Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1291
Revised: February 6, 2024
Accepted: March 29, 2024
Published online: June 15, 2024
Processing time: 196 Days and 0.8 Hours
Lingguizhugan (LGZG) decoction is a widely used classic Chinese medicine formula that was recently shown to improve high-fat diet (HFD)-induced insulin resistance (IR) in animal studies.
To assess the therapeutic effect of LGZG decoction on HFD-induced IR and explore the potential underlying mechanism.
To establish an IR rat model, a 12-wk HFD was administered, followed by a 4-wk treatment with LGZG. The determination of IR status was achieved through the use of biochemical tests and oral glucose tolerance tests. Using a targeted meta-bolomics platform to analyze changes in serum metabolites, quantitative real-time PCR (qRT-PCR) was used to assess the gene expression of the ribosomal protein S6 kinase beta 1 (S6K1).
In IR rats, LGZG decreased body weight and indices of hepatic steatosis. It effectively controlled blood glucose and food intake while protecting islet cells. Metabolite analysis revealed significant differences between the HFD and HFD-LGZG groups. LGZG intervention reduced branched-chain amino acid levels. Levels of IR-related metabolites such as tryptophan, alanine, taurine, and asparagine decreased significantly. IR may be linked to amino acids due to the contemporaneous increase in S6K1 expression, as shown by qRT-PCR.
Our study strongly suggests that LGZG decoction reduces HFD-induced IR. LGZG may activate S6K1 via metabolic pathways. These findings lay the groundwork for the potential of LGZG as an IR treatment.
Core Tip: This research examines the therapeutic properties of Lingguizhugan (LGZG) decoction, specifically focusing on its potential impact on insulin resistance (IR) and its associated mechanisms. The findings indicate that the LGZG decoction effectively controls the S6 kinase beta 1 metabolic pathway, enhancing IR, weight management, glucose tolerance, and liver steatosis. The findings of this study indicate that LGZG decoction can be used as a medical treatment for the control of IR. These results offer valuable clinical insights and propose avenues for future pharmacological investigations.
- Citation: Liu XM, Yuan SQ, Ning Y, Nie SJ, Wang XQ, Jia HY, Zheng XL. Therapeutic effects of Lingguizhugan decoction in a rat model of high-fat diet-induced insulin resistance. World J Diabetes 2024; 15(6): 1291-1298
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1291.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1291
Insulin resistance (IR), representing a diminished responsiveness of target organs to physiological insulin concentrations, has been considered a pivotal factor in the etiology of various maladies, notably obesity and metabolic syndrome[1,2]. Moreover, IR plays a significant role in the early stages of type 2 diabetes mellitus, which is becoming increasingly common worldwide, especially among younger people[3,4]. Therefore, the scientific justification for considering IR as a treatment approach is evident. Most pharmaceuticals, such as metformin, are categorized as antihyperglycemic medications to enhance IR in contemporary medical practice. These compounds mitigate the deleterious impact of prolonged hyperglycemia on insulin sensitivity, albeit with the potential for adverse consequences[5]. Hence, research to develop safer and more efficacious pharmacological interventions is imperative.
The Lingguizhugan (LGZG) decoction is a widely recognized herbal formula in traditional Chinese medicine that has demonstrated efficacy in preventing metabolic syndrome and alleviating obesity, hyperglycemia, and liver damage[6]. A clinical study provided evidence that LGZG decoction has significant effectiveness in improving weight, blood glucose levels, and glucose tolerance in obese individuals suffering from nonalcoholic fatty liver disease[7]. Consequently, LGZG decoction has emerged as a viable therapeutic intervention for IR.
Extensive research has been conducted on the complex mechanism of glucose catabolism, leading to the identification of metabolites such as branched-chain amino acids (BCAAs) that signify IR and obesity[8]. These findings highlight the critical function of ribosomal protein S6 kinase beta 1 (S6K1), which is triggered by foods such as amino acids, in increasing IR via insulin receptor substrate-1 (IRS-1) phosphorylation. Branched-chain fatty acids derived from BCAAs also contribute to increased glucose levels and S6K1 activation in hepatic tissues, impacting obesity and IR[9].
This study aimed to evaluate the therapeutic potential of LGZG and establish an IR model in obese rats by administering a high-fat diet (HFD). This investigation uses targeted metabolomics and quantitative real-time PCR (qRT-PCR) to discern the underlying mechanisms, thereby providing insights into how LGZG decoction influences HFD-induced IR.
Four Chinese medicinal herbs—Poria, cinnamon, Atractylodes, and licorice—were combined at a ratio of 12:9:6:6 to create the LGZG decoction. Beijing Kangrentang Pharmaceutical Co., Ltd., provided all of the herbs used. Supplementary Table 1 provides details on the components of the LGZG decoction[10]. The medications were previously analyzed by our team using mass spectrometry (MS; Supplementary Figure 1). The Chinese medicine herbs were proportionately dissolved in saline for administration, yielding a decoction concentration of 0.3625 g/mL. The high-fat feed was supplied by Xiaoshutai Biotechnology Co. in Beijing, China.
