Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.99200
Revised: September 30, 2024
Accepted: November 21, 2024
Published online: February 15, 2025
Processing time: 166 Days and 22.1 Hours
Numerous epidemiological studies have found that pesticide exposure is associated with the incidence of type 2 diabetes (T2D); however, the underlying mechanisms remain unknown. DNA methylation may play a role in this process.
To identify the genes associated with pesticide exposure and T2D by reviewing the current literature.
We systematically searched PubMed and Embase for relevant studies that examined the association between pesticide exposure and DNA methylation, and studies on DNA methylation and T2D through January 15, 2024.
We identified six genes (Alu, CABLES1, CDH1, PDX1, PTEN, PTPRN2) related to pesticide exposure and T2D. We also suggested future research directions to better define the role of DNA methylation in the association between pesticide exposure and T2D.
DNA methylation of specific genes may play a vital role in the association between pesticide exposure and T2D.
Core Tip: Previous studies have reported an association between pesticide exposure and the incidence of type 2 diabetes (T2D), but the underlying mechanisms have not been fully elucidated. In this article, we speculated that DNA methylation may be associated with pesticide exposure and T2D. We systematically reviewed the current literature to identify genes that are not only associated with pesticide exposure but also with T2D. Six genes (Alu, CABLES1, CDH1, PDX1, PTEN, PTPRN2) were identified.
- Citation: Zheng GJ, Fang ZE, Zhou BY, Zuo L, Chen X, Liu ML, Yu L, Jing CX, Hao G. DNA methylation in the association between pesticide exposures and type 2 diabetes. World J Diabetes 2025; 16(2): 99200
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/99200.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.99200
Type 2 diabetes (T2D) is a group of metabolic disorders characterized by a deficiency or failure to maintain normal glucose homeostasis, resulting from defects in insulin secretion and action[1]. Data from the International Diabetes Federation Diabetes Atlas (2021) showed that 537 million adults were living with diabetes (approximately 90%-95% had T2D), accounting for 10% of the world’s population[2]. This number is expected to rise to 643 million by 2030 and 783 million by 2045[3]. In China, the overall prevalence of diabetes is 11.2% in adults, and nearly 141 million patients have diabetes[4]. The rising burden of T2D has become a huge challenge for global health systems. As a complex multifactorial disease, T2D emerges from a combination of genetic and environmental factors[5].
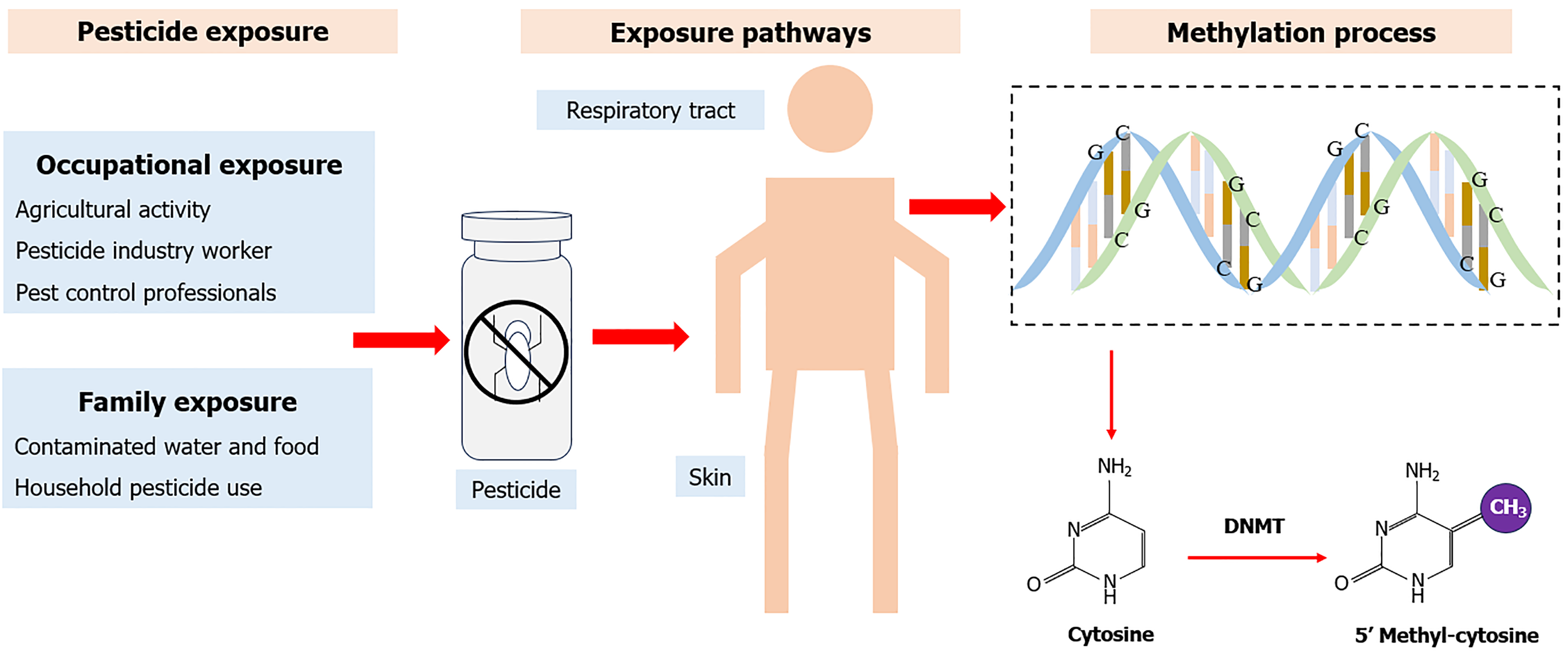
Pesticides are chemicals or biological substances primarily used in agriculture for pest, weed, and disease control, or public health to protect humans from vector-borne diseases such as malaria, dengue fever, and schistosomiasis[6]. Common pesticides include herbicides, fungicides, insecticides, and rodenticides according to their usages[7]. The main human pesticide exposure routes are the food chain, air, water, soil, flora, and faun[8]. After entering the body, pesticides are distributed throughout the body via the blood, and some eventually accumulate in human tissues[9]. Exposure to large pesticide doses over a short period can cause poisoning symptoms such as bronchospasm, vomiting, and hypotension[10]. In addition to these acute toxic effects, long-term pesticide exposure is linked to various diseases, including cancer, hormone disruption, asthma, allergies, hypersensitivity, congenital disabilities, and fetal death[11-16].
