TO THE EDITOR
Diabetes has become the third largest non-communicable disease after cardiovascular disease and cancer, significant impacting human health and imposing a heavy economic burden on families and societies worldwide. Currently, China has the largest number of diabetes patients, with type 2 diabetes mellitus (T2DM) being the most prevalent. T2DM is a chronic metabolic disease characterized by sustained hyperglycemia. The pathogenesis of T2DM involves either insufficient insulin secretion or insulin resistance (IR), or both[1]. With the development of 16S ribosomal ribonucleic acid gene sequencing, macro-genome sequencing and metabolomics, the composition, function and metabolic potential of the gut microbiome have been gradually investigated. Recently, numerous reports on the gut microbiome have emerged. The gut microbiome plays an important role in maintaining metabolic homeostasis in the body. Recent studies have revealed that the pathogenesis of T2DM is closely related to the gut microbiome[2]. The gut microbiome imbalances (e.g., lower abundance) alter the composition of the flora in patients with T2DM. Individuals with a lower abundance of the gut microbiome are more likely to exhibit IR. An increase in the abundance of the gut microbiome may improve glucose metabolism[3]. A review by Jeyaraman et al[4] in a recent issue of the World Journal of Diabetes detailed the mechanisms by which alterations in the gut microbiome affect IR and glucose metabolism, discussed the role of probiotic supplementation and fecal microbiota transplantation and other therapies developed on the basis of the gut microbiome in improving T2DM, and summarized the effects of common diabetes medications, diet, and exercise in lowering blood glucose levels by modulating the gut microbiome. Electroacupuncture (EA) therapy, developed from traditional acupuncture in Chinese medicine, achieves its therapeutic effects by applying minute amounts of low-frequency pulsed currents to needles piercing acupuncture points. Several studies have shown that EA therapy, a non-pharmacological intervention in traditional Chinese medicine with rapid onset of action, simplicity, and safety, can significantly improve IR and T2DM[5]. Due to its rapid onset of action, simplicity and safety, EA therapy is gradually gaining attention in clinical practice. Additionally, studies have confirmed that EA therapies may be involved in treating various diseases by modulating the gut microbiome[6,7]. Therefore, several recent reports have explored the efficacy of EA in alleviating T2DM from the perspective of the gut microbiome. However, the paper by Jeyaraman et al[4] has not yet discussed the effects of EA on the gut microbiome in T2DM. This letter aimed to provide an additional summary of this missing content, which provides additional theoretical support for the potential mechanisms underlying the application of EA for T2DM in clinical practice. This paper highlighted the ability of EA to significantly reduce blood glucose levels and improve IR in diabetic mice. The possible regulatory mechanism is closely related to the improvement of gut microbiota, providing high-quality experimental evidence for the clinical application of EA.
By reviewing several relevant papers published in recent years on the mechanism of action of EA to improve T2DM by modulating gut microbiome, it was found that the ability of EA to regulate blood glucose and improve IR by increasing the abundance of Firmicutes and decreasing the abundance of Bacteroidetes, as well as increasing the abundance and community diversity of gut microbiome in the intestines of T2DM mice[8-12]. Polycystic ovary syndrome (PCOS) and T2DM are common endocrine metabolic diseases. Both share important pathophysiologic mechanisms, namely IR and hyperinsulinemia. EA therapy has been found to restore the total gut microbial community to a normal physiological state and improve glucose tolerance in PCOS rats, playing a positive role in the regulation of glucose metabolism and IR[13].
