Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.101488
Revised: November 5, 2024
Accepted: December 26, 2024
Published online: March 15, 2025
Processing time: 127 Days and 2.6 Hours
Recent studies have indicated that triglyceride glucose (TyG)-waist height ratio (WHtR) and TyG-waist circumference (TyG-WC) are effective indicators for eva
To clarify the relation in TyG-WHtR, TyG-WC, and the risk of MACEs and overall mortality in T2DM patients.
Information for this investigation was obtained from Action to Control Car
After full adjustment for confounding variables, the highest baseline TyG-WHtR cohort respectively exhibited a 1.353-fold and 1.420-fold higher risk for MACEs and overall mortality, than the lowest quartile group. Similarly, the highest baseline TyG-WC cohort showed a 1.314-fold and 1.480-fold higher risk for MACEs and overall mortality, respectively. Each 1 SD increase in TyG-WHtR was significantly related to an 11.7% increase in MACEs and a 14.9% enhance in overall mortality. Each 1 SD increase in TyG-WC corresponded to an 11.5% in MACEs and a 16.6% increase in overall mortality. Including these two indexes in conventional models significantly improved the predictive power for MACEs and overall mortality.
TyG-WHtR and TyG-WC were promising predictors of MACEs and overall mortality risk in T2DM cases.
Core Tip: This study demonstrates that triglyceride-glucose-related indices, namely the triglyceride glucose (TyG)-waist height ratio (WHtR) and TyG-waist circumference (TyG-WC), are significant predictors of major adverse cardiovascular events (MACEs) and overall mortality in patients with type 2 diabetes mellitus (T2DM). Including TyG-WHtR and TyG-WC in conventional cardiovascular risk models significantly improves predictive accuracy for MACEs and mortality. These indices could be valuable for early cardiovascular risk stratification and targeted intervention in T2DM management.
- Citation: Liu MJ, Pei JY, Zeng C, Xing Y, Zhang YF, Tang PQ, Deng SM, Hu XQ. Triglyceride-glucose related indices as predictors for major adverse cardiovascular events and overall mortality in type-2 diabetes mellitus patients. World J Diabetes 2025; 16(3): 101488
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/101488.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.101488
In recent decades, the global population with diabetes has consistently increased, establishing diabetes as a significant global health issue[1,2]. According to a survey published in 2021, approximately 537 million people worldwide were estimated to have type 2 diabetes in 2021, and may rise by 46% to 783 million by 2045[2]. The morbidity and mortality rates from cardiovascular disease (CVD) are steadily climbing, posing severe threat to the human health. Tackling the increase in CVD has emerged as a major public health challenge of international importance[3,4]. CVD is a typical common complications in type 2 diabetes mellitus (T2DM) patients and the key cause of death among these patients[5,6]. Both cardiovascular mortality and all-cause mortality are obviously higher in people with diabetes[7]. Therefore, early and timely identification of additional cardiovascular and death risk factors in diabetes case is crucial to prevent, delay, or decrease the progression of diabetes and diabetes-related deaths.
Insulin resistance (IR) represents the reduced sensitivity of insulin-dependent organs and tissues to insulin, serving as the pathophysiological foundation of T2DM[8]. Individuals with IR are more likely to develop metabolic disorder like dyslipidemia, hypertension[9], all of which are obviously linked to adverse cardiovascular outcomes. IR is prevalent in clinical settings and serves as the "common soil" for chronic metabolic-related illness like T2DM, and ASCVD[10]. IR is also an obvious risk factor for the progression of T2DM and CVD[11]. IR plays a crucial role in CVD by inducing glucose metabolism disorders and lipid toxicity. It also leads to oxidative stress, the release of inflammatory factors, activation of the nervous system, dysfunction of vascular endothelial cells, decreased fibrinolytic activity, and platelet activation, which contribute to a range of CVDs[12-15]. Thus, long-term monitoring and intervention of IR may aid in the early treatment of major adverse cardiovascular events (MACEs) in T2DM patients. Currently, there is a lack of simple and accurate methods to assess IR in clinical settings. The hyperinsulinemic-euglycemic clamp test and glucose tolerance test are the gold standard tests for measuring IR; however, their high cost and invasive nature limit their use in large-scale clinical practice. The Homeostasis Model Assessment of IR is extensively applied to measure IR, but this index is of limited value in subjects receiving insulin therapy[16,17]. As a result, many alternative markers of IR was designed and compared to the hyperinsulin-normal glucose clamp assay and the intravenous glucose tolerance assay[18].
The triglyceride glucose (TyG) index is a composite marker that combines fasting triglycerides and glucose to evaluate IR. This emerging indicator has attracted attention due to its low cost and ease of use[19-22]. Recent studies have highlighted the significant clinical value of that in predicting MACEs in patients with either non-diabetic or diabetic baseline CVD[23-26]. In addition to metabolic biomarkers like triglycerides, body fat level and distribution are closely linked to IR. Hormones and cytokines released by fat cells can influence blood glucose response and insulin signaling[27-29]. Increasingly, research is investigating whether linking the TyG index with anthropometric obesity-associated factors, like waist height ratio (WHtR), and body mass index (BMI), can improve risk stratification for cardiometabolic outcomes. "Triglyceride-glucose-related indices" generally refer to various parameters derived from linking the TyG with body fat indicators, like TyG-waist circumference (WC). Several studies have highlighted the role of TyG-BMI to predict CVD events[30-32]. However, recent evidence points to a paradox in how BMI reflects obesity and mortality risk within a population[33,34]. Epidemiological studies indicate that individuals with a normal BMI can still exhibit abdominal obesity[35,36]. Conversely, an increased WC often predicts higher fat accumulation, reflecting the true burden of central obesity[37]. As a marker of visceral adipose tissue and an "early health risk", WHtR is recommended over BMI for fat assessment[38,39]. Moreover, WHtR has been shown to be a significantly better predictor of cardiometabolic health and mortality than other obesity-related measures, especially in individuals with diabetes[40,41]. Therefore, composite parameters derived from the TyG as well as obesity measures, specifically TyG-WHtR and TyG-WC, appear to better reflect IR situation and offer greater cost-effectiveness[42-44]. Some scholars found that linking the TyG and TyG-WHtR and TyG-WC, provides improved predictive effect for patient survival compared to the TyG index alone[45-47]. In summary, TyG-WHtR and TyG-WC are very excellent to predict the risk of MACEs. Nevertheless, there is few research on the association in them, and the risk and prognosis of MACEs in cases with diabetes. Utilizing data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) clinical trial and the corresponding follow-up study, this research aims to explore the association in these two indexes with MACE risk and overall mortality in T2DM patients.
This research performed post hoc test using data downloaded from the ACCORD/ACCORD Follow-On (ACCORDION) Trial. The ACCORD study was a multicenter clinical trial with a dual 2 × 2 factorial design. The fundamental principles, design, and main findings of the ACCORD study have been detailed in prior literature[48,49]. The ACCORD/ACCORDION study involved 10251 patients with T2DM. These patients either had a history of CVD or were at high risk of developing it. The trial took place at 77 sites in the United States and Canada from June 2001 to June 2009, with patients undergoing treatment and follow-up for an average of about five years. Participants who agreed to join the ACCORDION trial were followed for 3.5 years from 2011 to 2014 through clinic visits and phone calls.