Pesticide exposure has been found to be associated with the risk of T2D. This exposure may lead to reduced insulin sensitivity and impaired glucose tolerance, contributing to the onset and progression of diabetes[17]. Numerous in vivo studies have indicated that organophosphate pesticide (OPP) exposure can lead to hyperglycemia and glucose metabolism disruption[18]. Another study evaluated the effect of 180 days of monocrotophos exposure on insulin resistance in rats and observed weight gain in key white adipose tissue depots[19]. This association has also been found in epidemiological studies, most of which were observational. These studies predominantly used biological markers from blood samples or questionnaires to evaluate various types of pesticide exposure from environmental and occupational sources. Their results consistently indicate high pesticide exposure levels are associated with increased T2D risk[20]. Although the association between pesticide exposure and T2D risk has been proven in animal and epidemiological studies, the underlying mechanisms remain poorly understood. Therefore, it is suggested that DNA methylation may be involved in this process[21]. DNA methylation is an epigenetic mechanism that transfers the methyl group to the C5 position of cytosine to form 5-methylcytosine and regulates gene expression without changing the DNA sequence (Figure 1)[22]. Previous studies have shown that pesticide exposure is genome-wide and significantly associated with the methylation levels of some genes, such as CDKN2A[23], DNMT1[24], Satα[25], GSTp1[21], and IGF2[26]. DNA methylation is also a crucial component of the development of T2D[27]. Changes in methylation can influence gene expression[28] and affect insulin secretion[29].
Based on these results, we speculated that DNA methylation may be associated with pesticide exposure and T2D; our aim was to review the roles of DNA methylation in this association.
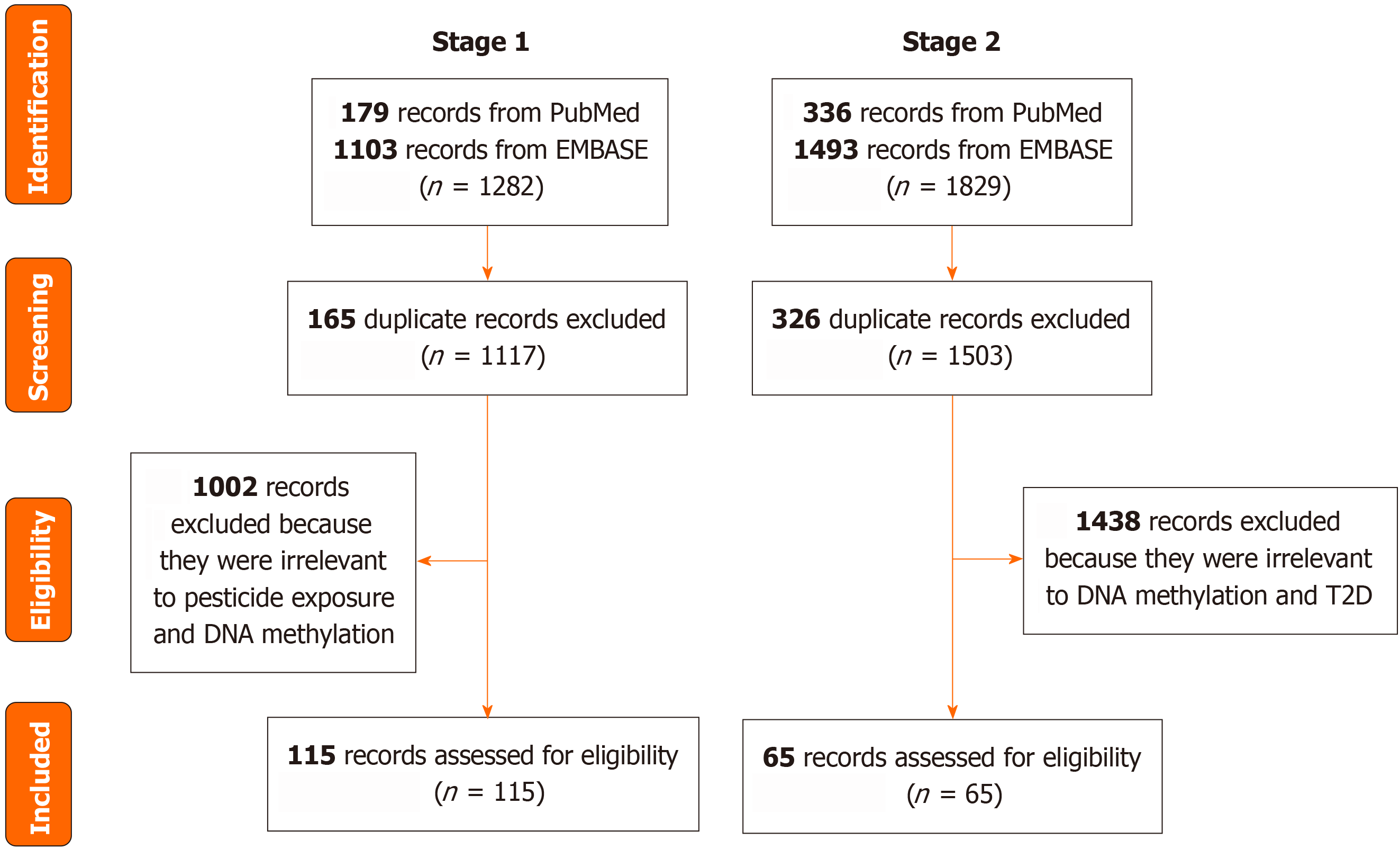
In the first stage, we systematically searched PubMed and Embase through January 15, 2024, for relevant studies that examined the association between pesticide exposure and DNA methylation. The exclusion criteria were: (1) Duplicate articles; and (2) Articles unrelated to pesticide exposure and DNA methylation.
