Published online Nov 15, 2025. doi: 10.4239/wjd.v16.i11.111472
Revised: July 22, 2025
Accepted: September 22, 2025
Published online: November 15, 2025
Processing time: 137 Days and 1.3 Hours
Cardiorespiratory fitness (CRF) is inversely associated with the risk of cardio
To assess the relationship of CRF with vascular function in type 2 diabetes.
Patients with type 2 diabetes who were aged ≥ 18 years and underwent an in
We included 343 patients with type 2 diabetes. CRF was positively correlated with VHI (β = 0.10, P = 0.047), particularly with ankle-brachial index and pulse wave velocity. The odds ratio (OR) of impaired vascular function was 0.44 [95% confidence interval (CI): 0.20-0.96] for the highest vs the lowest CRF category. For each one metabolic equivalent increase in CRF, the OR of impaired vascular function was 0.73 (95%CI: 0.57-0.93).
Higher CRF was associated with better vascular function and lower odds of impaired vascular function in patients with type 2 diabetes.
Core Tip: This study developed for the first time a new index for the assessment of vascular function, which incorporates measures related to microvascular function, macrovascular function, arterial stiffness, and vascular morphology. We found that higher cardiorespiratory fitness (CRF) was associated with better vascular health in type 2 diabetes, particularly with better macrovascular function. Our study provides evidence in support of the beneficial effect of exercise training, which is associated with improved CRF, in the management of cardiovascular diseases in patients with type 2 diabetes.
- Citation: Zhao ST, Zhu YM, Chen YY, Sun ZL, Qiu SH. Association between cardiorespiratory fitness and impaired vascular function in type 2 diabetes. World J Diabetes 2025; 16(11): 111472
- URL: https://www.wjgnet.com/1948-9358/full/v16/i11/111472.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i11.111472
Vascular function reflects the ability of efficient delivery of blood to peripheral organs and is key to the maintenance of vascular homeostasis[1]. Impairment in vascular function may contribute to the development of atherosclerosis via the mechanisms of impaired coronary perfusion, microvascular damage, and abnormal hemodynamics, which ultimately leads to increased risks of cardiovascular diseases[2]. Noninvasive measures, such as the ankle-brachial index (ABI), transcutaneous oxygen pressure (TcPO2), and carotid intima-media thickness (cIMT), are commonly used in clinical practice to assess vascular function in patients with type 2 diabetes. However, these measures may not provide a comprehensive assessment of vascular function, as they mainly refer to an individual component of vascular function. For instance, ABI is mainly considered an indicator of macrovascular function[3-5], while TcPO2 reflects microvascular function[6]. Moreover, despite a close correlation between ABI and TcPO2, there is the evidence that some patients with type 2 diabetes might have an abnormal ABI but show a normal TcPO2 in clinical practice. As a result, combining multiple indicators to develop a new index to enable a comprehensive assessment of vascular function is of clinical interest for patients with type 2 diabetes, who are at high risks for cardiovascular diseases[7].
Cardiorespiratory fitness (CRF) is an indicator of the ability of the circulatory and respiratory systems to deliver oxygen to the working muscles during exercise[8], and is proposed as the fifth vital sign in the assessment of overall health[9]. CRF is associated with increased risks of cardiovascular diseases in patients with type 2 diabetes. For example, studies showed that for every one-metabolic equivalent (MET) increase in CRF, the risks of coronary heart disease and heart failure were reduced by 21% and 20%, respectively[10,11]. However, to date there have been no studies examining the relationship between CRF and vascular function, which was shown to be implicated in the development of cardiovascular diseases[12], in patients with type 2 diabetes.
Therefore, the aim of this study was to assess the association between CRF and vascular function as well as its potential influencing factors based on the construction of a new index of vascular function by incorporating ABI, TcPO2, pulse wave velocity (PWV), and cIMT together in patients with type 2 diabetes.
This cross-sectional study enrolled patients with type 2 diabetes who were admitted to the Department of Endocrinology of Zhongda Hospital from 2015 to 2018. The study protocol was approved by the Ethics Review Committee of Zhongda Hospital (approval No. 2019ZDSYLL119-P01). Written informed consent was waived as the data analyzed in the present study were retrospectively collected.
