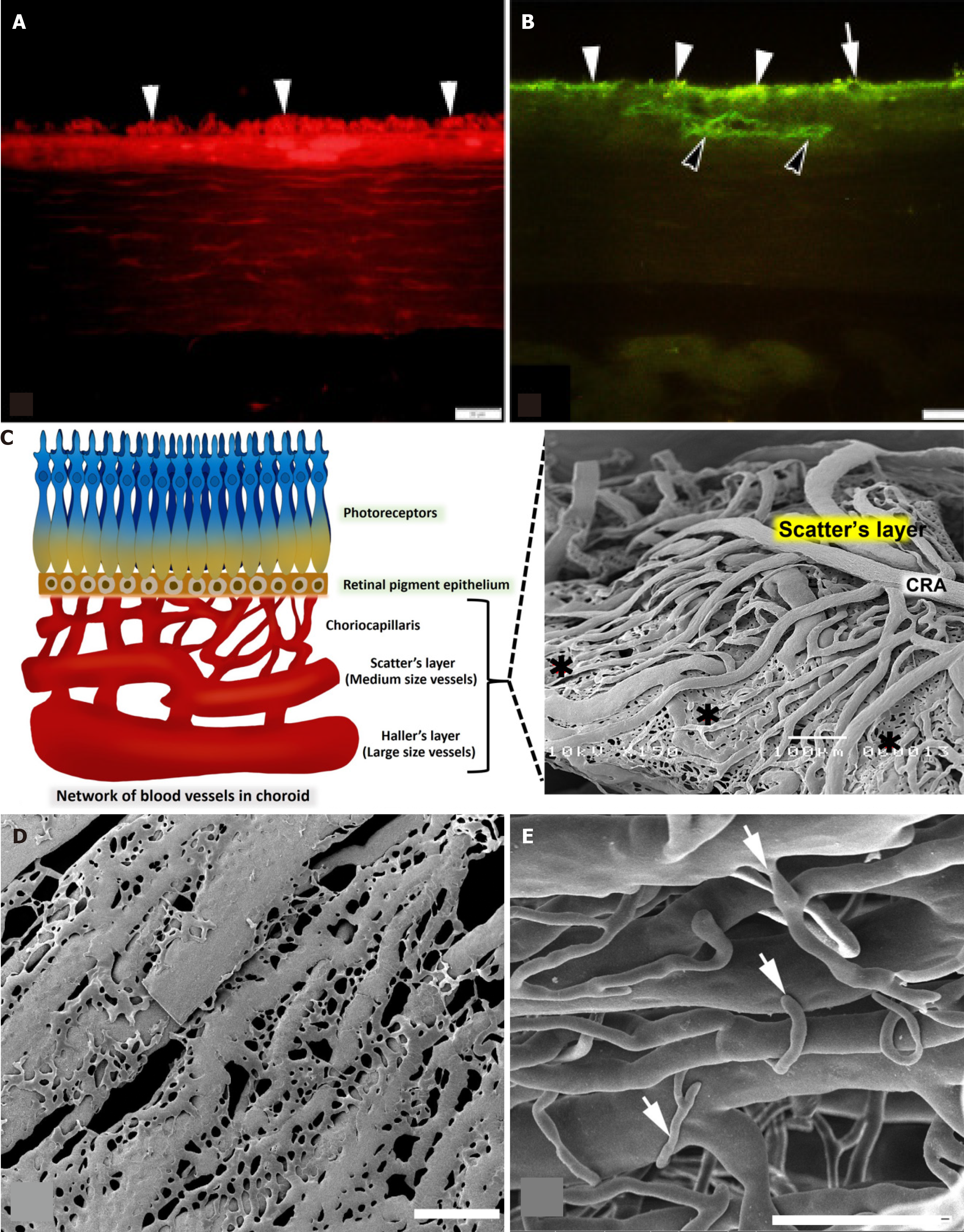
An illustration of immunoreactive VEGF in the choriocapillaris and the RPE (Figure 6A) and immunoreactive CD31 in the RPE, choriocapillaris, and blood vessel wall was observed in the diabetic group (Figure 6B).