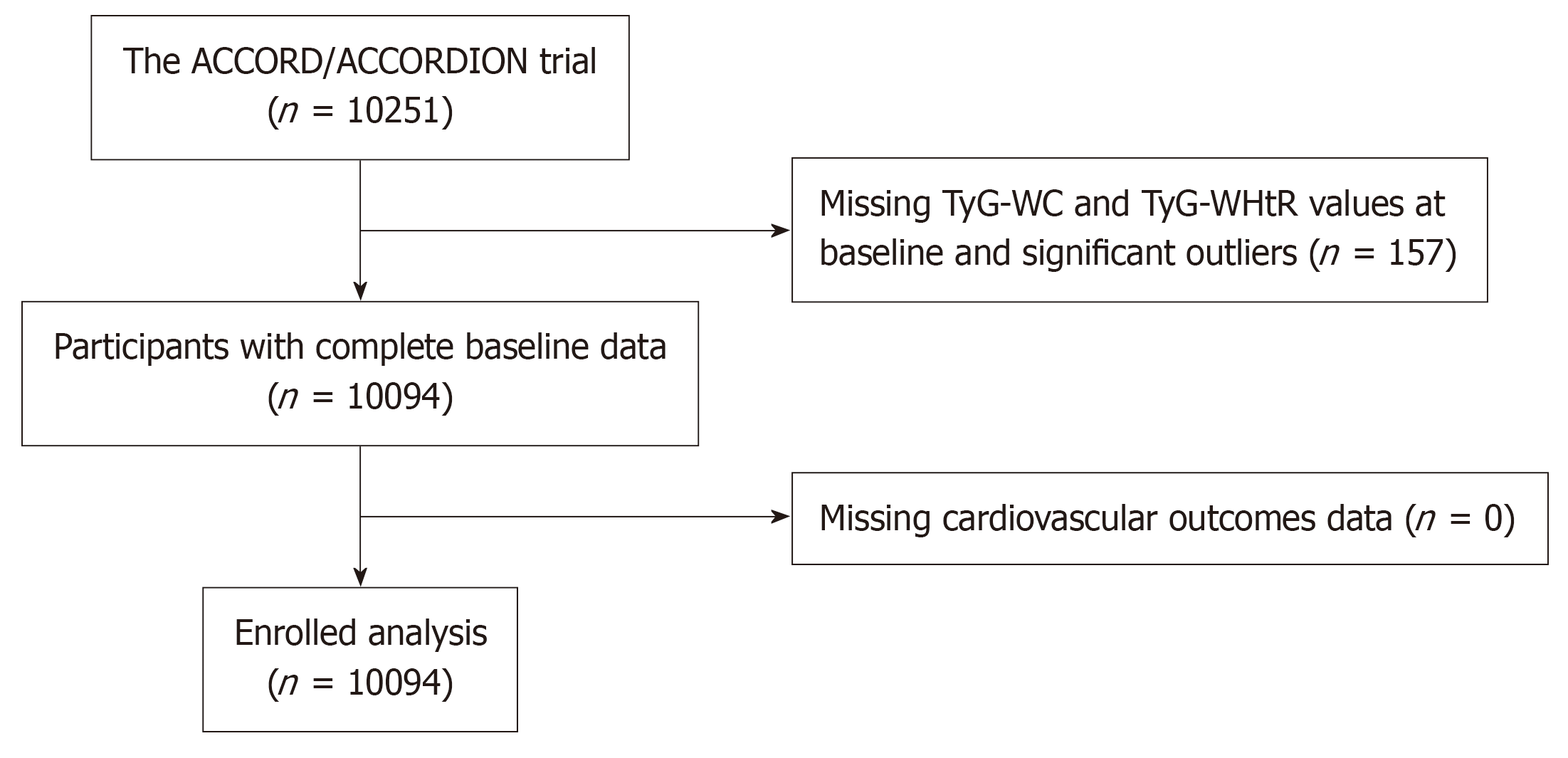
Data collected for this study included demographic and clinical features, such as age, sex, education, ethnicity, physical examination results, laboratory tests. Of the 10251 cases, 157 did not have TyG-WC and TyG-WHtR data, resulting in 10094 cases being included in present research (Figure 1). The specific calculations for these three indexes are as follows:
The primary endpoint was the occurrence of MACEs, which included common myocardial infarction, death from CVD, stroke. The secondary endpoint was total all-cause mortality.
The SPSS 26.0 tool was applied to conduct the Statistical analyses in this study, as well as the R (Vienna, Austria), and EmpowerStats software. Depending on the distribution of the variables, baseline features were expressed as mean ± SD, ratio, quartile ranges. The difference in continuous data were checked using ANOVA test, while difference in categorical data were checked based on the χ2 test. The Kaplan-Meier survival curve method was utilized to evaluate the cumulative risk of MACEs and overall mortality, with the log-rank test applied for checking differences of various groups.
Cox models were utilized to determine the relation of TyG-WC and TyG-WHtR with future MACEs and overall mortality. Prior to conducting the multivariate Cox proportional hazard analysis, univariate analysis assessed the association of all collected variables with future MACEs to identify potential confounding factors (Supplementary Table 1). Variables with significance in the univariate analysis, as well as those clinically significant with an estimated effect change greater than 10%, were evaluated for inclusion in the multivariate analyses. Each analysis utilized an unadjusted model and three progressively adjusted models to control for relevant confounders of MACEs. Model 1 included adjustments for sex, race, age, education, history of CVD, previous hypertension, previous hyperlipidemia, systolic blood pressure (SBP), diastolic blood pressure (DBP). Model 2 (mainly adjusted) built on Model 1 by adding covariates such as duration of diabetes, proteinuria, heart failure, smoking status, glycated hemoglobin (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C). Model 3 (fully adjusted) included all covariates from Model 2 plus the application of calcium channel blockers (CCB), beta-blockers, insulins, aspirin, statins, and cholesterol absorption inhibitors. The use of statins and cholesterol absorption inhibitors was specifically accounted for. The RCS analysis was applied to explore nonlinear correlations. If a nonlinear relationship was identified, the inflection point was further determined using a recursive method, and the effect size and confidence interval on the inflection point were determined using a two-segment Cox model. Subgroup and interaction analysis were performed to test the result robustness. The index of area under the curve (AUC) was employed to determine the additional predictive effect of TyG-WC and TyG-WHtR beyond conventional risk factors.
The basical profiling included 10094 participants, of whom 61.48% were male, with average age of 62.80 ± 6.64 years. The average TC level was 183.29 ± 41.85, LDL-C was 104.86 ± 33.89, TG were 190.26 ± 148.59, and fasting plasma glucose (FPG) was 175.21 ± 56.16. The TyG index was 9.49 ± 0.73, TyG-WC was 1014.05 ± 160.17, and TyG-WHtR was 5.97 ± 0.92. The case were classified into four groups based on the baseline TyG-WHtR: Quartile 1 (4.83 ± 0.37), Quartile 2 (5.62 ± 0.17), Quartile 3 (6.24 ± 0.19), and Quartile 4 (7.17 ± 0.50). Similarly, the cases were categorized via the quartile of TyG-WC: Quartile 1 (815.26 ± 67.01), Quartile 2 (955.95 ± 31.22), Quartile 3 (1062.40 ± 31.75), and Quartile 4 (1222.59 ± 85.99). Tables 1 and 2 summarize the baseline demographic, laboratory, and clinical features of patients in these four groups. Participants with higher TyG-WHtR were generally younger, with higher probability to be white and male, had lower education levels, consumed alcohol less frequently, and had shorter period of diabetes. Those in the higher quartiles of the two indexes also had a greater history of CVD, proteinuria and depression. These participants exhibited elevated levels of BMI, DBP, heart rate, FPG, TG, VLDL-C, and WC. These with higher TyG-WHtR were more prone to live alone and had higher rates of previous hypertension. Participants with elevated TyG-WHtR were more likely to use ARB/ACEI, β-blockers, thiazolidinediones and insulin, while they used statins less frequently. Those with higher TyG-WC tended to use ARB/ACEI, β-blockers, biguanides, thiazolidinediones and aspirin. These results indicate that higher TyG-WHtR in cases may relate to conventional risk factors.
