Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.112631
Revised: August 18, 2025
Accepted: September 3, 2025
Published online: October 15, 2025
Processing time: 75 Days and 14 Hours
Type 2 diabetes mellitus (T2DM) promotes a risk of the development of atherosclerosis and potentiates atherosclerotic cardiovascular events. Among these patients, chronic hyperglycemia, dyslipidemia, oxidative stress and systemic inflammation has been found as triggers for accelerating plaque formation. Additionally, conventionally used risk factors, such as age, overweight/obesity, hypertension, poor glycemic control, renal dysfunction, and metabolic distur
Core Tip: The integration of routinely used biomarkers of kidney dysfunction (urinary albumin-to-creatinine ratio) and metabolic abnormality (serum uric acid, high-density lipoprotein cholesterol) to predictive model may be practically useful to pre-screen the patients with type 2 diabetes mellitus at higher risk of carotid atherosclerotic plaque.
- Citation: Berezin AE. Early predictors of carotid atherosclerosis in patients with type 2 diabetes mellitus. World J Diabetes 2025; 16(10): 112631
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/112631.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.112631
Atherosclerosis is a common complication in patients with type 2 diabetes mellitus (T2DM), which is closely associated with huge economic burdens on patients’ families and resource of health systems[1,2]. Over the last two decades, the number of people living with both atherosclerosis and T2DM has significantly increased depending on global growth of T2DM prevalence[3]. In 2020, the estimated global prevalence rate of carotid atherosclerosis in people aged 30–79 years was 21.1%, equivalent to 815.8 million people worldwide[4]. To the best of our knowledge, asymptomatic carotid plaques tended to be more prevalent (72% vs 60%, P = 0.06) in new-onset T2DM compared to controls[5]. Patients with a new diagnosis of T2DM had more advanced preclinical carotid atherosclerosis than non-diabetic subjects even after controlling for traditional risk factors including age, sex, hypertension, dyslipidemia, and smoking[5]. Moreover, the prevalence of cardiovascular disease (CVD) including stroke, myocardial infarction, angina pectoris, heart failure (HF), ischemic heart disease, CVD, coronary heart disease, atherosclerosis, and cardiovascular (CV) death, among T2DM patients was 32.2% and 29.1% had atherosclerosis[6]. Although carotid plaques can predict future major adverse CV events (MACEs) in asymptomatic individuals with T2DM, their interactions with conventional and non-conventional CV risk factors remain inadequately defined.
In most population-based observational and clinical studies on the prevalence of carotid atherosclerosis, which used ultrasonography of the bilateral carotid arteries, the following criteria for atherosclerotic lesions were employed: (1) Increased carotid intima-media thickness (IMT) of 1.0 mm or more; (2) Focal IMT of 1.5 mm or more encroaching into the lumen; and (3) Local thickening of the carotid IMT of > 50% compared to the surrounding vessel wall[7]. However, criterion a may be preserved as increased carotid IMT of 1 mm or more since values of carotid IMT > 0.9 mm was considered abnormal according to by the European Society of Cardiology guideline[8]. According to these criteria, in general population carotid atherosclerosis is highly prevalent in men than in female and closely associated with conventional CV risk factors, i.e. elevated systolic blood pressure, fasting glucose levels, and several inflammatory biomarkers, such as monocyte count to high-density lipoprotein cholesterol (HDL-C) ratio, and triglyceride glucose body mass index (BMI)[9]. Among T2DM individuals[10-14], the predictors for carotid atherosclerosis were older age, hypertension, duration of T2DM, serum uric acid (SUA) and its ratio to serum albumin, fasting and post-prandial plasma glucose, inflammatory biomarkers [high-sensitive C-reactive protein (CRP), tumor necrosis factor-alpha, receptor for advanced end-glycation products, interferon gamma], low levels of bilirubin, N-terminal osteocalcin, as well as peri-renal fat thickness, but not visceral fat area, subcutaneous fat area or traditional metabolic risk factors[15-18].
