INTRODUCTION
Diabetic kidney disease (DKD) is a prevailing microvascular consequence associated with diabetes mellitus. It affects 25%-40% of the diabetic population[1] and is a prevalent cause of end-stage renal disease (ESRD) globally[2]. DKD pathophysiology is multifactorial, involving metabolic dysregulation, oxidative stress, and inflammatory responses, which interdependently exacerbate the disease[3,4]. Pathological changes in DKD renal tissues include tubular damage, glomerulosclerosis, and interstitial fibrosis[5]. Contemporary therapies for DKD emphasize the regulation of blood pressure and glucose levels, with inhibition of the renin–angiotensin–aldosterone system. While these methods may postpone DKD progression, they cannot entirely avert progression to ESRD. Consequently, it is essential to investigate novel treatment targets for DKD to improve quality of life for DKD patients and alleviate the related social and economic burdens.
Pyroptosis is an innovative process of intrinsic cell death activated via classical and noncanonical pathways. NOD-like receptor protein 3 (NLRP3) is a sensor protein that functions in signal detection within the traditional pyroptosis pathway, which is closely linked to DKD progression[6,7]. When receiving dangerous stimuli such as immune activation or environmental stresses, activated NLRP3 recruits apoptosis-associated speck-like protein containing a CARD and cysteinyl aspartate specific proteinase (caspase-1) together to form the NLRP3 inflammasome complex[8,9]. Activated NLRP3 inflammasome promotes caspase-1 cleavage in the complex to its active form cleaved-caspase-1. Cleaved-caspase-1 further promotes the formation of N-terminal structural domain of gasdermin D (GSDMD-N) and interleukin (IL)-1β/-18, leading to cell death and inflammatory responses[10,11]. NLRP3 is activated in HK-2 cells treated with elevated high glucose (HG), which increases cleaved-caspase-1 and IL-1β expression[12]. Blocking NLRP3 could mitigate NLRP3 inflammasome activation, which is protective against podocyte damage[13].
Renal fibrosis is one of the most critical processes in CKD, which results from metabolic dysfunction, oxidative stress and inflammatory response to ESRD[14,15]. It is mainly manifested by accumulating renal epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) and collagen deposition (collagen I and III), accompanied by secretion of transforming growth factor-β1 (TGF-β1)[16]. The signaling cascade pathways implicated in fibrosis formation in DKD are intricate and varied. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway is a critical factor in diabetic renal fibrosis[17-19]. More aggressive research into novel drugs or molecules that can inhibit NLRP3 activity and the PI3K/AKT pathway to mitigate pyroptosis and fibrosis may be an effective strategy for treating DKD.
The nuclear receptor subfamily 4 group A (NR4A) comprises a class of early response genes found in many tissues or cells that encode proteins. NR4A member 1 (NR4A1) is now the most recognized member of the NR4A family[20]. It is also termed an orphan nuclear receptor because it lacks a natural endogenous ligand. NR4A1 is widely involved in bioregulatory processes such as autophagy[21], apoptosis[22,23] and mitochondrial fission[24]. NR4A1 expression is raised in response to chronic hyperglycemic stimulation, and the suppression of NR4A1 reduces renal collagen fiber deposition in DKD mice[25]. NR4A1 downregulation hinders PI3K/AKT pathway activation in colorectal cancer cells[26]. The connection between NR4A1 and the PI3K/AKT pathway in diabetic renal fibrosis has yet to be clarified. Numerous studies have shown that NR4A1 suppression may restrict NLRP3 inflammasome-mediated pyroptosis and inflammation in numerous disease models[27,28]. The connection between NR4A1 and NLRP3-mediated pyroptosis in the setting of DKD is still ambiguous.
Based on the above evidence, we developed in vivo and in vitro DKD models in streptozotocin (STZ)-stimulated Sprague-Dawley rats and HG-activated HK-2 cells, respectively. This work sought to examine the influence of NR4A1 on diabetic renal pyroptosis and fibrosis, as well as the contribution of activated NLRP3 and the PI3K/AKT pathway in these pathogenic processes, to provide more effective targets for DKD treatment.
MATERIALS AND METHODS
Establishment of DKD rat models
Eighteen male specific pathogen-free Sprague–Dawley rats, weighing 180-220 g, aged 6-8 weeks, were obtained from Jinan Pengyue Co. (Jinan, China). The study protocol was approved by the Ethics Committee of Medical Science Research of the Affiliated Hospital of Jining Medical University (approval number: 2023-10-B008) and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals proposed by the Chinese National Institutes of Health. After 7 days of normal feeding, all rats were randomly assigned to the control (CON) and DKD groups. The DKD rats were administered a single intraperitoneal dose of STZ (60 mg/kg; Solarbio, Beijing, China), while those in the CON group were given the same volume of citrate buffer. Rats with tail vein blood glucose (BG) levels > 16.7 mmol/L, assessed twice 72 hours after STZ injection, were designated as type 1 diabetic rats. All rats were monitored for food consumption and urine output. BG, microalbumin (mALB) and body weight were quantified every 2 weeks. Approximately 6 weeks following diabetes induction, the DKD group of rats exhibited increased urine output, significant weight loss, elevated mALB and sustained BG ≥ 16.7 mmol/L. At this stage, the DKD model endpoint was considered to be achieved[29,30], and all rats were over anesthetized by intraperitoneal injection of 1 mL/kg 5% pentobarbital sodium.
Detection of biochemical indicators
BG levels from the tail vein were assessed with an BG meter (Roche Diagnostics, Basel, Switzerland). Twenty-four-hour urine samples were obtained from the metabolic cage, and mALB was quantified with a urine mALB kit. The heart was punctured for blood collection, and the level of serum creatinine (Scr) was determined after anesthesia induction. The kidney index was calculated by weighing the kidneys and body weight of each rat.
Pathomorphological examination
Rat kidney tissue specimens were collected and fixed in a 4% paraformaldehyde solution (Beyotime, Shanghai, China) for 24 hours. Paraffin-immersed slices were sliced into 5-μm segments. Hematoxylin and eosin (H&E), Masson's trichrome staining (Masson), and periodic acid-Schiff (PAS) were used to examine pathological modifications in the kidneys.
Cell culture and treatment
In the normal control (NC; 5.5 mmol/L glucose) group, HK-2 cells (ATCC CRL-2190) were obtained from Wuhan Pricella Biotechnology Co. Ltd. (China) and cultivated in HK-2 specialized medium enriched with 5.5 mmol/L glucose. Upon achieving 70% cell fusion, HK-2 cells were grown in a 45 mmol/L HG medium for 48 hours to simulate DKD (HG group).
