Published online Nov 15, 2025. doi: 10.4239/wjd.v16.i11.111698
Revised: August 11, 2025
Accepted: October 13, 2025
Published online: November 15, 2025
Processing time: 128 Days and 23.8 Hours
Achieving optimal glycemic control is a cornerstone of cardiovascular risk reduction in type 2 diabetes (T2D). However, the extent to which multifactorial interventions influence this relationship remains uncertain.
To evaluate the association between glycated hemoglobin (HbA1c) target achievement and long-term cardiovascular outcomes in patients receiving standard of care (SoC) or multifactorial intensive therapy (MT).
This post-hoc analysis of the nephropathy in diabetes type 2 cluster-randomized trial included 323 patients with T2D, albuminuria, and retinopathy (SoC: n = 139; MT: n = 184), who underwent a 4-year intervention phase. Outcomes were major adverse cardiovascular events (MACE) and all-cause mortality. Associations with HbA1c target achievement (≤ 7% vs > 7%) were assessed using Kaplan-Meier curves and shared frailty Cox regression models.
During a median follow-up of 12.1 years, 190 MACEs and 139 deaths occurred. Achievement of the HbA1c target was not associated with reduced mortality in either group. However, a significant reduction in MACEs was observed only among SoC patients achieving HbA1c ≤ 7% (P = 0.031), whereas no benefit was seen in the MT group (P = 0.645). In multivariable Cox regression models adjusted for cluster effect, in the MT group age [hazard ratio (HR) = 1.07, P < 0.001] and female sex (HR = 0.38, P < 0.001) were independent predictors of MACE, while in the SoC group only age (HR = 1.04, P = 0.009). For all-cause mortality, age (HR = 1.11, P < 0.001) and blood pressure control (HR = 0.55, P = 0.041) were significant predictors in the MT group, whereas age (HR = 1.06, P = 0.002) was independently associated with increased mortality in the SoC group.
In high-risk patients with T2D receiving standard care, achieving an HbA1c ≤ 7% was associated with fewer cardiovascular events only under standard care, but not with reduced mortality. This association was not observed in patients managed with a multifactorial strategy. These findings suggest that the prognostic value of glycemic control depends on the broader treatment context and highlight the central role of comprehensive risk factor management in microvascular-complicated T2D.
Core Tip: In high-risk patients with type 2 diabetes, achieving a glycated hemoglobin ≤ 7% was associated with fewer cardiovascular events only under standard care conditions, suggesting that the cardiovascular benefit of glycemic control may depend on treatment intensity. In contrast, younger age, female sex, and blood pressure control were more consistent predictors of improved outcomes. Low-density lipoprotein cholesterol control showed a borderline association with reduced mortality in the standard of care group only.
- Citation: Caturano A, Simeon V, Galiero R, Russo V, De Nicola L, Chiodini P, Rinaldi L, Vetrano E, Salvatore T, Conte C, Acierno C, Sardu C, Marfella R, Minutolo R, Sasso FC. Impact of achieving glycated hemoglobin targets on cardiovascular events/mortality: Post-hoc analysis of the nephropathy in diabetes type 2 trial. World J Diabetes 2025; 16(11): 111698
- URL: https://www.wjgnet.com/1948-9358/full/v16/i11/111698.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i11.111698
The prevalence of diabetes has risen significantly, from 108 million (4.7%) in 1980 to 537 million (10.5%) in 2021. Projections suggest that this will further increase to 1.31 billion by 2050[1]. Diabetes is a significant risk factor for cardiovascular disease (CVD), the leading cause of mortality in people living with diabetes, particularly in those with albuminuria and other microvascular complications[2,3]. A recent meta-analysis of 102 prospective studies shows that diabetes doubles the risk of cardiovascular (CV) events (including coronary heart disease, ischemic stroke, and vascular death), with even higher risks in those with microvascular complications or a long history of diabetes[4]. In recent years, CV risk assessment has evolved, moving beyond simple primary and secondary prevention categories. Current approaches focus on individualized risk profiles, emphasizing cumulative risk factors and organ-specific impairments rather than a one-size-fits-all model[5].
The nephropathy in diabetes type 2 (NID-2) study, conducted as a primary prevention trial in patients with type 2 diabetes (T2D) with albuminuria and diabetic retinopathy, highlighted that comprehensive, intensive treatment significantly reduces major adverse CV events (MACEs) and all-cause mortality, with no differences by sex for MACEs, while the reduction in mortality was more pronounced in women[6,7]. Notably, an elevated number of uncontrolled risk factors were associated with increased MACEs[8].
Disturbances in glucose metabolism significantly affect the progression of CVD in diabetes, particularly at fasting glucose levels above 126 mg/dL (7 mmol/L)[4]. While studies like Action to Control CV Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease (ADVANCE), Veterans Administration Diabetes Trial (VADT), and United Kingdom prospective diabetes study (UKPDS) have explored the impact of glycemic control on CV outcomes, conflicting results call for further research in targeted patient populations[9-12]. In fact, despite clear evidence that multifactorial therapy improves CV outcomes, uncertainty remains as to whether achieving glycated hemoglobin (HbA1c) targets provides additional benefit beyond comprehensive risk factor control. Most trials have either focused on glycemic control in isolation or lacked stratification by treatment intensity, leaving a critical gap in understanding how HbA1c target achievement interacts with multifactorial interventions in high-risk patients with microvascular complications. The present post-hoc analysis of the NID-2 trial addresses this gap by evaluating the prognostic value of HbA1c target achievement in the overall study population and within patients managed with either standard care or a structured multifactorial strategy. This approach allows us to assess whether glycemic control provides incremental benefit beyond comprehensive risk factor management.
This study is a post-hoc analysis of the NID-2 trial, an open-label, cluster-randomized clinical trial that included patients with T2D followed at 14 Italian diabetes clinics. A detailed description of the study design has been previously published[6]. Briefly, a preliminary survey confirmed that all participating physicians were well-informed on T2D management guidelines relevant to the study period[13-15]. Clinics were randomized to provide either a multifactorial intensive therapy (MT) or a standard of care (SoC) approach, with diagnoses of MACEs following internationally accepted diagnostic guidelines[16]. As this was a cluster-randomized trial, blinding of treatment allocation was not feasible. However, cardiologists, blinded to treatment arm assignments, assessed MACEs either within study centers or external hospitals where patients were referred for acute events. While both groups pursued the same guideline-recommended clinical targets at the time of study initiation, including HbA1c < 7%, systolic blood pressure < 130 mmHg, diastolic blood pressure < 80 mmHg, and low-density lipoprotein (LDL) cholesterol < 100 mg/dL[13-15], participants in the MT group received structured treatment based on a predefined algorithm, including standardized lifestyle interventions, stepwise antihypertensive therapy with renin-angiotensin system blockade, and protocol-driven use of statins and aspirin. In contrast, the SoC group was managed according to good clinical practice and at the discretion of treating physicians, without prespecified therapeutic algorithms[6]. The following references represent the original guidelines followed by the study protocol and applied by treating physicians in both groups and have been retained to accurately reflect the clinical standards in place during the trial[13-15].
The research protocol received ethical approval from the ethics committee of the University of Campania “Luigi Vanvitelli” (clinicaltrials.gov No. NCT00535925) and adheres to the principles outlined in the 1976 Declaration of Helsinki and its subsequent revisions. All participants provided informed consent. The original trial was conducted and reported according to CONSORT 2010. This post-hoc analysis adheres to CONSORT 2010 recommendations for secondary analyses.
In the MT group, glycemic control was monitored by measuring HbA1c levels and assessing daily glycemic profiles, with a minimum frequency of once every 15 days, to optimize therapy. Lifestyle modifications (diet and physical activity) were encouraged based on the American Diabetes Association guidelines and were regularly assessed using a patient diary[13]. Treatment adjustments were guided by HbA1c targets. If HbA1c values were between 7% and 8%, HbA1c was reassessed within two months and treatment modified if levels remained elevated. Values above 8% prompted immediate therapy modification. After any adjustment, HbA1c was re-evaluated every two to three months. In the SoC group, all enrolled patients were advised to adopt a healthy lifestyle. Physicians managed patients according to their clinical judgment, without the use of prespecified therapeutic algorithms and were free to modify therapy to achieve guideline-based clinical targets. The frequency of HbA1c monitoring in this group was not predefined and was left to the discretion of the treating physician, in line with routine clinical practice.
Eligible participants were patients with T2D aged 40 or older, with confirmed albuminuria (≥ 30 mg/24 hours), severe diabetic retinopathy, diabetes onset after age 30, and a follow-up period of at least 12 months at their center. Exclusion criteria included a history of myocardial infarction (MI) or stroke, and severe liver or heart failure. Between October 2005 and October 2008, a total of 395 patients were randomized: 207 to the MT group and 188 to the SoC group[6]. The intervention phase was planned to last four years and concluded in December 2011. After censoring patients who died (n = 27) or experienced a MACE during the intervention phase (n = 45), the final analysis included 323 patients (SoC: 139, MT: 184) (Supplementary Figure 1). The time origin (landmark) for survival analyses was set at the end of the intervention phase. Patients who died or experienced a MACE during this period were excluded from the primary analysis. In sensitivity analyses that included these early events, results were consistent with the main findings. These patients were further stratified based on HbA1c levels (≤ 7% vs > 7%). Follow-up was conducted until May 2019 to ensure comprehensive capture of primary outcome events. The sample size for this post-hoc analysis was determined by the design of the NID-2 trial[6]. HbA1c < 7% was a pre-specified clinical target in the original NID-2 protocol; however, the stratification of long-term outcomes according to HbA1c target achievement was not pre-specified. The study population therefore reflects the natural constraints of the parent trial rather than an a priori power calculation for the current endpoints.
The primary endpoint was a composite outcome of fatal and non-fatal MACEs, including CV mortality, non-fatal MI (confirmed by instrumental or enzymatic criteria), non-fatal stroke, coronary artery bypass grafting, revascularization procedures (e.g., percutaneous transluminal coronary angioplasty), and major lower limb amputation (an amputation occurring at or above the ankle joint). The secondary endpoint was all-cause mortality. Both endpoints were evaluated with stratification according to HbA1c target achievement (≤ 7% vs > 7%) at the end of the intervention phase.
Categorical variables are presented as frequencies and percentages, while continuous variables are described as median and interquartile range or mean and SD, depending on their distribution as assessed with the Shapiro-Wilk test. Comparisons within groups were analyzed using χ2 tests for categorical variables and either Student’s t-test or Wilcoxon rank test for continuous variables. Between-group comparisons used χ2 tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables. Time-to-event outcomes were analyzed with Kaplan-Meier curves. Associations between clinical variables and the risk of all-cause mortality or MACE were evaluated using Cox proportional hazards regression models with shared frailty terms to account for clustering by study site. Univariable models were first fitted, and variables with a P value < 0.100 were considered for inclusion in multivariable models. No covariates were forced a priori; the final models were built using a stepwise selection approach. Age consistently entered the models as it was the strongest predictor in univariable analyses. The proportional hazards assumption was assessed using Schoenfeld residuals and log-log survival plots. Global tests indicated no violations of proportionality in any of the multivariable models. A sensitivity analysis was conducted using the same univariable and multivariable Cox frailty models on the entire randomized population, including patients who experienced a MACE or death during the intervention phase, to test the robustness of the findings. As this is a post-hoc analysis of a completed randomized trial, no prospective power calculation was performed. Instead, we estimated the minimum detectable effect using the Schoenfeld approximation for Cox models. Given the number of observed events and the distribution of HbA1c groups, the overall analysis was powered to detect hazard ratios of approximately ≤ 0.67 or ≥ 1.50 for MACE (80% power, α = 0.05). For mortality and subgroup analyses, only larger effects could be detected. We therefore emphasize effect estimates and 95% confidence intervals (CI) as measures of precision. Restricted cubic splines were used to model the association between HbA1c (as a continuous variable) and the risk of MACE or all-cause mortality. Interaction terms between HbA1c and selected clinical covariates were also tested within Cox frailty models to explore possible effect modification. Given the exploratory, post-hoc nature of the subgroup analyses, no formal adjustment for multiple comparisons was performed. A two-sided P value < 0.05 was considered statistically significant. No specific outlier removal criteria were applied and the entire eligible dataset was used All statistical analyses were conducted in R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) using RStudio® software 2023.06.1 Build 524 (RStudio, Boston, MA, United States). The following R packages were used: Survival v3.8-3, dplyr v1.1.4, broom v1.0.9, survminer v0.5.0, ggplot2 v3.5.2, and splines v4.3.1.
A total of 323 patients were included, with 139 patients in the SoC group and 184 patients in the MT group. The mean duration of the intervention phase was 3.3 years, and the two groups were comparable in terms of age, sex, and body mass index. Table 1 summarizes the overall patient characteristics at the end of the intervention phase. During a median follow-up of 12.1 years (9.9-13.1 years), significantly fewer patients experienced MACEs in the MT group vs the SoC group (50.5% vs 69.8%, respectively; P < 0.001). A total of 139 deaths were recorded, with mortality rates being 48.2% in the SoC group and 39.1% in the MT group, respectively (P = 0.104) (Table 2).
| Variable | SoC group (n = 139) | MT group (n = 184) | P value |
| Age (years) | 71.0 ± 9.5 | 69.4 ± 9.1 | 0.141 |
| Sex | 0.229 | ||
| M/F | 57 (41)/82 (59) | 89 (48.4)/95 (51.6) | |
| Blood pressure (mmHg) | |||
| Systolic | 135.7 ± 15.4 | 127.4 ± 8.8 | < 0.001 |
| Diastolic | 78.9 ± 8.8 | 78.0 ± 5.7 | 0.295 |
| Blood pressure < 130/80 mmHg | 60 (45.1) | 121 (69.9) | < 0.001 |
| Laboratory tests | |||
| eGFR EPI-CKD (mL/minute) | 61.2 ± 22.4 | 61.2 ± 22.9 | 0.996 |
| Albuminuria (mg/day), median IQR | 85.7 (37.8-150.0) | 52.0 (11.1-165.5) | 0.013 |
| Albuminuria < 30 mg/day | 18 (12.9) | 57 (31.0) | < 0.001 |
| Fasting glucose (mg/dL), median IQR | 142.0 (123.0-171.5) | 140.0 (130.0-171.5) | 0.277 |
| HbA1c (%), median IQR | 7.1 (6.8-7.8) | 6.9 (6.5-7.2) | < 0.001 |
| HbA1c < 7% | 60 (43.2) | 119 (65.4) | < 0.001 |
| Total cholesterol (mg/dL), median IQR | 190.0 (170.0-211.5) | 171.0 (158.0-194.0) | < 0.001 |
| Total cholesterol < 175 mg/dL | 39 (28) | 98 (53.8) | < 0.001 |
| LDL cholesterol (mg/dL), median IQR | 118.2 (102.8-144.2) | 97.7 (85.6-118.6) | < 0.001 |
| LDL < 100 mg/dL | 26 (18.7) | 92 (50.5) | < 0.001 |
| Triglycerides (mg/dL), median IQR | 121.0 (94.0-168.8) | 140.0 (117.7-168.0) | < 0.001 |
| Therapy | |||
| Antihypertensive drugs, median IQR | 1 (1-3) | 2 (1-3) | < 0.001 |
| ACEi/ARBs | 139 (100.0) | 184 (100.0) | 1.000 |
| Diuretics | 63 (45.3) | 113 (61.4) | 0.047 |
| Calcium channel blockers | 43 (30.9) | 75 (40.8) | 0.070 |
| Beta blockers | 26 (18.7) | 37 (20.1) | 0.753 |
| Alpha blockers | 5 (3.6) | 10 (5.4) | 0.438 |
| Antihyperglycemic therapy | 0.814 | ||
| Diet | 4 (2.9) | 4 (2.2) | |
| Insulin | 43 (30.9) | 67 (36.4) | |
| Oral antihyperglycemics | 63 (45.3) | 81 (44.0) | |
| Combination therapy | 15 (10.8) | 24 (13.0) | |
| Missing | 14 (10.1) | 8 (4.4) | |
| Statins | 57 (41.0) | 81 (44.0) | 0.448 |
| Antiplatelet | 78 (56.1) | 135 (73.4) | < 0.001 |
| Variable | Overall population (n = 323) | SoC group (n = 139) | MT group (n = 184) | P value |
| MACE | 190 (58.8) | 97 (69.8) | 93 (50.5) | < 0.001 |
| Myocardial infarction | 38 (11.8) | 18 (12.9) | 20 (10.9) | 0.211 |
| TIA/stroke | 18 (5.6) | 10 (7.2) | 8 (4.3) | 0.885 |
| Revascularization | 10 (3.1) | 9 (6.5) | 1 (0.5) | 0.032 |
| Major amputation | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Cardiovascular death | 124 (38.4) | 60 (43.2) | 64 (34.8) | 0.126 |
| All-cause death | 139 (43.0) | 67 (48.2) | 72 (39.1) | 0.104 |
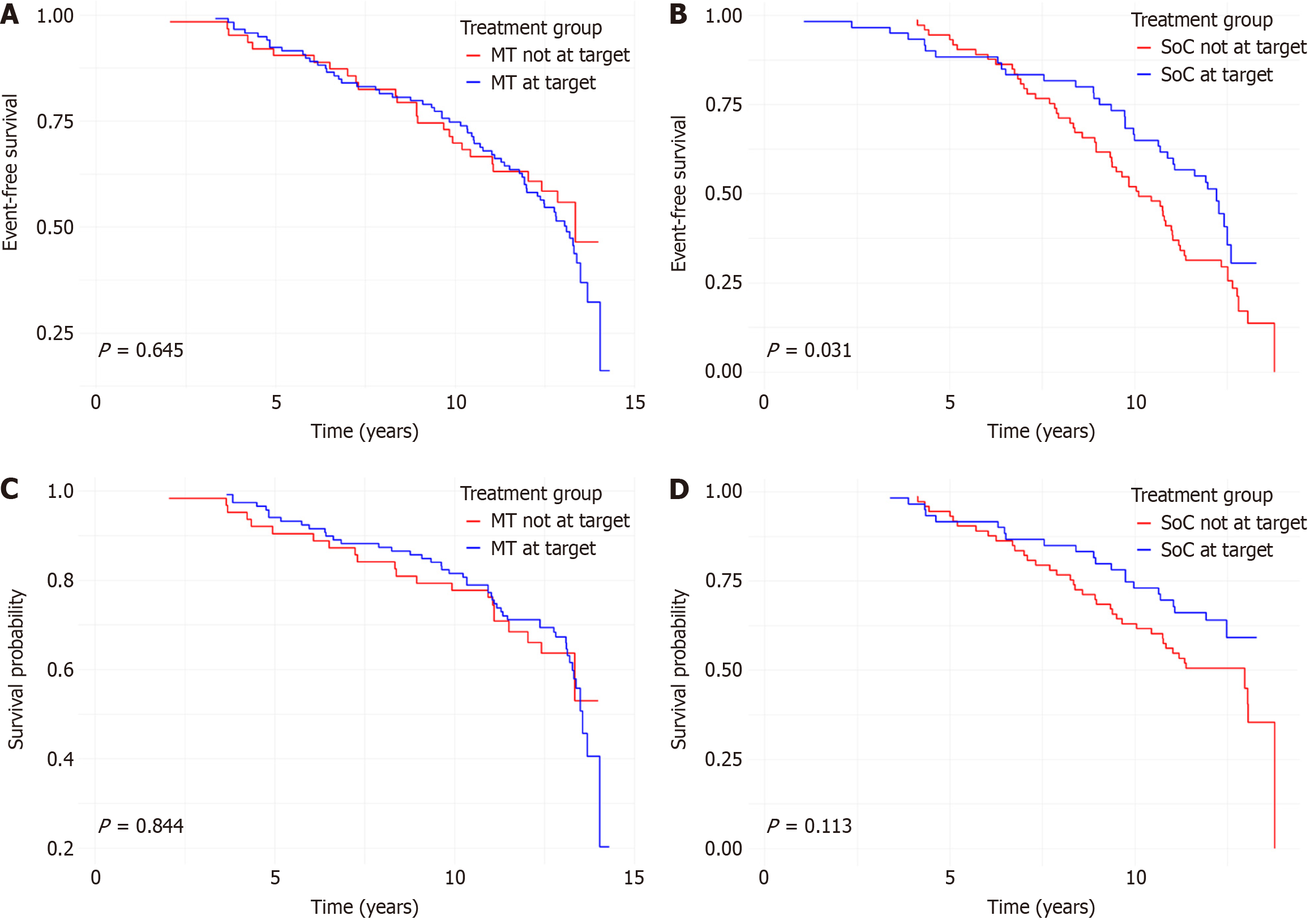
Kaplan-Meier survival analyses were conducted to compare MACE-free survival according to HbA1c target achievement. In the overall population, achieving the HbA1c target was associated with a significantly greater MACE-free survival during follow-up (P = 0.032) (Supplementary Figure 2). Subgroup analyses showed that this effect was driven by the SoC group (P = 0.031), while no significant difference was observed in the MT group (P = 0.645) (Figure 1A and B). No significant differences in mortality-free survival were observed based on HbA1c target achievement in the overall population (P = 0.054; Supplementary Figure 3) and in either group (MT: P = 0.844; SoC: P = 0.113; Figure 1C and D). At 12 years of follow-up, the cumulative incidence of MACE was 50.5% in the MT group and 69.8% in the SoC group. Corresponding rates of all-cause mortality were 39.1% and 48.2%, respectively.
In the overall population, age was the most robust predictor of MACEs, with each additional year increasing risk by 6% [hazard ratio (HR) = 1.06, 95%CI: 1.04-1.08; P < 0.001]. Female sex (HR = 0.51, 95%CI: 0.37-0.71; P < 0.001) and blood pressure at target (HR = 0.67, 95%CI: 0.46-0.97; P = 0.034) were also independently associated with reduced risk (Supplementary Table 1). When stratified by treatment group, age remained significantly associated with MACE in both arms. In the MT group, increasing age was associated with higher MACE risk (HR = 1.07, 95%CI: 1.04-1.09; P < 0.001), while female sex showed a strong protective effect (HR = 0.38, 95%CI: 0.23-0.61; P < 0.001) (Supplementary Table 2). In contrast, in the SoC group, only age was independently associated with MACE risk (HR = 1.04, 95%CI: 1.01-1.07; P = 0.009), while female sex showed a non-significant trend toward protection (Supplementary Table 3).
In the overall population, increasing age was the most prominent predictor of mortality, with each additional year associated with a 10% higher risk (HR = 1.10, 95%CI: 1.07-1.13; P < 0.001). A non-significant trend toward reduced mortality was observed in individuals with HbA1c ≤ 7% (HR = 0.68, 95%CI: 0.44-1.03; P = 0.069) (Supplementary Table 4). In the MT group, increasing age remained an independent predictor of mortality (HR = 1.11, 95%CI: 1.07-1.15; P < 0.001), and blood pressure at target was associated with a significant reduction in mortality risk (HR = 0.55, 95%CI: 0.30-0.98; P = 0.041) (Supplementary Table 5). In the SoC group, increasing age also predicted mortality risk (HR = 1.06, 95%CI: 1.02-1.11; P = 0.002), while achieving LDL cholesterol target was associated with a borderline protective effect (HR = 0.52, 95%CI: 0.26-1.01; P = 0.054) (Supplementary Table 6).
To assess the robustness of our findings and to address potential survivor bias, we conducted a sensitivity analysis on the overall population that included patients who had been excluded due to MACE or death during the intervention phase. Results from the expanded cohort were consistent with the main analysis. Age remained the strongest and most consistent predictor of both MACE (HR = 1.04, 95%CI: 1.02-1.06; P < 0.001) and all-cause mortality (HR = 1.08, 95%CI: 1.05-1.10; P < 0.001), while other associations, including those for female sex (MACE: HR = 0.54, 95%CI: 0.40-0.72; P < 0.001) were directionally similar and of comparable magnitude (Supplementary Tables 7 and 8).
To evaluate the impact of glycemic control across the full spectrum of HbA1c values, we modeled HbA1c as a continuous variable using restricted cubic spline regression. The resulting curve (Supplementary Figure 4) demonstrated a non-linear, U-shaped association between HbA1c and MACE. The lowest estimated risk was observed for HbA1c values between 6.28% and 7.65%, suggesting a protective effect within this range. In contrast, HbA1c values below 6.28% and above 7.65% were associated with a progressive increase in MACE risk, with the curve rising more steeply at the lower end. However, wide CIs at both extremes indicate reduced precision in these ranges.
A similar U-shaped pattern was observed in the association between HbA1c and all-cause mortality (Supplementary Figure 5). The lowest mortality risk occurred between 6.12% and 7.50%, while both lower and higher HbA1c values were linked to increasing HRs.
To assess whether the association between HbA1c and clinical outcomes was modified by other patient characteristics, we conducted interaction analyses between HbA1c values and a series of predefined clinical variables, using shared frailty Cox models. For both MACE (Supplementary Table 9) and all-cause mortality (Supplementary Table 10), no statistically significant interactions were observed. A borderline interaction was noted between HbA1c and female sex in predicting MACE (HR for interaction = 1.29, 95%CI: 0.98-1.71, P = 0.069), with a similar trend observed for mortality (HR for interaction = 1.32, 95%CI: 0.96-1.83, P = 0.091), although neither reached statistical significance.
This study investigated the impact of achieving HbA1c targets on MACEs and all-cause mortality in patients with complicated T2D who participated in a randomized controlled trial comparing SoC with MT. In the SoC group, achieving HbA1c < 7% was associated with a longer MACE-free survival, whereas in the MT group glycemic control did not significantly influence outcomes. This novel finding highlights the benefit of achieving HbA1c < 7% was confined to conventional care, while CV protection in the MT group independent of HbA1c target achievement. These results raise the hypothesis that once comprehensive risk factor control is achieved, including blood pressure, lipids, and albuminuria, the incremental contribution of HbA1c might become less pronounced, especially in patients with microvascular-complicated disease. This interpretation is consistent with our previous analysis showing a clear gradient of benefit according to the number of risk factors at target, and with evidence from STENO-2, UKPDS, and ACCORD, which emphasized the importance of multifactorial strategies and highlighted the limited impact of intensive glycemic control on CV outcomes in certain patient populations, particularly those at higher CV risk[8,9,12,17]. In patients with albuminuria, a marker of endothelial dysfunction and systemic inflammation, the structured multifactorial approach may exert additional protective effects, independent of glycemic control[18,19]. Hyperglycemia drives oxidative stress, chronic inflammation, and endothelial dysfunction, all contributing to atherosclerosis and CVD[20]. While tight glucose control may help mitigate these processes, its impact on CV outcomes appears to depend on the broader treatment context, as suggested by our findings. However, the relationship between glycemic control and CV events is unlikely to be linear, and individual factors such as comorbidities, age, and duration of diabetes are likely to modulate this effect[21,22]. Our spline analyses further support this interpretation, revealing a U-shaped association between HbA1c and both CV events and all-cause mortality. The lowest risk was observed at moderate HbA1c levels (approximately 6.2%-7.6%). Both lower and higher values were associated with increased hazard. Importantly, the lack of significant interaction between HbA1c and key clinical variables suggests that the association between glycemic control and outcomes was not significantly modified by the clinical variables examined, including age, sex, and baseline comorbidities. This reinforces the generalizability of the observed associations and supports a nuanced, yet uniform approach to HbA1c targets within multifactorial care. These findings align with results from landmark trials, such as ACCORD, VADT, ADVANCE and UKPDS[9-12]. For instance, ACCORD demonstrated that intensive glycemic control targeting HbA1c < 6.5% increased mortality, potentially due to hypoglycemic events in high-risk individuals. In contrast, UKPDS reported modest CV benefit in newly diagnosed patients with T2D. In keeping with these findings, our data suggest that, in patients with significant microvascular disease or microvascular-complicated diabetes, glycemic control alone may be insufficient, and the benefits of multifactorial intervention likely depend on comprehensive risk factor management[9-12]. Consistent with our findings, a recent meta-analysis of 11 randomized trials (51469 patients) reported that intensive glycemic control had a modest impact on non-fatal MI and microvascular endpoints, but not on overall CV outcomes, reinforcing the concept that glycemic control alone is insufficient to optimize long-term CV prognosis[23]. In contrast, long-term follow-up of the UKPDS (UKPDS 91) recently demonstrated that early intensive glycemic control at diagnosis was associated with a sustained, near-lifelong reduction in death and MI. Taken together, these data suggest that while early HbA1c lowering may impart durable CV benefits, in patients with microvascular-complicated disease such as those enrolled in NID-2, multifactorial risk management is paramount to improving outcomes[24].
Several factors may explain the lack of mortality benefit. Nearly 90% of all deaths were CV, suggesting that mortality occurred along the same pathophysiologic axis already targeted by intensive treatment. In this context, the MT group may have already achieved maximal protection through optimized control of blood pressure, lipids, and antithrombotic therapy, leaving limited room for incremental gains from tighter glycemic control. In contrast, in the SoC group, where treatment was less intensive, glycemic control may have been one of the few actively managed modifiable risk factors, thus having a greater relative impact. Importantly, none of the participants were treated with sodium-glucose cotransporter-2 inhibitors (SGLT-2i) or glucagon-like peptide-1 (GLP-1) receptor agonists at the time of enrollment and throughout the intervention phase, which concluded in December 2011. These drug classes have demonstrated CV and renal benefits independent of glycemic control, and their widespread use may alter the relative importance of HbA1c as a treatment target, shifting the focus even further toward organ protection and comprehensive cardiometabolic risk reduction[25-27]. Finally, competing risks such as infections, renal failure, or cancer[28] may have diluted the influence of glycemic control on survival over long-term follow-up, reinforcing the need for comprehensive, multimodal risk management rather than a singular focus on HbA1c.
The present analysis also observed that several non-glycemic factors, including age, sex, blood pressure, and LDL cholesterol, as independent predictors of CV events and all-cause mortality, highlighting their critical role in long-term risk stratification and management. Among these, increasing age consistently emerged as the strongest predictor across all models and subgroups. Each additional year of age was associated with a 6%-10% increase in the risk of both MACEs and mortality, aligning with extensive epidemiological evidence that identifies age as a dominant, non-modifiable risk factor for CVD in individuals with T2D. The cumulative impact of vascular aging, endothelial dysfunction, and comorbidities likely underlies this relationship[19,29]. Female sex was independently associated with a lower risk of MACEs in the overall population and within the MT group, but this protective effect was not observed in the SoC group. This discrepancy may reflect the role of structured, protocol-driven care in reducing long-standing sex-based disparities in CV management, a phenomenon well-documented in real-world settings, where women with T2D are often less likely to receive intensive risk factor control or cardioprotective therapies[3,7,19]. The lack of association in the SoC group may stem from variability in treatment intensity, while the observed benefit in the MT group supports the potential of standardized interventions to promote more equitable outcomes. These findings underscore the importance of applying evidence-based strategies uniformly, regardless of sex, particularly in high-risk populations. Blood pressure control was also strongly associated with outcomes: Achieving blood pressure targets was linked to a lower risk of MACEs in the overall cohort and to reduced all-cause mortality in the MT group. These results reinforce the established importance of blood pressure management in T2D, especially for individuals with microvascular complications such as albuminuria and retinopathy. Large trials such as ADVANCE and UKPDS have shown that intensive blood pressure control reduces both macrovascular and microvascular events in patients with diabetes[10,12]. The stronger association observed in the MT group may reflect higher adherence to structured antihypertensive protocols, in line with current guideline recommendations[26]. Together, these data support prioritizing protocol-driven blood pressure management as a central pillar of multifactorial care.
In the SoC group, achieving LDL cholesterol targets was associated with a borderline reduction in all-cause mortality, an association not observed in the MT group. Since statin prescription rates were similar between groups, this difference is unlikely to result from disparities in treatment. It is possible that in the MT group, where multiple risk factors were simultaneously targeted, the isolated effect of LDL control may have been diluted by the broader influence of comprehensive risk management. In the SoC group, the observed association may underscore the critical role of lipid control in mitigating atherothrombotic risk in patients not receiving intensive, multifactorial intervention[30].
Collectively, our findings support the concept that comprehensive CV risk reduction, encompassing blood pressure therapy and lipid-lowering treatment, plays a fundamental role in improving survival in high-risk patients with T2D. Mitigating these modifiable risk factors could translate into substantial gains in all-cause mortality reduction, particularly when CV risk predominates[26]. From a pathophysiological standpoint, this reinforces the multidimensional nature of vascular risk in diabetes[31]. Multifactorial therapy directly targets these pathways and may thus achieve broader protection[26]. Notably, the greater CV benefit observed in women receiving structured care highlights the importance of overcoming sex-based disparities in diabetes management and suggests that equitable application of guideline-based interventions may yield disproportionate benefits in historically undertreated groups.
Limitations of this study include its post-hoc design, which is subject to residual confounding and selection bias. Therefore, observed associations should not be interpreted as causal, and findings should be considered hypothesis-generating. The cluster-randomized design, though reflective of real-world clinical practice, lacks blind assignment and has reduced power compared to individually randomized trials. Furthermore, the absence of clinical and laboratory data collection during the follow-up phase prevents us from evaluating how changes in clinical parameters or therapeutic regimens over time may have influenced outcomes. This limitation may have resulted in non-differential exposure misclassification, potentially biasing associations toward the null. Similarly, the exclusion of patients who experienced MACE or death during the intervention phase may have introduced survival bias. However, findings remained consistent in a sensitivity analysis that included these early events (Supplementary Tables 7 and 8), supporting the robustness of the primary results. Another limitation is that the frequency of HbA1c monitoring in the SoC group was not predefined and was left to the discretion of treating physicians, likely resulting in less frequent assessments compared to the MT group. This difference in monitoring intensity may have influenced the timely optimization of glycemic control in the SoC group and could partly explain differences in achieving HbA1c targets and CV outcomes. The precise impact of this discrepancy cannot be determined due to lack of detailed monitoring data in the SoC group. However, this reflects routine clinical practice, where monitoring is often variable and depends on individual physician decisions, and thus enhances the real-world relevance of our findings. Additionally, the use of HbA1c thresholds as markers of glycemic control may not fully capture the complexity of optimal glycemic management, which is better reflected in individualized, patient-centered goals based on factors such as age, comorbidities, and treatment tolerance. Metrics such as time-in-range and glycemic variability, now recognized as important indicators of glycemic stability and CV risk, were not available during the study period. Their absence may have limited a more refined interpretation of treatment effectiveness. Moreover, this study includes multiple post-hoc subgroup analyses, which increase the risk of type I error. Although no adjustment for multiple comparisons was applied to preserve statistical power and reduce type II error, this limits the strength of causal inferences and requires cautious interpretation of these exploratory findings. Furthermore, the number of outcome events, especially within HbA1c subgroups, was limited. Our estimates indicate that only large effect sizes (roughly ≥ 35%-40% relative differences for MACE and ≥ 40%-50% for mortality) could be reliably detected. Consequently, subgroup findings should be considered exploratory, and interpretation should focus on point estimates with their CIs rather than statistical significance. Finally, none of the patients were treated with SGLT-2i or GLP-1 receptor agonists, therapies that are now considered SoC for CV and renal risk reduction. While these therapies became available during the follow-up period, which was censored in May 2019, the likelihood that a relevant number of participants were initiated on them was minimal, which limits their potential impact on long-term CV outcomes in this study. Nevertheless, our findings may not be generalizable to contemporary patients receiving SGLT2i/GLP-1 receptor agonist or to populations without microvascular complications. Future studies incorporating these agents are needed to better define the evolving role of glycemic targets within modern multifactorial care.
In this post-hoc analysis of the NID-2 trial, a multifactorial, protocol-driven intervention was associated with a significant reduction in MACEs compared to standard care, despite similar levels of glycemic control. In the survival analysis, HbA1c target achievement was associated with improved CV outcomes only in the SoC group; however, this association was not confirmed in multivariable analysis. Conversely, the benefit of the multifactorial approach appeared to stem from the consistent application of comprehensive risk factor management, rather than glycemic control alone. Risk was higher at both the lower and higher ends of HbA1c values, and the relationship between glycemic control and outcomes was broadly consistent across age, sex, and baseline comorbidities. In summary, our findings indicate that in patients with microvascular-complicated T2D, strict glycemic control alone may offer limited incremental benefit, whereas comprehensive, multifactorial risk management remains the cornerstone of long-term CV protection.
NID-2 study group investigators: Amelia U, Acierno C, Calatola P, Carbonara O, Caturano A, Conte G, Corigliano G, Corigliano M, D’Urso R, De Matteo A, De Nicola L, De Rosa N, Del Vecchio E, Di Giovanni G, Gatti A, Gentile S, Gesuè L, Improta L, Lampitella Jr A, Lampitella A, Lanzilli A, Lascar N, Masi S, Mattei P, Mastrilli V, Memoli P, Minutolo R, Nasti R, Pagano A, Pentangelo M, Pisa E, Rossi E, Adinolfi LE, Sasso FC, Sorrentino S, Torella R, Troise R, Trucillo P, Turco AA, Turco S, Zibella F, Zirpoli L.
| 1. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2437] [Cited by in RCA: 2616] [Article Influence: 872.0] [Reference Citation Analysis (18)] |
| 2. | Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019;21:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (1)] |
| 3. | Wright AK, Kontopantelis E, Emsley R, Buchan I, Mamas MA, Sattar N, Ashcroft DM, Rutter MK. Cardiovascular Risk and Risk Factor Management in Type 2 Diabetes Mellitus: A Population-Based Cohort Study Assessing Sex Disparities. Circulation. 2019;139:2742-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3857] [Cited by in RCA: 3569] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 5. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2765] [Article Influence: 553.0] [Reference Citation Analysis (0)] |
| 6. | Sasso FC, Pafundi PC, Simeon V, De Nicola L, Chiodini P, Galiero R, Rinaldi L, Nevola R, Salvatore T, Sardu C, Marfella R, Adinolfi LE, Minutolo R; NID-2 Study Group Investigators. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc Diabetol. 2021;20:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 7. | Minutolo R, Simeon V, De Nicola L, Chiodini P, Galiero R, Rinaldi L, Caturano A, Vetrano E, Sardu C, Marfella R, Sasso FC; NID-2 Study Group Investigators, Lampitella A Jr, Lampitella A, Lanzilli A, Lascar N, Masi S, Mattei P, Mastrilli V, Memoli P, Minutolo R, Nasti R, Pagano A, Pentangelo M, Pisa E, Rossi E, Sasso FC, Sorrentino S, Torella R, Troise R, Trucillo P, Turco AA, Turco S, Zibella F, Zirpoli L. Sex-difference of multifactorial intervention on cardiovascular and mortality risk in DKD: post-hoc analysis of a randomised clinical trial. Cardiovasc Diabetol. 2024;23:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Sasso FC, Simeon V, Galiero R, Caturano A, De Nicola L, Chiodini P, Rinaldi L, Salvatore T, Lettieri M, Nevola R, Sardu C, Docimo G, Loffredo G, Marfella R, Adinolfi LE, Minutolo R; NID-2 study group Investigators. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc Diabetol. 2022;21:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 9. | Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5679] [Article Influence: 315.5] [Reference Citation Analysis (0)] |
| 10. | ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4759] [Cited by in RCA: 4951] [Article Influence: 275.1] [Reference Citation Analysis (0)] |
| 11. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3358] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 12. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5376] [Article Influence: 298.7] [Reference Citation Analysis (1)] |
| 13. | American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28 Suppl 1:S4-S36. [PubMed] |
| 14. | European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2620] [Cited by in RCA: 2428] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 15. | De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, Ebrahim S, Faergeman O, Graham I, Mancia G, Manger Cats V, Orth-Gomér K, Perk J, Pyörälä K, Rodicio JL, Sans S, Sansoy V, Sechtem U, Silber S, Thomsen T, Wood D; Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1089] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 16. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 1800] [Article Influence: 257.1] [Reference Citation Analysis (1)] |
| 17. | Vaag AA. Glycemic control and prevention of microvascular and macrovascular disease in the Steno 2 study. Endocr Pract. 2006;12 Suppl 1:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Huang MJ, Wei RB, Zhao J, Su TY, Li QP, Yang X, Chen XM. Albuminuria and Endothelial Dysfunction in Patients with Non-Diabetic Chronic Kidney Disease. Med Sci Monit. 2017;23:4447-4453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ataga KI, Derebail VK, Caughey M, Elsherif L, Shen JH, Jones SK, Maitra P, Pollock DM, Cai J, Archer DR, Hinderliter AL. Albuminuria Is Associated with Endothelial Dysfunction and Elevated Plasma Endothelin-1 in Sickle Cell Anemia. PLoS One. 2016;11:e0162652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | González P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int J Mol Sci. 2023;24:9352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 336] [Reference Citation Analysis (0)] |
| 21. | Lin YC, Chen CW, Huang LY, Chen BL, Shao YJ, Huang CY. Glycemic levels and cardiovascular events in type 2 diabetes: A cohort study of drugs with different hypoglycemic potentials. Sci Rep. 2025;15:24852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Greenfield S, Billimek J, Pellegrini F, Franciosi M, De Berardis G, Nicolucci A, Kaplan SH. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Kunutsor SK, Balasubramanian VG, Zaccardi F, Gillies CL, Aroda VR, Seidu S, Khunti K. Glycaemic control and macrovascular and microvascular outcomes: A systematic review and meta-analysis of trials investigating intensive glucose-lowering strategies in people with type 2 diabetes. Diabetes Obes Metab. 2024;26:2069-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Adler AI, Coleman RL, Leal J, Whiteley WN, Clarke P, Holman RR. Post-trial monitoring of a randomised controlled trial of intensive glycaemic control in type 2 diabetes extended from 10 years to 24 years (UKPDS 91). Lancet. 2024;404:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 25. | Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 871] [Article Influence: 290.3] [Reference Citation Analysis (0)] |
| 26. | Kumar N, Kumar B, Ashique S, Yasmin S, Venkatesan K, Islam A, Ghosh S, Sahu A, Bhui U, Ansari MY. A critical review on SGLT2 inhibitors for diabetes mellitus, renal health, and cardiovascular conditions. Diabetes Res Clin Pract. 2025;221:112050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Edmonston D, Mulder H, Lydon E, Chiswell K, Lampron Z, Shay C, Marsolo K, Shah RC, Jones WS, Gordon H, Hwang W, Ayoub I, Ford D, Chamberlain A, Rao A, Fonseca V, Chang A, Ahmad F, Hung A, Hunt K, Butler J, Bosworth HB, Pagidipati N. Kidney and Cardiovascular Effectiveness of SGLT2 Inhibitors vs GLP-1 Receptor Agonists in Type 2 Diabetes. J Am Coll Cardiol. 2024;84:696-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Cavallari I, Bhatt DL, Steg PG, Leiter LA, McGuire DK, Mosenzon O, Im K, Raz I, Braunwald E, Scirica BM. Causes and Risk Factors for Death in Diabetes: A Competing-Risk Analysis From the SAVOR-TIMI 53 Trial. J Am Coll Cardiol. 2021;77:1837-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Meza CA, La Favor JD, Kim DH, Hickner RC. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int J Mol Sci. 2019;20:3775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 30. | Yang M, Kholmukhamedov A. Platelet reactivity in dyslipidemia: atherothrombotic signaling and therapeutic implications. Rev Cardiovasc Med. 2021;22:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JW. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 599] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/