Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.95102
Revised: August 28, 2024
Accepted: October 29, 2024
Published online: February 15, 2025
Processing time: 272 Days and 23.4 Hours
Adult-onset diabetes is most often considered to be type 2 diabetes. However, other types of diabetes can develop in adults, including exocrine pancreas disease-associated diabetes, also called type 3c diabetes. Differential diagnosis between these types of diabetes still remains a diagnostic challenge.
To define anthropometric and laboratory markers that will allow for early diag
The study group included 44 patients with pancreatogenic diabetes (26 with pancreatic cancer and 18 with chronic pancreatitis), while the control group consisted of 35 patients with type 2 diabetes. We analyzed several parameters, including sex, age, body mass index (BMI), fasting plasma glucose, fasting C-peptide and insulin with homeostasis model assessment of insulin resistance (HOMA-IR) index calculation, adrenomedullin, adiponectin and creatinine levels with epidermal growth factor receptor (eGFR) calculation. We also developed an equation, termed type 3c diabetes index, which utilized BMI, fasting insulin and adrenomedullin levels, and eGFR to better identify patients with type 3c diabetes.
Compared to patients with type 2 diabetes, patients with pancreatogenic diabetes had significantly lower BMI (25.11 ± 4.87 kg/m2vs 30.83 ± 5.21 kg/m2), fasting C-peptide (0.81 ± 0.42 nmol/L vs 1.71 ± 0.80 nmol/L), insulin (76.81 ± 63.34 pmol/L vs 233.19 ± 164.51 pmol/L) and HOMA-IR index, despite similar fasting plasma glucose levels. Patients with pancreatogenic diabetes also had lower adrenomedullin levels (0.41 ± 0.25 ng/mL vs 0.63 ± 0.38 ng/mL) but higher adiponectin levels (13.08 ± 7.20 μg/mL vs 8.28 ± 4.01 μg/mL) and eGFR levels (100.53 ± 21.60 mL/min/1.73 m2vs 85.14 ± 19.24 mL/min/1.73 m2). Finally, patients with pancreatogenic diabetes had significantly lower Type 3c diabetes index values.
Patients with pancreatogenic diabetes differ from patients with type 2 diabetes in anthropometric and laboratory parameters. The type 3c diabetes index had the highest discriminating value, above any single parameter.
Core Tip: Adult-onset diabetes is usually considered to be a type 2 diabetes mellitus. However also other types of diabetes, including diabetes in the course of exocrine pancreas diseases, are present at this age. Making an early, correct diagnosis is of utmost importance for the patient's future. Several anthropometric and laboratory parameters were found useful in differentiating these types of diabetes, and on their basis we developed an equation (type 3c diabetes index), which showed the highest sensitivity and specificity in identifying diabetes secondary to pancreatic pathology.
- Citation: Juza A, Kołodziej-Spirodek L, Gutkowski K, Partyka M, Dąbrowski M. Distinguishing exocrine pancreas disease-associated diabetes from type 2 diabetes based on anthropometric and metabolic parameters. World J Diabetes 2025; 16(2): 95102
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/95102.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.95102
Diabetes mellitus is a group of diseases characterized by hyperglycemia, which is associated with an increased risk of microvascular and macrovascular complications[1,2]. The most common form of adult-onset diabetes is type 2 diabetes mellitus (T2DM). However, there are several other types of diabetes, including those related to disorders of the exocrine pancreas such as chronic pancreatitis (CP) and pancreatic cancer (PC)[1-3]. This form of diabetes is classified as type 3c diabetes and is defined as dysfunction and loss of pancreatic islets because of diseases of the exocrine pancreas[4]. It is most commonly caused by CP, responsible for 79% of cases[5].
CP is characterized by repetitive episodes of pancreatic inflammation of variable lengths and intensities, leading to irreversible pancreatic tissue damage. Parenchymal and/or intraductal calcifications and pancreatic fibrosis are common in advanced CP. Development of exocrine and endocrine pancreatic insufficiency leads to malabsorption, diabetes and an increased risk of PC[4].
The most common primary malignant neoplasm of the pancreas is adenocarcinoma. Despite its low population incidence, it remains a diagnostic challenge due to its initial low-symptomatic course. Chronic inflammation is a risk factor for PC, which may also be mildly symptomatic. One of the first symptoms of pancreatic disease may be impaired glucose metabolism. It is estimated that up to 85% of patients with PC present with glucose metabolism disorders 1 to 2 years prior to cancer diagnosis in the form of impaired fasting glycemia, impaired glucose tolerance, or overt diabetes. Some data suggest that disrupted glucose metabolism is caused by defects in pancreatic cell proliferation, leading to dysfunction and/or loss of β-cells and insulin resistance. Importantly, in contrast to T2DM, progressive weight loss in PC is accompanied by hyperglycemia progression[6,7]. PC is often diagnosed using advanced imaging tests. Unfortunately, there are still no highly sensitive and specific diagnostic methods to facilitate early diagnosis and implementation of effective therapy enabling radical treatment. Numerous studies have focused on cancer risk factors, the coexistence of diabetes and prediabetes, and the identification of potential biochemical markers for early PC diagnosis[8-11], including adrenomedullin[12,13] and adiponectin[14,15].