Transfection of siRNA
siRNA directed against NR4A1 (si-NR4A1) and a negative control (si-Ctrl) were manufactured by Shanghai GenePharma Co. Ltd. (China). Lipofectamine 3000TM (Invitrogen, Carlsbad, CA, United States) was administered to HK-2 cells for 6 hours. The medium was substituted with HG-free or HG-containing medium to sustain the culture. Cells were collected for subsequent experiments after 24-36 hours.
Immunocytochemistry
When cell growth reached 60%-70%, the various treatments were administered to each group. Prior to overnight incubation at 4 °C with an anti-IL-1β antibody (Affinity, Jiangsu, China) or an anti-collagen III antibody (Affinity), the cells were fixed with a 4% paraformaldehyde solution (Beyotime). The cells were permeabilized with 0.5% Triton (Solarbio) and subsequently blocked with 10% goat serum (Beyotime). The cells were counterstained with DAPI (Beyotime) and treated with a fluorescent secondary antibody (Affinity) for 30 minutes to visualize the nuclei. A fluorescence microscope (Leica, Germany) was used to acquire the images.
Western blotting
RIPA lysate solution (Beyotime) was used to isolate the overall protein from rat kidney tissue and HK-2 cells. After BCA quantification, all protein were denatured by boiling, fractionated using SDS-PAGE, and moved onto polyvinylidene fluoride membranes. After addition of 5% skimmed milk powder for blocking, the membranes were immersed in primary antibody overnight at 4 °C. The membranes were immersed in secondary antibody (Affinity) for 1 hour. The bands were identified with an enhanced chemiluminescence detection kit (Proteintech, Wuhan, China) and assessed with ImageJ software. Supplementary Table 1 presents the specifics of the main antibody.
Quantitative real-time PCR
The RNA was isolated from rat kidney specimens and HK-2 cells with an RNA fast200 reagent (Feijie, Shanghai, China). cDNA was synthesized using a 5 × All-In-One RT MasterMix reagent (Abm, Nanjing, China). Quantitative real-time PCR (qRT-PCR) was conducted with a 2 × Ultra SYBR Mixture reagent (Cowin, Beijing, China). Relative gene expression was ascertained with the 2−ΔΔCt approach. The primer information (Huada Gene, Beijing, China) of the detected genes is presented in Supplementary Table 2.
Enzyme-linked immunosorbent assay
Rat serum was centrifuged at 12000 rpm for 15 minutes to remove particles and stored at -80 °C. All samples were rewarmed to room temperature and assayed using an Enzyme-linked immunosorbent assay (ELISA) kit (Uping Bio, Hangzhou, China) according to the manufacturer's instructions. Concentration values were calculated using a four-parameter logistic curve fitting method.
Statistical analysis
All data are stated as mean ± SD. Statistical analysis and charting were conducted with GraphPad Prism 9.5.0 software (San Diego, CA, United States). Student's t-test was implemented to compare the data of two groups, whereas one-way ANOVA was implemented to examine the data of several groups. P < 0.05 was deemed significant.
RESULTS
Metabolic indices and renal histopathological changes in DKD rats
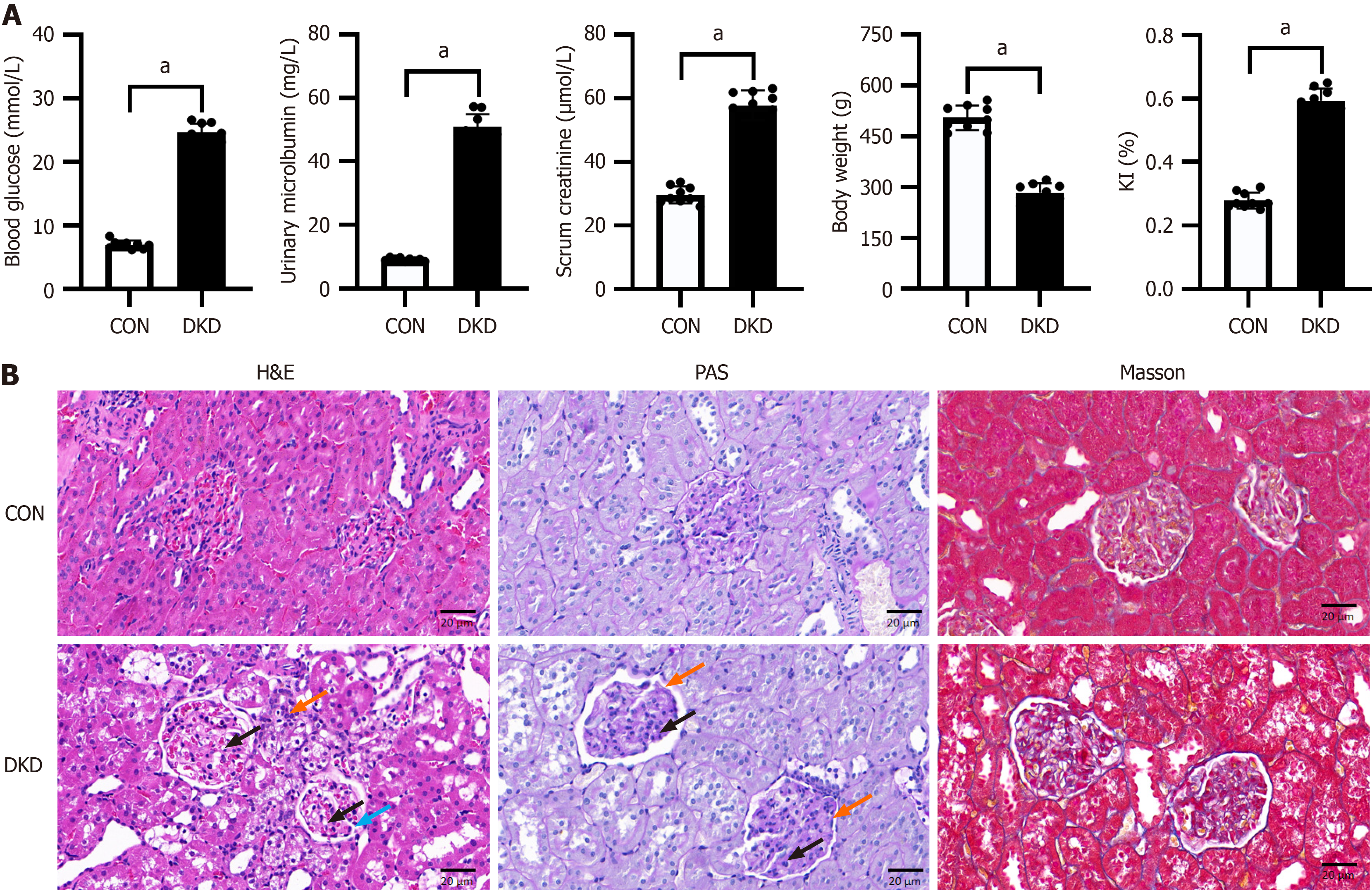
Contrasted with the CON group, rats in the DKD group showed elevated levels of BG, mALB and Scr, together with weight loss (Figure 1A), confirming the effective establishment of the DKD model. H&E and PAS staining revealed that the CON group possessed distinct, orderly glomeruli and tubules, devoid of inflammatory alterations. In contrast, the glomeruli in the DKD group had a lobular structure with vacuolated cytoplasm, hyperplasia of the mesangial matrix, basement membrane thickening, and inflammatory cell infiltration in the periphery. Afterward, the renal tissues of the DKD group had significantly elevated collagen fiber deposition, in contrast with the CON group, as seen by Masson's staining (Figure 1B). These findings were consistent with the pathophysiological process of DKD.