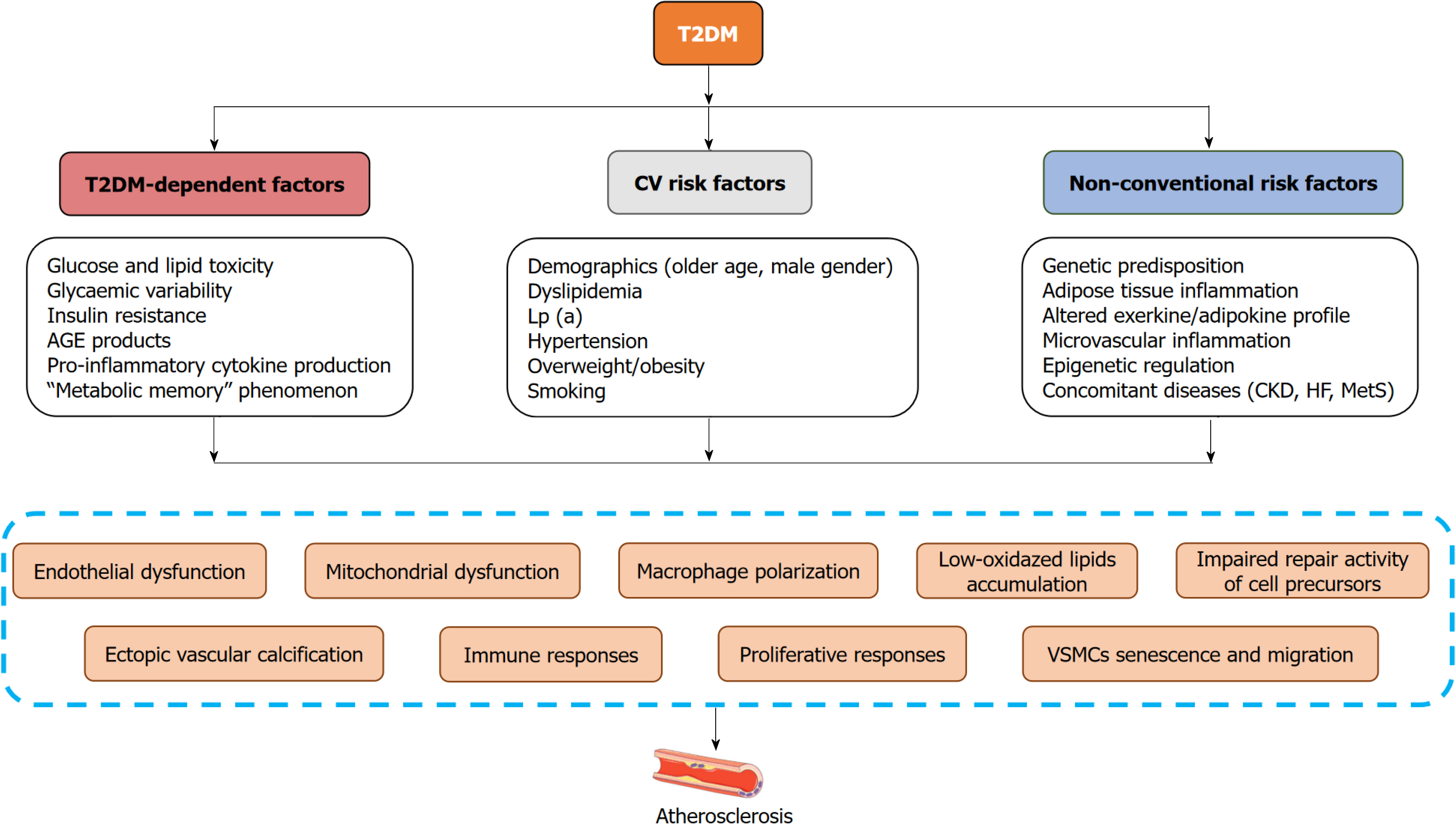
Thus, three main groups of risk factors have been identified as being involved in the formation and progression of carotid atherosclerosis in patients with T2DM (Figure 1). The first of these can be conditionally defined as T2DM-dependent factors, which include classic factors of tissue damages associated with glucose and lipid toxicity, mi
They contribute to the development of systemic inflammation, metabolic maladaptation known as metabolic memory, impaired tissue regeneration, hormonal and metabolic disturbances, vasculopathy and consequently accelerating atherosclerosis[20]. The second group of factors consists of classic CV risk factors, whose role in the formation and progression of systemic atherosclerosis has been well studied and is often incorporated into various prognostic scales [i.e., Cardiovascular Risk Score-2, Atherosclerotic Cardiovascular Disease Risk (ASCVD) Estimator and Reynolds Risk Score][21]. Moreover, these scores for ASCVD risk estimation are utilized as reliable tools to categorize plaque quality and to choose an intervention approach[22]. Additionally, a number of non-classical risk factors (the third group), such as genetic predisposition, altered epigenetic regulation of glucose and lipid homeostasis through an expression of nuclear factor-kappa B (NF-kB)-dependent proteins, pro-inflammatory genes, genes coding expression of adhesive molecules, glucose transporters, and adipose tissue dysfunction, the presence of concomitant diseases (metabolic syndrome, HF, chronic kidney disease) are markedly implicated in T2DM-associated vascular injury and ectopic vascular calcification[23-25]. Although carotid atherosclerosis is a risk factor for MACE among T2DM patients and its predictive and incremental value for MACE combined with traditional CV risk factors exhibits a high variability among studied populations[26-28]. Thus, plaque information when added to traditional risk factors can improve ASCVD risk prediction, but this discriminative value is sufficiently variable and requires to be clearly reported, for instance with reliable metabolic, renal, and vascular biomarkers. Without a doubt, these biomarkers should describe the key pathogenetic mechanisms of carotid atherosclerosis formation independently of traditional risk factors.
Regardless of the risk factors profile and a quality of glucose control, oxidative stress, lipid toxicity, inflammation and metabolic memory phenomenon are considered as the key triggers for initiating vascular damage and accelerating atherosclerosis in T2DM[29]. Further the local processes of plaque composition involved in the alteration of endothelial, calcium accumulation, proliferation and differentiation of vascular smooth muscle cells, polarizing macrophages with forming foam cells and consequently shaping plaque. These pathophysiological pathways are under regulation of a number of coexisting factors, including estrogens through bone-related protein and Lp(a), systemic inflammation, adipose tissue dysfunction, microRNAs and bioactive lipids[30]. They mainly act as modulators of phosphoinositide 3-kinase/protein kinase B/NF-kB, mammalian target of rapamycin/activator protein-1/vascular endothelial growth factor receptor pathway and regulate cell proliferation and differentiation, apoptosis/ferroptosis, expression of adhesion molecules and angiogenesis[31-34]. These pathogenetic mechanisms ultimately result in a metabolic sequence that affects the function of antigen-presenting cells, endothelial progenitor cells, and other cellular elements involved in plaque formation.