In the second stage, we systematically searched PubMed and Embase to identify genes related to T2D. A systematic review of the association between DNA methylation and T2D was performed, and articles were retrieved until April 2017[30]. We further searched and retrieved related articles up to January 2024. The exclusion criteria were: (1) Duplicate articles; and (2) Articles unrelated to DNA methylation and T2D (Figure 2). Table 1 presents the search strategy details.
| Stage | Database | MeSH1/explode2 | Title, abstract1/abstract, keywords2 | Retrieval period | |
| Stage 1 | PubMed, EMBASE | “DNA methylation” | AND | “DNA methylations” OR “methylation DNA” OR “methylations DNA” OR “Methylations” | Up to January 2024 |
| “Pesticides” | AND | “Fungicide” OR “herbicide” OR “insecticide” OR “molluscicide” OR “rodenticide” OR “carbamate” OR “pyrethroid” OR “chlorinated hydrocarbon” OR “agricultural chemical” OR “organochlorine” OR “organophosphorus” | |||
| Stage 2 | “Diabetes mellitus, type 2” | AND | “Diabetes mellitus noninsulin-dependent” OR “stable diabetes mellitus” OR “diabetes mellitus type ii” OR “diabetes mellitus maturity-onset” OR “maturity-onset diabetes mellitus” OR “diabetes mellitus slow onset” OR “type 2 diabetes mellitus” OR “diabetes mellitus adult-onset” | April 2017 to January 2024 | |
In the first stage, we retrieved 1282 articles (179 from PubMed and 1103 from Embase). After excluding 165 duplicated records, and 1002 irrelevant articles, 115 articles were identified (Supplementary Table 1). In the second stage, 1829 articles were retrieved (336 from PubMed and 1493 from EMBASE). After excluding 326 duplicated records, and 1438 irrelevant articles, 65 articles were identified (Supplementary Table 1). Finally, we identified six genes [Alu, CABLES1, cadherin 1 gene (CDH1), pancreatic and duodenal homeobox 1 gene (PDX1), phosphatase and tensin homolog gene (PTEN), protein tyrosine phosphatase receptor type N2 gene (PTPRN2)] whose methylation was associated with pesticide exposure and T2D (Table 2).