| | Overall | Q1 (n = 2524) | Q2 (n = 2523) | Q3 (n = 2523) | Q4 (n = 2524) | P value |
| TyG-WHtR | 5.97 ± 0.92 | 4.83 ± 0.37 | 5.62 ± 0.17 | 6.24 ± 0.19 | 7.17 ± 0.50 | < 0.001 |
| Age (years) | 62.80 ± 6.64 | 63.56 ± 7.04 | 63.10 ± 6.73 | 62.63 ± 6.50 | 61.92 ± 6.16 | < 0.001 |
| Sex | < 0.001 | |||||
| Male | 6206 (61.48) | 1706 (67.59) | 1661 (65.83) | 1525 (60.44) | 1314 (52.06) | |
| Female | 3888 (38.52) | 818 (32.41) | 862 (34.17) | 998 (39.56) | 1210 (47.94) | |
| Race | < 0.001 | |||||
| White | 6302 (62.43) | 1201 (47.58) | 1489 (59.02) | 1677 (66.47) | 1935 (76.66) | |
| Non-white | 3792 (37.57) | 1323 (52.42) | 1034 (40.98) | 846 (33.53) | 589 (23.34) | |
| Education | < 0.001 | |||||
| Less than high school graduate | 1492 (14.79) | 364 (14.44) | 389 (15.42) | 400 (15.87) | 339 (13.44) | |
| High school grad (or GED) | 2662 (26.39) | 668 (26.50) | 657 (26.05) | 645 (25.59) | 692 (27.43) | |
| Some college or technical school | 3311 (32.82) | 736 (29.19) | 818 (32.43) | 839 (33.28) | 918 (36.39) | |
| College graduate or more | 2622 (25.99) | 753 (29.87) | 658 (26.09) | 637 (25.27) | 574 (22.75) | |
| CVD History | 3556 (35.23) | 848 (33.60) | 931 (36.90) | 856 (33.93) | 921 (36.49) | 0.022 |
| Duration of diabetes (years) | 10.79 ± 7.58 | 11.70 ± 7.99 | 11.07 ± 7.68 | 10.30 ± 7.29 | 10.08 ± 7.26 | < 0.001 |
| Previous hypertension | 7609 (75.38) | 1847 (73.18) | 1908 (75.62) | 1899 (75.27) | 1955 (77.46) | 0.006 |
| Previous hyperlipidemia | 7065 (69.99) | 1713 (67.87) | 1792 (71.03) | 1784 (70.71) | 1776 (70.36) | 0.058 |
| Proteinuria | 2001 (19.83) | 443 (17.55) | 443 (17.56) | 527 (20.90) | 588 (23.30) | < 0.001 |
| Heart failure | 484 (4.80) | 102 (4.04) | 94 (3.73) | 107 (4.24) | 181 (7.17) | < 0.001 |
| Depression | 2394 (23.72) | 409 (16.20) | 510 (20.21) | 631 (25.02) | 844 (33.45) | < 0.001 |
| Living alone | 2039 (20.20) | 460 (18.23) | 496 (19.67) | 519 (20.57) | 564 (22.35) | 0.003 |
| Smoking | < 0.001 | |||||
| Yes | 5886 (58.31) | 1391 (55.11) | 1483 (58.78) | 1474 (58.42) | 1538 (60.94) | |
| No | 4208 (41.69) | 1133 (44.89) | 1040 (41.22) | 1049 (41.58) | 986 (39.06) | |
| Alcohol | < 0.001 | |||||
| Yes | 2414 (23.92) | 637 (25.24) | 655 (25.97) | 612 (24.28) | 510 (20.21) | |
| No | 7676 (76.08) | 1887 (74.76) | 1867 (74.03) | 1909 (75.72) | 2013 (79.79) | |
| BMI (kg/m2) | 32.21 ± 5.40 | 27.09 ± 3.43 | 30.63 ± 3.63 | 33.62 ± 3.91 | 37.49 ± 4.21 | < 0.001 |
| SBP (mmHg) | 136.36 ± 17.10 | 136.11 ± 17.04 | 136.41 ± 16.81 | 136.64 ± 17.21 | 136.30 ± 17.36 | 0.823 |
| DBP (mmHg) | 74.89 ± 10.66 | 73.41 ± 10.67 | 74.31 ± 10.31 | 75.66 ± 10.66 | 76.18 ± 10.78 | < 0.001 |
| Heart rate, bpm | 72.66 ± 11.74 | 70.82 ± 11.31 | 71.81 ± 11.58 | 73.47 ± 11.82 | 74.54 ± 11.90 | < 0.001 |
| FPG (mg/dL) | 175.21 ± 56.16 | 154.52 ± 52.23 | 168.84 ± 51.57 | 179.59 ± 54.19 | 197.88 ± 57.38 | < 0.001 |
| HbA1C (%) | 8.30 ± 1.06 | 8.16 ± 1.06 | 8.24 ± 1.02 | 8.32 ± 1.03 | 8.48 ± 1.09 | < 0.001 |
| TC (mg/dL) | 183.29 ± 41.85 | 173.74 ± 37.60 | 179.77 ± 39.06 | 184.93 ± 40.26 | 194.72 ± 47.02 | < 0.001 |
| TG (mg/dL) | 190.26 ± 148.59 | 113.74 ± 57.49 | 166.67 ± 93.30 | 201.97 ± 120.21 | 278.64 ± 217.96 | < 0.001 |
| VLDL-C (mg/dL) | 36.56 ± 24.38 | 22.69 ± 11.33 | 32.88 ± 16.77 | 39.16 ± 20.68 | 51.52 ± 33.22 | < 0.001 |
| LDL-C (mg/dL) | 104.86 ± 33.89 | 104.48 ± 31.87 | 105.21 ± 33.52 | 104.97 ± 33.83 | 104.77 ± 36.22 | 0.717 |
| eGFR (mL/minute/1.73 m2) | 91.05 ± 27.18 | 90.82 ± 24.23 | 91.06 ± 29.91 | 91.03 ± 27.48 | 91.30 ± 26.79 | 0.941 |
| WC (cm) | 106.74 ± 13.64 | 92.87 ± 8.68 | 102.75 ± 8.36 | 110.47 ± 8.81 | 120.85 ± 9.98 | < 0.001 |
| WHtR | 0.63 ± 0.08 | 0.54 ± 0.04 | 0.60 ± 0.04 | 0.65 ± 0.04 | 0.72 ± 0.05 | < 0.001 |
| Medications | ||||||
| ARB/ACEI | 6992 (69.27) | 1678 (66.48) | 1738 (68.89) | 1754 (69.52) | 1822 (72.19) | < 0.001 |
| CCB | 1929 (19.11) | 457 (18.11) | 469 (18.59) | 488 (19.34) | 515 (20.40) | 0.178 |
| β-Blockers | 3041 (30.21) | 641 (25.45) | 725 (28.84) | 807 (32.07) | 868 (34.47) | < 0.001 |
| Biguanides | 6455 (63.96) | 1563 (61.93) | 1618 (64.13) | 1644 (65.19) | 1630 (64.58) | 0.085 |
| Thiazolidinediones | 2228 (22.07) | 527 (20.88) | 503 (19.94) | 598 (23.71) | 600 (23.77) | < 0.001 |
| Sulfonylureas | 5401 (53.51) | 1374 (54.44) | 1372 (54.38) | 1329 (52.70) | 1326 (52.54) | 0.351 |
| Insulins | 3522 (34.89) | 822 (32.57) | 858 (34.01) | 908 (35.99) | 934 (37.00) | 0.004 |
| Statins | 6417 (63.81) | 1644 (65.39) | 1636 (65.23) | 1601 (63.58) | 1536 (61.03) | 0.004 |
| Aspirin | 5497 (54.70) | 1341 (53.36) | 1344 (53.61) | 1436 (57.12) | 1376 (54.71) | 0.030 |
| Cholesterol absorption inhibitors | 205 (2.04) | 46 (1.83) | 58 (2.31) | 52 (2.07) | 49 (1.95) | 0.658 |
| MACEs | 1791 (17.74) | 367 (14.54) | 458 (18.15) | 467 (18.51) | 499 (19.77) | < 0.001 |
| Total mortality | 1914 (18.96) | 420 (16.64) | 457 (18.11) | 484 (19.18) | 553 (21.91) | < 0.001 |
| | Overall | Q1 (n = 2524) | Q2 (n = 2523) | Q3 (n = 2523) | Q4 (n = 2524) | P value |
| TyG-WC | 1014.05 ± 160.17 | 815.26 ± 67.01 | 955.95 ± 31.22 | 1062.40 ± 31.75 | 1222.59 ± 85.99 | < 0.001 |