In the choroidal walls of the eye, there is a PCA in the Hallter’s layer that connects to the choroid artery in the Scatter’s layer and divides into a network of capillaries known as the choriocapillaris. This network helps to transport blood throughout the eye. The choriocapillaris connects to the retina through the RPE and certain photoreceptor cells. Large-caliber vessels make up the outermost layer of the choroid, which is also referred to as Haller’s layer. Sattler’s layer is the name given to the innermost layer of the choroid (Figure 6C), which is made up of vessels that are much smaller in size. Many anastomotic capillaries are what make up the choriocapillaris of the choroid, which is the part of the choroid that runs the deepest within the chest. For venous drainage to occur from the choriocapillaris, the vortex veins are the prin
In contrast, the arrangement of choriocapillaris in diabetic rats, was not dense or untidy. There was a connection in the choriocapillaris that was wilted and appeared as thin fringes with tube breaks. Within a particular region, the choriocapillaris had ruptured and linked to one another, resulting in the formation of a flatter sheet (Figure 6D). In addition, the sprouting of blood vessels was more identifiable in the choroidal arteries in Sattler’s layer (Figure 6E).
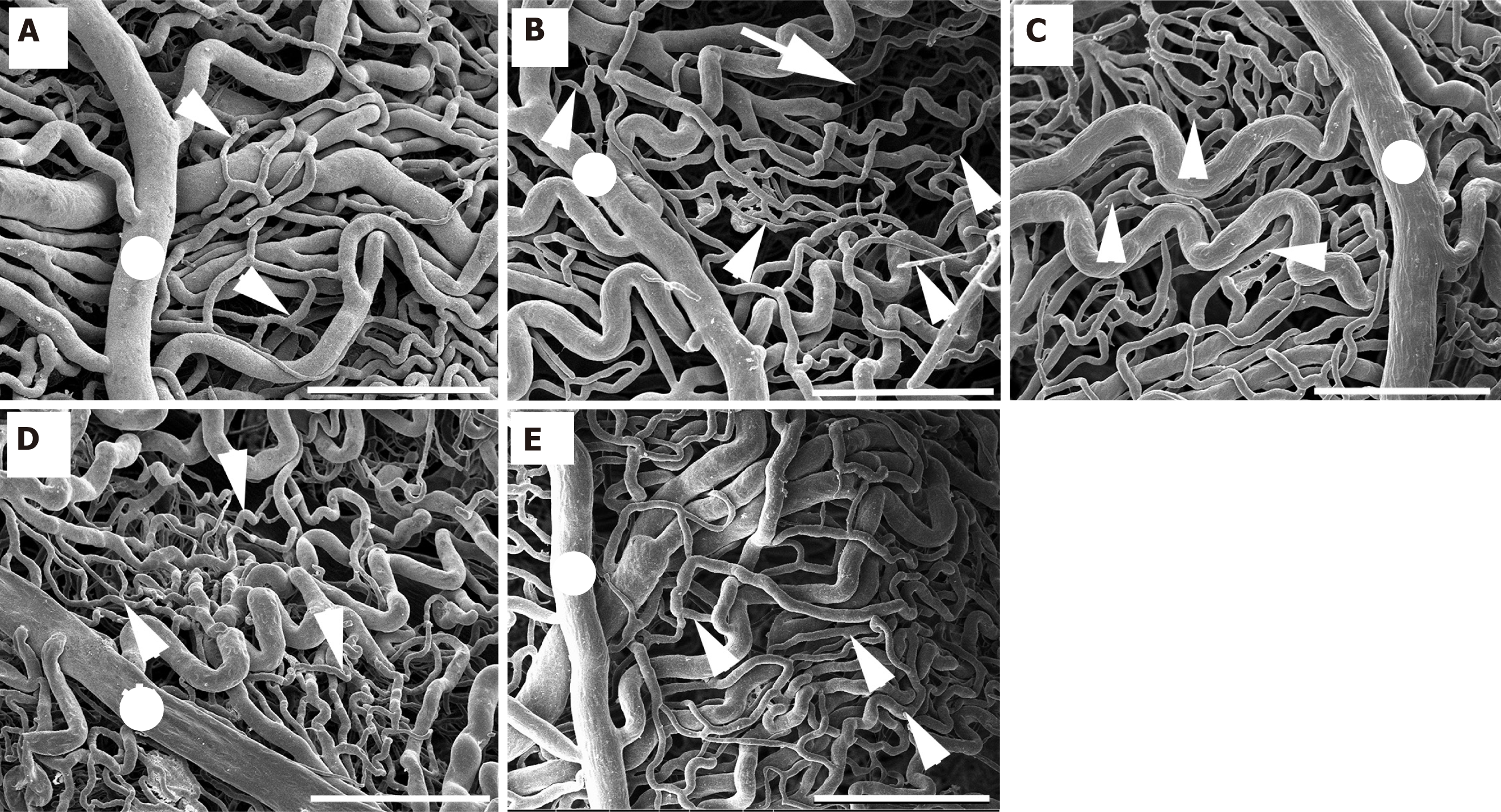
Upon examination, it was discovered that the choroidal artery had branched out into the choriocapillaris in an organized fashion. In control rats, they were stacked in a thick manner, and there was no evidence of blood vessel fracture (Figure 7). The average diameters of the choroidal artery and choriocapillaris in control rats were 89.73 μm ± 2.82 μm and 10.52 μm ± 0.72 μm, respectively (Table 5). In addition to being clearly visible under sparse vessels, the choriocapillaris alterations were more noticeable in diabetic rats. It was elongated and decreased in quantity, and it was also plainly visible. In the blood vessels that were destroyed in the diabetic group, choroidal arteries had a small diameter, stenosis was observed, and chori-ocapillaris shrank (Figure 7). The average diameters of the choroidal artery and choriocapillaris in diabetic rats were 60.31 μm ± 2.71 μm and 4.68 μm ± 0.65 μm, respectively, as shown in Table 5.
| Groups | Diameters of blood vessels (μm), mean ± SE | |
| Choroidal arteries | Choriocapillaris | |
| Control | 89.73 ± 2.82 | 10.52 ± 0.72 |
| DM | 60.31 ± 2.71 | 4.68 ± 0.65 |