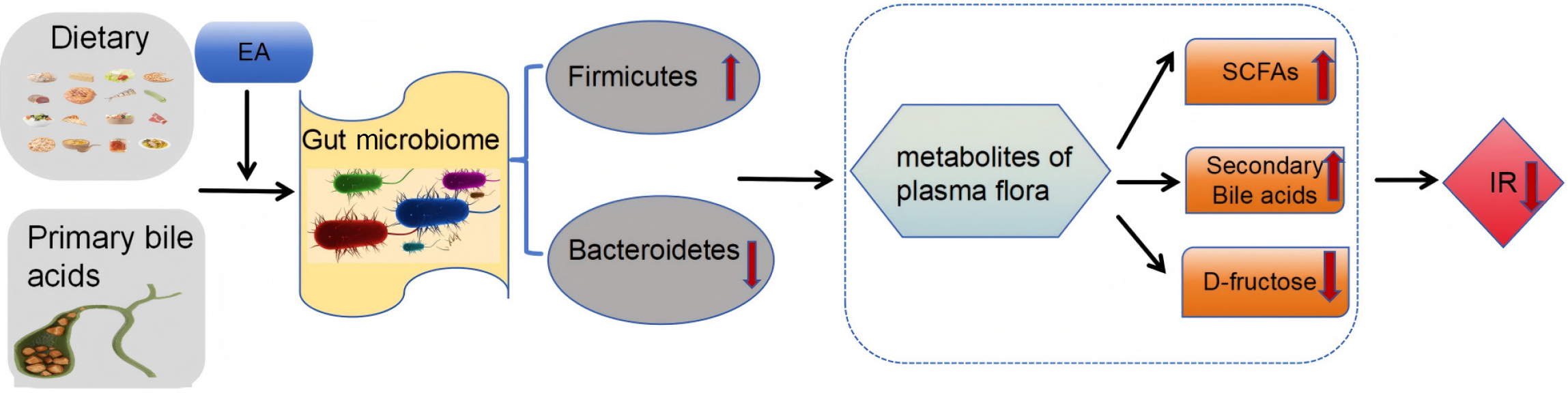
Zhang et al[11] revealed that EA increased the abundance of Lactobacillus and Blautia in the thick-walled phylum Firmicutes of T2DM mice by remodeling the structure of the gut microbiome, thereby enhancing the content of gut microbiome-associated metabolites, specifically short-chain fatty acids (SCFAs). SCFAs mainly include acetic acid, propionic acid and butyric acid, which are the main products of dietary fiber fermentation by the gut microbiome. SCFAs improve the acidic environment of the colon, inhibit the growth of harmful bacteria, and prevent intestinal dysfunction. Furthermore, SCFAs have been shown to exert anti-inflammatory effects in diabetes by inhibiting histone deacetylase[14]. A low-grade inflammatory state is an important contributor to IR[15]. Zhang et al[11] conducted Spearman correlation analysis on gut microbiome composition and inflammation-related indexes before and after the EA intervention to validate that serum lipopolysaccharide (LPS) levels and mucosal inflammation in intestinal tissues could be reduced by restoring the structure of the gut microbiome. Therefore, they proposed that EA could remodel the gut microbiome to increase intestinal SCFAs, ultimately reducing inflammatory and improving IR in T2DM patients. Bile acids are classified as primary and secondary. The conversion of primary bile acids to secondary bile acids is accomplished by the gut microbiome. Bile acids are also crucial in regulating glucose and lipid metabolism[16]. One important signaling pathway is that secondary bile acids activate the G-protein coupled bile acid receptor 5 (TGR5), promoting the secretion of glucagon-like peptide-1 (GLP-1) from intestinal L cells to regulate glucose metabolism[17,18]. TGR5 is a transmembrane G protein-coupled receptor that binds to extracellular ligands and transduces extracellular signals to intracellular cascades, playing important roles in energy metabolism, inflammatory responses, and cancer therapy through multiple pathways[19]. Pan et al[8] explored the effects of EA on gut microbiome and fecal bile acid metabolism in T2DM mice. They found that EA treatment increased the abundance of gut microbiome, decreasing primary bile acids, increased secondary bile acids, and elevated TGR5/GLP1 levels in small intestine of T2DM mice. Correlation analysis of different bile acids with differential bacterial communities revealed that the gut microbiome plays crucial role in bile acid metabolism. These interactions function as key signaling molecules in blood glucose regulation[8]. In summary, EA therapy, as a non-pharmacological treatment, can promote the production of gut microbiome-related metabolites, such as bile acids and SCFAs, by elevating the abundance of gut microbiome, ultimately improving glucose regulation and insulin sensitivity.