Although previous studies have widely assessed the risk factors associated with atherosclerosis, it remains unclear whether the integrated biomarkers of kidney diseases and metabolic condition are predictively applicable to T2DM patients with carotid atherosclerosis. The study by Shi and Li[35], published in this issue of the World Journal of Diabetes, was to evaluate the possible associations of the conventional biomarkers of renal dysfunction [urinary albumin-to-creatinine ratio (UACR)] and metabolic indicator (SUA) with subclinical carotid plaque formation that was detected as IMT ≥ 1.5 mm in patients with known T2DM. The authors established that age, fasting plasma glucose, glycated hemoglobin (HbA1c), systolic blood pressure, serum creatinine, UACR, SUA, and HDL-C were independent predictors of carotid atherosclerosis. In a retrospective single-center study, Shi and Li[35] provided important clinical evidence of an association between asymptomatic carotid atherosclerosis and routine, easy-to-use biomarkers, i.e. UACR and SUA, which can be utilized for the early identification of individuals at risk. Since monitoring renal function and uric acid levels is routinely used to assess the condition of patients with diabetes mellitus, one of the main advantages of this study is undoubtedly the simplicity and cost-effectiveness of prescreening based on the above biomarkers. This approach does not require additional economic costs or staff training, and can also be used to analyze previously obtained data. The extent to which the new discriminatory model for the preliminary detection of carotid atherosclerosis is applicable in populations of patients with varying ASCVD risk remains to be established. Although in previous clinical studies SUA and UACR demonstrated positive correlations with target organ damage and MACEs in diabetes mellitus, they are not specific biomarkers of metabolic disorders[36,37]. In this context, it is undoubtedly necessary to validate the study results and compare the findings with those from other studies that have investigated alternative inflammatory markers, novel lipid-related indices and imaging-based predictors. Indeed, the linear associations between various insulin-based (Homeostasis Model Assessment of Insulin Resistance) and non-insulin-based IR (i.e., MeTabolic Score for Insulin Resistance) indices, lipid abnormalities defined as Castelli's risk indices-I, II and subclinical atherosclerosis were found in the CHIEF Atherosclerosis Study[38]. In contrast with the findings reported by Shi and Li[35], the indices of IR correlated with carotid IMT in patients without hyperuricemia, whereas in those with elevated SUA did not. In several studies and meta-analysis, triglyceride-glucose (TyG) index and TyG-BMI, novel surrogate indicators of IR, were found to be positively associated with extension of multifocal atherosclerosis and the risk of ASCVD in people without ASCVDs at baseline[38,39]. Interestingly, SUA has revealed moderate interaction with IR and arterial stiffness in a large cohort of newly diagnosed, never-treated hypertensive patients[40]. Moreover, SUA to HDL ratio has been suggested as a promising predictive factor for metabolic syndrome and ASCVD outcomes in T2DM[41,42], but its discriminative