Figure 1 Metabolic indices and renal pathological alterations in diabetic kidney disease rats.
A: Comparison of blood glucose, urinary microalbumin, blood creatinine, body weight, and renal index of rats; B: Representative images of histopathological changes in the rat kidneys. Hematoxylin and eosin: Lobular structure (blue arrow), vacuolated cytoplasm (black arrow), inflammatory cell infiltration in the periphery (orange arrow); Periodic acid-Schiff: Hyperplasia of the mesangial matrix (black arrow), basement membrane thickening (orange arrow). Scale bar = 20 μm, n = 9. aP < 0.05 vs control. CON: Control; DKD: Diabetic kidney disease; KI: Kidney index.
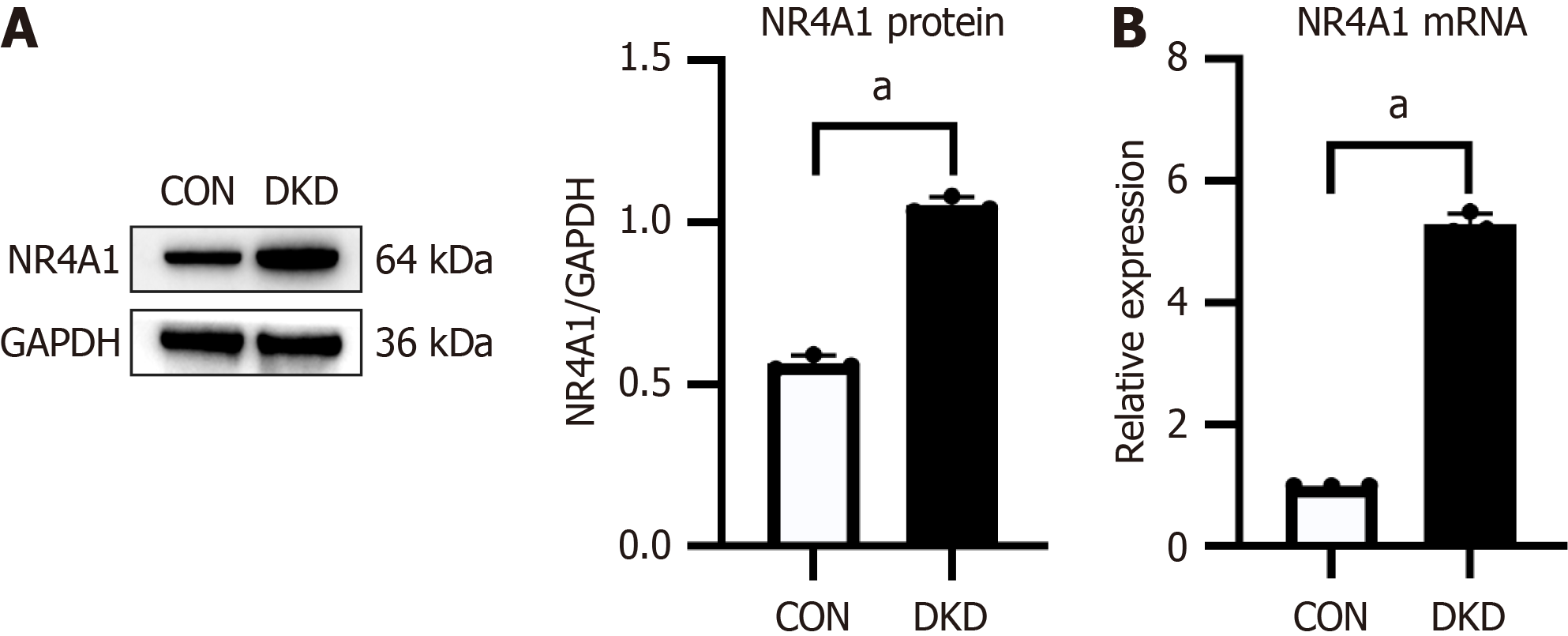
Upregulation of NR4A1 in DKD rats
The translational and transcriptional levels of NR4A1 in rat kidney tissues were assessed by western blotting and qRT-PCR, respectively. NR4A1 protein and mRNA levels in the DKD group were significantly elevated compared with those in the CON group (Figure 2), indicating that NR4A1 was overexpressed in the renal tissue of DKD rats.
Figure 2 Nuclear receptor subfamily 4 group A member 1 expression was upregulated in kidney tissue of rats with diabetic kidney disease.
A: Representative western blotting bands and integrated density study of nuclear receptor subfamily 4 group A member 1 (NR4A1) in rat kidney tissues; B: Quantitative real-time PCR of NR4A1 mRNA expression in rat kidney tissues, n = 3. aP < 0.05 vs control. CON: Control; DKD: Diabetic kidney disease; NR4A1: Nuclear receptor subfamily 4 group A member 1.