Another study found that EA not only improved IR and markers of diabetes in T2DM mice by modulating the richness of the gut microbiomes, but also improved blood glucose levels by lowering the content of D-fructose, metabolites of plasma flora[12]. Although D-fructose does not directly enhance glucose-induced insulin release, it can inhibit intestinal α-glucosidase and increase GLP-1 secretion after oral sucrose administration, thus delaying glucose absorption and impairing glucose tolerance and IR[20]. Nutrient supply is required for the growth of gut microbes, while D-fructose influences intestinal glucose absorption. This finding links plasma fructose metabolism to the abundance of gut microbes, revealing a potential mechanism by which EA alleviates T2DM by decreasing fructose levels in plasma flora metabolites and thereby affecting gut microbe abundance[12] (as shown in Figure 1).
Figure 1 Mechanisms by which electroacupuncture improves insulin resistance via metabolites of plasma flora.
EA: Electroacupuncture; SCFAs: Short-chain fatty acids; IR: Insulin resistance.
The intestinal tract is an important barrier in the human body. Gut microbiome, functioning as a relatively complete ecosystem, can influence intestinal mucosal permeability[21,22]. The abnormal metabolic state of T2DM patients results in a disruption of gut microbiome homeostasis, destruction of intestinal epithelial cellular structure, and increased intestinal mucosal permeability, ultimately leading to a state of low-grade inflammation[23]. Wang et al[10] found that EA treatment may protect the intestinal barrier by regulating the abundance and community diversity of the gut microbiome and reduce the permeability of the intestinal mucosa, thus effectively preventing large amounts of LPS from entering the body circulation, thereby improving the systemic inflammatory state and ultimately ameliorating IR in T2DM mice. Notably, inflammatory cytokines have been shown to activate phosphorylation of the inhibitor of nuclear factor-κB kinase β (IKKβ)/nuclear factor-κB (NF-κB)-c-Jun N-terminal kinase (JNK)-insulin receptor substrate (IRS-1) pathway[24,25], which in turn inhibits the phosphorylation of protein kinase B (AKT) and induces IR[26]. An et al[9] developed an interstitial cells of Cajal (ICC)-deficient mouse model to explore whether ICC-dependent colonic motility mediates the regulation of gut microbiome homeostasis by EA. The study revealed that EA promotes colonic peristalsis by maintaining ICC, which regulates the abundance and community diversity of gut microbiome, enhances the intestinal barrier, and consequently reduces the inflammatory state and improves insulin sensitivity, ultimately lowering blood glucose levels. The possible mechanism is related to IKKβ/NF-κB-JNK-IRS-1-AKT signaling[9].
CONCLUSIONS AND PERSPECTIVES
The current study confirms that EA, as a traditional Chinese medicine therapy, effectively ameliorates T2DM and IR. A disturbed gut microbiome is one of the key mechanisms triggering T2DM. On the one hand, EA therapy can regulate the abundance and community diversity of gut microbiome and restore gut microbiome homeostasis in T2DM by affecting the production of microbial metabolites such as bile acids, SCFAs, and D-fructose, which in turn improves the inflammatory microenvironment and richness of gut microbiome, and ultimately correcting abnormal glucose and lipid metabolism. On the other hand, EA can promote colonic peristalsis and regulate the structure of gut microbiome by maintaining ICC, thereby reducing the permeability of the intestinal mucosa, decreasing the entry of inflammatory factors into the blood circulation, and ultimately ameliorating IR.
However, EA therapies may differ in their stimulatory effects and therapeutic efficacy on the organism depending on the specific points of action and EA parameters (frequency, intensity, and duration). This paper does not specifically discuss the potential impact of these factors on treatment outcomes. Future studies should incorporating these variables into the experimental design to provide more precise guidance for clinical practice. In addition, clinical studies on EA to regulate gut microbiome in T2DM patients have not yet been conducted. It is expected that further clinical studies can be conducted for the treatment of diabetes by EA from the perspective of the gut microbiome, based on individual patient differences, reproducibility of treatment, and stability of long-term efficacy. This will provide a noval strategy and robust scientific basis for using EA to modulate the gut microbiome in T2DM treatment, which is expected to provide a safe, effective treatment with few side effects for patients with T2DM or those who do not respond to conventional hypoglycemic agents, as well as an effective preventive modality for people with high risk of diabetes.