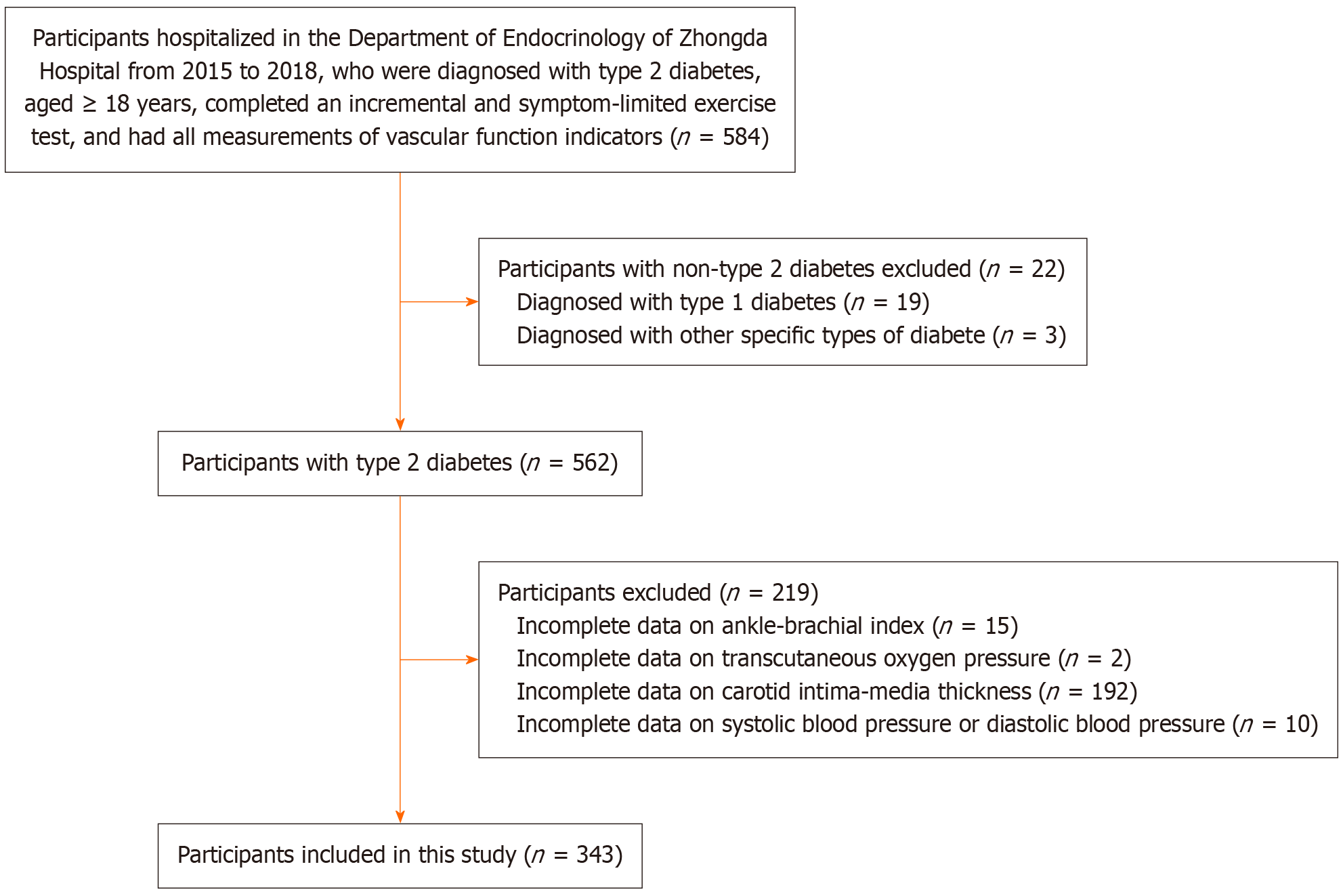
In this study we included participants who were diagnosed with type 2 diabetes, aged ≥ 18 years, completed an incremental and symptom-limited exercise test, and had all measurements of ABI, TcPO2, PWV, and cIMT. Participants were excluded if they: (1) Were diagnosed with type 1 diabetes or other specific types of diabetes; (2) Were pregnant women; and (3) Had missing information on any of the measures of vascular function. In total, 343 patients with type 2 diabetes were included in the study (Figure 1).
An incremental and symptom-limited exercise test was conducted using a cycling ergometer to assess CRF under the supervision of an expert physician. Patients started pedaling on command at a cadence of 50-60 rpm with the work load set to be “25 W” for females and “50 W” for males. The work load was increased by 25 W every 3 minutes, and the test was terminated when symptoms were reported (e.g., fatigue, dyspnea, or others). Peak oxygen uptake was measured by the COSMED K4b2 system, and CRF was determined as the peak oxygen uptake/weight (kg)/3.5 and expressed in the unit of MET.
General information including gender, age, and history of drinking and smoking was collected from the electronic medical records. Height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured using standard protocols. Body mass index (BMI) was calculated as weight (kg)/height (m2). Hypertension was defined as
Fasting blood samples were obtained to measure glycated hemoglobin A1c (HbA1c), total cholesterol, triglycerides (TG), high-density lipoprotein-cholesterol (HDL), low-density lipoprotein-cholesterol (LDL), and creatine. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease Chinese modified formula: 175 × creatine-1.234 × age- 0.179 × (female × 0.79)[13].
ABI: Patients were instructed to lie in the supine position. A pneumatic cuff was applied to the arms and ankles, and the SBPs were measured at the brachial, posterior tibial and dorsalis pedis arteries in both limbs using a hand-held continuous-wave Doppler probe. The ABI is defined as the ratio of ankle arterial pressure to brachial arterial pressure for each limb. In this study, the lower ABI value was selected for analysis. In case this was available for only one limb, the value from that limb was used for analysis.
TcPO2: Patients were asked to lie in the supine position, and the measurement of TcPO2 was performed at the dorsolateral site of both feet. The electrodes were applied to the skin and the measurements were obtained after calibration and preheating of the electrodes to approximately 44 °C. The lower TcPO2 value was selected for analysis. If the value was available for only one foot, it was then used for analysis.
cIMT: CIMT was determined using high-resolution Doppler ultrasound scanning approximately 2 cm proximal and distal to the dilated common carotid artery. In this study the averages from both sides were calculated for analysis.
PWV: According to the Reference Values for Arterial Stiffness Collaboration[14], PWV was estimated using a validated formula based on age and mean blood pressure (MBP): 9.587 - 0.402 × age + 4.560 × 10-3 × age2 - 2.621 × 10-5 × age2 × MBP + 3.176 × 10-3 × age × MBP - 1.832 × 10-2 × MBP, in which MBP was calculated as DBP + 0.4 × (SBP - DBP). The formula was derived from the reference population (individuals presenting cardiovascular risk factors that included type 2 diabetes) and was validated in patients with type 2 diabetes due to its close relationship with all-cause mortality[15,16].
With reference to the approach for the construction of the Healthy Aging Score[17], vascular function was assessed by the construction of the vascular health index (VHI), which is defined as a composite score of ABI, TcPO2, PWV, and cIMT. For this, we assigned a score of 1 (worst) to 3 (best) points for each of the measures based on their tertiles. This composite score ranges from 4 to 16 points, with a higher score indicative of better vascular function. In this study, we defined a VHI of < 8 points as impaired vascular function. The details of the construction of the VHI are shown in Supplementary Table 1 and the performance of VHI over the individual vascular function indicators in identifying macrovascular dysfunction (defined as ABI < 1.0) and microvascular dysfunction (defined as TcPO2 < 60 mmHg)[18,19] by the area under the curves is shown in Supplementary Figure 1.
Continuous data are presented as the median and interquartile range (25th, 75th percentile) or the means and standard deviations, based on the normality test. Categorical data are shown as numbers and percentages (%). The nonparametric test or χ2 test was used to compare the differences between groups, where appropriate. The VHI was analyzed using two approaches: (1) Being categorized as impaired and normal vascular function groups; and (2) Being treated as a con
Subgroup analysis was conducted to assess the influences of age (≥ 60 years vs < 60 years), gender (male vs female), duration of diabetes (< 1 years, 1-10 years, vs > 10 years), history of smoking (yes vs no), history of drinking (yes vs no), BMI (≥ 28 kg/m2, 24-28 kg/m2, vs < 24 kg/m2), and LDL (≥ 2.6 mmol/L vs < 2.6 mmol/L), on the association between CRF and the odds of impaired vascular function. Sensitivity analysis was performed by excluding participants with missing data and re-categorizing CRF from tertiles to quartiles. All the analyses were performed using Stata (version 14.0, College Station, TX, United States), and a 2-sided P < 0.05 was considered statistically significant.
Table 1 shows the characteristics of the included 343 participants with type 2 diabetes. Their mean age was 53.3 ± 9.8 years and the majority of them were male (63.6%). Compared to patients with normal vascular function, those with impaired vascular function had higher age, SBP, and BMI (all P < 0.05) and lower eGFR (P < 0.05). Moreover, CRF was lower in patients with impaired vascular function [5.9 (5.0, 6.7) MET vs 5.2 (4.5, 6.0) MET, P < 0.001]. In contrast, participants with the highest tertile of CRF had the largest VHI (8.9 ± 1.4 vs 8.2 ± 1.6, P < 0.01; Supplementary Figure 2).
| Variables | Total | Normal vascular function | Impaired vascular function | P value |
| Sample size | 343 | 255 | 88 | |
| Age (years) | 54 (48, 59) | 52 (45, 57) | 59 (55, 65) | < 0.001 |
| Gender | 0.45 | |||
| Male | 218 (63.6) | 165 (64.7) | 53 (60.2) | |
| Female | 125 (36.4) | 90 (35.3) | 35 (39.8) | |
| Smoking | 0.64 | |||
| Yes | 128 (37.3) | 97 (38.0) | 31 (35.2) | |
| No | 215 (62.7) | 158 (62.0) | 57 (64.8) | |
| Drinking | 0.42 | |||
| Yes | 89 (25.9) | 69 (27.1) | 20 (22.7) | |
| No | 254 (74.1) | 186 (72.9) | 68 (77.3) | |
| Hypertension | < 0.001 | |||
| Yes | 186 (54.2) | 123 (48.2) | 63 (71.6) | |
| No | 157 (45.8) | 132 (51.8) | 25 (28.4) | |
| SBP (mmHg) | 120 (120, 130) | 120 (115, 126) | 120 (112, 124) | < 0.001 |
| DBP (mmHg) | 80 (70, 80) | 80 (70, 80) | 80 (70, 80) | 0.83 |
| BMI (kg/m2) | 25.0 (22.8, 30.1) | 24.9 (22.5, 27.1) | 25.9 (24.0, 28.6) | 0.02 |
| HbAlc (%)1 | 9.4 (7.8, 11.2) | 9.4 (7.8, 11.3) | 9.0 (7.5, 11.0) | 0.25 |
| TG (mmol/L)1 | 1.71 (1.06, 2.59) | 1.79 (1.07, 2.68) | 1.57 (1.05, 2.25) | 0.23 |
| TC (mmol/L)1 | 4.9 (4.2, 5.7) | 5.0 (4.2, 5.7) | 4.8 (4.3, 5.8) | 0.54 |
| HDL (mmol/L)1 | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.4) | 0.37 |
| LDL (mmol/L)1 | 3.0 (2.5, 3.6) | 3.0 (2.5, 3.5) | 3.0 (2.6, 3.9) | 0.49 |
| eGFR (mL/minute/1.73 m2)1 | 90.9 (79.6, 104.0) | 92.6 (81.3, 105.3) | 87.9 (77.0, 98.7) | 0.02 |
| CRF (MET) | 5.7 (4.8, 6.6) | 5.9 (5.0, 6.7) | 5.2 (4.5, 6.0) | < 0.001 |
Table 2 shows the linear relationship between CRF and VHI. In the crude model (model 1), CRF was significantly and positively correlated with the VHI (β = 0.20, P < 0.001). The relationship remained significant after controlling for different variables (model 2 and model 3, both P < 0.05). Furthermore, after controlling for multivariable factors, CRF was positively correlated with TcPO2 but inversely with PWV (β = 0.13 and -0.11, respectively; model 3). However, there was no significant association of CRF with ABI or cIMT (both P > 0.05).
| Variables | Model 1 | Model 2 | Model 3 | |||
| Sβ | P value | Sβ | P value | Sβ | P value | |
| VHI | 0.20 | < 0.001 | 0.10 | 0.045 | 0.10 | 0.047 |
| ABI | 0.07 | 0.20 | 0.04 | 0.53 | 0.08 | 0.18 |
| TcPO2 | 0.16 | 0.004 | 0.16 | 0.01 | 0.13 | 0.03 |
| PWV | -0.24 | < 0.001 | -0.08 | 0.02 | -0.11 | < 0.001 |
| cIMT | -0.07 | 0.21 | -0.04 | 0.53 | -0.03 | 0.58 |
Table 3 shows the association between CRF and impaired vascular function. In the crude model (model 1), the ORs for impaired vascular function in the middle and the highest tertiles of CRF vs the lowest tertile were 0.58 (95%CI: 0.33-1.01) and 0.34 (95%CI: 0.18-0.64), respectively. These associations were slightly changed after controlling for different variables (that is, model 2 and model 3). Dose-response analysis showed that there was no significant evidence of a departure from the non-linear relationship (Pnon-linearity = 0.06) regarding the association between CRF and odds of impaired vascular function. Subsequent analysis showed that the OR for impaired vascular function was 0.73 (95%CI: 0.57-0.93) per one-MET increase in CRF (Table 3).
| Variable | No. of cases/total | Model 1 | Model 2 | Model 3 | |||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| CRF tertiles (MET) | |||||||
| Lowest (< 5.2) | 42/117 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Middle (5.2-6.2) | 29/119 | 0.58 (0.33-1.01) | 0.06 | 0.73 (0.40-1.35) | 0.32 | 0.68 (0.35-1.32) | 0.25 |
| Highest (> 6.2) | 17/107 | 0.34 (0.18-0.64) | 0.001 | 0.48 (0.24-0.98) | 0.04 | 0.44 (0.20-0.96) | 0.04 |
| Per 1-MET increase | 88/343 | 0.69 (0.57-0.84) | < 0.001 | 0.75 (0.61-0.94) | 0.01 | 0.73 (0.57-0.93) | 0.01 |
Table 4 shows the subgroup analysis for the association between CRF and the odds of impaired vascular function. It appears that this association was more pronounced in some subgroups, e.g., among patients with older age, males, non-smokers, and non-drinkers, after multivariable-adjustment (all P < 0.05). However, none of the variables, which included age, gender, duration of diabetes, history of smoking, history of drinking, BMI, and LDL, showed any significant moderating effects (all Pinteraction > 0.05). Sensitivity analysis showed that the associations remained comparable in general upon the exclusion of participants with missing data (Supplementary Table 2) or re-categorizing CRF into quartiles (Supplementary Table 3).
| Variables | Model 1 | Model 2 | Model 3 | Pinteraction1 | |||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age (years) | 0.68 | ||||||
| < 60 | 0.77 (0.60-0.98) | 0.04 | 0.76 (0.59-0.99) | 0.04 | 0.74 (0.54-1.00) | 0.05 | |
| ≥ 60 | 0.67 (0.47-0.96) | 0.03 | 0.66 (0.45-0.95) | 0.03 | 0.58 (0.36-0.92) | 0.02 | |
| Gender | 0.30 | ||||||
| Male | 0.62 (0.47-0.82) | 0.001 | 0.66 (0.50-0.88) | 0.004 | 0.62 (0.45-0.87) | 0.01 | |
| Female | 0.79 (0.57-1.09) | 0.15 | 0.84 (0.59-1.19) | 0.32 | 0.80 (0.54-1.20) | 0.28 | |
| Duration of diabetes (years)2 | 0.55 | ||||||
| < 1 | 0.96 (0.60-1.55) | 0.87 | 0.74 (0.52-1.04) | 0.08 | 0.66 (0.46-0.95) | 0.03 | |
| 1-10 | 0.98 (0.58-1.68) | 0.95 | 0.75 (0.51-1.09) | 0.13 | 0.67 (0.45-0.99) | 0.04 | |
| ≥ 10 | 1.40 (0.54-3.61) | 0.48 | 0.76 (0.46-1.26) | 0.29 | 0.56 (0.36-0.88) | 0.01 | |
| Smoking | 0.82 | ||||||
| Yes | 0.75 (0.54-1.05) | 0.09 | 0.76 (0.54-1.06) | 0.11 | 0.75 (0.47-1.20) | 0.23 | |
| No | 0.66 (0.51-0.85) | 0.001 | 0.70 (0.53-0.93) | 0.01 | 0.63 (0.46-0.87) | 0.003 | |
| Drinking | 0.73 | ||||||
| Yes | 0.80 (0.55-1.16) | 0.25 | 0.79 (0.53-1.18) | 0.25 | 0.81 (0.50-1.31) | 0.40 | |
| No | 0.65 (0.51-0.83) | 0.001 | 0.69 (0.53-0.89) | 0.01 | 0.66 (0.50-0.88) | 0.01 | |
| BMI (kg/m2) | 0.19 | ||||||
| < 24 | 0.66 (0.47-0.92) | 0.01 | 0.67 (0.46-0.98) | 0.04 | 0.57 (0.36-0.89) | 0.01 | |
| 24-28 | 0.85 (0.62-1.17) | 0.33 | 0.91 (0.63-1.31) | 0.61 | 0.86 (0.55-1.35) | 0.52 | |
| ≥ 28 | 0.56 (0.35-0.89) | 0.01 | 0.67 (0.40-1.10) | 0.12 | 0.61 (0.33-1.14) | 0.12 | |
| LDL (mmol/L) | 0.72 | ||||||
| < 2.6 | 0.64 (0.45-0.93) | 0.02 | 0.57 (0.37-0.89) | 0.01 | 0.51 (0.30-0.85) | 0.01 | |
| ≥ 2.6 | 0.71 (0.56-0.90) | 0.01 | 0.78 (0.61-1.00) | 0.05 | 0.75 (0.56-1.00) | 0.05 | |
To our knowledge, this study is the first cross-sectional analysis to examine the relationship between CRF and vascular function in patients with type 2 diabetes by developing a new index. Our results showed a close relationship between CRF and vascular function, as indicated by the positive association of CRF with VHI as well as by the inverse association of higher CRF with lower odds of impaired vascular function in type 2 diabetes. This association was independent of differences in age, gender, history of hypertension, history of smoking, history of drinking, and BMI.
Previous studies have shown that increased physical activity is associated with favorable ABI and PWV; however, these significant associations do not remain consistent across different subgroups of populations[20-23]. Moreover, the relationship between physical activity and cIMT remains controversial, with some studies showing that increased physical activity was associated with decreases in cIMT, while others reported no significant association[24,25]. Notably, physical activity in these studies was mainly measured by self-reported questionnaires, which might be subject to recall or social desirability bias[26]. In contrast, CRF, which is closely related to physical activity, was objectively measured using an incremental and symptom-limited exercise test in the present study. Our subsequent analysis showed that CRF was positively correlated with TcPO2, and negatively correlated with PWV in patients with type 2 diabetes. However, we did not find any significant association with ABI or cIMT.
Of note, in this study we constructed a new composite index of VHI for the evaluation of vascular function by integrating ABI (representative of macrovascular function), TcPO2 (representative of microvascular function), PWV (representative of arterial stiffness), and cIMT (representative of vascular morphology) together. This is of clinical relevance, as it provides a comprehensive approach to assess vascular health from different aspects. Moreover, we found that this index was superior to all its components in identifying macrovascular and microvascular dysfunction as evidenced by its discriminability in the receiver operating characteristic curves (Supplementary Figure 1). Similar to our study, a previous study developed a grading system named “Beijing Vascular Health Stratification” for vascular health assessment by combining four vascular measures together, which included cardio-ankle vascular index, ABI, carotid femoral-PWV, and carotid radial-PWV[27,28]. However, this grading system did not include any measures that reflect microvascular function (that is, TcPO2) or vascular morphology (that is, cIMT). Furthermore, it did not provide clear evidence that the grading system outperformed its component in predicting cardiovascular outcomes[27,28].
In this study, we found that CRF was positively correlated with VHI, and was associated with a decrease in the odds of impaired vascular function in type 2 diabetes dose-dependently. However, our categorial analysis showed that the middle tertile of CRF (5.2-6.2 METs) or the middle quartile (4.9-5.7 METs) was not associated with any decreased odds of impaired vascular function. Together with the evidence that the risk of cardiovascular diseases was significantly lower in individuals with a CRF of ≥ 7.9 METs than those with a CRF of < 7.9 METs[29], it appears likely that there may exist a threshold effect for the preventive effect of CRF. However, it should be noted that a lack of improvement in CRF following the exercise intervention does not necessarily entail a lack of improvement in vascular function among patients with type 2 diabetes[30].
Our study is the first to explore the association between CRF and various indicators of vascular function in patients with type 2 diabetes. Moreover, both the CRF and the indicators of vascular function including ABI, TcPO2 and cIMT were measured directly and objectively, in particular, CRF was quantified by the gold standard method using a gas exchanger based on an incremental and symptom-limited exercise test. Furthermore, vascular function was analyzed by creating a new index with the integration of different items related to microvascular function, macrovascular function, morphology, and arterial stiffness.
Our study also has some limitations. First, due to the nature of the cross-sectional study, the causality of CRF with impaired vascular function cannot be inferred and the reverse causation cannot be ruled out either. Second, the sample size of our study was relatively small, but was larger than most of studies that focused on the analysis of TcPO2[31,32]. Third, arterial stiffness was assessed by estimating PWV, and it is unclear whether CRF shows any association with flow-mediated dilatation in Chinese patients with type 2 diabetes, which is another measure which reflects vascular function, particularly arterial stiffness[33]. Fourth, although the formula for PWV estimation was validated in individuals with cardiovascular risk factors that included type 2 diabetes[15,16], the lack of a population-specific formula (e.g., for patients with type 2 diabetes only) might potentially affect its association with CRF. Fifth, despite the effort to control for multivariable factors, we could not completely exclude the possibility of unmeasured effects from some factors such as medication use. Finally, our study did not include patients who had contraindications in performing the incremental and symptom-limited exercise test, e.g., patients with diabetic ulcerations. This may potentially limit the generalization of our findings to a broader spectrum of patients with type 2 diabetes (e.g., those who cannot perform the exercise test).
Our study shows that higher CRF was associated with better vascular function as well as with lower odds of impaired vascular function in patients with type 2 diabetes. This is of clinical importance, as it could provide some evidence or explanations in support of the beneficial effect of exercise training, which is associated with improved CRF, in the management of cardiovascular diseases in patients with type 2 diabetes.
| 1. | Tomiyama H. Vascular function: a key player in hypertension. Hypertens Res. 2023;46:2145-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, Harding IC, Ebong EE, Cameron SJ, Stewart AG, Weng J. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol Rev. 2021;73:924-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 733] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 3. | Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 4. | Teoh WL, Price JF, Williamson RM, Payne RA, Van Look LA, Reynolds RM, Frier BM, Wilkinson IB, Webb DJ, Strachan MW; ET2DS Investigators. Metabolic parameters associated with arterial stiffness in older adults with Type 2 diabetes: the Edinburgh Type 2 diabetes study. J Hypertens. 2013;31:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Gow ML, Varley BJ, Nasir RF, Skilton MR, Craig ME. Aortic intima media thickness in children and adolescents with type 1 diabetes: A systematic review. Pediatr Diabetes. 2022;23:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Fagher K, Katzman P, Löndahl M. Transcutaneous oxygen pressure as a predictor for short-term survival in patients with type 2 diabetes and foot ulcers: a comparison with ankle-brachial index and toe blood pressure. Acta Diabetol. 2018;55:781-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Yun JS, Ko SH. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism. 2021;123:154838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 8. | Qiu S, Cai X, Yang B, Du Z, Cai M, Sun Z, Zügel M, Michael Steinacker J, Schumann U. Association Between Cardiorespiratory Fitness and Risk of Type 2 Diabetes: A Meta-Analysis. Obesity (Silver Spring). 2019;27:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653-e699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1646] [Article Influence: 164.6] [Reference Citation Analysis (0)] |
| 10. | Lyerly GW, Sui X, Church TS, Lavie CJ, Hand GA, Blair SN. Maximal exercise electrocardiography responses and coronary heart disease mortality among men with diabetes mellitus. Circulation. 2008;117:2734-2742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Pandey A, Patel KV, Bahnson JL, Gaussoin SA, Martin CK, Balasubramanyam A, Johnson KC, McGuire DK, Bertoni AG, Kitzman D, Berry JD; Look AHEAD Research Group. Association of Intensive Lifestyle Intervention, Fitness, and Body Mass Index With Risk of Heart Failure in Overweight or Obese Adults With Type 2 Diabetes Mellitus: An Analysis From the Look AHEAD Trial. Circulation. 2020;141:1295-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1616] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 13. | Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937-2944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1596] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 14. | Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338-2350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1545] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 15. | Vlachopoulos C, Terentes-Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, Sfikakis PP, Tousoulis D. Association of Estimated Pulse Wave Velocity With Survival: A Secondary Analysis of SPRINT. JAMA Netw Open. 2019;2:e1912831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Solini A, Orsi E, Vitale M, Garofolo M, Resi V, Bonora E, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, Penno G, Pugliese G; Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Independent association of estimated pulse-wave velocity with all-cause mortality in individuals with type 2 diabetes. QJM. 2024;117:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Jaspers L, Schoufour JD, Erler NS, Darweesh SK, Portegies ML, Sedaghat S, Lahousse L, Brusselle GG, Stricker BH, Tiemeier H, Ikram MA, Laven JS, Franco OH, Kavousi M. Development of a Healthy Aging Score in the Population-Based Rotterdam Study: Evaluating Age and Sex Differences. J Am Med Dir Assoc. 2017;18:276.e1-276.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686-e725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 464] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 19. | China Diabetic Cellular and Interventional Therapy Technology Alliance for Diabetic Foot. [Clinical Guidelines for Comprehensive Interventional Diagnosis and Treatment of Diabetic Foot (Fourth Edition)]. Zhongguo Jieru Yingxiang Yu Zhiliaoxue. 2018;15:3-12. [DOI] [Full Text] |
| 20. | Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Housley E, Leng GC, Donnan PT, Fowkes FG. Physical activity and risk of peripheral arterial disease in the general population: Edinburgh Artery Study. J Epidemiol Community Health. 1993;47:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Tanaka H, Palta P, Folsom AR, Meyer ML, Matsushita K, Evenson KR, Aguilar D, Heiss G. Habitual physical activity and central artery stiffening in older adults: the Atherosclerosis Risk in Communities study. J Hypertens. 2018;36:1889-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Ahmadi-Abhari S, Sabia S, Shipley MJ, Kivimäki M, Singh-Manoux A, Tabak A, McEniery C, Wilkinson IB, Brunner EJ. Physical Activity, Sedentary Behavior, and Long-Term Changes in Aortic Stiffness: The Whitehall II Study. J Am Heart Assoc. 2017;6:e005974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Kwaśniewska M, Jegier A, Kostka T, Dziankowska-Zaborszczyk E, Rębowska E, Kozińska J, Drygas W. Long-term effect of different physical activity levels on subclinical atherosclerosis in middle-aged men: a 25-year prospective study. PLoS One. 2014;9:e85209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Walker TJ, Heredia NI, Lee M, Laing ST, Fisher-Hoch SP, McCormick JB, Reininger BM. The combined effect of physical activity and sedentary behavior on subclinical atherosclerosis: a cross-sectional study among Mexican Americans. BMC Public Health. 2019;19:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Hebert JR. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 745] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 27. | Liu H, Zhou X, Liu J, Huang W, Zhao N, Wang H. Rationale and design of the application value of Beijing Vascular Health Stratification (BVHS): predictive value of combined assessment of vascular structure and function for cardiovascular events in general Chinese population. BMC Cardiovasc Disord. 2021;21:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Liu H, He YD, Liu JB, Huang W, Zhao N, Zhao HW, Zhou XH, Wang HY. [Predictive value of vascular health indicators on newly cardiovascular events: Preliminary validation of Beijing vascular health stratification system]. Beijing Da Xue Xue Bao Yi Xue Ban. 2020;52:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1966] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (1)] |
| 30. | Hetherington-Rauth M, Magalhães JP, Júdice PB, Melo X, Sardinha LB. Vascular improvements in individuals with type 2 diabetes following a 1 year randomised controlled exercise intervention, irrespective of changes in cardiorespiratory fitness. Diabetologia. 2020;63:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | de Meijer VE, Van't Sant HP, Spronk S, Kusters FJ, den Hoed PT. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J Vasc Surg. 2008;48:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Dinesh R, Vinod KV, Ramkumar G. Comparison of resting/postexercise ankle-brachial index and transcutaneous partial pressure of oxygen for noninvasive diagnosis of peripheral artery disease in type 2 diabetes mellitus. Med J Armed Forces India. 2023;79:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, Schumann U. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/