| Age (years) | 62.80 ± 6.64 | 63.61 ± 7.09 | 63.26 ± 6.68 | 62.68 ± 6.54 | 61.66 ± 6.07 | < 0.001 |
| Sex | < 0.001 | |||||
| Male | 6206 (61.48) | 1323 (52.42) | 1527 (60.52) | 1617 (64.09) | 1739 (68.90) | |
| Female | 3888 (38.52) | 1201 (47.58) | 996 (39.48) | 906 (35.91) | 785 (31.10) | |
| Race | < 0.001 | |||||
| White | 6302 (62.43) | 1075 (42.59) | 1445 (57.27) | 1778 (70.47) | 2004 (79.40) | |
| Non-white | 3792 (37.57) | 1449 (57.41) | 1078 (42.73) | 745 (29.53) | 520 (20.60) | |
| Education | < 0.001 | |||||
| Less than high school graduate | 1492 (14.79) | 420 (16.66) | 415 (16.46) | 370 (14.67) | 287 (11.38) | |
| High school grad (or GED) | 2662 (26.39) | 713 (28.28) | 640 (25.39) | 646 (25.61) | 663 (26.28) | |
| Some college or technical school | 3311 (32.82) | 715 (28.36) | 799 (31.69) | 850 (33.70) | 947 (37.53) | |
| College graduate or more | 2622 (25.99) | 673 (26.70) | 667 (26.46) | 656 (26.01) | 626 (24.81) | |
| CVD history | 3556 (35.23) | 821 (32.53) | 872 (34.56) | 924 (36.62) | 939 (37.20) | 0.002 |
| Duration of diabetes (years) | 10.79 ± 7.58 | 11.82 ± 8.06 | 11.11 ± 7.72 | 10.37 ± 7.21 | 9.86 ± 7.17 | < 0.001 |
| Previous hypertension | 7609 (75.38) | 1895 (75.08) | 1896 (75.15) | 1868 (74.04) | 1950 (77.26) | 0.059 |
| Previous hyperlipidemia | 7065 (69.99) | 1712 (67.83) | 1774 (70.31) | 1786 (70.79) | 1793 (71.04) | 0.050 |
| Proteinuria | 2001 (19.83) | 432 (17.12) | 460 (18.23) | 519 (20.58) | 590 (23.38) | < 0.001 |
| Heart failure | 484 (4.80) | 107 (4.24) | 85 (3.37) | 121 (4.80) | 171 (6.77) | < 0.001 |
| Depression | 2394 (23.72) | 433 (17.16) | 519 (20.57) | 631 (25.03) | 811 (32.13) | < 0.001 |
| Living alone | 2039 (20.20) | 516 (20.44) | 514 (20.38) | 497 (19.70) | 512 (20.29) | 0.908 |
| Smoking | < 0.001 | |||||
| Yes | 5886 (58.31) | 1257 (49.80) | 1449 (57.43) | 1532 (60.72) | 1648 (65.29) | |
| No | 4208 (41.69) | 1267 (50.20) | 1074 (42.57) | 991 (39.28) | 876 (34.71) | |
| Alcohol | 0.002 | |||||
| Yes | 2414 (23.92) | 540 (21.39) | 629 (24.94) | 650 (25.78) | 595 (23.58) | |
| No | 7676 (76.08) | 1984 (78.61) | 1893 (75.06) | 1871 (74.22) | 1928 (76.42) | |
| BMI (kg/m2) | 32.21 ± 5.40 | 27.32 ± 3.80 | 30.80 ± 3.91 | 33.52 ± 4.07 | 37.20 ± 4.21 | < 0.001 |
| SBP (mmHg) | 136.36 ± 17.10 | 136.82 ± 17.10 | 136.56 ± 17.39 | 136.27 ± 17.23 | 135.81 ± 16.67 | 0.156 |
| DBP (mmHg) | 74.89 ± 10.66 | 73.40 ± 10.60 | 74.49 ± 10.41 | 75.37 ± 10.78 | 76.29 ± 10.64 | < 0.001 |
| Heart rate, bpm | 72.66 ± 11.74 | 71.20 ± 11.25 | 72.08 ± 11.71 | 72.87 ± 11.73 | 74.50 ± 12.01 | < 0.001 |
| FPG (mg/dL) | 175.21 ± 56.16 | 154.21 ± 53.15 | 168.08 ± 50.86 | 179.83 ± 52.30 | 198.70 ± 58.37 | < 0.001 |
| HbA1c (%) | 8.30 ± 1.06 | 8.18 ± 1.05 | 8.25 ± 1.04 | 8.29 ± 1.04 | 8.47 ± 1.07 | < 0.001 |
| TC (mg/dL) | 183.29 ± 41.85 | 176.93 ± 38.09 | 180.96 ± 40.52 | 183.75 ± 41.18 | 191.52 ± 45.89 | < 0.001 |
| TG (mg/dL) | 190.26 ± 148.59 | 116.86 ± 69.17 | 162.72 ± 90.13 | 201.83 ± 119.22 | 279.62 ± 216.65 | < 0.001 |
| VLDL-C (mg/dL) | 36.56 ± 24.38 | 23.21 ± 12.74 | 32.12 ± 16.35 | 39.43 ± 21.35 | 51.50 ± 32.60 | < 0.001 |
| LDL-C (mg/dL) | 104.86 ± 33.89 | 105.98 ± 32.30 | 106.31 ± 34.13 | 104.31 ± 33.70 | 102.85 ± 35.27 | < 0.001 |
| eGFR (mL/minute/1.73 m2) | 91.05 ± 27.18 | 91.55 ± 29.26 | 90.87 ± 29.19 | 89.95 ± 23.73 | 91.84 ± 26.11 | 0.074 |
| WC (cm) | 106.74 ± 13.64 | 91.52 ± 7.73 | 102.48 ± 6.70 | 110.63 ± 7.10 | 122.32 ± 8.99 | < 0.001 |
| WHtR | 0.63 ± 0.08 | 0.55 ± 0.05 | 0.61 ± 0.05 | 0.65 ± 0.05 | 0.71 ± 0.06 | < 0.001 |
| Medications | ||||||
| ARB/ACEI | 6992 (69.27) | 1674 (66.32) | 1736 (68.81) | 1752 (69.44) | 1830 (72.50) | < 0.001 |
| CCB | 1929 (19.11) | 475 (18.82) | 493 (19.54) | 457 (18.11) | 504 (19.97) | 0.353 |
| β-Blockers | 3041 (30.21) | 647 (25.71) | 715 (28.37) | 795 (31.64) | 884 (35.12) | < 0.001 |
| Biguanides | 6455 (63.96) | 1542 (61.09) | 1638 (64.92) | 1618 (64.16) | 1657 (65.65) | 0.004 |
| Thiazolidinediones | 2228 (22.07) | 513 (20.32) | 516 (20.45) | 572 (22.68) | 627 (24.84) | < 0.001 |
| Sulfonylureas | 5401 (53.51) | 1370 (54.28) | 1335 (52.91) | 1342 (53.21) | 1354 (53.65) | 0.784 |
| Insulins | 3522 (34.89) | 834 (33.04) | 878 (34.80) | 888 (35.20) | 922 (36.53) | 0.075 |
| Statins | 6417 (63.81) | 1630 (64.91) | 1595 (63.37) | 1622 (64.57) | 1570 (62.38) | 0.221 |
| Aspirin | 5497 (54.70) | 1302 (51.89) | 1343 (53.38) | 1432 (57.05) | 1420 (56.48) | < 0.001 |
| Cholesterol absorption inhibitors | 205 (2.04) | 50 (1.99) | 55 (2.19) | 51 (2.03) | 49 (1.95) | 0.940 |
| MACEs | 1791 (17.74) | 366 (14.50) | 426 (16.88) | 484 (19.18) | 515 (20.40) | < 0.001 |
| Total mortality | 1914 (18.96) | 406 (16.09) | 447 (17.72) | 484 (19.18) | 577 (22.86) | < 0.001 |
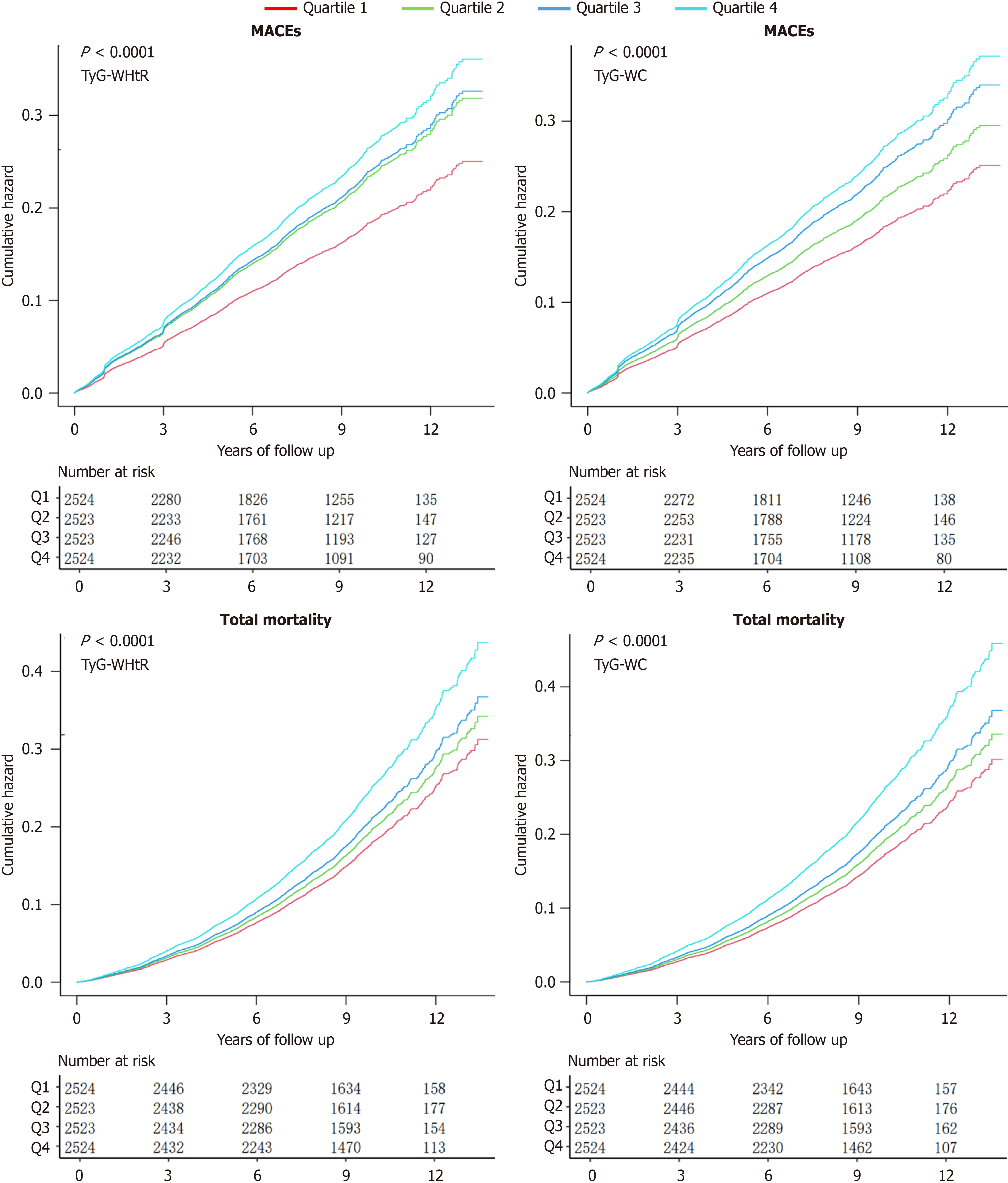
In a median follow-up of 8.82 years, 1791 (17.74%) T2DM participants experienced MACEs, and 1914 (18.96%) patients died. Kaplan-Meier curves were employed to evaluate the cumulation risk for MACE and total mortality. The Kaplan-Meier analysis results suggested that higher TyG-WHtR levels were related to a higher cumulative risk of future MACE and total mortality (log-rank test, MACEs: P < 0.0001; Figure 2).