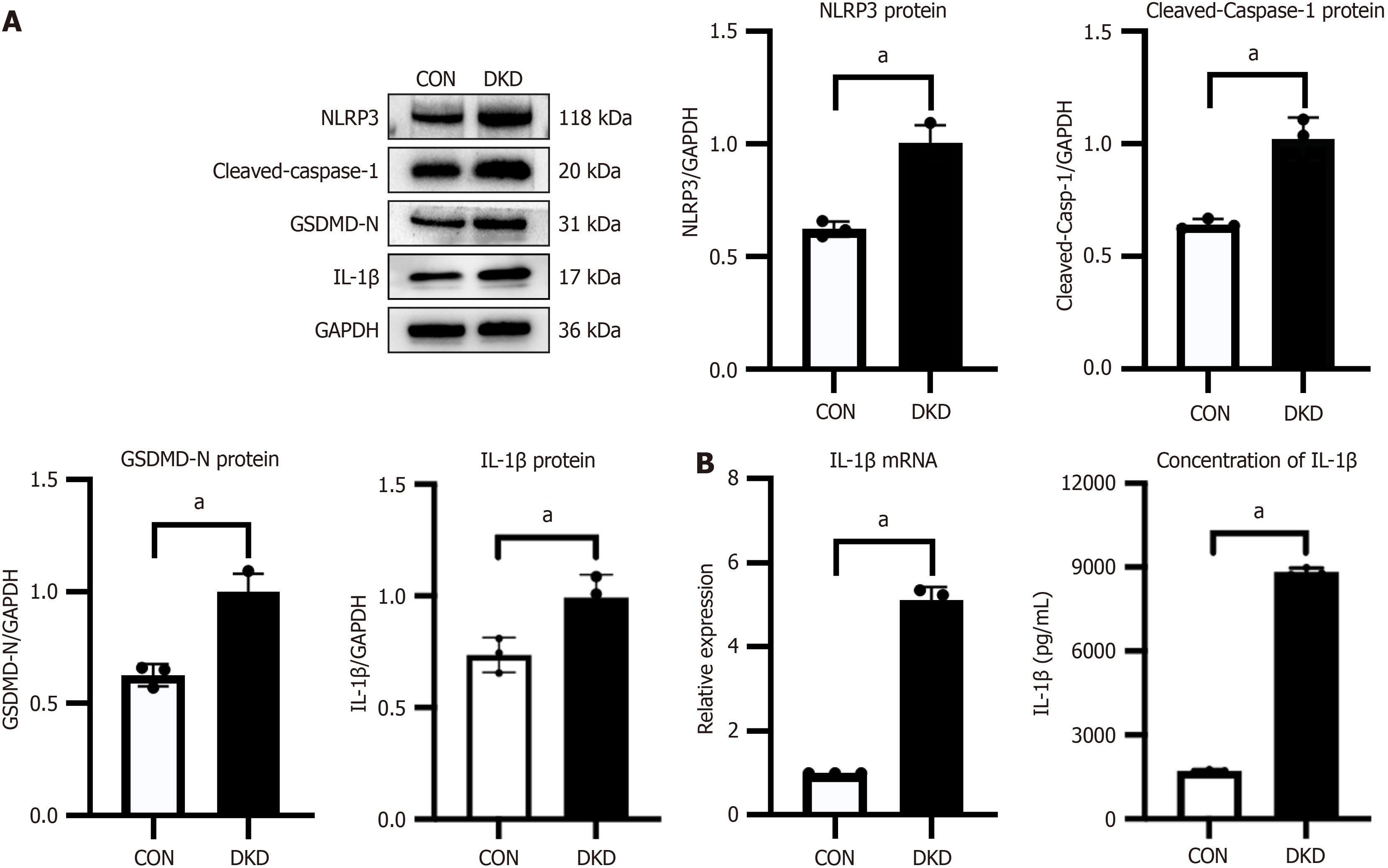
Elevated levels of markers of NLRP3-mediated pyroptosis in DKD rats
Western blotting was performed to estimate the NLRP3 and pyroptosis-associated protein expression in rat kidney tissue samples. The DKD group exhibited significant elevation of NLRP3, cleaved-caspase-1, IL-1β and GSDMD-N protein expression compared with the CON group (Figure 3A). The DKD group had an increase in IL-1β mRNA levels, pro-inflammatory factor. Meanwhile, a significant increase in IL-1β secretion was detected in DKD rats serum by the ELISA assay (Figure 3B). The findings indicated that NLRP3-mediated pyroptosis contributed to DKD etiology.
Figure 3 Markers of NOD-like receptor protein 3-mediated pyroptosis were increased in rats with diabetic kidney disease.
A: Representative western blotting bands and integrated density analysis of NOD-like receptor protein 3, cleaved-caspase-1, and gasdermin D N-terminal structural domain in rat kidney tissues; B: Quantitative real-time PCR of interleukin (IL)-1β mRNA expression in rat kidney tissues and concentration of IL-1β in rat serum, n = 3. aP < 0.05 vs control. CON: Control; DKD: Diabetic kidney disease; GSDMD-N: Gasdermin D N-terminal structural domain; IL-1β: Interleukin-1β; NLRP3: NOD-like receptor protein 3.
Upregulation of the markers of PI3K/AKT pathway-mediated fibrosis in DKD rats
The critical protein expression levels in the PI3K/AKT pathway and fibrosis markers were assessed in rat kidneys using western blotting, to ascertain the degree of STZ-stimulated kidney fibrosis and the potential mechanisms involved. The findings indicated that the DKD group had significant elevation of PI3K and AKT phosphorylation levels compared with the CON group (Figure 4A). The DKD group had a significant increase in TGF-β1 and collagen III levels (Figure 4B).
Figure 4 Markers of PI3K/AKT signaling pathway-mediated fibrosis were upregulated in rats with diabetic kidney disease.
A: Representative western blotting and integrated density study of AKT, p-AKT, PI3K, and p-PI3K in rat kidney tissues; B: Representative western blotting and integrated density study of TGF-β1 and collagen III in rat kidney tissue, n = 3. aP < 0.05 vs control. AKT: Protein kinase B; CON: Control; DKD: Diabetic kidney disease; PI3K: Phosphoinositide 3-kinase; TGF-β1: Transforming growth factor β1.