| DM + GB | 81.18 ± 3.56 | 8.88 ± 0.70 |
| DM + GM | 80.31 ± 3.83 | 7.76 ± 0.82 |
| DM + GR | 79.82 ± 4.73 | 9.54 ± 0.78 |
| P value | < 0.001 | < 0.001 |
In contrast, it was found that rats in the DM + GB group, DM + GM group, and DM + GR group were exposed to glabridin, gymnemic acid, and glyburide, respectively. It was found that blood vessels increased in diameter and were close to normal. The average diameters of the choroidal artery and choriocapillaris in the DM + GB group were 81.18 μm ± 3.56 μm and 8.88 μm ± 0.70 μm, respectively. The average diameters of the choroidal artery and choriocapillaris in the DM + GM group were 80.31 μm ± 3.83 μm and 7.76 μm ± 0.82 μm, respectively. The average diameters of the choroidal artery and choriocapillaris in the DM + GR group were 79.82 μm ± 4.73 μm and 9.54 μm ± 0.78 μm, respectively, as shown in Table 5.
From the results of this study, after the end of the 8-week experiment, rats in the diabetes group had higher blood sugar levels and significantly de-creased body weight. Compared to the control group, consistent with the results of a 2021 study by Sandech et al[17], the effects of blood sugar levels and body weight were studied. STZ at a dose of 60 mg/kg body weight induces diabetes in rats. The experiment was conducted for a period of 8 weeks. It was found that the blood sugar level of the diabetic rats increased, and the body weight of the rats decreased with statistical significance compared to normal rats due to STZ. Because of its low cost and the fact that it has less adverse effects than other medications, it is one of the diabetogenic medications. Specifically, the process of the alkylation of DNA is linked to the specific harmful impact that it has on β-cells that are located within the pancreas[23]. Because of this, the production of the hormone insulin is slowed, which is a consequence of the action. This is accomplished via binding with glucose transporter 2 (GLUT2) to distribute STZ, which can enter cells. The DNA strand is then modified by the addition of a methyl group, which leads to the destruction of beta cells and necrosis. The result causes certain conditions in the body, namely hypoinsulinemia and hyperglycemia, and as a result, the body has higher blood sugar levels[24]. One of the obvious specific characteristics of diabetes is a decrease in body weight. As a result of the loss or deterioration of proteins in the body, the body fluid of diabetic rats decreased[25].