| Gene | Function | Pesticide exposure & DNA methylation1 | DNA methylation & T2D2 | |||
| Type of pesticide | Methylation status | Location | Methylation status | Location | ||
| Alu | A member of the short interspersed repetitive DNA elements family of repetitive elements | Persistent organic pollutants | ↓[32-35] | - | ↓[49] | - |
| CABLES1 | Located on 18q11.2, encodes a protein involved in cell cycle regulation through interactions with several cyclin-dependent kinases | Atrazine | ↑[31] | Promoter | ↓[42] | Body |
| CDH1 | Located on 16q22.1, encodes a classical cadherin of the cadherin superfamily | Imidacloprid | ↓[39] | Promoter | ↑[43] | Body |
| Persistent organic pollutants | ↑[40] | Promoter | ||||
| PDX1 | Located on 13q12.2, the protein encoded by this gene is a transcriptional activator of several genes, including insulin, somatostatin, glucokinase, islet amyloid polypeptide, and glucose transporter type 2 | Fonofos, parathion, terbufos | ↑[36] | Promoter | ↑[45] | Intronic region |
| PTEN | Located on 10q23.31, the protein encoded by this gene is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase | Diazinon | ↑[37] | Promoter | ↓[46] | Promoter |
| PTPRN2 | Located on 7q36.3, encodes a protein with sequence similarity to receptor-like protein tyrosine phosphatases | Pesticide | ↓[38] | Body | ↓ | Body[47]/CpG island[48] |
Candidate and genome-wide epigenetic studies have identified several genes whose methylation status is altered when exposed to pesticides (Supplementary Table 1). We showed the changes in the methylation of various genes linked to T2D after exposure to different pesticides.
Alterations in methylation status were observed in animal experiments (Supplementary Table 1). In a whole-genome study, zebrafish embryos were exposed to varying concentrations of atrazine from 1 to 72 hours after fertilization. The results from whole-genome bisulfite sequencing found that hypermethylation in the promoter region of CABLES1 had occurred[31].
Most methylation status changes were observed in humans (Supplementary Table 1). Among these, Alu elements, an indicator of global DNA methylation, have been studied extensively. Research conducted on Greenlandic Inuit populations has reported that high plasma concentrations of persistent organic pollutants (POPs) were related to global DNA hypomethylation[32]. This association was also observed in healthy Koreans, where low-dose exposure to POPs, particularly organochlorine pesticides (OCPs), correlated with hypomethylation of global DNA[33]. Moreover, another Korean study further highlighted the sex difference in the relationship between global DNA hypomethylation and POPs exposure, showing that this association was stronger in males, particularly concerning specific POPs such as pp’-DDE, PCB52, and PCB101[34]. Additionally, Huen et al[35]suggested that prenatal exposure to POPs may be associated with Alu hypomethylation in the blood of Mexican-American children, with lower Alu methylation levels at 9 years of age than in the fetal stage. In addition to genome-wide epigenetic studies, several candidate gene studies have identified important human genes. Zhang et al[36] reported that the promoter region of the PDX1 was hypermethylated following in vitro exposure to fonofos, parathion, and terbufos. In another experiment, the PTEN demonstrated hypomethylation in the promoter region with in vitro exposure to diazinon[37]. A study involving 1561 participants further explored the relationship between occupational pesticide exposure and genome-wide DNA methylation, revealing that high pesticide exposure was significantly associated with hypomethylation of the PTPRN2 in the gene body[38]. For the CDH1, results regarding pesticides and methylation status are controversial. A cellular experiment investigated the epigenetic impact of insecticides on the early differentiation of mouse embryonic stem cells and found that these cells exposed to imidacloprid exhibited hypomethylation of CDH1 in its promoter region[39]. However, in a cross-sectional study among Koreans, Lee et al[40] found that low-dose exposure to POPs was positively related to the hypermethylation of CDH1. This incon
The important role of DNA methylation in the onset of T2D has been determined in previous studies, and our results show that most of these studies were conducted in humans. In terms of study design, they included candidate genes, genome-wide associations, and observational studies (including cross-sectional, case-control, and cohort studies). Of the 65 studies included, only three were animal experiments: One focused on rats with T2D, and the others were conducted in mice (Supplementary Table 2).
All studies related to the aforementioned genes that exhibited methylation changes with pesticide exposure were conducted in humans. In a study conducted in Thailand, Thongsroy et al[41] reported that Alu methylation levels were significantly lower in patients with T2D than in the general population, correlating with elevated hemoglobin A1c levels. Rönn et al[42] further explored the impact of epigenetic changes in human pancreatic islets on insulin secretion and T2D development. They reported that hypomethylation in the gene body of CABLES1 and its reduced expression was observed in islets from patients with T2D vs controls with a lower risk of T2D. Baca et al[43] investigated the changes in DNA methylation in the visceral adipose tissue of 19 females. Their results demonstrated that cg19834745 in CDH1 exhibited hypermethylation in samples from women with obesity and T2D, and this methylation change was further validated. In addition, PDX1, an important intracellular factor in β cells[44], was suggested to be hypermethylated in the gene body among T2D cases[45]. An observational study involving 100 participants reported that PTEN expression was significantly upregulated, and the methylation level in its promoter region decreased in Uyghur patients with mild T2D[46]. Furthermore, Wang et al[47] found that PTPRN2 was hypomethylated in the placenta of patients with gestational diabetes mellitus. This result was also reported in a study on adipose tissues from individuals with obesity and patients with T2D[48]. Collectively, these studies highlight the intricate relationship between epigenetic modifications and the pathophysiology of T2D.