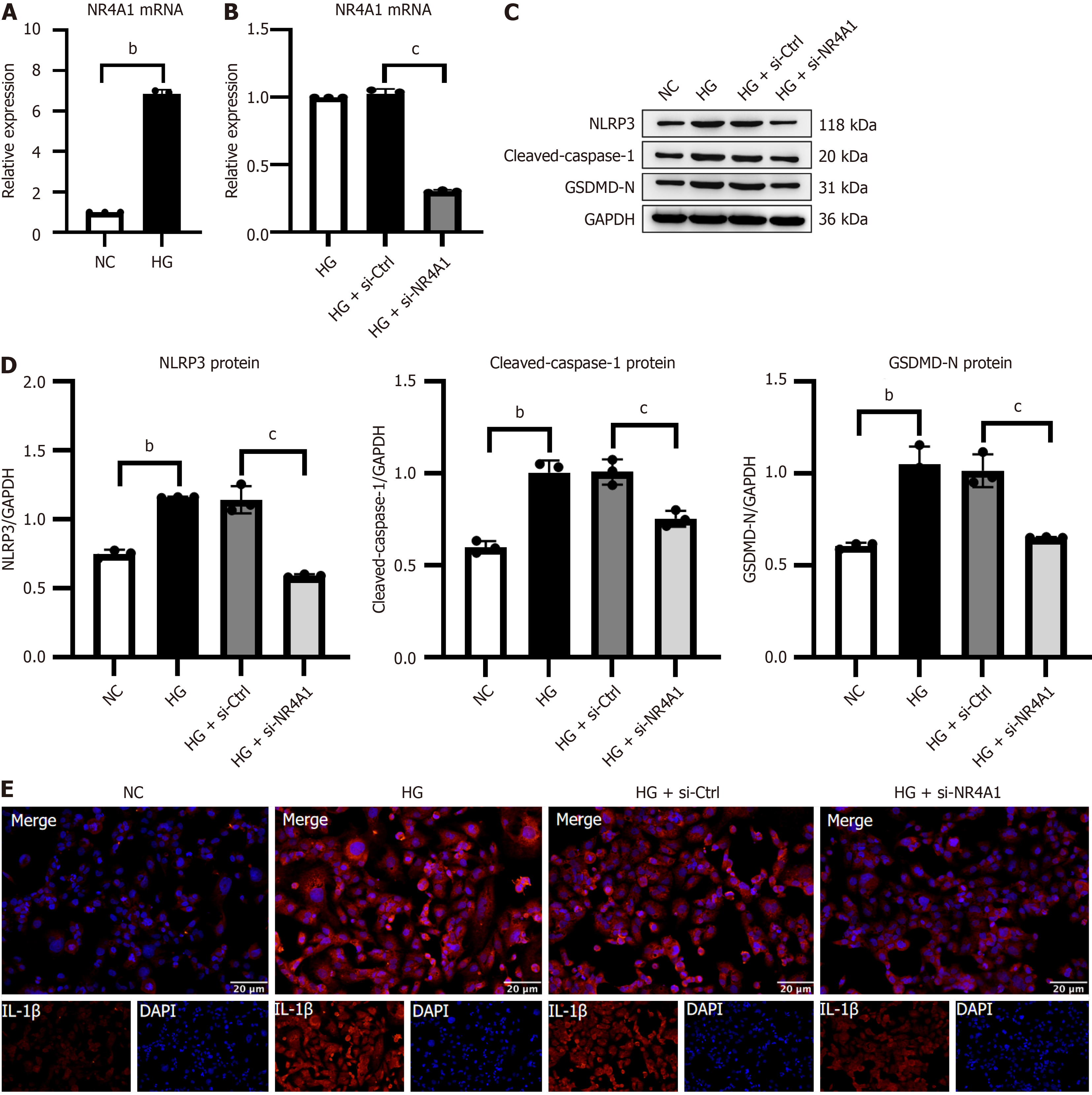
Silencing NR4A1 attenuated NLRP3-mediated pyroptosis in vitro
Chronic hyperglycemia promotes DKD development. We generated an in vitro DKD model using the traditional HG intervention in HK-2 cells to confirm NR4A1 expression. NR4A1 silencing was induced in HK-2 cells using NR4A1 siRNA interference to examine the impact of NR4A1 downregulation on NLRP3-associated pyroptosis.
Initially, qRT-PCR revealed that the HG group had significant elevation in NR4A1 mRNA levels compared with the NC group (Figure 5A). The HG+si-NR4A1 group had significantly mitigated NR4A1 mRNA level compared with the HG + si-Ctrl group. There was no significant variation in NR4A1 Levels between the HG and HG + si-Ctrl groups, demonstrating that the siNR4A1 HK-2 cell model was successfully established and that the transfection process did not cause any other significant disturbances (Figure 5B). Additionally, immunocytochemistry and western blotting showed that the HG group had significantly elevated NLRP3 expression and pyroptosis-linked GSDMD-N, cleaved-caspase-1 and IL-1β compared with the NC group. The HG + si-NR4A1 group possessed an inverted expressions as opposed to the HG + si-Ctrl group (Figure 5C-E).
Figure 5 Silencing of NR4A1 attenuated NOD-like receptor protein 3-mediated pyroptosis in high glucose-stimulated HK-2 cells.
A and B: Quantitative real-time PCR of NR4A1 mRNA expression and siRNA transfection efficiency in HK-2 cells; C and D: Illustrative western blotting and integrated density study of NOD-like receptor protein 3, cleaved-caspase-1, and gasdermin D N-terminal structural domain in HK-2 cells; E: Illustrative immunocytochemistry images of interleukin-1β in HK-2 cells. Scale bar = 20 μm, n = 3. bP < 0.05 vs NC, cP < 0.05 vs high glucose + negative control. GSDMD-N: Gasdermin D N-terminal structural domain; NC: Normal control; HG: High glucose; NLRP3: NOD-like receptor protein 3; NR4A1: Nuclear receptor subfamily 4 group A member 1; si-NR4A1: SiRNA targeting nuclear receptor subfamily 4 group A member 1.