Results from this study show that after the end of the 8-week experiment, diabetic rats treated with glabridin at a dose of 40 mg/kg (DM + GB) showed a statistically significant decrease in blood sugar levels when compared to the rats in the diabetic group. The administration of glabridin at a dose of 40 mg/kg body weight to rats induced with diabetes by injecting STZ at a dose of 60 mg/kg body weight was able to increase levels of antioxidants SOD and reduce levels of free radicals MDA, which causes the body to reduce oxidative stress, thus helping to reduce blood sugar levels[18,19]. In addition, this study shows that at the end of the 8-week experiment, diabetic rats treated with gymnemic acid at a dose of 400 mg/kg body weight had a reduction in blood sugar levels compared to control rats. A study involving the administration of gymnemic acid at 400 mg/kg body weight to rats induced with diabetes by STZ injection at a dose of 60 mg/kg body weight for a period of 60 days found that the blood sugar levels of diabetic rats were significantly reduced compared to untreated diabetic rats[17]. The structure of the gymnemic acid molecule is somewhat comparable to that of the glucose molecule. Therefore, it can bind to the receptor instead of the sugar molecule in the taste buds on the tongue, inhibiting the taste of sweetness. It also binds to receptors in the small intestine and can delay the absorption of glucose into the small intestine[16]. In addition, the administration of gymnemic acid can increase the number of beta cells in the pancreas. This is the reason that gymnemic acid can reduce blood sugar levels.
In the results of a study comparing the histological changes of cells and choroidal tissues in the eyeball using HE and Masson’s trichome staining, it was found that the thickness of the choroid layer in the diabetic rats was significantly increased when compared to the control rats. This is consistent with research published in 2020 by Endo et al[26] who studied the thickness of the choroid layer in diabetic patients. The thickening of the choroidal layer is caused by the increased permeability of the choriocapillaris. Considering that the choroid is responsible for the blood flow to the eye, the autonomic nervous system plays a significant role in the autoregulation of the choroidal blood flow. During the early stages of DR, sympathetic innervation was engaged, which resulted in increased choroidal circulation, which ultimately led to an increase in the thickness of the choroid. At the same time, blood vessels in the choroid layer caused a significantly increased thickness of the blood vessel wall, especially in the connective tissue layer or the tunica adventitia, when compared to the control group rats. Hyperglycemia is directly responsible for the acceleration of the atherosclerotic process, which in turn leads to the development of endothelial dysfunction. This dysfunction, in turn, leads to vasoconstriction, proinflammatory processes, and prothrombotic processes, all of which contribute to the development and rupture of blood vessels[27].
Diabetes may be an independent factor that contributes to the thickening of the choroid, and subsequent progression of DR may result in the reduction in choroid thickening. This may manifest as a thicker choroidal layer during the first stage of DR, which then gradually thins out as the disease progresses[28]. The increased permeability of the choriocapillaris is what causes the thickening of the choroidal layer. Angiogenesis and cytokines that are caused by inflammation and oxidative stress may be the cause of the thickening of the choroidal layer in the early stages of DR. This is because there is a possibility that these cytokines produce an excessive quantity of expression. These cytokines include platelet-derived growth factor, monocyte chemotactic protein-1, VEGF, pigment epithelium-derived factor, and insulin-like growth factor 1, among others. Using Masson’s trichrome staining to examine the choroidal arteries from the choroidal tissues of diabetic rats revealed that the vessel wall had become thicker and there were more collagen fibers deposited in the wall of the vessel. A narrowing of the lumen was also observed in rats with diabetes. The buildup of collagen can result in the stenosis of the arteries. Recent collagen synthesis has the potential to act as a substrate, which can result in luminal narrowing[29]. A narrowing of the arteries that provide blood to the body, particularly the head, face, and brain, is referred to as arterial stenosis[30]. In diabetic patients, vascular problems are the result of the deterioration of the vascular wall[30]. Since these histopathological alterations cause blood vessels to become rigid, they are unable to respond to either exogenous or endogenous stimuli, which prevents them from being able to efficiently regulate blood flow[31].