Many studies have reported an association between pesticide exposure and the incidence of T2D; however, the under
The current understanding of the underlying biological mechanisms linking gene methylation to T2D remains limited, and deeper insights are still under investigation. A previous study reported that Alu hypomethylation is related to elevated blood glucose levels in patients with T2D[49]. Hypomethylation associated with hyperglycemia can induce DNA damage and contribute to insulin resistance, reduced glucose uptake, and increased hemoglobin A1c levels, thereby promoting the progression of T2D[41]. Additionally, the hypomethylation of CABLES1 is accompanied by reduced expression. This is negatively correlated with insulin resistance markers and hyperglycemia and positively associated with insulin sensitivity markers, high-density lipoprotein cholesterol levels, and glucose uptake in adipocytes[50]. Currently, there is little research on the association between CDH1 and T2D, and the mechanisms underlying this potential relationship remain to be explored. PDX1 is a transcription factor essential for the development and function of islet cells[44]. An association between DNA methylation of PDX1 and reduced activity in T2D islets has been observed in previous studies[44,51]. DNA methylation may reduce PDX1 protein and mRNA levels, leading to impaired expression of GLUT2 (an important β-cell gene) and insulin, ultimately causing hyperglycemia development[52]. Moreover, high glucose concentrations can increase DNA methylation, leading to decreased insulin secretion and the development of diabetes[53]. PTEN is not only involved in tumorigenesis but is also associated with the onset of diabetes. Previous human studies have indicated that methylation of the PTEN promoter may downregulate or silence PTEN in metabolic syndrome[54], leading to disrupted insulin signaling and increased insulin resistance[55]. Animal studies have also provided insights. In a mouse study, PTEN affected glucose tolerance by influencing adipose tissue, muscles, and beta cells[56]. Increased PTEN expression can exacerbate insulin resistance by inhibiting the intracellular insulin signaling pathway[57]. PTPRN2 is crucial for insulin secretion in response to glucose stimulation and is recognized as an autoantigen in diabetes[58]. PTPRN2 is related to the onset of diabetes among Goto-Kakizaki rats, and its overexpression or inhibition can alter glucose tolerance in these rodents[59]. In humans, it may be involved in various metabolic functions and influence the development of obesity and T2D[60]. However, the underlying mechanism remains unclear.
To better delineate the role of DNA methylation in the association between pesticide exposure and T2D, several factors should be considered.
Effects of novel pesticides: The mechanisms of traditional pesticides such as OPPs, OCPs, and pyrethroids increasing T2D risk have been well investigated. Studies have indicated that OPPs can activate the hypothalamus-pituitary-adrenal axis, increasing plasma corticosterone and gluconeogenesis and producing high blood glucose levels in mice[61,62]. Chronic exposure to OPPs can induce oxidative stress[63], damaging islet cells. However, conventional pesticides have been banned worldwide since the 1980s, corresponding to the emergence and use of novel pesticides[64]. Further studies, such as animal experiments and longitudinal studies, on these new pesticides are required to better understand their effects.
Cause and consequence: Unlike DNA sequence stability, epigenetic modifications are dynamic and reversible[65]. It is difficult to determine whether the identified DNA methylation changes are the causes and consequences of T2D using a cross-sectional design. Therefore, to address these problems, it is necessary to conduct more longitudinal designs or Mendelian randomization studies that consider potential confounding factors (such as demographics, lifestyle, genetic factors, and environmental exposure).
Cell and tissue heterogeneity: Current studies have mainly used various samples such as cells, cord blood, venous blood, and peripheral blood to test the concentrations of pesticides and investigate the status of DNA methylation because of their easy access. However, as lipophilic chemicals, pesticides mainly accumulate in tissues, especially adipose tissue, after entering the body[66]. The concentrations of pesticides in blood may not accurately reflect the concentrations of pesticides in tissues, and DNA methylation patterns may differ between cells and tissues. Because human specimens, such as adipose tissue, cannot be obtained from living bodies, more animal experiments should be conducted to study pesticide accumulation and methylation status in tissues.
More direct evidence is needed: To date, no studies have directly investigated pesticide exposure, DNA methylation, and T2D. Direct evidence will be beneficial for revealing the role of DNA methylation in the relationship between pesticide exposure and T2D.
Other factors, such as DNA methylation and its change over time; inheritance of DNA methylation: Bjornsson et al[67] showed that global methylation levels change over time and that genetic factors influence methylation maintenance. Another study found that maternal methylation of some genes during pregnancy correlated with the weight of newborns[68]. This means that the inheritance of DNA methylation can affect the growth of subsequent generations. In addition, unhealthy lifestyles such as unbalanced diets and lack of exercise over an individual’s life cycle can alter epigenomes in tissues and consequently influence the occurrence of T2D[69]. Therefore, well-designed family studies with multiple measurements should be considered to investigate the role of DNA methylation in the association between pesticide exposure and T2D risk.