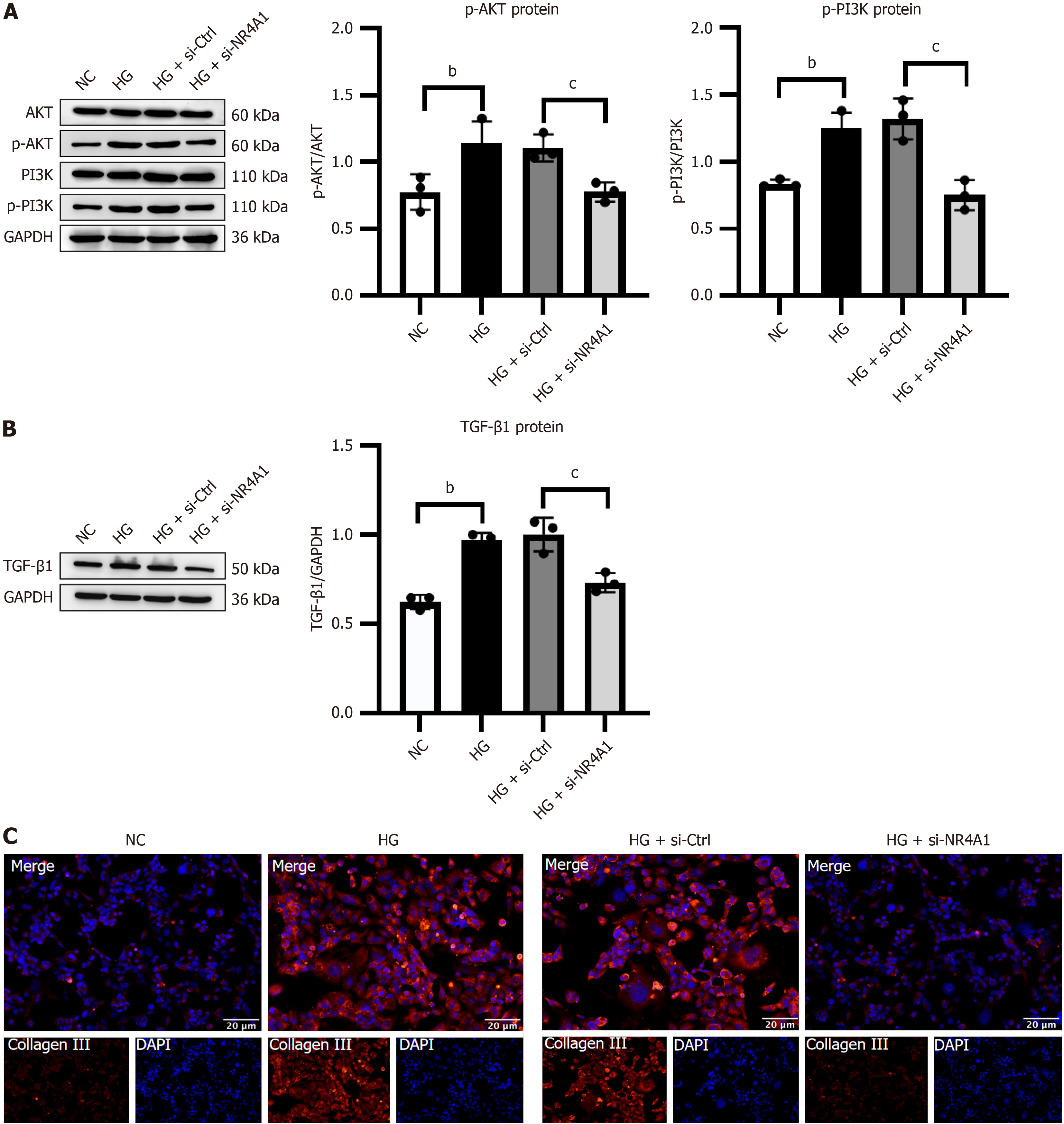
Silencing NR4A1 attenuated PI3K/AKT pathway-mediated fibrosis in vitro
NR4A1 was silenced in HK-2 cells to investigate its effect on the PI3K/AKT pathway and fibrosis. Immunocytochemistry and western blotting demonstrated that the HG group had a significant rise in p-PI3K/PI3K, p-AKT/AKT, TGF-β1, and collagen III expression compared with the NC group. The HG+si-NR4A1 group showed a significant decline in their expression compared with the HG + si-Ctrl group (Figure 6).
Figure 6 Silencing of nuclear receptor subfamily 4 group A member 1 attenuated phosphoinositide 3-kinase/protein kinase B pathway-mediated fibrosis in high glucose-activated HK-2 cells.
A: Illustrative Western blotting bands and integrated density analysis of protein kinase B (AKT), p-AKT, phosphoinositide 3-kinase (PI3K), and p-PI3K in HK-2 cells; B: Representative western blotting and integrated density analysis of transforming growth factor-β1 in HK-2 cells; C: Illustrative immunocytochemistry images of collagen III in HK-2 cells. Scale bar = 20 μm, n = 3. bP < 0.05 vs normal control, cP < 0.05 vs high glucose + negative control. HG: High glucose; NC: Normal control; NR4A1: Nuclear receptor subfamily 4 group A member 1; AKT: Protein kinase B; PI3K: Phosphoinositide 3-kinase; si-NR4A1: SiRNA targeting nuclear receptor subfamily 4 group A member 1; TGF-β1: Transforming growth factor-β1.
DISCUSSION
NR4A1 is a transcriptional regulator within the nuclear receptor superfamily, extensively present throughout many tissues and organs in the body. It has garnered attention for its role in controlling several disorders, including DKD. Several recent studies have provided different opinions on NR4A1 expression in DKD. By analyzing the sequencing data, Joshi et al[31] found that NR4A1 was upregulated in samples of patients with DKD and had a high correlation with DKD. Similarly, Sheng et al[25] revealed that NR4A1 mRNA expression was significantly elevated in renal tissue from diabetes animal models. In contrast, Wang et al[32] identified a significant decline in NR4A1 expression in an in vitro DKD model using HK-2 cells. In the present study, an STZ-stimulated DKD rat model and an HG-stimulated HK-2 cell model were successfully established, and indicated that NR4A1 was overexpressed in vivo and in vitro. DKD pathogenesis is complex and NR4A1 is likely to be a novel causative factor. In terms of pathway mechanisms, NR4A1 may promote renal interstitial fibrosis by regulating the phosphorylation of p38 mitogen-activated protein kinase[33]. Wang et al[32] demonstrated that Jumonji domain containing 1A may be an important upstream regulator of NR4A1. The process of renal tubular epithelial mesenchymal fibrosis in HK-2 cells was accelerated by the JMD1A/NR4A1 signaling activation, which was stimulated via glycosylation end products. However, these mechanisms represent only a small fraction of the potential pathogenic mechanisms of NR4A1. This investigation mainly ascertained the regulatory consequences of NR4A1 on DKD pyroptosis and fibrosis, and the possible mechanisms involved in these processes were validated and discussed.