This study used induced diabetic rats as subjects and used SEM to show that the choroidal arteries were changed in a way that was not seen in any of the other five groups of rats. Choroidal damage is increasing in rats with diabetes mellitus. The choroid micro-vasculature is made up of choroid arteries, choriocapillaris, short-running arterioles and venules, and choroid arteries. The choroid arteries and the choriocapillaris were observed to have tortuosity, shrinkage, and widespread constriction. The choriocapillaris, which is the smallest vessel, was the most severely affected by the damage. In diabetic eyes, the choriocapillaris can become blocked, the blood vessels can change shape with more vascular tortuosity, the blood vessels can drop out, there can be areas of vascular non-perfusion, and the choroidal neovascularization can occur. These findings were strikingly comparable to those observed in a previous study. Hiday at and fine[32], who were the first to propose the idea of diabetic choroidopathy, discovered capillary dropout and choroidal neovascularization in the enucleated eyes of diabetic patients. They used light and electron microscopy to make their observations. When the choriocapillaris is damaged, it can cause substantial damage to the function of the retinal tissue, particularly in the macula fovea.
All the gaps between the capillaries were not regular. Both the arterioles and the venules exhibited a significant amount of loss. Given the observations, there is indication that choroidal neovascularization has taken place. Higher blood glucose levels are the main cause of microvascular complications in diabetes, such as retinopathy, nephropathy, and neuropathy. Numerous studies have demonstrated that hyperglycemia has a detrimental effect on the endothelium’s functioning and causes pathological alterations that are associated with diabetes. In endothelial cells, hyperglycemia activates four primary molecular signaling systems. These mechanisms are in the cell membrane. Some of these turn on protein kinase C (PKC), speeding up the hexosamine pathway, making more advanced glycation end-products, and speeding up the polyol pathway[33]. PKC, for instance, affects the activation of a variety of growth factors and alters the activity of vasoactive factors in the context of diabetes microvascular problems. Vasoactive factors include vasodilators like nitric oxide as well as vasoconstrictors like angiotensin II and endothelin 1. These vasoactive factors are responsible for relaxing blood vessels.
VEGF plays a significant role in the process of neural regeneration and angiogenesis that takes place after an ischemic stroke[34]. In addition, it has been demonstrated that VEGF, which is the main regulator of angiogenesis, may directly modulate the size of lumens. Lumen diameter is a reaction to blood pressure and blood flow, which influence the delivery of oxygen and immune surveillance that can occur. Furthermore, VEGF and Ang I are capable of inducing hyp
The mechanism, in conjunction with the decreased permeability of Bruch’s membrane, which is observed with advancing age, causes a reduction in the amount of oxygen that is supplied to the retina as an individual ages[35]. However, this can also result in choroidal neovascularization. Retinal and RPE cells are responsible for the release of VEGF, which results in the dilation of the choroidal capillaries and an increase in blood flow. Diabetes, in general, is associated with decreased cellular proliferation and endothelial cells dysfunction, which causes angiogenesis to be impaired[36]. The pathogenesis of DR has been linked to several pro-angiogenic cytokines, such as insulin-like growth factor I and platelet-derived growth factor. However, VEGF is generally acknowledged as the most important cytokine in the process of driving DR. Ren-in-angiotensin and peroxisome proliferator-activated receptor gamma are two more pathways that are taken into consideration. Reactive oxygen species (ROS) levels rise, which creates interleukin (IL)-1, IL-6, IL-8, monocyte chemoattractant protein-1, inducible nitric oxide synthase, interferon-γ inducible protein 10, matrix metalloproteinases (especially MMP9), and tumor necrosis factor-α. These ROS cause inflammation at the cellular level[37]. Intercellular adhesion molecule 1 (CD54) or E-selectin (CD62E); vascular cell adhesion protein 1 or CD106; and platelet-endothelial cell adhesion molecule 1 or CD31 are examples of endothelial adhesion molecules that are in-creased in endothelial cells. CD31 is considered a potential target for atherosclerosis since it is considered a proinflammatory and, thus, a proatherosclerotic molecule. Additionally, CD31 is involved in a wide variety of other biological processes, such as angiogenesis, apoptosis, platelet aggregation, and thrombosis[38]. It is important for the vascular endothelium to be able to survive and respond properly to different types of mechanical, immune, and metabolic stresses[39].