Our results demonstrate for the first time that the DNA methylation of six genes (Alu, CABLES1, CDH1, PDX1, PTEN, PTPRN2) may play a crucial role in the association between pesticide exposure and T2D. This study provides insights into the mechanisms underlying the relationship between pesticide exposure and the onset of T2D from an epigenetic perspective. More well-designed longitudinal studies and animal experiments are needed to fill the remaining gaps in the literature concerning pesticide exposure and T2D.
We sincerely acknowledge the authors of all the articles involved in this systematic review.
| 1. | Marchetti P, Lupi R, Del Guerra S, Bugliani M, D'Aleo V, Occhipinti M, Boggi U, Marselli L, Masini M. Goals of treatment for type 2 diabetes: beta-cell preservation for glycemic control. Diabetes Care. 2009;32 Suppl 2:S178-S183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 2. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1255] [Cited by in RCA: 1581] [Article Influence: 121.6] [Reference Citation Analysis (6)] |
| 3. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th ed. Brussels: International Diabetes Federation; 2021. [PubMed] |
| 4. | Liu X, Zhang L, Chen W. Trends in economic burden of type 2 diabetes in China: Based on longitudinal claim data. Front Public Health. 2023;11:1062903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 239] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 6. | Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health. 2016;4:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 622] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 7. | Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Sci Total Environ. 2017;575:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 912] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 8. | Amaral AF. Pesticides and asthma: challenges for epidemiology. Front Public Health. 2014;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8:1402-1419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1114] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 10. | Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 711] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 11. | Ernst P. Pesticide exposure and asthma. Am J Respir Crit Care Med. 2002;165:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hernández AF, Parrón T, Alarcón R. Pesticides and asthma. Curr Opin Allergy Clin Immunol. 2011;11:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Alavanja MC, Bonner MR. Occupational pesticide exposures and cancer risk: a review. J Toxicol Environ Health B Crit Rev. 2012;15:238-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Roberts JR, Karr CJ; Council On Environmental Health. Pesticide exposure in children. Pediatrics. 2012;130:e1765-e1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Araki A, Miyashita C, Mitsui T, Goudarzi H, Mizutani F, Chisaki Y, Itoh S, Sasaki S, Cho K, Moriya K, Shinohara N, Nonomura K, Kishi R. Prenatal organochlorine pesticide exposure and the disruption of steroids and reproductive hormones in cord blood: The Hokkaido study. Environ Int. 2018;110:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Ferreira TS, Carneiro APS, Mancuzo EV. Occupational exposure to pesticides and chronic hypersensitivity pneumonia: a case report. Rev Bras Med Trab. 2021;19:249-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Chen T, Liu X, Zhang J, Wang L, Su J, Jing T, Xiao P. Associations of chronic exposure to a mixture of pesticides and type 2 diabetes mellitus in a Chinese elderly population. Chemosphere. 2024;351:141194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Czajka M, Matysiak-Kucharek M, Jodłowska-Jędrych B, Sawicki K, Fal B, Drop B, Kruszewski M, Kapka-Skrzypczak L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ Res. 2019;178:108685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Nagaraju R, Joshi AK, Rajini PS. Organophosphorus insecticide, monocrotophos, possesses the propensity to induce insulin resistance in rats on chronic exposure. J Diabetes. 2015;7:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Evangelou E, Ntritsos G, Chondrogiorgi M, Kavvoura FK, Hernández AF, Ntzani EE, Tzoulaki I. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ Int. 2016;91:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 21. | Rusiecki JA, Beane Freeman LE, Bonner MR, Alexander M, Chen L, Andreotti G, Barry KH, Moore LE, Byun HM, Kamel F, Alavanja M, Hoppin JA, Baccarelli A. High pesticide exposure events and DNA methylation among pesticide applicators in the agricultural health study. Environ Mol Mutagen. 2017;58:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 3279] [Article Influence: 252.2] [Reference Citation Analysis (0)] |