Three multivariate regression models were used to analysis the correlation between these two indexes', while the occurrence of outcome events (Tables 3 and 4). Model 1 adjusts for sex, race, age, history of CVD, previous hypertension, previous hyperlipidemia, SBP. Model 2 further adjusts for the duration of diabetes, proteinuria, heart failure, smoking, HbA1c, TC, LDL-C, and estimated glomerular filtration rate on top of the factors in Model 1. Model 3 adds the use of drugs (CCB, thiazolidinediones, insulins, aspirin, statins, and cholesterol absorption inhibitors) to that in Model 2.
| Outcome | Events/n | Non-adjusted | Model 1 | Model 2 | Model 3 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| MACEs | |||||||||
| TyG-WHtR | 1.147 (1.091, 1.206) | < 0.0001 | 1.184 (1.123, 1.249) | < 0.0001 | 1.146 (1.081, 1.214) | < 0.0001 | 1.128 (1.064, 1.196) | < 0.0001 | |
| Q1 | 367/2524 | Reference | Reference | Reference | Reference | ||||
| Q2 | 458/2523 | 1.273 (1.110, 1.461) | 0.0006 | 1.216 (1.059, 1.397) | 0.0056 | 1.196 (1.040, 1.376) | 0.0121 | 1.179 (1.025, 1.357) | 0.0211 |
| Q3 | 467/2523 | 1.303 (1.137, 1.494) | 0.0001 | 1.336 (1.162, 1.536) | < 0.0001 | 1.291 (1.120, 1.488) | 0.0004 | 1.266 (1.097, 1.460) | 0.0012 |
| Q4 | 499/2524 | 1.442 (1.260, 1.650) | < 0.0001 | 1.526 (1.326, 1.757) | < 0.0001 | 1.408 (1.212, 1.635) | < 0.0001 | 1.353 (1.164, 1.574) | < 0.0001 |
| Per 1 SD | 1.135 (1.084, 1.188) | < 0.0001 | 1.169 (1.113, 1.227) | < 0.0001 | 1.134 (1.075, 1.196) | < 0.0001 | 1.117 (1.059, 1.180) | < 0.0001 | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Total mortality | |||||||||
| TyG-WHtR | 1.141 (1.088, 1.198) | < 0.0001 | 1.230 (1.168, 1.295) | < 0.0001 | 1.177 (1.113, 1.246) | < 0.0001 | 1.163 (1.099, 1.231) | < 0.0001 | |
| Q1 | 420/2524 | Reference | Reference | Reference | Reference | ||||
| Q2 | 457/2523 | 1.09 (0.96, 1.25) | 0.1802 | 1.07 (0.94, 1.23) | 0.2912 | 1.06 (0.92, 1.21) | 0.4310 | 1.04 (0.91, 1.19) | 0.5849 |
| Q3 | 484/2523 | 1.175 (1.031, 1.339) | 0.0156 | 1.266 (1.108, 1.446) | 0.0005 | 1.214 (1.060, 1.391) | 0.0052 | 1.187 (1.035, 1.361) | 0.0140 |
| Q4 | 553/2524 | 1.399 (1.232, 1.588) | < 0.0001 | 1.625 (1.423, 1.855) | < 0.0001 | 1.474 (1.279, 1.698) | < 0.0001 | 1.420 (1.231, 1.638) | < 0.0001 |
| Per 1 SD | 1.130 (1.080, 1.181) | < 0.0001 | 1.210 (1.154, 1.269) | < 0.0001 | 1.162 (1.104, 1.224) | < 0.0001 | 1.149 (1.091, 1.211) | < 0.0001 | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Outcome | Events/n | Non-adjusted | Model 1 | Model 2 | Model 3 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| MACEs | |||||||||
| TyG-WC (per 100 units) | 1.102 (1.070, 1.134) | < 0.0001 | 1.100 (1.067, 1.135) | < 0.0001 | 1.080 (1.044, 1.117) | < 0.0001 | 1.070 (1.034, 1.108) | 0.0001 | |
| Q1 | 366/2524 | Reference | Reference | Reference | Reference | ||||
| Q2 | 426/2523 | 1.176 (1.023, 1.353) | 0.0226 | 1.13 (0.98, 1.31) | 0.0838 | 1.10 (0.96, 1.27) | 0.1712 | 1.09 (0.94, 1.25) | 0.2551 |
| Q3 | 484/2523 | 1.354 (1.182, 1.551) | < 0.0001 | 1.300 (1.131, 1.496) | 0.0002 | 1.245 (1.080, 1.436) | 0.0026 | 1.225 (1.061, 1.413) | 0.0056 |
| Q4 | 515/2524 | 1.481 (1.295, 1.693) | < 0.0001 | 1.471 (1.276, 1.695) | < 0.0001 | 1.370 (1.179, 1.592) | < 0.0001 | 1.314 (1.129, 1.529) | 0.0004 |
| Per 1 SD | 1.168 (1.115, 1.223) | < 0.0001 | 1.165 (1.109, 1.225) | < 0.0001 | 1.131 (1.071, 1.194) | < 0.0001 | 1.115 (1.056, 1.178) | 0.0001 | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| Total mortality | |||||||||
| TyG-WC (per 100 units) | 1.105 (1.075, 1.136) | < 0.0001 | 1.136 (1.102, 1.171) | < 0.0001 | 1.109 (1.073, 1.146) | < 0.0001 | 1.101 (1.065, 1.138) | < 0.0001 | |
| Q1 | 406/2524 | Reference | Reference | Reference | Reference | ||||
| Q2 | 447/2523 | 1.11 (0.97, 1.27) | 0.1183 | 1.11 (0.97, 1.27) | 0.1448 | 1.08 (0.94, 1.24) | 0.2769 | 1.06 (0.93, 1.22) | 0.3817 |
| Q3 | 484/2523 | 1.219 (1.068, 1.391) | 0.0033 | 1.241 (1.084, 1.422) | 0.0018 | 1.183 (1.030, 1.359) | 0.0171 | 1.170 (1.018, 1.344) | 0.0272 |
| Q4 | 577/2524 | 1.520 (1.339, 1.726) | < 0.0001 | 1.689 (1.475, 1.933) | < 0.0001 | 1.535 (1.330, 1.770) | < 0.0001 | 1.480 (1.282, 1.710) | < 0.0001 |
| Per 1 SD | 1.173 (1.122, 1.226) | < 0.0001 | 1.227 (1.169, 1.288) | < 0.0001 | 1.180 (1.120, 1.244) | < 0.0001 | 1.166 (1.106, 1.230) | < 0.0001 | |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |||||
In Model 3, the cumulative risk of MACEs enhanced with TyG-WHtR and TyG-WC, even after thorough adjustment for potential confounders. In comparison to the lowest quartile, the risks of MACEs and overall mortality were 1.353 and 1.420 times higher, in the top quartile of baseline TyG-WHtR, and 1.314 and 1.480 times higher, respectively, in the highest quartile of baseline TyG-WC. When treating TyG-WHtR and TyG-WC as continuous variables, and related to a 12.8% increase in MACEs risk (HR 1.128, 95%CI: 1.064-1.196) and a 16.3% increase in total mortality risk (HR 1.163, 95%CI: 1.099-1.231). Similarly, each 100-unit enhancement in TyG-WC related to a 7.0% increase in MACEs risk (95%CI: 1.034-1.108) and 10.1% in overall mortality risk (95%CI: 1.065-1.138). The risk of MACEs and overall mortality for TyG-WHtR (MACEs: P < 0.0001) and TyG-WC (MACEs: P for trend = 0.0001; overall mortality: P < 0.0001) demonstrated a significant upward trend. Furthermore, every 1 SD enhancement in TyG-WHtR was significantly related to a 12% (P < 0.0001) increase in MACEs and a 15% (P < 0.0001) increase in overall mortality. Every 1 SD enhancement in TyG-WC was obviously linked to a 12% (P = 0.0001) increase in MACEs and a 17% (P < 0.0001) increase in overall mortality.