Additionally, the beneficial therapeutic impact of gymnemic acid, which is the active component generated by Gymnema sylvestre, has been examined, and it was discovered that gymnemic acid was present in every region of the plant. Rats that were given gymnemic acid had lower levels of ROS and higher levels of an-tioxidants like glutathione, glutathione peroxidase, catalase, and MDA, and these levels decreased[40]. The glycolysis pathway relies heavily on the enzyme known as gymnemic acid. It has been discovered that it interacts with glyceraldehyde-3-phosphate dehydrogenase. A favorable therapeutic action of glabridin isolated from licorice is that it increases the activity of SOD considerably, while simultaneously lowering the levels of MDA in the liver, kidney, and pancreas. The compound known as glabridin is a powerful anti-inflammatory drug, antioxidant, and free radical scavenger[41]. So, the hypoglycemic effects of glabridin and gymnemic acid in rats that were given STZ may be linked to the antioxidant effects of these two compounds, at least in part. It is important to note that the retinal structures and functions are closely linked. The blood retinol barrier degradation is significantly influenced by the elevated VEGF level that is induced by ROS. The structural alterations (the loss of intercellular junctions, pericyte apoptosis) and functional changes (the changes in blood flow, the increase in vessel permeability) that are mediated by oxidative stress are correlated and both contribute to the progre
This study demonstrates that diabetic rats with high blood sugar levels are more likely to exhibit pathological alterations in the choroid of the eye than rats without diabetes. It was found that when animals were given glabridin at a dose of 40 mg/kg and gymnemic acid at a dose of 400 mg/kg, the levels of VEGF and CD31 proteins decreased. This treatment also supported the recovery process. Glabridin and gymnemic acid are re-sponsible for the histological condition that affects the tissues and blood vessels in the choroidal layer. It has the impact of lowering the choroid thickness, reducing the thickness of the wall of choroid vessels, and lowering neovascularization, all of which are significant contributors to the development of diabetes in the eyes. The use of glabridin, an important extract from licorice, and gymnemic acid, an important extract from Gymnema sylvestre, to treat, ease, or prevent diabetic choroid disease is therefore something that should be implemented as it constitutes an additional therapy alternative. In addition, obtaining a wide range of information and expertise on this topic will be advantageous to patients in the future. The results of the current study indicate that additional research is necessary to evaluate the mechanism and pharmacological actions of glabridin and gymnemic acid in DR, as well as to determine whether they may be beneficial as a therapeutic agent in the treatment of diabetes.
| 1. | Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 632] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 2. | Liu R, Li L, Shao C, Cai H, Wang Z. The Impact of Diabetes on Vascular Disease: Progress from the Perspective of Epidemics and Treatments. J Diabetes Res. 2022;2022:1531289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 4. | Hecht I, Yeshurun I, Bartov E, Bar A, Burgansky-Eliash Z, Achiron A. Retinal layers thickness changes following epiretinal membrane surgery. Eye (Lond). 2018;32:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Keskin Ç, Dilekçi ENA, Üçgül AY, Üçgül RK, Toprak G, Cengiz D. Choroidal vascularity index as a predictor for the development of retinopathy in diabetic patients. J Endocrinol Invest. 2024;47:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Qin Y, Wang S, Liu Y, Li X, Sun X. Higher choroidal thickness and lower choriocapillaris blood flow signal density based on optical coherence tomography angiography in diabetics. Sci Rep. 2021;11:5799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris Nonperfusion is Associated With Poor Visual Acuity in Eyes With Reticular Pseudodrusen. Am J Ophthalmol. 2017;174:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 9. | Khimmaktong W, Petpiboolthai H, Sriya P, Anupunpisit V. Effects of curcumin on restoration and improvement of microvasculature characteristic in diabetic rat's choroid of eye. J Med Assoc Thai. 2014;97 Suppl 2:S39-S46. [PubMed] |
| 10. | Rodrigues AC, Schellini SA, Gregório EA, Spadella CT, Padovani CR. Choroidal vasculature in diabetic rats. J Submicrosc Cytol Pathol. 2004;36:327-331. [PubMed] |
| 11. | Johnson MA, Lutty GA, McLeod DS, Otsuji T, Flower RW, Sandagar G, Alexander T, Steidl SM, Hansen BC. Ocular structure and function in an aged monkey with spontaneous diabetes mellitus. Exp Eye Res. 2005;80:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts--technique and applications: updated review. Scanning Microsc. 1990;4:889-940; discussion 941. [PubMed] |