Pyroptosis has gained considerable attention in recent years. Its main morphological features are inflammasome activation, serous membrane pore formation, and cytomembrane rupture. It is regarded as an inflammatory cell death program[13]. There are two main mechanisms for the initiation of pyroptosis; canonical and noncanonical pathways. In the former case, the NLRP3 inflammasome is triggered in response to glucose, fatty acids, cholesterol and hypoxia[34], triggering the caspase-1-dependent classical pyroptosis pathway. In contrast, the noncanonical pyroptosis signal is mainly triggered via caspase-4/5/11 activation due to stimulation by lipopolysaccharides (LPS) in the bacterial cell wall[8]. There is increasing evidence that the classical pyroptosis pathway, triggered by NLRP3 inflammasome activation, contributes to DKD pathogenesis[35,36]. NLRP3, caspase-1 and IL-1β expression is significantly elevated in DKD rat kidneys and HK-2 cells[37,38]. NLRP3-mediated pyroptosis is not an independent pathological process and is closely linked to several pathways. Aberrant mitochondrial activity that enhances the mitochondrial reactive oxygen species (mROS) formation is a significant catalyst of pyroptosis. Trimethylamine oxide may facilitate DKD via stimulating the mROS–NLRP3 axis, leading to pyroptosis[39]. Hu et al[40] found that the vascular endothelial growth factor receptor 2 (VEGFR2)/NLRP3 signaling pathway is implicated in HG-induced podocyte pyroptosis. VEGFR2 directly interacts with the pyroptosis-initiating signal, NLRP3, whereas rutaecarpine provides protection to podocytes by specifically targeting and suppressing VEGFR2. Consistent with the above findings, this study observed the metabolic signature of key molecules involved in pyroptosis mediated by NLRP3 activation both in vivo and in vitro, including cleaved-caspase-1 and mature GSDMD-N and IL-1β.
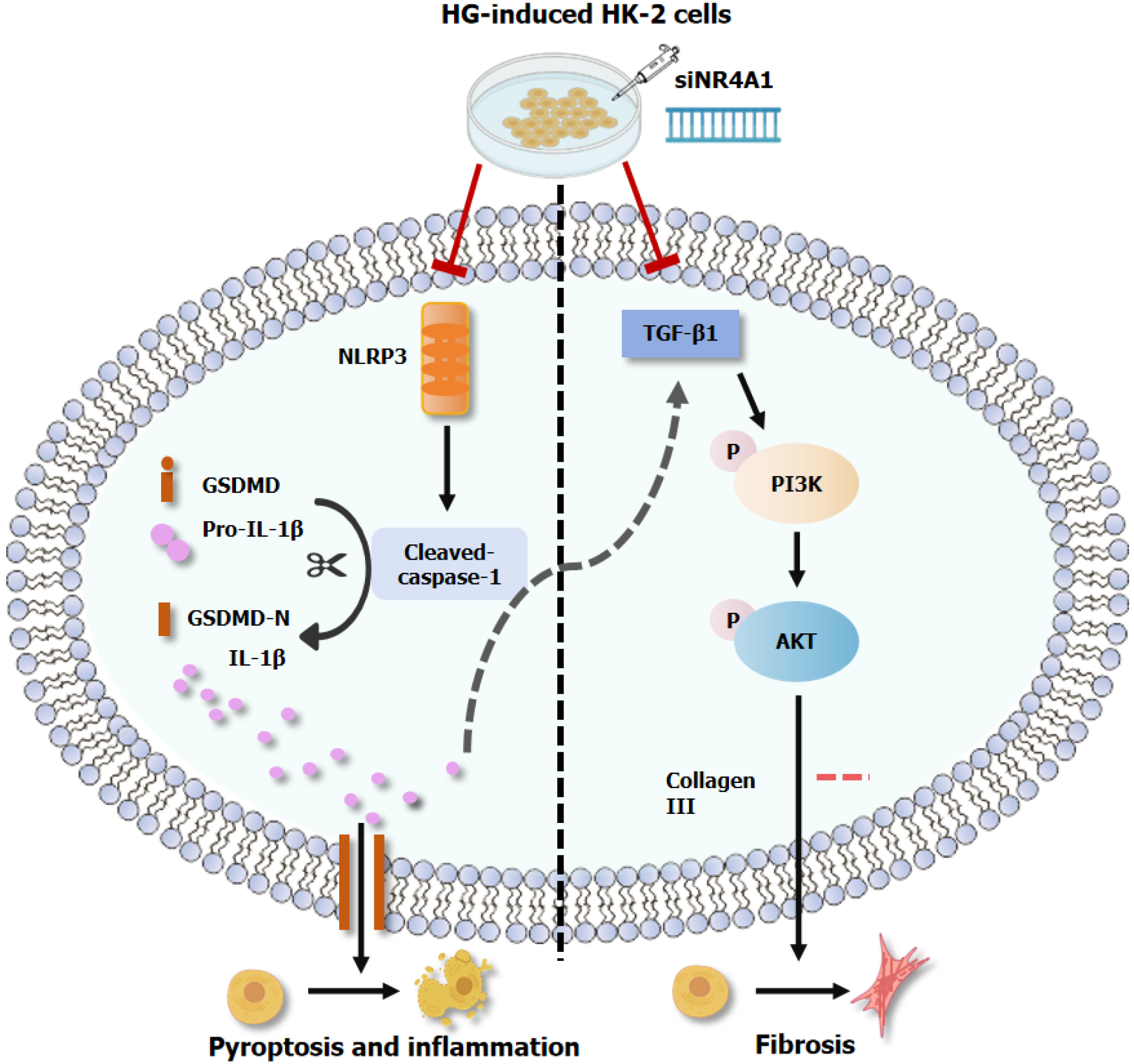
Previous studies have indicated that NR4A1 deficiency accelerates atherogenesis by intensifying NLRP3 inflammasome-driven pyroptosis and inflammation[41]. Hu et al[28] found that NR4A1 knockdown ameliorated adriamycin-induced myocardial injury by reducing the expressions of IL-1β and GSDMD through inhibition of NLRP3 inflammasome activity. A recent study[42] confirmed that NR4A1 may function as an intracellular LPS sensor. First, LPS induced the formation of serous membrane pores in macrophages via the caspase-11-dependent nonclassical pyroptosis pathway, and mitochondrial DNA flowed into the cytoplasm through the membrane pores. NR4A1 in the cytoplasm then senses and binds LPS, which further activates the NLRP3 inflammasome under the regulation of mitochondrial DNA and mediates caspase-1-dependent classical pyroptosis. Nevertheless, the inhibition of NR4A1 in vivo protected the host from developing an immune response to endotoxin. This finding also overturns the conventional impression that NR4A1 is an orphan nuclear receptor. In this study, HK-2 cells were treated with siRNA to obtain a stable si-NR4A1 cell line. Through further experiments, the silencing of NR4A1 resulted in a reduction in NLRP3-associated protein expression. These outcomes demonstrated for the first time that NR4A1 downregulation can attenuate HG-stimulated pyroptosis in HK-2 cells via inhibiting NLRP3 activation, which bridged the research gap between NR4A1 and pyroptosis in DKD.