| 23. | Desaulniers D, Xiao GH, Lian H, Feng YL, Zhu J, Nakai J, Bowers WJ. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int J Toxicol. 2009;28:294-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Wang C, Zhang J, Chen Y, Zuo Z. DNA hypomethylation induced by tributyltin, triphenyltin, and a mixture of these in Sebastiscus marmoratus liver. Aquat Toxicol. 2009;95:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Consales C, Toft G, Leter G, Bonde JP, Uccelli R, Pacchierotti F, Eleuteri P, Jönsson BA, Giwercman A, Pedersen HS, Struciński P, Góralczyk K, Zviezdai V, Spanò M. Exposure to persistent organic pollutants and sperm DNA methylation changes in Arctic and European populations. Environ Mol Mutagen. 2016;57:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Kim S, Cho YH, Lee I, Kim W, Won S, Ku JL, Moon HB, Park J, Kim S, Choi G, Choi K. Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: A CHECK cohort study. Environ Int. 2018;119:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Kaimala S, Ansari SA, Emerald BS. DNA methylation in the pathogenesis of type 2 diabetes. Vitam Horm. 2023;122:147-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Nadiger N, Veed JK, Chinya Nataraj P, Mukhopadhyay A. DNA methylation and type 2 diabetes: a systematic review. Clin Epigenetics. 2024;16:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (19)] |
| 29. | Raciti GA, Desiderio A, Longo M, Leone A, Zatterale F, Prevenzano I, Miele C, Napoli R, Beguinot F. DNA Methylation and Type 2 Diabetes: Novel Biomarkers for Risk Assessment? Int J Mol Sci. 2021;22:11652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Walaszczyk E, Luijten M, Spijkerman AMW, Bonder MJ, Lutgers HL, Snieder H, Wolffenbuttel BHR, van Vliet-Ostaptchouk JV. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA(1c) levels: a systematic review and replication in a case-control sample of the Lifelines study. Diabetologia. 2018;61:354-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Wang S, Bryan C, Xie J, Zhao H, Lin LF, Tai JAC, Horzmann KA, Sanchez OF, Zhang M, Freeman JL, Yuan C. Atrazine exposure in zebrafish induces aberrant genome-wide methylation. Neurotoxicol Teratol. 2022;92:107091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116:1547-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 34. | Lee MH, Cho ER, Lim JE, Jee SH. Association between serum persistent organic pollutants and DNA methylation in Korean adults. Environ Res. 2017;158:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Huen K, Yousefi P, Bradman A, Yan L, Harley KG, Kogut K, Eskenazi B, Holland N. Effects of age, sex, and persistent organic pollutants on DNA methylation in children. Environ Mol Mutagen. 2014;55:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Zhang X, Wallace AD, Du P, Kibbe WA, Jafari N, Xie H, Lin S, Baccarelli A, Soares MB, Hou L. DNA methylation alterations in response to pesticide exposure in vitro. Environ Mol Mutagen. 2012;53:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H, Jafari N, Zheng Y, Xie H, Soares MB, Kibbe WA, Hou L. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol. 2012;34:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | van der Plaat DA, de Jong K, de Vries M, van Diemen CC, Nedeljković I, Amin N, Kromhout H; Biobank-based Integrative Omics Study Consortium, Vermeulen R, Postma DS, van Duijn CM, Boezen HM, Vonk JM. Occupational exposure to pesticides is associated with differential DNA methylation. Occup Environ Med. 2018;75:427-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Wang W, Ito T, Otsuka S, Nansai H, Abe K, Nakao Y, Ohgane J, Yoneda M, Sone H. Epigenetic effects of insecticides on early differentiation of mouse embryonic stem cells. Toxicol In Vitro. 2021;75:105174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Lee JY, Lee KM, Lee DH, Kim DS. Association of low-dose exposure to persistent organic pollutants with E-cadherin promoter methylation in healthy Koreans. Biomarkers. 2018;23:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Thongsroy J, Mutirangura A. Decreased Alu methylation in type 2 diabetes mellitus patients increases HbA1c levels. J Clin Lab Anal. 2023;37:e24966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Rönn T, Ofori JK, Perfilyev A, Hamilton A, Pircs K, Eichelmann F, Garcia-Calzon S, Karagiannopoulos A, Stenlund H, Wendt A, Volkov P, Schulze MB, Mulder H, Eliasson L, Ruhrmann S, Bacos K, Ling C. Genes with epigenetic alterations in human pancreatic islets impact mitochondrial function, insulin secretion, and type 2 diabetes. Nat Commun. 2023;14:8040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 43. | Baca P, Barajas-Olmos F, Mirzaeicheshmeh E, Zerrweck C, Guilbert L, Sánchez EC, Flores-Huacuja M, Villafán R, Martínez-Hernández A, García-Ortiz H, Contreras-Cubas C, Centeno-Cruz F, Orozco L. DNA methylation and gene expression analysis in adipose tissue to identify new loci associated with T2D development in obesity. Nutr Diabetes. 2022;12:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 44. | Liu J, Lang G, Shi J. Epigenetic Regulation of PDX-1 in Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2021;14:431-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 45. | Volkov P, Bacos K, Ofori JK, Esguerra JL, Eliasson L, Rönn T, Ling C. Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis. Diabetes. 2017;66:1074-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 46. | Yin L, Cai WJ, Chang XY, Li J, Zhu LY, Su XH, Yu XF, Sun K. Analysis of PTEN expression and promoter methylation in Uyghur patients with mild type 2 diabetes mellitus. Medicine (Baltimore). 2018;97:e13513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Wang WJ, Huang R, Zheng T, Du Q, Yang MN, Xu YJ, Liu X, Tao MY, He H, Fang F, Li F, Fan JG, Zhang J, Briollais L, Ouyang F, Luo ZC. Genome-Wide Placental Gene Methylations in Gestational Diabetes Mellitus, Fetal Growth and Metabolic Health Biomarkers in Cord Blood. Front Endocrinol (Lausanne). 