potency for carotid IMT in asymptomatic patients at higher risk of ASCVD remains uncertain. Because this parameter can be a valuable indicator in facilitating the early identification of individuals at elevated ASCVD risk, the incorporation of routine SUA to HDL ratio monitoring into clinical practice could support the early identification of high-risk individuals, facilitate timely interventions, and reduce the burden of CV and metabolic diseases[43]. The relationship between carotid atherosclerosis and novel inflammatory markers was investigated, including the platelet-to-lymphocyte ratio, the neutrophil-to-lymphocyte ratio, the lymphocyte-to-monocyte ratio, the platelet-to-neutrophil ratio, neutrophil-to-lymphocyte-platelet ratio, systemic immune-inflammation index, systemic inflammation response index and aggregate index of systemic inflammation, has been investigated in numerous clinical studies[43]. These new inflammatory markers were not only significantly correlated with carotid IMT, but were also affordable and simple to detect. To note, conventionally used in routine clinical practice high-sensitivity CRP along with certain inflammatory cytokines, such as interleukins (IL-2, IL-6, IL-8), tumor necrosis factor-alpha, interferon-gamma, monocyte chemoattractant protein-1, have revealed the association with carotid IMT, degree of carotid stenosis and recently symptomatic carotid atherosclerosis[44-46]. Last, but not least, computed tomography angiography, magnetic resonance angiography and 18F-fluorodeoxyglucose positron emission tomography scans are proven valuable tools for evaluating patients with carotid atherosclerosis and perhaps with plaque vulnerability, while they have demonstrated weaker predictive ability for ASCVD events in asymptomatic individuals in comparison with symptomatic patients[47]. All these findings mentioned above suggest the discovery of alternative characterization of atherosclerosis risk beyond classical CV risk factors, inflammatory indices and neuroimaging features in asymptomatic individuals.
Another issue that remains unresolved in the study is the lack of information regarding the discriminatory potential of the new model for patients with target lipid levels who are receiving adequate statin therapy or combined lipid-lowering therapy, antihypertensive treatment, or management of HF. These treatments could significantly influence vascular outcomes and biomarker levels. Indeed, recent clinical studies have shown that statins, ezetimibe and the protein convertase subtilisin/kexin type 9 significantly improve blood pressure control and a signature of circulating biomarkers reflecting inflammation, oxidative stress and kidney function, as well as decreasing the risk of carotid atherosclerosis progression among asymptomatic individuals and patients with known ASCVD[48-51]. Future treatment modalities targeting carotid plaque modification may prevent related vascular complications and carotid plaque assessment may be used a surrogate risk marker for management of CVDs.
Not only was this aspect of implementing a biomarker model for predicting carotid atherosclerosis in patients with T2DM left out of the discussion, it is also likely to be a significant limitation due to the retrospective nature of the study. Nevertheless, the results obtained by the authors are undoubtedly highly attractive due to their practical value, offering prospects for future research in this area.