In this study, we aimed to analyze associations between the anthropometric and laboratory parameters of patients with diabetes in the course of PC and/or CP vs T2DM. The secondary objective was to analyze differences between patients with PC and CP.
Between July 1, 2016 and June 30, 2019, 88 patients diagnosed with PC (n = 55) or CP (n = 33) were hospitalized in the Gastroenterology and Hepatology Clinic of the University Clinical Hospital in Rzeszów, Poland. New-onset or short-lasting (up to 6 months) diabetes was present in 26 subjects with PC and 18 with CP, and these patients constituted the study group. The control group consisted of 35 consecutive patients with T2DM attending the Diabetic Outpatient Clinic in the University Clinical Hospital in Rzeszów, Poland. Overall, 79 patients with diabetes were included in our analysis. Additional analyses included all 88 patients with PC and CP.
The analyzed parameters included sex, age, body mass index (BMI), fasting plasma glucose, fasting insulin and C-peptide concentration with homeostasis model assessment of insulin resistance (HOMA-IR) index calculation, adrenomedullin, adiponectin and creatinine levels with epidermal growth factor receptor (eGFR) calculation. Body weight and height were measured fasting using an electronic scale. BMI was calculated according to the following formula:
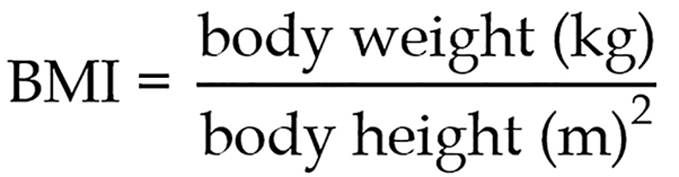
HOMA-IR index was calculated using the following equation:
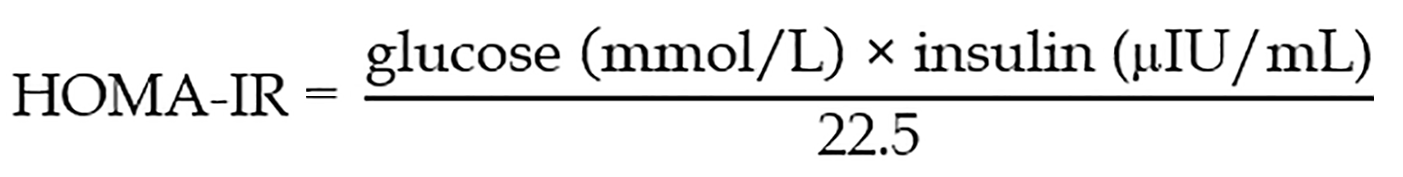
Waist circumference was measured midway between the lower edge of the costal arch and the upper iliac crest. Hip circumference was measured at the level of the greater trochanter of the femur and then waist/hip ratio was calculated.
Adiponectin levels were analyzed in the Laboratory of the Natural and Medical Center for Innovative Research of the University of Rzeszów by ELISA using a Tecan Infinite M200 plate reader and Human Adiponectin ELISA reagent (BioVendor, Brno, Czech Republic). Adrenomedullin levels were determined in the Laboratory of the Clinical Neuroendocrinology Department of the Postgraduate Medical Center in Warsaw using the Adrenomedullin (Human) - EIA Kit (Phoenix Pharmaceuticals Inc., Burlingame, CA, United States). Measurements were performed on a Biochrom Anthos Zenyth 340 Microplate Reader (Biochrom Ltd., Cambridge, United Kingdom). The remaining laboratory parameters were analyzed in the central laboratory of the University Clinical Hospital in Rzeszów. Hematology analysis was performed using the Sysmex XN 1000 Automatic Analyzer (Sysmex Inc., Lincolnshire, IL, Unites States). Biochemical tests were performed on the Vitros 4600 analyzer (Cardinal Health, Dublin, OH, United States). In all cases, blood samples were collected in the morning, between 8:00 and 9:00 after at least 12 hours of fasting.
Statistical analysis of the data was performed using SigmaPlot for Windows, version 12.5 (Systat Software Inc., San Jose, CA, United States). The normality of data distribution was checked using Shapiro-Wilk and Kolmogorov-Smirnov tests. Continuous variables were summarized using descriptive statistics, and they are presented as mean and SD and as median and interquartile range. Differences between the groups were analyzed using an unpaired two-tailed Student’s t-test or by a Mann–Whitney rank sum test where appropriate. Categorical variables were analyzed using χ2 test with the Yates continuity correction applied.
Due to the small sample size, we performed analysis of statistical power for all P values < 0.05. Results with a power below the required 0.800 are marked with an asterisk in the tables. Variables significantly associated with pancreatogenic diabetes in the univariate analysis were included in the multiple logistic regression analysis to identify markers independently associated with exocrine pancreas disease-associated diabetes. Due to the strong co-linearity of some variables (C-peptide, insulin and HOMA-IR index), we performed Backward Stepwise Regression analysis and identified four independent markers of pancreatogenic diabetes, namely BMI, fasting insulin and adrenomedullin levels, and eGFR. Using these variables, we created an equation termed the type 3c diabetes index.
We used the log of each parameter in the formula due to its abnormal distribution in the analyzed populations:
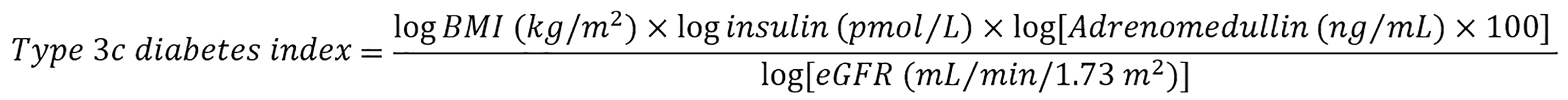
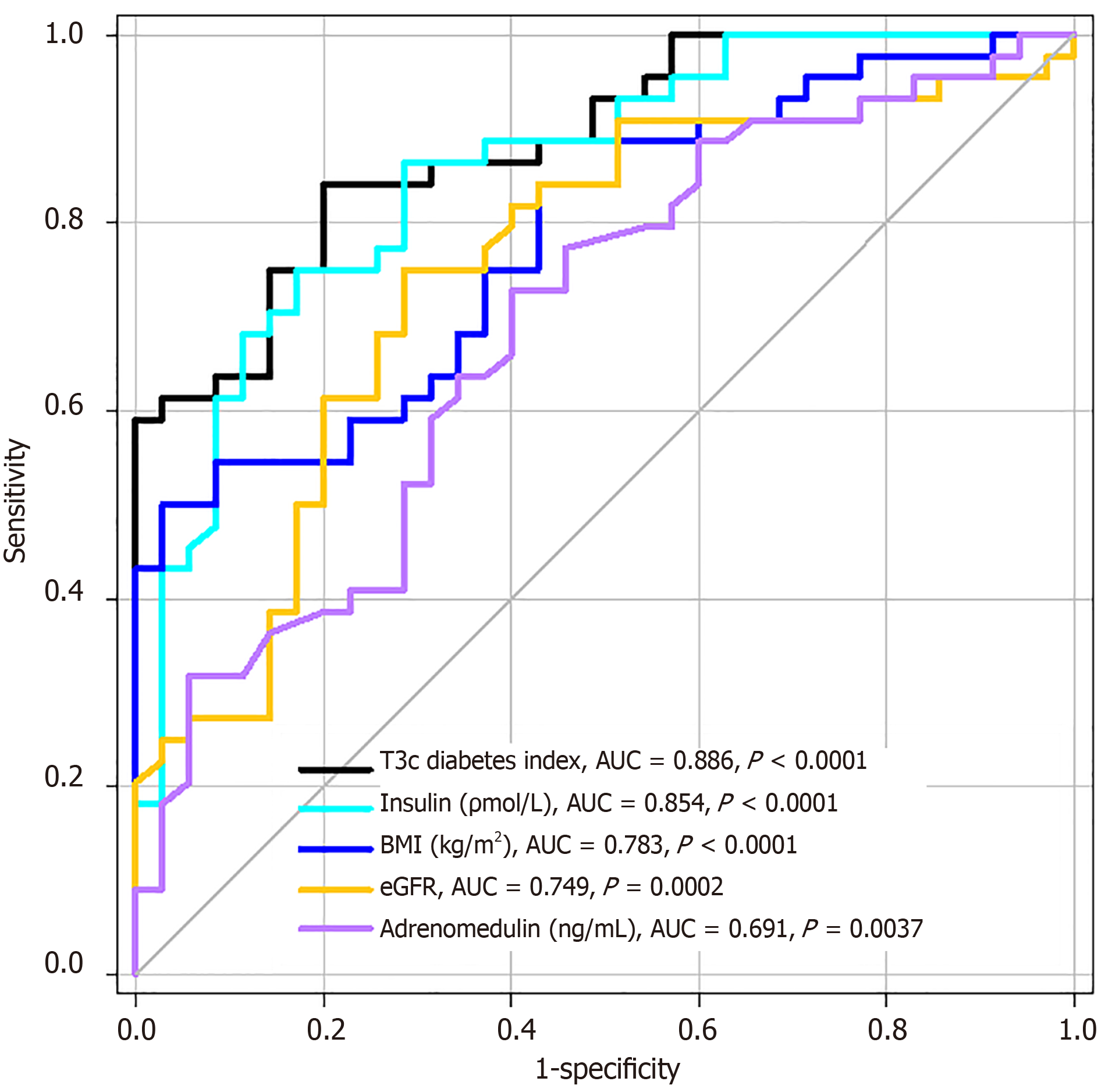
To check its utility in distinguishing T2DM from pancreatogenic diabetes, we performed receiver operating characteristic (ROC) curve analysis to calculate and compare areas under curve (AUC) of our type 3c diabetes index and each single variable independently and to identify the best cut-off points (the highest sum of sensitivity and specificity) for all analyzed parameters.
The study was approved by the Bioethics Committee of the Regional Medical Chamber in Rzeszów (Resolution No. 57/B/2015 issued on June 26, 2015). All study participants were informed about the study purpose and the study procedures before signing written consent.
Out of 79 patients with diabetes, 44 had diabetes associated with PC or CP (study group) and 35 had T2DM (control group). Demographic and anthropometric characteristics of the two groups are presented in Table 1. In most cases in the study group, diabetes was diagnosed upon oral glucose tolerance test results. The primary aim of our analysis was to identify differences between these groups in order to more accurately diagnose diabetes in the course of PC and CP. The majority of patients with PC or CP were men, while the majority of patients with T2DM were women. However, the difference did not reach statistical significance. Age was not significantly different between the groups. Subjects with pancreatogenic diabetes had significantly lower BMI and lower prevalence of obesity and overweight (Table 1).
| Parameter | PC + CP, n = 44 | T2DM, n = 35 | P value |
| Sex | |||
| Female | 16 (36.4) | 21 (60.0) | 0.062 |
| Male | 28 (63.6) | 14 (40.0) | |
| Age in years | 60.0 ± 9.7 | 60.7 ± 6.9 | 0.828 |
| BMI (kg/m2) | 25.11 ± 4.87 | 30.83 ± 5.21 | < 0.001a |
| Obesity, ≥ 30 | 5 (11.4) | 17 (48.6) | < 0.001a |
| Overweight, 25-29.9 | 16 (36.4) | 15 (42.9) | |
| Normal + underweight, < 25 | 23 (52.3) | 3 (8.6) |
Fasting insulin, C-peptide levels, and HOMA-IR were significantly higher in T2DM patients despite comparable fasting glycemia between the two groups. Adrenomedullin levels were significantly higher in the T2DM group, while adiponectin levels and eGFR were higher in patients with pancreatic pathology. Differences between the study and control groups in our empirically developed type 3c diabetes index were highly significant (full P value < 0.000000001; Table 2).
| Parameter | PC + CP, n = 44 | T2DM, n = 35 | P value | ||
| mean ± SD | Median (IQR) | mean ± SD | Median (IQR) | ||
| Fasting plasma glucose (mmol/L) | 7.22 ± 2.87 | 6.22 (5.36-8.00) | 7.13 ± 1.99 | 6.50 (5.94-7.50) | 0.341 |
| Insulin (pmol/L) | 76.81 ± 63.34 | 58.54 (37.78-98.06) | 233.19 ± 164.51 | 185.76 (95.07-347.57) | < 0.001a |
| C-peptide (nmol/L) | 0.81 ± 0.42 | 0.77 (0.47-1.07) | 1.71 ± 0.80 | 1.69 (1.46-2.04) | < 0.001a |
| HOMA-IR | 3.70 ± 3.25 | 2.67 (1.44-5.10) | 11.21 ± 9.82 | 8.24 (3.73-15.14) | < 0.001a |
| Adrenomedullin (ng/mL) | 0.41 ± 0.25 | 0.35 (0.23-0.50) | 0.63 ± 0.38 | 0.51 (0.32-0.86) | 0.004b |
| Adiponectin (μg/mL) | 13.08 ± 7.20 | 11.58 (7.96-17.53) | 8.28 ± 4.01 | 6.73 (5.71-10.01) | < 0.001a |
| Type 3c diabetes index | 1.93 ± 0.63 | 1.87 (1.62-2.31) | 3.04 ± 0.71 | 3.03 (2.50-3.55) | < 0.001a |
| Creatinine (μmol/L) | 63.7 ± 41.6 | 55.7 (48.6-63.7) | 75.1 ± 23.9 | 66.3 (59.2-76.0) | < 0.001a,c |
| eGFR (mL/min/1.73 m2) | 100.53 ± 21.60 | 100.10 (95.25-110.23) | 85.14 ± 19.24 | 91.00 (74.60-98.00) | < 0.001a |
In analysis of the ROC curve, the type 3c diabetes index had the largest AUC. Furthermore, its AUC was significantly higher than any single parameter alone, with the exception of insulin (Figure 1). The optimal cut-off points to identify patients with Type 3c diabetes (the highest sum of sensitivity and specificity) for analyzed parameters with odds ratio calculation and accompanying 95% confidence intervals are presented in Table 3.
| Parameter | Probability of pancreatogenic diabetes | ||||
| Cut-off point | Sensitivity (%) | Specificity (%) | OR (95%CI) | P value | |
| Type 3c diabetes index | < 2.40 | 84.1 | 80.0 | 21.14 (6.65-67.24) | < 0.001a |
| Insulin (pmol/L) | < 85.00 | 75.0 | 82.9 | 14.50 (4.77-44.12) | < 0.001a |
| BMI (kg/m2) | < 24.6 | 50.0 | 97.1 | 16.50 (3.52-77.34) | < 0.001a |
| eGFR (mL/min/1.73 m2) | > 96.0 | 75.0 | 71.4 | 7.50 (2.75-20.42) | < 0.001a |
| Adrenomedullin (ng/mL) | < 0.49 | 72.7 | 60.0 | 4.00 (1.55-10.32) | 0.007b |
In patients with pancreatogenic diabetes, we identified demographic and laboratory parameters that were significantly different between patients with PC-related diabetes vs CP-related diabetes (Table 4 and 5). Compared to patients with CP, patients with PC were significantly older. There was no difference in sex distribution in the PC group, but there was a statistically significant enrichment of men in the CP group. BMI and prevalence of overweight and obesity were not significantly different. However, HOMA-IR, adrenomedullin levels and eGFR were significantly lower in the PC group compared to the CP group. Finally, the type 3c diabetes index was significantly lower in patients with PC compared to the CP group. However, multiple logistic regression analysis revealed that age was the only significant factor that distinguished between people with PC vs CP. Moreover, statistical power of analysis was above 0.800 only for adrenomedullin (Table 5).
| Parameter | Pancreatic cancer, n = 26 | Chronic pancreatitis, n = 18 | P value | ||
| mean ± SD | Median (IQR) | mean ± SD | Median (IQR) | ||
| Fasting plasma glucose (mmol/L) | 6.61 ± 2.23 | 5.97 (5.38-7.17) | 8.11 ± 3.48 | 6.83 (5.31-9.39) | 0.104 |
| C-peptide (nmol/L) | 0.86 ± 0.42 | 0.79 (0.56-1.12) | 0.75 ± 0.41 | 0.76 (0.36-0.92) | 0.407 |
| Insulin (pmol/L) | 60.49 ± 6.73 | 46.74 (35.98-69.03) | 100.29 ± 77.09 | 61.74 (45.77-152.72) | 0.061 |
| HOMA-IR | 2.79 ± 2.39 | 2.36 (1.27-3.12) | 5.00 ± 3.92 | 3.24 (2.27-7.10) | 0.026a,b |
| Adrenomedullin (ng/mL) | 0.33 ± 0.18 | 0.27 (0.20-0.47) | 0.54 ± 0.28 | 0.45 (0.35-0.65) | 0.004c |
| Adiponectin (μg/mL) | 14.27 ± 8.06 | 11.89 (8.46-20.50) | 11.14 ± 5.17 | 10.50 (6.98-13.34) | 0.294 |
| Type 3c diabetes index | 1.76 ± 0.59 | 1.77 (1.41-2.13) | 2.18 ± 0.61 | 2.01 (1.77-2.75) | 0.028a,b |
| Creatinine (µmol/L) | 60.11 ± 14.14 | 55.69 (52.16-64.53) | 68.95 ± 63.65 | 57.46 (44.20-62.76) | 0.390 |
| eGFR (mL/min/1.73 m2) | 94.18 ± 13.94 | 98.15 (91.85-101.38) | 109.71 ± 27.27 | 111.30 (100.50-128.00) | < 0.001b,d |
When we analyzed the entire group of patients with PC (n = 55), 50 subjects presented with dysglycemia (diabetes and prediabetes), while 20 patients in the entire CP group (n = 33) had dysglycemia (P = 0.002). In addition, eGFR was significantly lower in the PC group. We did not identify any other significant differences between these two groups.
PC still remains a major clinical challenge. According to data from the 2019 Polish National Cancer Registry, it accounted for 2.2% and 2.3% of all incident malignant neoplasms in males and females, respectively. Furthermore, it was responsible for 4.5% and 5.7% of cancer deaths in males and females, respectively[16]. In the first decade of the 21st century, the 5-year survival rate for PC in Poland was 8.9% for men and 9.1% for women[17]. Data from the United States show that PC is the third cause of cancer death. PC prognosis is the worst among all malignant neoplasms with a 5-year survival rate of 10%. At the time of diagnosis, 80%-85% of patients are at an advanced stage of disease, preventing the use of radical treatment[18]. Among non-modifiable inherited susceptibility PC risk factors, male sex, and age are listed. Modifiable risk factors for PC include obesity, especially abdominal, and smoking[19,20]. Patients with new-onset diabetes and CP are considered at high risk for PC. T2DM lasting more than 5 years is associated with a moderately elevated risk of PC (up to 1.5-fold), while diabetes lasting up to 1 year from diagnosis is associated with 5.4 times higher risk of PC. Moreover, PC itself can induce diabetes, and 0.8%-1.0% of patients over 50 with newly diagnosed diabetes have PC[19].
The prevalence of CP in the United States from 2001-2013 ranged from 25.4 to 98.7 cases per 100000 people. Alcohol abuse and smoking are important risk factors for CP. In this population of patients, the prevalence of diabetes varies from 25% to 80%, with onset 10-20 years after CP diagnosis[4]. CP is a known risk factor for PC, and the incidence of PC in this group is on average 5%. The incidence of PC among patients with hereditary pancreatitis reaches up to 40% throughout patients’ lifespan[21]. Limitations of imaging methods create a diagnostic challenge for patients with CP, as it can be difficult to differentiate between inflammation and a neoplastic tumor[22,23]. Endoscopic ultrasound, particularly contrast-enhanced endoscopic ultrasound, has a sensitivity of 89% and specificity of 84% in the diagnosis of PC[24]. However, this method has several limitations resulting from acoustic artifacts, making the final diagnosis challenging[24,25].
An early symptom of ongoing inflammatory or neoplastic processes in the pancreas is impaired glucose metabolism. In this study, 90.9% of patients with PC and 60.6% of subjects with CP had impaired glucose metabolism. Adult-onset diabetes is usually considered to be T2DM. However, other types of diabetes can develop in adults, including diabetes associated with exocrine pancreatic diseases, mainly CP and PC. In fact, it is still challenging to distinguish between type 3c diabetes and T2DM. Several clinical factors and biomarkers, such as adrenomedullin and adiponectin, have been studied as tests to differentiate between T2DM and pancreatogenic diabetes[5,26]. Such differential diagnosis is of utmost importance because it enables early and radical treatment of PC, which can improve survival rates of this potentially lethal disease.
The objective of our study was to identify simple and easily accessible anthropometric and serological parameters that can be used to distinguish between patients with pancreatogenic diabetes and T2DM. We examined markers of glucose metabolism (fasting plasma glucose, insulin and C-peptide levels with calculation of HOMA-IR) and kidney function (creatinine level with calculation of eGFR) as laboratory parameters and adrenomedullin and adiponectin as potential biomarkers. Patients with diabetes related to PC and CP had significantly lower BMI, insulin levels and C-peptide levels compared to patients with T2DM, despite comparable fasting glycemia, which resulted in a lower HOMA-IR in this group. Moreover, patients with PC and CP had significantly lower adrenomedullin and higher eGFR levels. To account for all of these altered values, we combined fasting insulin concentration, BMI, adrenomedullin level and eGFR into one equation to generate the type 3c diabetes index. This index had the largest AUC compared to any single parameter that we analyzed, and its value (< 2.40) could be used to identify patients with pancreatogenic diabetes with very high sensitivity and specificity.
The lower BMI in patients with pancreatogenic diabetes can be explained by malabsorption due to either exocrine and endocrine insufficiency[4]. In patients with PC, it can also be associated with advanced neoplastic processes leading to paraneoplastic cachexia[27].
We found significantly lower insulin and C-peptide levels in patients with pancreatogenic diabetes. Decreased β-cell mass may contribute to PC or CP-associated hyperglycemia[4,6]. Several potential mediators of impaired function of β-cells in PC-related diabetes have been identified, including adrenomedullin and possibly other hormones and cytokines[6,26,28]. β-cell function can be indirectly affected by elevated concentrations of free fatty acids due to increased lipolysis or by hyperglycemia and insulin resistance caused by decreased muscle mass[6]. In CP-related diabetes, ongoing chronic inflammatory processes cause the formation of main duct stones and strictures, pseudocyst formation and parenchymal fibrosis and calcification. This leads to loss of active pancreatic parenchyma and ultimately endocrine insufficiency[4,29].
Adrenomedullin is a hormone secreted by enteroendocrine cells throughout the gastrointestinal tract, including the mucosal epithelium, glandular duct cells, neuroendocrine cells, and smooth muscle cells between the oral cavity and the rectum[12]. Adrenomedullin exerts its anti-inflammatory properties by suppressing pro-inflammatory cytokine production in the intestinal mucosa, stimulating mucosal epithelial repair, and improving vascular and lymphatic function and intestinal barrier function. Adrenomedullin levels are highly concentrated in the adrenal medulla and its receptors are widely expressed across many tissues, including the pancreas. In the pancreas, adrenomedullin inhibits insulin secretion, thus causing a dose-dependent increase in blood glucose levels[28]. Adrenomedullin plasma levels correlate with age, BMI, presence of heart failure, grade of kidney function impairment[30] and the grade of hypertension[31]. In studies conducted in the United States and Italy, plasma adrenomedullin concentration was significantly higher in patients with PC compared to healthy controls[32,33]. Moreover, the adrenomedullin receptor is overexpressed in PC cells and its activation stimulates angiogenesis and lymphangiogenesis in cancer tissue[13,34-36]. In contrast, patients with pancreatogenic diabetes in our study had lower adrenomedullin levels compared to the control group. However, the control group was comprised of patients with T2DM, which could account for the difference between published studies and our current study. Lower adrenomedullin levels, especially in patients with PC, could be beneficial in terms of cancer growth and progression. A planned prospective study (Registration No. NCT02456051) of patients with diabetes lasting up to 2 years was expected to shed some light on whether adrenomedullin levels could be a predictive factor for the development of PC in newly diagnosed diabetes but was unfortunately terminated prematurely due to low recruitment rates[36].
Adiponectin is a polypeptide hormone primarily synthesized by mature adipocytes of peripheral adipose tissue but is also generated to a lesser extent by cardiomyocytes, hepatocytes, skeletal muscle cells, and vascular endothelium[37,38]. The main targets of adiponectin action are the liver, skeletal muscle, adipose tissue, pancreatic β-cells and endothelial cells. Adiponectin’s physiological effects include stimulation of fatty acid oxidation in liver and skeletal muscle, reduced hepatic gluconeogenesis, increased glucose uptake and utilization in muscle cells and adipocytes, increased β-cell survival and improved endothelial function. Moreover, it exerts anti-inflammatory effects[37]. Thus, adiponectin improves insulin sensitivity and inhibits hepatic glucose production, playing an indispensable role in protection from T2DM. Low adiponectin levels have been reported in obesity, T2DM, hypertension, coronary artery disease and stroke[37,38]. Interestingly, patients with PC and CP have higher adiponectin levels compared to healthy controls[39,40]. Thus, patients with PC and CP had higher adiponectin concentrations compared to T2DM controls. A nested case-control study of 468 patients with PC and 1080 matched controls from five prospective United States’ cohorts (i.e., Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative, and Women’s Health Study) reported that adiponectin levels were inversely associated with PC risk[41].
Our study has several limitations. The first and the most important is that it was a single-center study, resulting in a relatively small number of study participants. Therefore, some of the tests had low statistical power, not allowing us to exclude the possibility that some results were affected by random chance. Due to limited resources, we could not analyze other promising and potentially relevant biomarkers of PC apart of adrenomedullin and adiponectin[26]. The cross-sectional design of our study did not allow us to draw conclusions regarding causal relationships; thus, variables associated with type 3c diabetes can only be considered as markers and not risk factors for pancreatogenic diabetes. Nevertheless, our study also has some strengths. Our analysis only considered patients with new-onset or short-lasting (up to 6 months) diabetes. We developed an equation (type 3c diabetes index) that can be useful in the differential diagnosis of adult-onset diabetes, distinguishing pancreatogenic diabetes from T2DM. This tool can improve patient prognosis, especially in PC where early diagnosis enables radical treatment at non-advanced disease stages. However, this index also has an important limitation. Our results may not be fully applicable when different tests than those used here are utilized because reference values for adrenomedullin vary by kit. To overcome this, we used the log of adrenomedullin value multiplied by 100.
Differential diagnosis between pancreatogenic diabetes and T2DM remains challenging. In our study, we aimed to identify anthropometric and laboratory parameters that can distinguish patients with type 3c diabetes from those with T2DM. We found that lower BMI, C-peptide, insulin and adiponectin levels, and higher adiponectin and eGFR may be helpful in distinguishing patients with pancreatogenic diabetes from those with T2DM. Our type 3c diabetes index demonstrated great utility in the identification of pancreatogenic diabetes compared to other analyzed parameters. Thus, patients with new-onset or short-lasting diabetes, with normal weight and low type 3c diabetes index (or its components) should be screened for pancreatic pathologies. Larger studies are necessary to confirm our findings.
| 1. | World Health Organization. Classification of diabetes mellitus. Aug 6, 2021. [cited 18 August 2024]. Available from: https://iris.who.int/bitstream/handle/10665/325182/9789241515702-eng.pdf. |
| 2. | Araszkiewicz A, Bandurska-stankiewicz E, Borys S, Broncel M, Budzyński A, Cyganek K, Cypryk K, Cyranka K, Czupryniak L, Dzida G, Dziedzic T, Franek E, Gajewska D, Gawrecki A, Górska M, Gumprecht J, Idzior-waluś B, Jarosz-chobot P, Kalarus Z, Karczewska-kupczewska M, Klupa T, Kokoszka A, Korzon-burakowska A, Kowalska I, Krętowski A, Kwiendacz H, Majkowska L, Małecki M, Mamcarz A, Matejko B, Matyjaszek-matuszek B, Mianowska B, Mrozikiewicz-rakowska B, Myśliwiec M, Nabrdalik K, Narkiewicz K, Sieradzki J, Skupień J, Solnica B, Stompór T, Strojek K, Szadkowska A, Szypowska A, Uruska A, Wender-ożegowska E, Witek P, Wolnik B, Wyleżoł M, Wylęgała E, Zmysłowska A, Zozulińska-ziółkiewicz D. Standards of Care in Diabetes. The position of Diabetes Poland – 2024. Curr Top Diab. 2024;3:1-348. [DOI] [Full Text] |
| 3. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S20-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 1065] [Article Influence: 532.5] [Reference Citation Analysis (4)] |
| 4. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (1)] |
| 5. | Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC). Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 6. | Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Cui Y, Andersen DK. Diabetes and pancreatic cancer. Endocr Relat Cancer. 2012;19:F9-F26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Herreros-Villanueva M, Gironella M, Castells A, Bujanda L. Molecular markers in pancreatic cancer diagnosis. Clin Chim Acta. 2013;418:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Yang J, Li H, Wu Y, Zhang H, Chen W. Tumor markers CA19-9, CA242 and CEA in the diagnosis of pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:11683-11691. [PubMed] |
| 10. | Pekarek L, Fraile-Martinez O, Garcia-Montero C, Saez MA, Barquero-Pozanco I, Del Hierro-Marlasca L, de Castro Martinez P, Romero-Bazán A, Alvarez-Mon MA, Monserrat J, García-Honduvilla N, Buján J, Alvarez-Mon M, Guijarro LG, Ortega MA. Clinical Applications of Classical and Novel Biological Markers of Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Jacks BE, Ekpemiro CU, Adeosun AA, Ogbonna UO, Ogundiran FT, Babalola F, Onyechi NP, Ajayi OO, Boms MG, Nwanguma AN, Udo UA, Okobi OE, Ohikhuai EE, Evbayekha EO. Molecular Markers of Pancreatic Cancer: A 10-Year Retrospective Review of Molecular Advances. Cureus. 2022;14:e29485. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Martínez-Herrero S, Martínez A. Adrenomedullin: Not Just Another Gastrointestinal Peptide. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Vázquez R, Riveiro ME, Berenguer-Daizé C, O'Kane A, Gormley J, Touzelet O, Rezai K, Bekradda M, Ouafik L. Targeting Adrenomedullin in Oncology: A Feasible Strategy With Potential as Much More Than an Alternative Anti-Angiogenic Therapy. Front Oncol. 2020;10:589218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Wang Q, Wang H, Ding Y, Wan M, Xu M. The Role of Adipokines in Pancreatic Cancer. Front Oncol. 2022;12:926230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 15. | Chang ML, Yang Z, Yang SS. Roles of Adipokines in Digestive Diseases: Markers of Inflammation, Metabolic Alteration and Disease Progression. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Didkowska J, Wojciechowska U, Olasek P, dos Santos FC, Michałek I. Nowotwory złośliwe w Polsce w 2019 roku, Cancer in Poland in 2019. [cited 18 August 2024]. Available from: https://onkologia.org.pl/sites/default/files/publications/2022-05/Nowotwory_2019.pdf. |