2022;13:875180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Rodríguez-Rodero S, Menéndez-Torre E, Fernández-Bayón G, Morales-Sánchez P, Sanz L, Turienzo E, González JJ, Martinez-Faedo C, Suarez-Gutiérrez L, Ares J, Díaz-Naya L, Martin-Nieto A, Fernández-Morera JL, Fraga MF, Delgado-Álvarez E. Altered intragenic DNA methylation of HOOK2 gene in adipose tissue from individuals with obesity and type 2 diabetes. PLoS One. 2017;12:e0189153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Thongsroy J, Patchsung M, Mutirangura A. The association between Alu hypomethylation and severity of type 2 diabetes mellitus. Clin Epigenetics. 2017;9:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Hetty S, Vranic M, Kamble PG, Lundqvist MH, Pereira MJ, Eriksson JW. CABLES1 expression is reduced in human subcutaneous adipose tissue in obesity and type 2 diabetes but may not directly impact adipocyte glucose and lipid metabolism. Adipocyte. 2023;12:2242997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Yang BT, Dayeh TA, Volkov PA, Kirkpatrick CL, Malmgren S, Jing X, Renström E, Wollheim CB, Nitert MD, Ling C. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol Endocrinol. 2012;26:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 52. | Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 710] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 53. | Pinzón-Cortés JA, Perna-Chaux A, Rojas-Villamizar NS, Díaz-Basabe A, Polanía-Villanueva DC, Jácome MF, Mendivil CO, Groot H, López-Segura V. Effect of diabetes status and hyperglycemia on global DNA methylation and hydroxymethylation. Endocr Connect. 2017;6:708-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Hashemi M, Rezaei H, Eskandari-Nasab E, Kaykhaei MA, Taheri M. Association of promoter methylation and 32-bp deletion of the PTEN gene with susceptibility to metabolic syndrome. Mol Med Rep. 2013;7:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Li YZ, Di Cristofano A, Woo M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb Perspect Med. 2020;10:a036137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 57. | Tang X, Powelka AM, Soriano NA, Czech MP, Guilherme A. PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J Biol Chem. 2005;280:22523-22529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Smyth LJ, Kilner J, Nair V, Liu H, Brennan E, Kerr K, Sandholm N, Cole J, Dahlström E, Syreeni A, Salem RM, Nelson RG, Looker HC, Wooster C, Anderson K, McKay GJ, Kee F, Young I, Andrews D, Forsblom C, Hirschhorn JN, Godson C, Groop PH, Maxwell AP, Susztak K, Kretzler M, Florez JC, McKnight AJ. Assessment of differentially methylated loci in individuals with end-stage kidney disease attributed to diabetic kidney disease: an exploratory study. Clin Epigenetics. 2021;13:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Meng Y, Cui Y, Zhang W, Fu S, Huang L, Dong H, Du H. Integrative Analysis of Genome and Expression Profile Data Reveals the Genetic Mechanism of the Diabetic Pathogenesis in Goto Kakizaki (GK) Rats. Front Genet. 2018;9:724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Lee S. The association of genetically controlled CpG methylation (cg158269415) of protein tyrosine phosphatase, receptor type N2 (PTPRN2) with childhood obesity. Sci Rep. 2019;9:4855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Rezg R, Mornagui B, Benahmed M, Chouchane SG, Belhajhmida N, Abdeladhim M, Kamoun A, El-fazaa S, Gharbi N. Malathion exposure modulates hypothalamic gene expression and induces dyslipedemia in Wistar rats. Food Chem Toxicol. 2010;48:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Acker CI, Nogueira CW. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere. 2012;89:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, Parthasarathy A, Babu DD, Thumburaj LJ, Freddy AJ, Dinakaran V, Puhari SS, Rekha B, Christy YJ, Anusha S, Divya G, Suganya K, Meganathan B, Kalyanaraman N, Vasudevan V, Kamaraj R, Karthik M, Jeyakumar B, Abhishek A, Paul E, Pushpanathan M, Rajmohan RK, Velayutham K, Lyon AR, Ramasamy S. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Xuan Z, Ma Y, Zhang J, Zhu J, Cai M. Dissolved legacy and emerging organochlorine pesticides in the Antarctic marginal seas: Occurrence, sources and transport. Mar Pollut Bull. 2023;187:114511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA Methylation in the Mammalian Genome. Cell. 2016;167:233-247.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 893] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 66. | González-Casanova JE, Pertuz-Cruz SL, Caicedo-Ortega NH, Rojas-Gomez DM. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. Biomed Res Int. 2020;2020:7453786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Ekström TJ, Harris TB, Launer LJ, Eiriksdottir G, Leppert MF, Sapienza C, Gudnason V, Feinberg AP. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299:2877-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 68. | Kheirkhah Rahimabad P, Arshad SH, Holloway JW, Mukherjee N, Hedman A, Gruzieva O, Andolf E, Kere J, Pershagen G, Almqvist C, Jiang Y, Chen S, Karmaus W. Association of Maternal DNA Methylation and Offspring Birthweight. Reprod Sci. 2021;28:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Ling C. Epigenetic regulation of insulin action and secretion - role in the pathogenesis of type 2 diabetes. J Intern Med. 2020;288:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |