Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.101779
Revised: November 15, 2024
Accepted: December 5, 2024
Published online: February 15, 2025
Processing time: 95 Days and 1.3 Hours
Insulin therapy plays a crucial role in managing diabetes. Regulatory guidelines mandate assessing the pharmacokinetics (PK) and pharmacodynamics (PD) of new insulin formulations with euglycemic clamp techniques before entry into the market. Typically, blood glucose (BG) levels are maintained at 5% below baseline to suppress endogenous insulin secretion in healthy volunteers. However, in scenarios where BG baseline is relatively low, maintaining it at 5% below baseline can increase hypoglycemic risk. Consequently, we adjusted to maintain it at 2.5% below a baseline of < 4.00 mmol/L. It remains uncertain whether this adjustment impacts endogenous insulin inhibition or the PD of study insulin.
To evaluate and compare the PD and C-peptide status using two different target BG setting methods.
Data came from euglycemic clamp trials assessing the PK/PD of insulin aspart (IAsp) in healthy participants. Target BG was set at 2.5% below baseline for those with a basal BG of < 4.00 mmol/L (group A), and at 5% below baseline for others (group B). The area under the curve (AUC) of IAsp (AUCIAsp, 0-8 h) and GIR from 0 to 8 hours (AUCGIR, 0-8 h) was used to characterize the PK and PD of IAsp, respec
Out of 135 subjects, 15 were assigned to group A and 120 to group B; however, group B exhibited higher basal C-peptide (1.59 ± 0.36 vs 1.32 ± 0.42 ng/mL, P = 0.006). Following propensity score matching to adjust for basal C-peptide differences, 71 subjects (15 in group A and 56 in group B) were analyzed. No significant differences were observed in demographics, IAsp dosage, or clamp quality. Group B showed significantly higher baseline (4.35 ± 0.21 vs 3.91 ± 0.09 mmol/L, P < 0.001), target (4.13 ± 0.20 vs 3.81 ± 0.08 mmol/L, P < 0.001), and clamped (4.10 ± 0.17 vs 3.80 ± 0.06 mmol/L, P < 0.001) BG levels. Both groups exhibited comparable C-peptide suppression (32.5% ± 10.0% vs 35.6% ± 12.1%, P = 0.370) and similar IAsp activity (AUCGIR, 0-8 h: 1433 ± 400 vs 1440 ± 397 mg/kg, P = 0.952) under nearly equivalent IAsp exposure (AUCIAsp, 0-8 h: 566 ± 51 vs 571 ± 85 ng/mL × h, P = 0.840).
Maintaining BG at 2.5% below a baseline of < 4.00 mmol/L did not compromise the endogenous insulin suppression nor alter the observed pharmacodynamic effects of the study insulin.
Core Tip: Insulin therapy is of great importance in anti-diabetes treatment. This study focuses on the preclinical assessment of insulin preparation using the euglycemic clamp technique in healthy subjects. Typically, blood glucose is usually maintained below the individual’s baseline throughout the clamp to inhibit endogenous insulin secretion. The hypoglycemic risk would increase if the BG baseline is relatively low. This retrospective cohort study enrolled euglycemic clamp trials evaluating the pharmacokinetics and pharmacodynamics of insulin aspart to explore optimal methodologies for conducting euglycemic clamps under these circumstances.
- Citation: Liu H, Li T, Chen XL, Yu HL, Yu YR. Impact of setting distinct target blood glucose levels on endogenous insulin suppression and pharmacodynamics of insulin preparations. World J Diabetes 2025; 16(2): 101779
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/101779.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.101779
The euglycemic clamp technique is regarded as the gold standard method for evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of insulin formulations[1]. This method typically involves the recruitment of healthy volunteers for the study purpose[2-4]. Various strategies, such as induced hyperinsulinemia from continuous intravenous insulin infusion[5-7] and somatostatin infusion[8,9], have been employed to suppress the endogenous insulin secretion in these individuals. Over the past decades, one effective approach has been to maintain blood glucose (BG) levels below baseline for normoglycemia throughout the duration of the euglycemic clamp, with BG reductions of 5 mg/dL[10-12] or 10%[13,14] commonly implemented by researchers.
Normal fasting BG levels typically range from 3.9 to 6.1 mmol/L in healthy subjects[15]. It is not unusual in clinical settings to encounter relatively low BG baseline levels, such as < 4.00 mmol/L. Euglycemic clamp studies with a BG baseline of ≤ 3.50 mmol/L are generally not initiated due to the risk of hypoglycemic events[11]. One incidence of hypoglycemic event is considered indicative of a failed clamp procedure[16,17]. Setting a target BG level at 0.28 mmol/L (approximately 5%) below a relatively low baseline is deemed inappropriate because of the heightened risk of hypoglycemia. Based on our experience, setting a target BG level at approximately 2.5% below baseline when the BG baseline does not exceed 4.00 mmol/L is considered safer in clinical practice, effectively minimizing the risk of hypoglycemic incidents.
Previous studies suggest that lower sub-baseline target BG levels may result in a more pronounced suppression of endogenous insulin (e.g., greater inhibition of postdose C-peptide at target BG levels of 9 mg/dL below baseline[18] compared to 5 mg/dL below baseline[19]). However, it remains uncertain whether a higher sub-baseline target BG level, such as 2.5% instead of 5% below a baseline of less than 4.00 mmol/L, might affect the status of endogenous insulin suppression and the PK/PD evaluation of insulin formulations. In this study, we conducted a retrospective analysis of euglycemic clamp trials assessing the PK/PD of insulin aspart (IAsp) in healthy volunteers to address this question.
This study was a retrospective cohort analysis of two clinical trials with registration IDs of CTR20220854 and CTR20222843, respectively, with details on the website http://www.chinadrugtrials.org.cn/index.html. Healthy participants who underwent an 8-hour euglycemic clamp following a 0.2-IU/kg subcutaneous injection of IAsp were considered eligible. BG baseline was established using the average value of three BG assessments taken at -30, -20, and -10 minutes before dosing. The subjects were divided into group A (BG baseline < 4.00 mmol/L) and group B (BG baseline ≥ 4.00 mmol/L) based on the BG baseline levels. Comparative analyses were conducted regarding demographic data, euglycemic clamp metrics (including degree of C-peptide suppression), and PK/PD evaluations of IAsp. The study was conducted in the Early Phase Clinical Trial Center at Sichuan University West China Hospital. The study protocol was approved by the ethics committee of West China Hospital of Sichuan University. All participants provided written informed consent, and the studies were conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, and local regulations.
Subjects were recruited by the investigators at the West China Hospital of Sichuan University. Volunteers with an age of 18-45 years and a body mass index (BMI) of 19.0-26.0 kg/m2 were considered eligible. Experiments were performed only after an informed consent form was signed by the participants. A robust assessment was conducted to ascertain the health of each of the volunteers, which included gathering both current and historical medical data, performing physical examinations, monitoring vital signs (such as temperature, heart rate, blood pressure, and respiratory rate), recording a 12-lead electrocardiogram, and executing various laboratory tests including a complete blood count, liver and renal function tests, a coagulation profile, an oral glucose tolerance test, and urine analysis. Individuals who had taken any experimental medicinal products within 30 days or five times its half-life (whichever period was longer) before dosing, those with historical or current comorbid conditions that could significantly affect the drug's absorption, metabolism, or excretion, heavy smokers consuming more than 10 cigarettes daily, participants unable to abstain from smoking for the duration of the study, or those with a known allergy to IAsp or any of its components were excluded from the study.
Strenuous exercise was forbidden from 3 days before dosing. Subjects came to the clinical research unit the day before the dosing day and were provided with dinner. They were then required to abstain from any food or beverages for 10 hours before dosing. On the morning of the dosing day, participants' body weights were recorded following a bowel movement and urination. Venepunctures were performed on the cephalic vein at the wrist and the median cubital vein for blood sampling and a 20% dextrose infusion, respectively. The blood sampling channel was continuously heated in a warm blanket at 50-60 °C to collect venous blood. After the baseline data collection, a dose of 0.2 IU/kg of IAsp was administered subcutaneously into the abdominal wall. BG levels were measured every 5 minutes from 0 to 4 hours, and 10 minutes from 4 to 8 hours postdosing at the bedside. An infusion of 20% dextrose was initiated when BG levels decreased by at least 0.28 mmol/L from baseline. The glucose infusion rate (GIR) was adjusted as necessary by the investigators to maintain the BG around approximately 2.5% below baseline for subjects with a basal BG level under 4.00 mmol/L (group A), or 5% below baseline for those with a basal BG level of 4.00 mmol/L or higher (group B). The clamp would discontinue when GIR fell to zero for at least 30 minutes after 6 hours following drug administration.
BG levels were analyzed in a 0.1-mL whole blood sample with a glucose analyzer (Biosen C_line, EKF Diagnostics, Barleben, Germany) using an automated glucose oxidase method. The analyzer's precision was noted at 1.5%, with a measurement range of 0.5 to 50 mmol/L.
Plasma IAsp concentrations were determined at -30, 10, 20, 30, 40, 50, 60, 75, 90, 105, 120, 180, 240, 300, 360, and 480 minutes using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method with a lower limit quantification of 0.20 ng/mL.
An electrochemiluminescence method with a lower limit quantification of 0.01 ng/mL was used to determine the C-peptide levels at -0.5, -0.17, 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 8 hours. The C-peptide baseline was defined as the mean value of two C-peptide measurements predosing.
The IAsp exposure was expressed by the area under the curve (AUC) of IAsp from 0 to 8 hours (AUCIAsp, 0-8 h) and the activity of IAsp was represented by the AUC of GIR from 0 to 8 hours (AUCGIR, 0-8 h).
The AUCIAsp, 0-8 h and AUCGIR, 0-8 h were calculated using a trapezoidal method to present the main endpoint of PK or PD, respectively. The coefficient of variation of BG (CVBG) as previously delineated[20] was employed to describe the quality of the euglycemic clamp. Data is expressed as the mean ± SD for normal distribution variables, and median (range) for abnormal distribution variables. Inter-group comparisons were conducted utilizing either a Student’s t-test or a Mann-Whitney U test, depending on the normality of data distribution. Fisher's exact test was applied to ascertain differences in sex distribution. Given the significant disparity in basal C-peptide levels between the two groups (Table 1), a propensity score matching (PSM) method with an approximate ratio of 1:4 was implemented to correct this imbalance. Statistical analyses were performed using SPSS software (version 27.0), considering a two-tailed P value of less than 0.05 as statistically significant.
| Before PSM | After PSM | |||||
| Group A | Group B | P value | Group A | Group B | P value | |
| N | 15 | 120 | - | 15 | 56 | - |
| Age (years)1 | 24.7 ± 3.0 | 27.3 ± 5.3 | 0.073 | 24.7 ± 3.0 | 25.4 ± 4.1 | 0.565 |
| Weight (kg)1 | 61.0 ± 7.0 | 61.2 ± 6.6 | 0.916 | 61.0 ± 7.0 | 61.4 ± 6.8 | 0.859 |
| Height (cm)1 | 170.6 ± 6.6 | 169.1 ± 7.6 | 0.442 | 170.6 ± 6.6 | 169.3 ± 7.1 | 0.522 |
| BMI (kg/m2)1 | 20.9 ± 1.7 | 21.4 ± 1.9 | 0.33 | 20.9 ± 1.7 | 21.4 ± 1.7 | 0.367 |
| Dosage (IU)1 | 12.1 ± 1.5 | 12.2 ± 1.4 | 0.81 | 12.1 ± 1.5 | 12.3 ± 1.4 | 0.711 |
| Female, n (%) | 1, 6.7 | 15, 12.5 | 0.442 | 1, 6.7 | 6, 10.7 | 0.541 |
| Basal BG (mmol/L)1 | 3.91 ± 0.09 | 4.39± 0.24 | < 0.001 | 3.91 ± 0.09 | 4.35 ± 0.21 | < 0.001 |
| Target BG (mmol/L)1 | 3.81 ± 0.08 | 4.17 ± 0.23 | < 0.001 | 3.81 ± 0.08 | 4.13 ± 0.20 | < 0.001 |
| Clamped BG (mmol/L)1 | 3.80 ± 0.06 | 4.13 ± 0.19 | < 0.001 | 3.80 ± 0.06 | 4.10 ± 0.17 | < 0.001 |
| CVBG (%)1 | 4.04 ± 0.79 | 3.97 ± 0.84 | 0.755 | 4.04 ± 0.79 | 3.96 ± 0.97 | 0.78 |
| AUCIAsp, 0-8 h (ng/mL × h)1 | 566 ± 51 | 562 ± 85 | 0.86 | 566 ± 51 | 571 ± 85 | 0.84 |
| AUCGIR, 0-8 h (mg/kg)1 | 1433 ± 400 | 1465 ± 396 | 0.764 | 1433 ± 400 | 1440 ± 397 | 0.952 |
| Basal C-peptide (ng/mL)1 | 1.32 ± 0.42 | 1.59 ± 0.36 | 0.006 | 1.32 ± 0.42 | 1.47 ± 0.34 | 0.161 |
| Mean postdose C-peptide (ng/mL)1 | 0.90 ± 0.34 | 1.04 ± 0.31 | 0.088 | 0.90 ± 0.34 | 0.94 ± 0.26 | 0.6 |
| C-peptide reduction (%)1 | 32.5 ± 10.0 | 34.6 ± 12.2 | 0.527 | 32.5 ± 10.0 | 35.6 ± 12.1 | 0.37 |
Table 1 illustrates that 135 participants were deemed eligible for this study. After stratification, groups A (BG baseline < 4.0 mmol/L) and B (BG baseline ≥ 4.0 mmol/L) comprised 15 and 120 subjects, respectively. No significant differences were observed in age, body weight, height, BMI, or sex distribution between the groups. However, a notable discrepancy in basal C-peptide between the two groups was detected. Since one of the aims of this study was to explore the C-peptide changes throughout the clamp procedure between the two groups and considering that the differences in basal C-peptide might influence the change of postdose C-peptide, a PSM method was utilized to equilibrate basal C-peptide differences across the groups.
After PSM, groups A and B consisted of 15 and 56 participants, respectively. The average ages for groups A and B were 24.7 and 25.4 years, respectively. Post-matching assessments showed no significant differences in body weight, height, BMI, or sex distribution between the two groups.
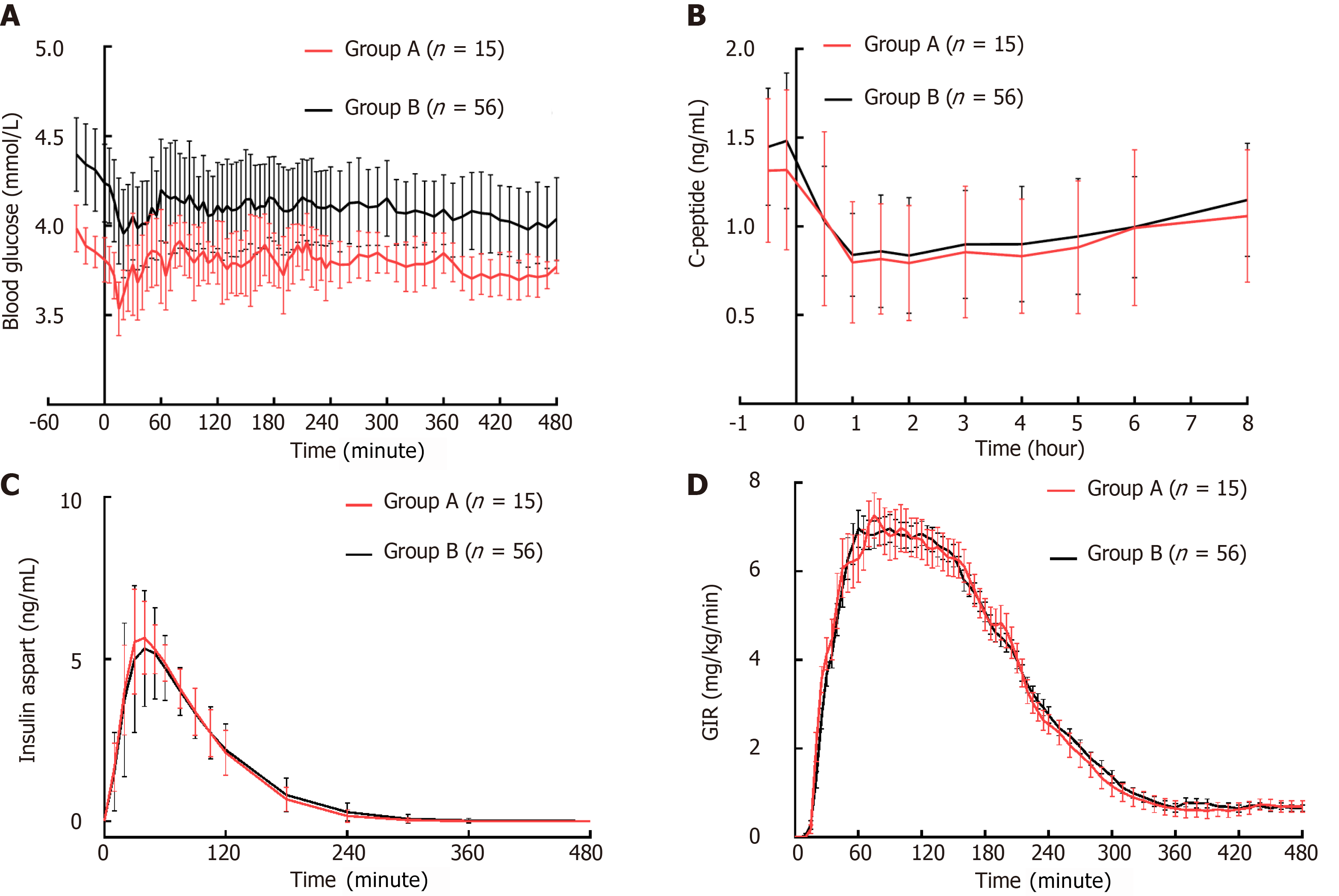
In group A, the mean BG baseline was recorded at 3.91 ± 0.09 mmol/L. The target and clamped BG levels were comparable, at 3.81 ± 0.08 mmol/L and 3.80 ± 0.06 mmol/L respectively, demonstrating no significant difference (P = 0.643). In group B, the baselines of mean BG were 4.39 ± 0.24 mmol/L and 4.35 ± 0.21 mmol/L before and after PSM, respectively. The clamped mean BG levels closely approached the target levels both before (4.13 ± 0.19 vs 4.17 ± 0.23 mmol/L, P = 0.095) and after PSM (4.10 ± 0.17 vs 4.13 ± 0.20 mmol/L, P = 0.296). Group B consistently exhibited higher basal BG, target BG, and clamped BG values compared to group A (Figure 1A). The CVBG remained consistent across both groups, irrespective of matching for basal C-peptide levels, with an overall CVBG of less than 5%.
Initially, the basal mean C-peptide level was significantly higher in group B (1.59 ± 0.36 vs 1.32 ± 0.42 ng/mL, P = 0.006). After PSM, the basal mean C-peptide levels were comparable between the two groups (1.32 ± 0.42 vs 1.47 ± 0.34 ng/mL, P = 0.161), with both groups experiencing a mean C-peptide reduction of approximately 32% to 36% from baseline (Figure 1B).
The overall IAsp exposures were comparable between the two groups before (566 ± 51 vs 562 ± 85 ng/mL × h, P = 0.860) and after (566 ± 51 vs 571 ± 85 ng/mL × h, P = 0.764) PSM. The time profiles of IAsp between the two groups after matching for basal C-peptide are shown in Figure 1C. Similarly, the total glucose-lowering effects measured by AUCGIR, 0-8 h were comparable before (1433 ± 400 vs 1465 ± 396 mg/kg, P = 0.764) and after PSM (1433 ± 400 vs 1440 ± 397 mg/kg, P = 0.952). The time-action profiles of IAsp are shown in Figure 1D.
The euglycemic clamp technique invariably necessitates the expertise of experienced investigators. The occurrence of hypoglycemia indicates a failure of the euglycemic clamp procedure[16,17], and unsuppressed endogenous insulin secretion could render the pharmacodynamic data unreliable[21,22]. Typically, the suppression of endogenous insulin secretion can be accomplished by consistently maintaining BG levels beneath baseline in healthy participants[10-14]. The degree of suppression of endogenous insulin secretion was directly correlated with the reduction in BG levels from baseline up to a certain threshold[18,19]. Commonly, a BG level maintained at 5% below baseline was employed in the majority of the studies[10-12,23,24]. Nonetheless, sustaining BG at 5% below baseline in subjects with a comparatively lower BG baseline (e.g., < 4.00 mmol/L) could exaggerate the risk of hypoglycemia. This study reveals that over 10% (11.1%) of euglycemic clamps commenced with a BG baseline of lower than 4.00 mmol/L. Therefore, it is crucial to deliberate on how to effectively balance the prevention of hypoglycemia with the sufficient inhibition of endogenous insulin secretion in euglycemic clamp settings characterized by a relatively low BG baseline.
This investigation encompassed euglycemic clamp studies that engaged healthy subjects to evaluate the PK/PD of IAsp. According to the basal BG levels, they were divided into two groups (group A: Basal BG < 4.00 mmol/L; group B: Basal BG ≥ 4.00 mmol/L). The target BG level was established at 2.5% below baseline for group A and 5% below baseline for group B. The study further examined variations in endogenous insulin secretion and their effect on PK/PD eva
After balancing for basal C-peptide, similar C-peptide baseline and postdose C-peptide levels were noted in both groups. Numerous factors could affect the suppression of endogenous insulin, with the quality of the clamp potentially playing a significant role[20]. In this study, BG levels were consistently maintained at around target levels in both groups, with a CVBG below 5%, suggesting a uniformly high quality across the euglycemic clamp studies. Moreover, exogenous insulin levels would inhibit the secretion of endogenous insulin[25-28]. The comparable impact of exogenous IAsp on the suppression of endogenous insulin secretion can be deduced from comparable values of AUCIAsp, 0-8 h and similar time profiles of IAsp. The primary differences observed between the groups were in their basal and clamped BG levels. The study revealed a comparable reduction in mean C-peptide from baseline, demonstrating that setting a BG target at 2.5% below a relatively low baseline of less than 4.00 mmol/L still effectively suppresses endogenous insulin secretion.
The concentration of IAsp was determined by a validated LC-MS/MS method which would be not affected by endogenous insulin. The PK endpoint largely depends on the administered insulin dose and the metabolic processes in the subjects. In this study, comparable insulin doses and similar time profiles of IAsp were observed between the two groups, resulting in a similar GIR response. Maintaining BG at 2.5% below baseline when the baseline was less than 4.00 mmol/L appeared not to affect the PK/PD assessments of insulin preparations.
The present study first introduced a different target BG setting method when the BG baseline was relatively low to ensure the successful performance of euglycemic clamps with a reduction of the hypoglycemic risk during the procedure. This study also had several limitations: It included only a small number of participants in group A; it focused solely on the PK/PD assessments of IAsp, a type of rapid-acting insulin, that may not be applicable to other types of insulin preparations such as long-acting, intermediate-acting, and premixed insulin preparations; it enrolled significantly fewer female participants, which could affect generalizability of the results across sex; and only one level (2.5% below baseline) with a relatively low baseline was evaluated in this study, and other levels (e.g., 3% or 3.5% below baseline) might be evaluated in the future. Further studies with a larger sample size conducted with other types of insulin preparations are warranted to provide more robust evidence.