Fibrosis is the predominant pathological alteration in DKD, resulting in nephrosclerosis, scarring, and deterioration of renal function in affected individuals. This is an inevitable and serious outcome in the steady progression of DKD. TGF-β1 is expressed in almost all renal cell types and macrophages and is a key fibrotic factor in DKD advancement. It can promote renal fibrosis by participating in processes such as enhancing collagen synthesis, inhibiting ECM degradation, and promoting the dedifferentiation of proximal tubular or endothelial cells[43]. Meng[44] observed that the TGF-β1 pathway was triggered in an animal model of DKD, whereas kidney fibrosis was significantly attenuated after TGF-β1 inhibition. There is considerable evidence that the TGF-β1/PI3K/AKT axis activation is involved in EMT development in renal tubular epithelial cells[45], fibrosis in rat lung tissue[46], and atrophic gastritis[47]. TGF-β1 stimulation resulted in the upregulation of collagen I and α-smooth muscle actin in renal pericytes and HK-2 cells, with an elevated phosphorylation level of PI3K/AKT[48,49]. Similarly, TGF-β1 was raised in HG-induced rat kidney cells and accelerated renal fibrosis through PI3K/AKT activation[48]. In contrast, Zhai et al[50] observed that the PI3K/AKT pathway was suppressed via adding the TGF-β1 inhibitor SB431542 to HG-intervened podocytes. Accordingly, TGF-β1 enhances the profibrotic cascade response by stimulating the PI3K/AKT pathway, which is a significant pathway involved in renal fibrosis. The outcomes of this investigation revealed that the TGF-β1 and collagen III expression was upregulated both in vivo and in vitro, along with a significant rise in the PI3K/AKT phosphorylation level. However, when NR4A1 was silenced in vitro, the above changes were significantly reversed. These results may indicate that fibrotic changes were present in both STZ-stimulated DKD rats and the HG-treated HK-2 cells. NR4A1 inhibition had a protective effect on DKD fibrosis, which agreed with the outcomes of a previous investigation[33]. There is increasing evidence that PI3K/AKT inhibition significantly attenuates fibrosis in DKD rat kidneys and HK-2 cells[19,51]. While NR4A1 activation facilitated bone marrow mesenchymal stromal cell proliferation and differentiation via enhancing PI3K/AKT signaling[52]. Similarly, hypoxia-induced NR4A1 suppressed Dicer/Let-7i-5p and stimulated EMT in colorectal cancer cells via triggering the PI3K/AKT pathway[26]. Here, we report the first demonstration of a positive regulatory link between NR4A1 and the PI3K/AKT pathway in DKD. Downregulation of NR4A1 may reduce TGF-β1 activity, consequently obstructing the PI3K/AKT pathway and mitigating HG-stimulated fibrosis in HK-2 cells.
Recent research indicated that NLRP3-mediated pyroptosis may facilitate pulmonary fibrosis via TGF-β1 activation and its linked pathway PI3K/AKT/mTOR[53]. It is uncertain whether NLRP3 participates in diabetic renal fibrosis via modulating the PI3K/AKT pathway.
Renal tubular epithelial cells and podocytes contain the NLRP3 inflammasome, playing a crucial contribution in initiating several aspects of renal inflammation and fibrosis[54]. Upon activation, the NLRP3 inflammasome initiates the traditional pyroptosis pathway and augments the inflammatory mediators expression by secreting proinflammatory molecules encompassing IL-1β/-18, hence facilitating renal sterile inflammation and fibrosis[38,55,56]. As a result of the chronic inflammatory condition associated with diabetes, intrinsic renal cells, encompassing tubular epithelial cells, endothelial cells, and pericytes, secrete elevated cytokines levels, encompassing TGF-β1 and connective tissue growth factor. These cytokines promote myofibroblast development and enhance renal interstitial fibrosis via producing collagen I/III, fibronectin, and other components[16]. Wu et al[56] recently reported that the knockdown of NLRP3 inhibited TGF-β1 generation and attenuated STZ-induced renal fibrosis in diabetic mice. Therefore, the high IL-1β, TGF-β1 and collagen III expression in this study may, to some extent, have been attributed to NLRP3 activation. It is reasonable to speculate that NLRP3 is involved in DKD fibrosis by promoting the IL-1β release to further activate TGF-1β and its associated PI3K/AKT signaling pathway. The exact association between pyroptosis and fibrosis in DKD requires further investigation. Furthermore, it is essential to ascertain the direct regulatory effects of NR4A1 gene on NLRP3 and PI3K/AKT. The mechanism will be verified in our future studies using immunoprecipitation, chromatin immunoprecipitation, and a luciferase reporter gene assay at the protein and transcriptional levels.
Studies of targeted NR4A1 inhibitors for the treatment of DKD have not been clarified. However, NR4A1 inhibitors have shown promising results in a variety of cancer models. Bis-indole-derived compounds (CDIMs) are a class of NR4A1 inhibitors that have shown clear antitumor growth properties in cellular models of breast, liver and lung cancer[57]. Resveratrol induces many effects in cancer similar to CDIMs. It acts as an NR4A1 receptor antagonist to inhibit NR4A1-dependent transactivation in lung cancer cells[58]. Although these drugs have not been validated for DKD treatment, these results still provide some theoretical clues to the feasibility of NR4A1-targeted drug development. However, considering the cytotoxicity and nephrotoxicity shown by CDIMs in the antitumor process, whether these drugs can be used to treat DKD and the safety of the drugs remains a major challenge. The present study demonstrated that NR4A1 silencing in vitro markedly suppressed the expression of molecules associated with pyroptosis and fibrosis. The lack of validation of NR4A1-inhibiting drugs in other animal studies or clinical trials represents a potential limitation that should be addressed in future research.