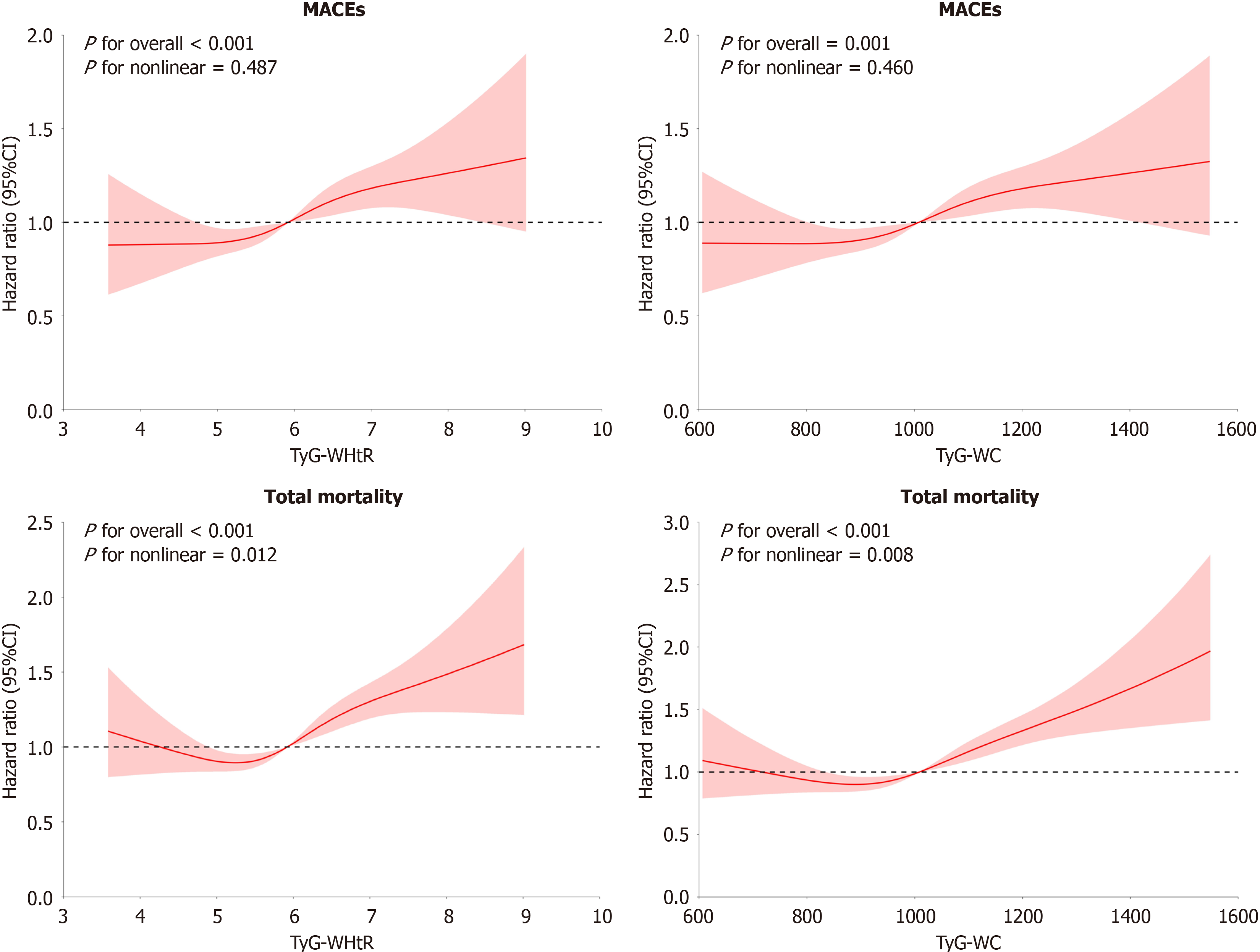
We further utilized a RCS curve to assess the potential association in TyG-WHtR, TyG-WC, and MACEs risk and overall mortality in T2DM cases, as Figure 3. It was found that these two indexes had a non-linear association with overall mortality in T2DM patients (TyG-WHtR: P for overall < 0.001; TyG-WC: P for nonlinear = 0.008). Additionally, TyG-WHtR and TyG-WC demonstrated an approximate linear association with the risk of MACEs in T2DM patients (TyG-WHtR: P < 0.001, TyG-WC: P for nonlinear = 0.460; Figure 3). In addition, the inflection points for overall mortality were calculated using a recursive method, yielding values of 5.062 and 870.1, respectively. Effect sizes and confidence intervals for both sides of these inflection points were then estimated based on Cox hazard regression model. Beyond the inflection point, each 1-unit decrease in TyG-WHtR was related to a 22.3% decrease in overall mortality risk [hazard ratio (HR) = 1.223, 95%CI: 1.146-1.306]. Similarly, every 100-unit decrease in TyG-WC reduced the risk of overall mortality by 22.3% (HR = 1.223, 95%CI: 1.146-1.306; Table 5).
| One linear-regression model | Inflection point (K) | < K, effect 1 | > K, effect 2 | P value for LRT | |
| TyG-WHtR | 1.163 (1.099, 1.231); P < 0.0001 | 5.062 | 0.802 (0.626, 1.026); P = 0.0797 | 1.223 (1.146, 1.306); P < 0.0001 | 0.004 |
| TyG-WC | 1.101 (1.065, 1.138); P < 0.0001 | 870.1 | 0.891 (0.781, 1.017); P = 0.0880 | 1.137 (1.094, 1.182); P < 0.0001 | 0.002 |
To test the robustness and further investigate the relationship among TyG-WHtR, TyG-WC, and outcome events, we performed subgroup analyses. The analysis was stratified by age, sex, race, history of CVD, heart failure, previous hypertension, diabetes time (< 10 and ≥ 10 years), HbA1c (< 8.1% and ≥ 8.1%), and insulin use. There were minimal interactions between them, and MACEs and total mortality. Regarding the association in TyG-WHtR, TyG-WC, as well as MACEs, TyG-WC emerged as a valuable predictor of future MACEs in T2DM cases with heart failure. For the relation in TyG-WHtR, and total mortality, TyG-WHtR showed a higher predictive ability for total mortality in T2DM patients without history of CVD, while TyG-WC had a higher predictive ability for total mortality in T2DM cases with HbA1c < 8.1% (Supplementary Tables 2, 3, 4 and 5).