| 13. | Patanè G, Piro S, Anello M, Rabuazzo AM, Vigneri R, Purrello F. Exposure to glibenclamide increases rat beta cells sensitivity to glucose. Br J Pharmacol. 2000;129:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Oh CS, Kohanim S, Kong FL, Song HC, Huynh N, Mendez R, Chanda M, Edmund Kim E, Yang DJ. Sulfonylurea receptor as a target for molecular imaging of pancreas beta cells with (99m)Tc-DTPA-glipizide. Ann Nucl Med. 2012;26:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Petit P, Loubatières-Mariani MM. Potassium channels of the insulin-secreting B cell. Fundam Clin Pharmacol. 1992;6:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Patel K, Gadewar M, Tripathi R. Pharmacological and analytical aspects of gymnemic acid: a concise report. Asian Pac J Trop Dis. 2012;2:414-416. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Sandech N, Jangchart R, Komolkriengkrai M, Boonyoung P, Khimmaktong W. Efficiency of Gymnema sylvestre-derived gymnemic acid on the restoration and improvement of brain vascular characteristics in diabetic rats. Exp Ther Med. 2021;22:1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Komolkriengkrai M, Nopparat J, Vongvatcharanon U, Anupunpisit V, Khimmaktong W. Effect of glabridin on collagen deposition in liver and amelioration of hepatocyte destruction in diabetes rats. Exp Ther Med. 2019;18:1164-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Wu F, Jin Z, Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7:1278-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Komolkriengkrai M, Jangchart R, Sandech N, Vongvatcharanon U, Khimmaktong W. Beneficial effects of gymnemic acid on three-dimensional vascular architecture and expression of vascular endothelial growth factor of intrarenal segmental and interlobar arteries in diabetic rat kidney. FFHD. 2022;12:340. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Gandhi GR, Sasikumar P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Fu J, Wei C, Zhang W, Schlondorff D, Wu J, Cai M, He W, Baron MH, Chuang PY, Liu Z, He JC, Lee K. Gene expression profiles of glomerular endothelial cells support their role in the glomerulopathy of diabetic mice. Kidney Int. 2018;94:326-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 23. | Andonova M, Dzhelebov P, Trifonova K, Yonkova P, Kostadinov N, Nancheva K, Ivanov V, Gospodinova K, Nizamov N, Tsachev I, Chernev C. Metabolic Markers Associated with Progression of Type 2 Diabetes Induced by High-Fat Diet and Single Low Dose Streptozotocin in Rats. Vet Sci. 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1264] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 25. | Sarkhail P, Rahmanipour S, Fadyevatan S, Mohammadirad A, Dehghan G, Amin G, Shafiee A, Abdollahi M. Antidiabetic effect of Phlomis anisodonta: effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacol Res. 2007;56:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Endo H, Kase S, Takahashi M, Saito M, Yokoi M, Sugawara C, Katsuta S, Ishida S, Kase M. Relationship between diabetic macular edema and choroidal layer thickness. PLoS One. 2020;15:e0226630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Li Y, Xiao Y, Gao W, Pan J, Zhao Q, Zhang Z. Gymnemic acid alleviates inflammation and insulin resistance via PPARδ- and NFκB-mediated pathways in db/db mice. Food Funct. 2019;10:5853-5862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Wang W, Liu S, Qiu Z, He M, Wang L, Li Y, Huang W. Choroidal Thickness in Diabetes and Diabetic Retinopathy: A Swept Source OCT Study. Invest Ophthalmol Vis Sci. 2020;61:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Enache AL, Slujitoru AS, Pintea IL, Stocheci CM, Mateescu GO, Gheorghişor I. Histological and immunohistochemical aspects of cerebral vessels of the elderly. Rom J Morphol Embryol. 2012;53:1043-1050. [PubMed] |
| 32. | Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985;92:512-522. [PubMed] |
| 33. | Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, Dailey G, Gerich J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA. 1995;273:1855-1861. [PubMed] |
| 34. | Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 36. | Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 360] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 37. | Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 539] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 38. | Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Ibrahim A, Onyike E, Nok AJ, Umar IA. Combined Effect on Antioxidant Properties of Gymnema Sylvestre and Combretum Micranthum Leaf Extracts and the Relationship to Hypoglycemia. ESJ. 2017;13:266. [DOI] [Full Text] |
| 41. | Li C, Li T, Zhu M, Lai J, Wu Z. Pharmacological properties of glabridin (a flavonoid extracted from licorice): A comprehensive review. J Funct Foods. 2021;85:104638. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Tiwari P, Mishra BN, Sangwan NS. Phytochemical and pharmacological properties of Gymnema sylvestre: an important medicinal plant. Biomed Res Int. 2014;2014:830285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/