Setting a target BG at 2.5% below a relatively low BG baseline of less than 4.00 mmol/L to minimize hypoglycemic risk appears to provide comparable suppression of endogenous insulin secretion without affecting the PK/PD assessments of insulin preparations compared to those with a target BG of 5% below a baseline of ≥ 4.00 mmol/L. It may be advisable to maintain BG levels at 2.5% rather than 5% below baseline when the basal BG is less than 4.00 mmol/L. Future studies with larger sample sizes and different types of insulins are warranted to examine the generalizability of these results.
This retrospective analysis incorporated data from two clinical trials (CTR20220854 and CTR20222843), sponsored by Chongqing Chenan Biopharmaceutical Co., Ltd. and Jiangsu Hengrui Pharmaceuticals Co., Ltd. However, these sponsors did not partake in the study design, data interpretation, or manuscript preparation.
| 1. | Bequette BW. Glucose clamp algorithms and insulin time-action profiles. J Diabetes Sci Technol. 2009;3:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | U.S.. Food And Drug Administration. Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs — General Considerations. Mar 2014. [cited September 26, 2024]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-and-bioequivalence-studies-submitted-ndas-or-inds-general-considerations. |
| 3. | European Medicines Agency. Non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues - Scientific guideline. [cited September 26, 2024]. Available from: https://www.ema.europa.eu/en/non-clinical-clinical-development-similar-biological-medicinal-products-containing-recombinant-human-insulin-insulin-analogues-scientific-guideline. |
| 4. | Koksharova E, Drai R, Noskov S, Dorotenko A, Protsenko E, Radaeva K, Arefeva A, Gefen M, Galstyan G, Makarenko I. Clinical Pharmacology of GP40321 (Insulin Glulisine Biosimilar): Pharmacokinetic and Pharmacodynamic Comparability in a Hyperinsulinemic-Euglycemic Clamp Procedure. Clin Pharmacol Drug Dev. 2024;13:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Pan Q, Wang X, Li W, Chen X, Zhuang Y, Zhou Q, Huang Y, Zhou Y, Lan L, Wang Z, Wang W, Hong J, Hao WH, Yang YT, Guo L. Pharmacokinetics, pharmacodynamics, and safety of prandial oral insulin (N11005) in healthy subjects. Front Endocrinol (Lausanne). 2023;14:1172327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Karakaplan ND, Song Y, Laurenti MC, Vella A, Jensen MD. Suppression of Endogenous Insulin Secretion by Euglycemic Hyperinsulinemia. J Clin Endocrinol Metab. 2024;109:e596-e601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Bratusch-Marrain PR, Waldhäusl WK. Suppression of basal, but not of glucose-stimulated insulin secretion by human insulin in healthy and obese hyperinsulinemic subjects. Metabolism. 1985;34:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ipp E, Sinai Y, Bar-Oz B, Nesher R, Cerasi E. Somatostatin impairs clearance of exogenous insulin in humans. Diabetes. 1987;36:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wu MS, Ho LT. The pancreatic glucagon and C-peptide secretion during hyperinsulinemia in euglycemic glucose clamp with or without somatostatin infusion in normal man. Horm Metab Res. 1987;19:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | de la Peña A, Seger M, Soon D, Scott AJ, Reddy SR, Dobbins MA, Brown-Augsburger P, Linnebjerg H. Bioequivalence and comparative pharmacodynamics of insulin lispro 200 U/mL relative to insulin lispro (Humalog®) 100 U/mL. Clin Pharmacol Drug Dev. 2016;5:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Leohr JK, Dellva MA, LaBell E, Coutant DE, Linnebjerg H. Evaluation of the Pharmacokinetic Profile of Ultra Rapid Lispro Administered Subcutaneously at Different Injection Sites. Clin Ther. 2022;44:836-847. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | de la Peña A, Riddle M, Morrow LA, Jiang HH, Linnebjerg H, Scott A, Win KM, Hompesch M, Mace KF, Jacobson JG, Jackson JA. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care. 2011;34:2496-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Heise T, Nosek L, Spitzer H, Heinemann L, Niemöller E, Frick AD, Becker RH. Insulin glulisine: a faster onset of action compared with insulin lispro. Diabetes Obes Metab. 2007;9:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Hompesch M, Hawryluk A, Hernandez M, Uchil B, Wilmington A, Peterson L. Pharmacokinetic and pharmacodynamic bioequivalence between regular human insulin (rDNA origin) in 0.9% sodium chloride ready-to-use infusion 1 U/mL and 100 U/mL concentrate diluted to 1 U/mL in healthy males. Diabetes Obes Metab. 2021;23:2679-2686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Menke A, Rust KF, Savage PJ, Cowie CC. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in U.S. population subgroups: NHANES 2005-2010. Ann Epidemiol. 2014;24:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Heinemann L. Time-action profiles of insulin preparations. Mainz: Verlag Kirchheim; 2004. http://www.profil-research.de/downloads/publikationen/buch.pdf. |
| 17. | Heise T, Zijlstra E, Nosek L, Heckermann S, Plum-Mörschel L, Forst T. Euglycaemic glucose clamp: what it can and cannot do, and how to do it. Diabetes Obes Metab. 2016;18:962-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Bhatia A, Tawade S, Mastim M, Kitabi EN, Gopalakrishnan M, Shah M, Yeshamaina S, Gobburu J, Sahib M, Thakur D, Prasanna Kumar KM. Comparative evaluation of pharmacokinetics and pharmacodynamics of insulin glargine (Glaritus(®)) and Lantus(®) in healthy subjects: a double-blind, randomized clamp study. Acta Diabetol. 2018;55:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Sørensen LP, Brock B, Mengel A, Rungby J, Moller N, Nielsen S, Vølund A, Schmitz O. Similarity of pharmacodynamic effects of a single injection of insulin glargine, insulin detemir and NPH insulin on glucose metabolism assessed by 24-h euglycaemic clamp studies in healthy humans. Diabet Med. 2010;27:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 20. | Benesch C, Heise T, Klein O, Heinemann L, Arnolds S. How to Assess the Quality of Glucose Clamps? Evaluation of Clamps Performed With ClampArt, a Novel Automated Clamp Device. J Diabetes Sci Technol. 2015;9:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Liu H, Yu H, Sun L, Qiao J, Li J, Tan H, Yu Y. Effects of Unsuppressed Endogenous Insulin on Pharmacokinetics and/or Pharmacodynamics of Study Insulin in the Healthy: A Retrospective Study. Clin Pharmacol Drug Dev. 2022;11:930-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Liu H, Yu H, Qiao J, Sun L, Li J, Tan H, Yu Y. Oscillations of C-peptide in the euglycemic clamp and their effect on the pharmacodynamic assessment of insulin preparations. Fundam Clin Pharmacol. 2021;35:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Arnolds S, Rave K, Hövelmann U, Fischer A, Sert-Langeron C, Heise T. Insulin glulisine has a faster onset of action compared with insulin aspart in healthy volunteers. Exp Clin Endocrinol Diabetes. 2010;118:662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Sinha VP, Choi SL, Soon DK, Mace KF, Yeo KP, Lim ST, Howey DC. Single-dose pharmacokinetics and glucodynamics of the novel, long-acting basal insulin LY2605541 in healthy subjects. J Clin Pharmacol. 2014;54:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Ohgawara H, Tasaka Y, Kosaka K, Shizume K. Effect of exogenous bonito insulin on endogenous insulin secretion in dogs. Endocrinol Jpn. 1974;21:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Comparison of the inhibitory effect of insulin and hypoglycemia on insulin secretion in humans. Metabolism. 2000;49:950-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Murayama Y, Kawai K, Nakamura S, Yamashita K. The endogenous insulin secretion was suppressed during insulin therapy in NIDDM patients. Diabetes Res Clin Pract. 1990;9:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Argoud GM, Schade DS, Eaton RP. Insulin suppresses its own secretion in vivo. Diabetes. 1987;36:959-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/