| 17. | Wojciechowska U, Didkowska J. Changes in five-year relative survival rates in Poland in patients diagnosed in the years 1999–2010. Nowotw. J Oncol. 2017;67:349-358. [DOI] [Full Text] |
| 18. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 12202] [Article Influence: 2440.4] [Reference Citation Analysis (7)] |
| 19. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (1)] |
| 20. | Ke TM, Lophatananon A, Muir KR. Risk Factors Associated with Pancreatic Cancer in the UK Biobank Cohort. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Benzel J, Fendrich V. Familial Pancreatic Cancer. Oncol Res Treat. 2018;41:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic Pancreatitis or Pancreatic Tumor? A Problem-solving Approach. Radiographics. 2019;39:1965-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | D'Onofrio M, Biagioli E, Gerardi C, Canestrini S, Rulli E, Crosara S, De Robertis R, Floriani I. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med. 2014;35:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Iglesias-García J, Lindkvist B, Lariño-Noia J, Domínguez-Muñoz JE. The role of EUS in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma. Rev Esp Enferm Dig. 2012;104:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 26. | Hart PA, Andersen DK, Petrov MS, Goodarzi MO. Distinguishing diabetes secondary to pancreatic diseases from type 2 diabetes mellitus. Curr Opin Gastroenterol. 2021;37:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Roy A, Sahoo J, Kamalanathan S, Naik D, Mohan P, Kalayarasan R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J Gastroenterol. 2021;27:4939-4962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 28. | Nagpal SJS, Kandlakunta H, Her T, Sharma A, Sannapaneni S, Smyrk TC, Velamala P, Garg SK, Rakshit K, Majumder S, Chari S, Matveyenko A. Pancreatic ductal adenocarcinoma is associated with a unique endocrinopathy distinct from type 2 diabetes mellitus. Pancreatology. 2020;20:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322:2422-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 340] [Article Influence: 48.6] [Reference Citation Analysis (2)] |
| 30. | Bell D, Gordon BJ, Lavery A, Megaw K, Kinney MO, Harbinson MT. Plasma levels of intermedin (adrenomedullin-2) in healthy human volunteers and patients with heart failure. Peptides. 2016;76:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Hu W, Zhou PH, Zhang XB, Xu CG, Wang W. Plasma concentrations of adrenomedullin and natriuretic peptides in patients with essential hypertension. Exp Ther Med. 2015;9:1901-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 32. | Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ, Rumie Vittar NB, de Andrade M, Mukhopadhyay D, Petersen GM, Fernandez-Zapico ME, Logsdon CD, Chari ST. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology. 2012;143:1510-1517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Francesco D, Claudio L, Laura A, Rocca Mara L, Paolo A, Giovanni R. Adrenomedullin in pancreatic carcinoma: A case-control study of 22 patients. Integr Cancer Sci Therap. 2016;3:390-392. [DOI] [Full Text] |
| 34. | Ramachandran V, Arumugam T, Hwang RF, Greenson JK, Simeone DM, Logsdon CD. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Ramachandran V, Arumugam T, Langley R, Hwang RF, Vivas-Mejia P, Sood AK, Lopez-Berestein G, Logsdon CD. The ADMR receptor mediates the effects of adrenomedullin on pancreatic cancer cells and on cells of the tumor microenvironment. PLoS One. 2009;4:e7502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Antolino L, Rocca M, Todde F, Catarinozzi E, Aurello P, Bollanti L, Ramacciato G, D'Angelo F. Can pancreatic cancer be detected by adrenomedullin in patients with new-onset diabetes? The PaCANOD cohort study protocol. Tumori. 2018;104:312-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Fang H, Judd RL. Adiponectin Regulation and Function. Compr Physiol. 2018;8:1031-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 38. | Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 39. | Dranka-Bojarowska D, Lekstan A, Olakowski M, Jablonska B, Lewinski A, Musialski P, Sobczyk W, Kapalka A, Lampe P. The assessment of serum concentration of adiponectin, leptin and serum carbohydrate antigen-19.9 in patients with pancreatic cancer and chronic pancreatitis. J Physiol Pharmacol. 2015;66:653-663. [PubMed] |
| 40. | Gasiorowska A, Talar-Wojnarowska R, Kaczka A, Borkowska A, Czupryniak L, Małecka-Panas E. Subclinical Inflammation and Endothelial Dysfunction in Patients with Chronic Pancreatitis and Newly Diagnosed Pancreatic Cancer. Dig Dis Sci. 2016;61:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, Rifai N, Pollak MN, Cochrane BB, Kaklamani V, Lin JH, Manson JE, Fuchs CS, Wolpin BM. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/