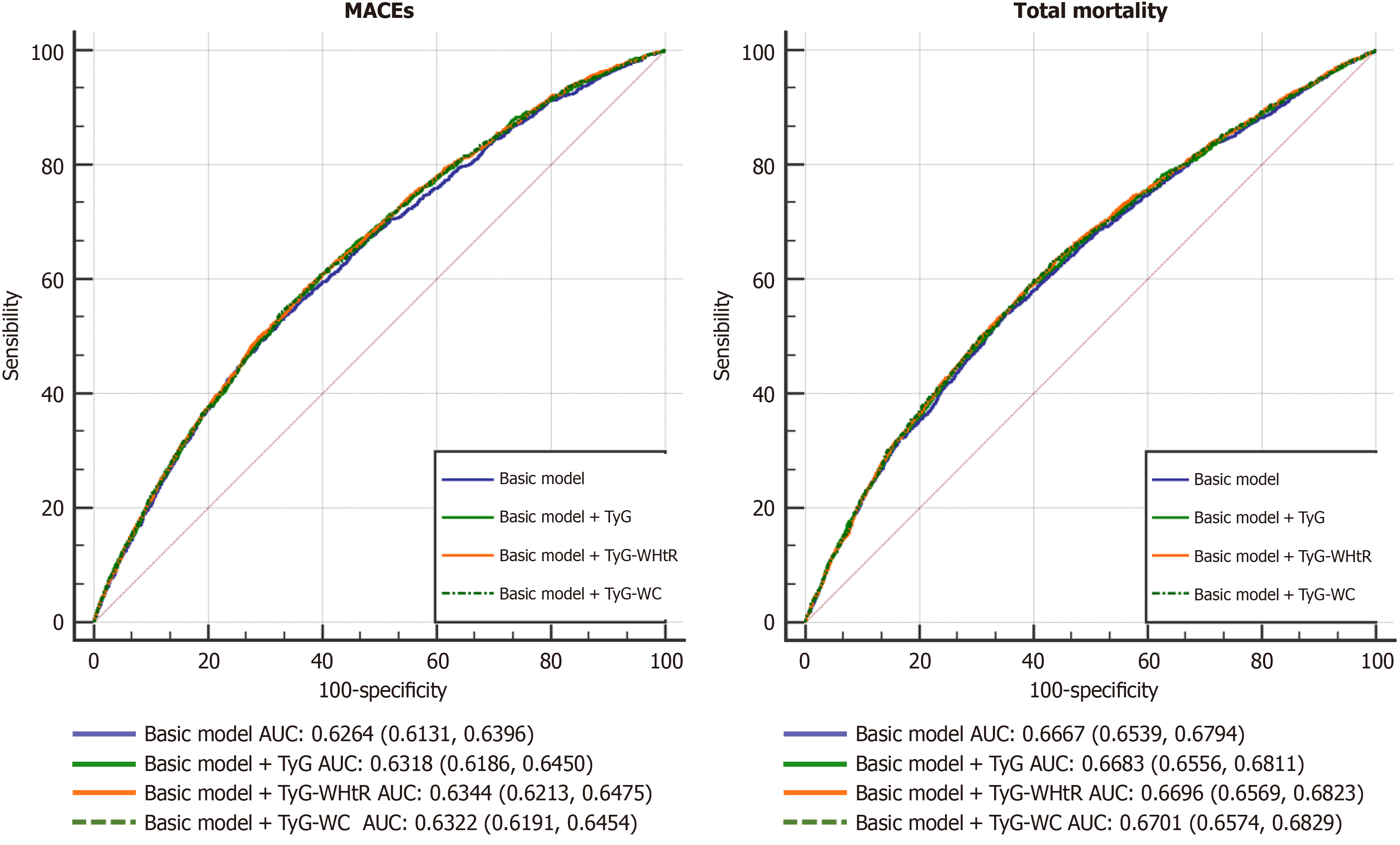
To determine the predictive performance of these two indexes for future MACEs and total mortality, we analyzed the receiver operating characteristic curve. For predicting MACEs, compared with the traditional model, the AUC increased after incorporating the levels of them. Among them, TyG-WHtR (95%CI: 0.6213-0.6475) had the max predictive performance for MACEs in T2DM patients, followed by TyG (95%CI: 0.6186-0.6450). For predicting total mortality, the AUC increased with the levels of the two indexes added to the traditional model. These results indicated that combining the traditional model with TyG-WHtR improved the prediction efficiency for future MACEs and total mortality in T2DM patients (Table 6 and Figure 4).
| AUC (95%CI) | P value | NRI (95%CI) | P value | IDI (95%CI) | P value | |
| MACEs | ||||||
| Basic model | 0.6264 (0.6131, 0.6396) | Reference | Reference | |||
| Basic model + TyG | 0.6318 (0.6186, 0.6450) | 0.0039 | 0.0660 (0.0380, 0.1000) | < 0.0001 | 0.0030 (0.0010, 0.0060) | < 0.0001 |
| Basic model + TyG-WHtR | 0.6344 (0.6213, 0.6475) | 0.0012 | 0.0770 (0.0450, 0.1060) | < 0.0001 | 0.0040 (0.0020, 0.0070) | < 0.0001 |
| Basic model + TyG-WC | 0.6322 (0.6191, 0.6454) | 0.0043 | 0.0610 (0.0280, 0.0850) | < 0.0001 | 0.0030 (0.0010, 0.0060) | < 0.0001 |
| Total mortality | ||||||
| Basic model | 0.6667 (0.6539, 0.6794) | Reference | Reference | |||
| Basic model + TyG | 0.6683 (0.6556, 0.6811) | 0.0324 | 0.0340 (0.0020, 0.0630) | 0.0400 | 0.0010 (0.0000, 0.0020) | 0.1200 |
| Basic model + TyG-WHtR | 0.6696 (0.6569, 0.6823) | 0.0038 | 0.0520 (0.0200, 0.0800) | < 0.0001 | 0.0020 (0.0000, 0.0040) | 0.0070 |
| Basic model + TyG-WC | 0.6701 (0.6574, 0.6829) | 0.0025 | 0.0410 (0.0110, 0.0690) | < 0.0001 | 0.0020 (0.0000, 0.0050) | 0.0070 |
In present research, we explored the relation in triglyceride-glucose-related indices and MACEs in T2DM patients. To our knowledge, this is the first investigation linking TyG-WHtR and TyG-WC with MACEs in T2DM patients within the ACCORD/ACCORDION cohort. Our findings suggest that baseline TyG-WHtR and TyG-WC are promising predictors of MACEs and overall mortality in this population. After adjusting for confounders, elevated levels of them were independently related to increased future MACEs and all-cause mortality in T2DM patients. These results provide new insights for preventing CVD-related deaths in T2DM patients and may inform future strategies for CVD prevention and treatment in this group.
The T2DM and its complications significantly affect both quality of life and life expectancy[50]. It is closely related to CVDs, with poor prognoses closely tied to cardiovascular complications, leading to the majority of diabetic patients ultimately succumbing to these issues. In the median follow-up of 8.82 years, 17.74% of T2DM patients suffer MACEs and 18.96% died. These rates are higher than those previously reported, likely due to the fact that most ACCORD and ACCORDION patients were at high risk for CVD.
A recent study suggests that the TyG-WHtR to be an excellent predictor of CVD mortality compared to that of only TyG[45]. Ren et al[51] found that changes in TyG-WHtR were independently related to CVD risk among Chinese individuals with age higher than 454. Another study identified significant associations among the two indexes, and mortality in individuals with metabolic syndrome[52]. This evidence suggests that the two indices may serve as valuable prognostic predictors. However, research on the association of them with MACEs and death in T2DM patients remains limited. A recent analysis of triglyceride-glucose-associated factors in the NHANES database revealed significant associations with CVD risk in individuals with prediabetes or diabetes. After adjusting for multiple variables, the study found that TyG-WHtR [odds ratio (OR) 1.63] and TyG-WC (OR 1.13) were obviously related to CVD risk. However, it did not examine outcome measures such as MACEs risk and overall mortality, and our study builds upon this previous work[53].
Our study is the first to associate them with MACEs and total mortality in T2DM patients within the ACCORD study. In the analysis of 10094 T2DM patients, higher baseline levels of TyG-WHtR and TyG-WC relation with higher risk of future MACEs and total mortality. After regulating major confounders, these relation remained significant. In comparison to the lowest quartile, the risks of MACEs and total mortality were 1.353-fold and 1.420-fold higher, in the top quartile of TyG-WHtR, and 1.314-fold and 1.480-fold higher in the highest quartile of baseline TyG-WC. The fully adjusted RCS analysis demonstrated a non-linear association in baseline TyG-WHtR, and overall mortality risk in T2DM patients. The inflection points were identified as 5.062 for TyG-WHtR and 870.1 for TyG-WC, which has significant clinical implications. This information may assist in clinical consultations and support strategies for preventing cardiovascular mortality in T2DM patients. Reducing WHtR, WC, TG, via lifestyle interventions may decrease the risk of cardiovascular and total mortality. It is recommended to maintain TyG-WHtR below 5.062 and TyG-WC below 870.1. Further stratification and interaction analyses largely corroborated the main findings. This also indicates that the research results of this article have high reference value and can provide support for optimizing treatment plans for such patients, while effectively improving their prognosis
Our results indicate that these two indexes are valuable for predicting the risk of MACEs and total mortality in T2DM cases. Lowering TyG-WHtR and TyG-WC levels significantly decreased the risk of these outcomes. Thus, monitoring TyG-WHtR and TyG-WC levels during the early clinical diagnosis and treatment of diabetic patients, maintaining a healthy TyG index, effective weight management, and appropriate waist circumference can help prevent cardiovascular complications and reduce mortality in T2DM patients. Moreover, incorporating them into traditional models with established risk factors obviously enhanced the predictive performance for MACEs and death risk in T2DM cases, with TyG-WHtR exhibiting the highest overall predictive ability.
This study's strength lies in being the first within the ACCORD study to investigate the effect of them for future MACEs and total mortality in T2DM patients. After comprehensive adjustment for cardiovascular risk factors, we conducted an extensive and continuously follow-up of MACEs and overall mortality in a relatively large cohort, objectively evaluating TyG-WHtR and TyG-WC as biomarkers. The findings have significant clinical implications and potential applications.
However, there are limitations to our study. First, this research is a post hoc analysis. The Cox analysis, unmeasured or residual confounders may still be present, such as changes in dietary habits and lifestyle. Future studies, should aim to collect comprehensive information, including dietary patterns and daily activities, to minimize factors that may impact the reliability of obtained results. Second, it does not establish a causal association in baseline TyG-WC with MACEs risk in T2DM patients. Future research should include well-designed, longitudinal, and propensity score-matched prospective intervention studies. Third, relying on predictive indices instead of directly estimating insulin levels presents a limitation in itself. Finally, the exclusion of individuals with incomplete baseline data is a limitation of this study. Although missing data accounted for only 1.53%, this could introduce selection bias and may impact the conclusion generalizability. Futures researches should consider utilizing more complete datasets to enhance the applicability of the research.
In conclusion, our study found that changes in TyG-WHtR and TyG-WC were independently associated with MACEs and total mortality in this population. Baseline TyG-WHtR and TyG-WC are promising predictors of MACEs and total mortality in T2DM patients. Our findings highlight the clinical utility of TyG-WHtR and TyG-WC as valuable biomarkers for assessing cardiovascular risk and overall mortality. The implications of our study are significant; incorporating TyG-WHtR and TyG-WC into routine assessments may facilitate early intervention, potentially reducing MACEs and all-cause mortality in T2DM patients while improving outcomes. Additionally, focusing on modifiable triglyceride-glucose indices allows clinicians to develop targeted strategies. This research provides valuable insights into the clinical management of T2DM patients and the evaluation of their risk for MACEs and overall mortality.
The authors gratefully acknowledge the ACCORD/ACCORDION study group and the NHLBI BioLINCC. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the ACCORD/ACCORDION study authors or the NHLBI BioLINCC.
| 1. | Chan JCN, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, Lau ESH, Eliasson B, Kong APS, Ezzati M, Aguilar-Salinas CA, McGill M, Levitt NS, Ning G, So WY, Adams J, Bracco P, Forouhi NG, Gregory GA, Guo J, Hua X, Klatman EL, Magliano DJ, Ng BP, Ogilvie D, Panter J, Pavkov M, Shao H, Unwin N, White M, Wou C, Ma RCW, Schmidt MI, Ramachandran A, Seino Y, Bennett PH, Oldenburg B, Gagliardino JJ, Luk AOY, Clarke PM, Ogle GD, Davies MJ, Holman RR, Gregg EW. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396:2019-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 493] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Claude Mbanya J, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. Erratum to "IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045" [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res Clin Pract. 2023;204:110945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 3. | Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J Am Coll Cardiol. 2022;80:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1283] [Reference Citation Analysis (0)] |
| 4. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5792] [Article Influence: 965.3] [Reference Citation Analysis (2)] |
| 5. | Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 6. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1480] [Article Influence: 185.0] [Reference Citation Analysis (2)] |
| 7. | Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, Wilson PWF, Phillips LS. Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J Am Heart Assoc. 2019;8:e011295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 8. | Lee SH, Park SY, Choi CS. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab J. 2022;46:15-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 643] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 9. | Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 635] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 10. | Muzurović E, Mikhailidis DP, Mantzoros C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism. 2021;119:154770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 11. | Adeva-Andany MM, Martínez-Rodríguez J, González-Lucán M, Fernández-Fernández C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr. 2019;13:1449-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 12. | Louie JZ, Shiffman D, McPhaul MJ, Melander O. Insulin resistance probability score and incident cardiovascular disease. J Intern Med. 2023;294:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 13. | Abdul-Ghani MA, Jayyousi A, DeFronzo RA, Asaad N, Al-Suwaidi J. Insulin Resistance the Link between T2DM and CVD: Basic Mechanisms and Clinical Implications. Curr Vasc Pharmacol. 2019;17:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. 2023;51:3000605231164548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 197] [Reference Citation Analysis (0)] |
| 15. | Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 1430] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 16. | Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, Assi HI. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 2022;23:786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 821] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 17. | Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15-E26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1112] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 19. | Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 20. | Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16:102581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 359] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 21. | Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 639] [Article Influence: 159.8] [Reference Citation Analysis (1)] |
| 22. | He J, Song C, Yuan S, Bian X, Lin Z, Yang M, Dou K. Triglyceride-glucose index as a suitable non-insulin-based insulin resistance marker to predict cardiovascular events in patients undergoing complex coronary artery intervention: a large-scale cohort study. Cardiovasc Diabetol. 2024;23:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 23. | Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum Á, Barbarash O, Chifamba J, Diaz ML, Gulec S, Ismail N, Iqbal R, Kelishadi R, Khatib R, Lanas F, Levitt NS, Li Y, Mohan V, Mony PK, Poirier P, Rosengren A, Soman B, Wang C, Wang Y, Yeates K, Yusuf R, Yusufali A, Zatonska K, Rangarajan S, Yusuf S. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4:e23-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 225] [Reference Citation Analysis (0)] |
| 24. | Yao Y, Wang B, Geng T, Chen J, Chen W, Li L. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999-2018. Cardiovasc Diabetol. 2024;23:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 25. | Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 26. | Liu C, Liang D. The association between the triglyceride-glucose index and the risk of cardiovascular disease in US population aged ≤ 65 years with prediabetes or diabetes: a population-based study. Cardiovasc Diabetol. 2024;23:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3763] [Article Influence: 198.1] [Reference Citation Analysis (2)] |
| 28. | Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov Today. 2022;27:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res. 2020;126:1549-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 710] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 30. | Huo RR, Zhai L, Liao Q, You XM. Changes in the triglyceride glucose-body mass index estimate the risk of stroke in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. 2023;22:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 87] [Reference Citation Analysis (0)] |
| 31. | Li F, Wang Y, Shi B, Sun S, Wang S, Pang S, Wu X. Association between the cumulative average triglyceride glucose-body mass index and cardiovascular disease incidence among the middle-aged and older population: a prospective nationwide cohort study in China. Cardiovasc Diabetol. 2024;23:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 97] [Reference Citation Analysis (0)] |
| 32. | Xiao S, Zhang Q, Yang HY, Tong JY, Yang RQ. The association between triglyceride glucose-body mass index and all-cause and cardiovascular mortality in diabetes patients: a retrospective study from NHANES database. Sci Rep. 2024;14:13884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 33. | Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. 2016;17:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 34. | Strulov Shachar S, Williams GR. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 35. | Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P; American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 701] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 36. | Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS; INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 1932] [Article Influence: 92.0] [Reference Citation Analysis (1)] |
| 37. | Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Ashwell M, Gibson S. Waist-to-height ratio as an indicator of 'early health risk': simpler and more predictive than using a 'matrix' based on BMI and waist circumference. BMJ Open. 2016;6:e010159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 39. | Butt JH, Petrie MC, Jhund PS, Sattar N, Desai AS, Køber L, Rouleau JL, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray JJV. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: revisiting the obesity paradox. Eur Heart J. 2023;44:1136-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 40. | Lo K, Huang YQ, Shen G, Huang JY, Liu L, Yu YL, Chen CL, Feng YQ. Effects of waist to height ratio, waist circumference, body mass index on the risk of chronic diseases, all-cause, cardiovascular and cancer mortality. Postgrad Med J. 2021;97:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Parente EB, Mutter S, Harjutsalo V, Ahola AJ, Forsblom C, Groop PH. Waist-height ratio and waist are the best estimators of visceral fat in type 1 diabetes. Sci Rep. 2020;10:18575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Yan S, Wang D, Jia Y. Comparison of insulin resistance-associated parameters in US adults: a cross-sectional study. Hormones (Athens). 2023;22:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 43. | Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Sun C, Cheung M, Viikari JS, Dwyer T, Raitakari OT. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1187] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 44. | Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS One. 2016;11:e0149731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 425] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 45. | Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, Liu L, Ming Z, Tao X, Li Y. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. 2024;23:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 357] [Reference Citation Analysis (0)] |
| 46. | Xia X, Chen S, Tian X, Xu Q, Zhang Y, Zhang X, Li J, Wu S, Wang A. Association of triglyceride-glucose index and its related parameters with atherosclerotic cardiovascular disease: evidence from a 15-year follow-up of Kailuan cohort. Cardiovasc Diabetol. 2024;23:208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 47. | Zhuang Y, Qiu L, Han D, Qiao Z, Wang F, Jiang Q, An Q, Li Y, Shangguan J, Bi X, Shen D. The association between triglyceride-glucose index and related parameters and risk of cardiovascular disease in American adults under different glucose metabolic states. Diabetol Metab Syndr. 2024;16:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 48. | Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH Jr, Byington RP, Rosenberg YD, Friedewald WT; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 729] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 49. | Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD; ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i-33i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 454] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 50. | Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1082] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 51. | Ren Q, Huang Y, Liu Q, Chu T, Li G, Wu Z. Association between triglyceride glucose-waist height ratio index and cardiovascular disease in middle-aged and older Chinese individuals: a nationwide cohort study. Cardiovasc Diabetol. 2024;23:247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 52. | Wei X, Min Y, Song G, Ye X, Liu L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc Diabetol. 2024;23:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 93] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 53. | Zheng D, Cai J, Xu S, Jiang S, Li C, Wang B. The association of triglyceride-glucose index and combined obesity indicators with chest pain and risk of cardiovascular disease in American population with pre-diabetes or diabetes. Front Endocrinol (Lausanne). 2024;15:1471535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/