Asymptomatic carotid plaque in T2DM is associated with older age, increased BMI, biomarkers of poor glycemic control (HbA1c, fasting glucose), kidney dysfunction (UACR), and metabolic abnormalities (HDL-C, SUA). However, conventionally used risk factors, such as age, overweight/obesity, hypertension, poor glycemic control, renal dysfunction, and metabolic disturbances frequently underestimate the patients at the risk of asymptomatic carotid atherosclerosis, while it may be a target for further interventions to prevent vascular complications. Routine measurements of these parameters, especially SUA and UACR, could be practically useful to pre-screen the patients with T2DM at higher risk of carotid plaque formation.
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6382] [Article Influence: 911.7] [Reference Citation Analysis (12)] |
| 2. | Fu J, Deng Y, Ma Y, Man S, Yang X, Yu C, Lv J, Wang B, Li L. National and Provincial-Level Prevalence and Risk Factors of Carotid Atherosclerosis in Chinese Adults. JAMA Netw Open. 2024;7:e2351225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Wu TW, Chou CL, Cheng CF, Lu SX, Wang LY. Prevalences of diabetes mellitus and carotid atherosclerosis and their relationships in middle-aged adults and elders: a community-based study. J Formos Med Assoc. 2022;121:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, Fowkes FGR, Fowkes FJI, Rudan I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721-e729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 559] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 5. | Catalan M, Herreras Z, Pinyol M, Sala-Vila A, Amor AJ, de Groot E, Gilabert R, Ros E, Ortega E. Prevalence by sex of preclinical carotid atherosclerosis in newly diagnosed type 2 diabetes. Nutr Metab Cardiovasc Dis. 2015;25:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1469] [Article Influence: 183.6] [Reference Citation Analysis (2)] |
| 7. | Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, Fuster V. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, Christodorescu RM, Daskalopoulou SS, Ferro CJ, Gerdts E, Hanssen H, Harris J, Lauder L, McManus RJ, Molloy GJ, Rahimi K, Regitz-Zagrosek V, Rossi GP, Sandset EC, Scheenaerts B, Staessen JA, Uchmanowicz I, Volterrani M, Touyz RM; ESC Scientific Document Group. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. 2024;45:3912-4018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1002] [Article Influence: 501.0] [Reference Citation Analysis (0)] |
| 9. | Dai G, Cai X, Ye C, Zhang Y, Guan R. A cross-sectional study of factors associated with carotid atherosclerosis. Front Physiol. 2024;15:1434173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Li W, Wang Y, Ouyang S, Li M, Liu R, Zhang Y, Liu X, Li T, Liu S. Association Between Serum Uric Acid Level and Carotid Atherosclerosis and Metabolic Syndrome in Patients With Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2022;13:890305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 11. | Lin CC, Li CI, Liu CS, Lin CH, Yang SY, Li TC. Association of carotid atherosclerosis markers with all-cause and cardiovascular disease-specific mortality in persons with type 2 diabetes: a causal mediation analysis with glucose variation. Acta Diabetol. 2024;61:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Jin CH, Wang JW, Ke JF, Li JB, Li MF, Li LX. Low-normal serum unconjugated bilirubin levels are associated with late but not early carotid atherosclerotic lesions in T2DM subjects. Front Endocrinol (Lausanne). 2022;13:948338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Yin Y, Zhang L, Zhang J, Jin S. Predictive value of uric acid to albumin ratio for carotid atherosclerosis in type 2 diabetes mellitus: A retrospective study. PLoS One. 2025;20:e0320738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Pleskovič A, Letonja MŠ, Vujkovac AC, Nikolajević Starčević J, Gazdikova K, Caprnda M, Gaspar L, Kruzliak P, Petrovič D. C-reactive protein as a marker of progression of carotid atherosclerosis in subjects with type 2 diabetes mellitus. Vasa. 2017;46:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Li W, Liu X, Liu L, Zhang L, Li M, Liu R, Li T, Chen E, Liu S. Relationships of Serum Bone Turnover Markers With Metabolic Syndrome Components and Carotid Atherosclerosis in Patients With Type 2 Diabetes Mellitus. Front Cardiovasc Med. 2022;9:824561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Guo XL, Wang JW, Tu M, Wang W. Perirenal fat thickness as a superior obesity-related marker of subclinical carotid atherosclerosis in type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1276789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 17. | Li J, Shangguan H, Chen X, Ye X, Zhong B, Chen P, Wang Y, Xin B, Bi Y, Zhu D. Advanced glycation end product levels were correlated with inflammation and carotid atherosclerosis in type 2 diabetes patients. Open Life Sci. 2020;15:364-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Scott DA, Ponir C, Shapiro MD, Chevli PA. Associations between insulin resistance indices and subclinical atherosclerosis: A contemporary review. Am J Prev Cardiol. 2024;18:100676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 19. | Solis-Herrera C, Triplitt C, Cersosimo E, DeFronzo RA. Pathogenesis of Type 2 Diabetes Mellitus. 2021 Sep 27. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 20. | Berezin A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab Syndr. 2016;10:S176-S183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Mewborn EK, Tolley EA, Wright DB, Doneen AL, Stanfill AG. Atherosclerotic Cardiovascular Disease Risk Scores are Associated with Carotid Intima-Media Thickness. Clin Nurs Res. 2025;34:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, Ballantyne CM. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Siebel AL, Fernandez AZ, El-Osta A. Glycemic memory associated epigenetic changes. Biochem Pharmacol. 2010;80:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ Res. 2010;107:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Queiroz M, Sena CM. Perivascular adipose tissue: a central player in the triad of diabetes, obesity, and cardiovascular health. Cardiovasc Diabetol. 2024;23:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Scilletta S, Di Marco M, Miano N, Capuccio S, Musmeci M, Bosco G, Di Giacomo Barbagallo F, Martedì M, La Rocca F, Vitale A, Scicali R, Piro S, Di Pino A. Cardiovascular risk profile in subjects with diabetes: Is SCORE2-Diabetes reliable? Cardiovasc Diabetol. 2025;24:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Raitakari OT, Magnussen CG, Juonala M, Kartiosuo N, Pahkala K, Rovio S, Koskinen JS, Mykkänen J, Laitinen TP, Kähönen M, Nuotio J, Viikari JSA. Subclinical atherosclerosis in young adults predicting cardiovascular disease: The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2024;393:117515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Winckler K, Wiinberg N, Jensen AK, Thorsteinsson B, Lundby-Christensen L, Heitmann BL, Jensen GB, Tarnow L. Progression in risk factors during 36 years of follow-up and prediction of carotid intima-media thickness in a large cohort of adults with and without diabetes. Scand J Clin Lab Invest. 2020;80:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Ménégaut L, Laubriet A, Crespy V, Leleu D, Pilot T, Van Dongen K, de Barros JP, Gautier T, Petit JM, Thomas C, Nguyen M, Steinmetz E, Masson D. Inflammation and oxidative stress markers in type 2 diabetes patients with Advanced Carotid atherosclerosis. Cardiovasc Diabetol. 2023;22:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 30. | Certo M, Rahimzadeh M, Mauro C. Immunometabolism in atherosclerosis: a new understanding of an old disease. Trends Biochem Sci. 2024;49:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Naguib M, Ali N, ElSaraf N, Rashed L, Azzam H. Does Serum Osteocalcin Level Affect Carotid Atherosclerosis in Post-Menopausal Diabetic Females? A Case-Control Study. Int J Gen Med. 2022;15:4513-4523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Wei W, Liu H, Qiu X, Zhang J, Huang J, Chen H, Qiu S, Lin R, Li S, Tu M. The association between serum adropin and carotid atherosclerosis in patients with type 2 diabetes mellitus: a crosssectional study. Diabetol Metab Syndr. 2022;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Kärberg K, Forbes A, Lember M. Visfatin and Subclinical Atherosclerosis in Type 2 Diabetes: Impact of Cardiovascular Drugs. Medicina (Kaunas). 2023;59:1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Wang S, Lu J, You Q, Huang H, Chen Y, Liu K. The mTOR/AP-1/VEGF signaling pathway regulates vascular endothelial cell growth. Oncotarget. 2016;7:53269-53276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Shi L, Li NJ. Comprehensive analysis of risk factors associated with carotid plaque in patients with type 2 diabetes mellitus. World J Diabetes. 2025;16:104180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Nonterah EA, Boateng D, Crowther NJ, Klipstein-Grobusch K, Oduro AR, Agongo G, Mohamed SF, Boua PR, Choma SSR, Norris SA, Tollman SM, Bots ML, Ramsay M, Grobbee D. Carotid Atherosclerosis, Microalbuminuria, and Estimated 10-Year Atherosclerotic Cardiovascular Disease Risk in Sub-Saharan Africa. JAMA Netw Open. 2022;5:e227559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Chen L, Zhu Z, Ye S, Zheng M. The Serum Uric Acid to Serum Creatinine Ratio is an Independent Risk Factor for Diabetic Kidney Disease. Diabetes Metab Syndr Obes. 2022;15:3693-3703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 38. | Lin YP, Hsu YC, Tsai KZ, Kwon Y, Lin GM. Insulin Resistance Indices and Carotid Intima-media Thickness in Physically Fit Adults: CHIEF Atherosclerosis Study. Endocr Metab Immune Disord Drug Targets. 2023;23:1442-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Zhang Y, Wang M, Cai X, Jin A, Jing J, Wang S, Meng X, Li S, Zhou Q, Wang X, Wei T, Wang Y, Pan Y. Associations of noninsulin-based insulin resistance indices with presence and extent of multiterritorial atherosclerosis: A cross-sectional study. J Clin Lipidol. 2025;19:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 41. | Kocak MZ, Aktas G, Erkus E, Sincer I, Atak B, Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras (1992). 2019;65:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 42. | Yi Y, Luo Q, Chen J, Chen Z, Aydemir HA, Chen P, Tang J, Luo F, Fang Z. Association between the uric acid-to-HDL-cholesterol ratio (UHR) and the risk of cardiovascular disease and dyslipidemia: a population-based study. Lipids Health Dis. 2025;24:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 43. | Cui Y, Zhang W. Long-term cardiovascular risk and mortality associated with uric acid to HDL-C ratio: a 20-year cohort study in adults over 40. Sci Rep. 2025;15:14242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 44. | Liao M, Liu L, Bai L, Wang R, Liu Y, Zhang L, Han J, Li Y, Qi B. Correction: Correlation between novel inflammatory markers and carotid atherosclerosis: A retrospective case-control study. PLoS One. 2025;20:e0327051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Coveney S, McCabe JJ, Murphy S, Belton O, Gray C, Cassidy T, Dolan E, de Gaetano M, Harbison J, Horgan G, Marnane M, Merwick A, Noone I, Williams DJ, Kelly PJ. Dose-Dependent Association of Inflammatory Cytokines with Carotid Atherosclerosis in Transient Ischaemic Attack: Implications for Clinical Trials. Cerebrovasc Dis. 2022;51:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Mayer FJ, Binder CJ, Wagner OF, Schillinger M, Minar E, Mlekusch W, Tsiantoulas D, Goliasch G, Hoke M. Combined Effects of Inflammatory Status and Carotid Atherosclerosis: A 12-Year Follow-Up Study. Stroke. 2016;47:2952-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Nyárády BB, Dósa E, Kőhidai L, Pállinger É, Gubán R, Szőnyi Á, Kiss LZ, Bagyura Z. Associations between Various Inflammatory Markers and Carotid Findings in a Voluntary Asymptomatic Population Sample. Int J Mol Sci. 2024;25:9656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Maes L, Versweyveld L, Evans NR, McCabe JJ, Kelly P, Van Laere K, Lemmens R. Novel Targets for Molecular Imaging of Inflammatory Processes of Carotid Atherosclerosis: A Systematic Review. Semin Nucl Med. 2024;54:658-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Silla A, Fogacci F, Punzo A, Hrelia S, Simoni P, Caliceti C, Cicero AFG. Treatment with PCSK9 Inhibitor Evolocumab Improves Vascular Oxidative Stress and Arterial Stiffness in Hypercholesterolemic Patients with High Cardiovascular Risk. Antioxidants (Basel). 2023;12:578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 50. | Sjölander M, Carlberg B, Norberg M, Näslund U, Ng N. Prescription of Lipid-Lowering and Antihypertensive Drugs Following Pictorial Information About Subclinical Atherosclerosis: A Secondary Outcome of a Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2121683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Huang R, Mills K, Romero J, Li Y, Hu Z, Cao Y, Huang H, Xu Y, Jiang L. Comparative effects of lipid lowering, hypoglycemic, antihypertensive and antiplatelet medications on carotid artery intima-media thickness progression: a network meta-analysis. Cardiovasc Diabetol. 2019;18:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/