Thirty male Sprague-Dawley rats (SPF grade), aged 5 wk and weighing between 200 g and 220 g, were obtained from Sprague-Dawley Biotechnology Co. (Beijing, China). After three days of adaptive feeding, the rats were randomly divided into three groups: The normal diet (ND) group, the HFD group, and the HFD-LGZG treatment group, each comprising 10 rats. The ND group received standard chow, while the HFD and HFD-LGZG groups were fed a HFD. Throughout the 12-wk modeling period, the rats had unlimited access to food and water, and their body weights were measured weekly. After 12 wks, blood glucose and glucose tolerance tests were performed, which resulted in a re-evaluation of the results. The rats in the HFD-LGZG group were administered LGZG (1.64 g/kg), while the rats in the HFD group were given saline. The gavage doses were adjusted based on the body surface area of the rats in comparison to that of humans. Weekly food intake was recorded. After 16 wks, the rats were subjected to a 12-h fast followed by an oral glucose tolerance test (OGTT) at a dose of 2 g/kg. At the end of the experiment, sodium pentobarbital (2%, 45 mg/kg, intravenously) was used to induce anesthesia, and tissues samples of liver, serum, pancreas, and feces were collected for further experiments. The animal model preparation and experimental protocols are shown in Supplementary Figure 2. The animal experiment was approved by the Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine (Approval No. 2024020), and all procedures were performed following the National Institutes of Health of China's guidelines for the care and use of laboratory animals.
After collecting the specimens, fixed sections of the liver and pancreas were subjected to HE staining to visualize the pathological changes. Biochemical kits obtained from Nanjing Jiancheng Bioengineering Research Institute (Shenzhen, China) and Myriad Biotechnology were used to measure serum levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Additionally, the serum insulin concentration was measured using an ELISA kit from Wuhan Xavier Biotechnology Co., Ltd.
Double-labeling immunofluorescence analysis was conducted on pancreatic sections. The specimens were examined and imaged using a fluorescence microscope, with positively expressed regions identified through corresponding red or green fluorescence. Insulin was specifically labeled in red, while glucagon-like peptide-1 receptor (GLP-1R) was labeled in green.
qRT-PCR was performed with a Foregene RNA Extraction Kit (Cat. No. RE-03014) to extract total RNA from liver samples. The specimens were liver tissues stored at a temperature of -80°C, and 20-40 mg of tissue was used for each extraction. The RNA extraction procedure involved the use of DNA-cleaning columns and RNA-only columns as essential Foregene RNA Extraction Kit elements. After homogenization and centrifugation to remove DNA, the RNA was further purified using RNA-only columns with specific buffers and centrifugation techniques, ensuring the complete removal of any remaining contaminants. The final RNA solution was obtained by elution with heated ddH2O.
A Foregene Reverse Transcription Kit (Cat. No. QP-01011/01012/01013/01014) was used to transform RNA into complementary DNA (cDNA) according to the instructions provided in the manual. The cDNA templates for PCR were generated by diluting the samples to a concentration of 1 ng/μL. The qRT-PCR assays were conducted using Jena PCR devices. The reaction mixture comprised 2 × Real PCR EasyTM Mix-SYBR (10 μL), forward and reverse primers (0.4 μL), cDNA template (2 μL), and DNase-Free ddH2O (7.2 μL), resulting in a total volume of 20 μL. The PCR cycling procedure consisted of an initial denaturation step at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 55°C for 10 s, and extension at 72°C for 20 s. Moreover, the 2-ΔΔCt method was used to calculate the expression of the relevant target genes. The list of primers utilized in the study can be found in Supplementary Table 2.
To detect amino acids in the serum, 50 μL of the serum sample was measured and added to 450 μL of MS water, followed by vortexing to create a 10-fold diluted sample. Next, 50 mL of the diluted sample was added to 200 mL of precipitant, which consisted of acetonitrile and methanol in a 1:1 ratio, along with the mixed internal standard. After vortexing and mixing, the sample was left to stand on ice for 30 min. The sample was subsequently centrifuged at 12000 rpm at 4°C for 10 min. Finally, all the supernatants were collected using liquid chromatography-MS for analysis.
Ultra-performance liquid chromatography-electrospray ionization tandem MS (UPLC-ESI-MS/MS) was used to analyze short-chain fatty acids (SCFAs) quantitatively in the serum samples. The samples were first stored at -80°C, after which the serum samples were thawed at room temperature before being subjected to UPLC-ESI-MS/MS analysis. A 150 μL aliquot of each sample was pipetted and mixed with 150 μL of a 50% acetonitrile and water solution (v/v). This mixture was ultrasonicated in an icewater bath for 10 min. After centrifugation at 12000 rpm for 10 min at 4°C, 80 μL of the resulting supernatant was carefully transferred into a vial. Following derivatization, the samples were combined with 40 μL of a 200 mM 3-NPH solution in a 50% acetonitrile configuration (v/v) and 40 μL of a 120 mM ED-6% pyridine solution in a 50% acetonitrile configuration (v/v). The reaction proceeded at 40°C for 30 min, followed by cooling on ice for 1 min. The samples were then stored in brown vials at -80°C until analysis. The UPLC-ESI-MS/MS analytical method utilizing triple quadrupole MS in multiple reaction detection (MRM) mode was used for qualitative and quantitative detection of target metabolites. Concentrations were determined considering sampling and dilution characteristics, followed by determination of the absolute content of each metabolite in the sample.
The statistical analysis was performed with the software GraphPad Prism version 8.0.1. One-way ANOVA was used to evaluate differences between three groups with normally distributed data. Furthermore, the mean ± SD was calculated. A t test was used to compare differences in the metabolites between the HFD and HFD-LGZG groups. A difference between the two groups was considered significant at a level of P < 0.05.
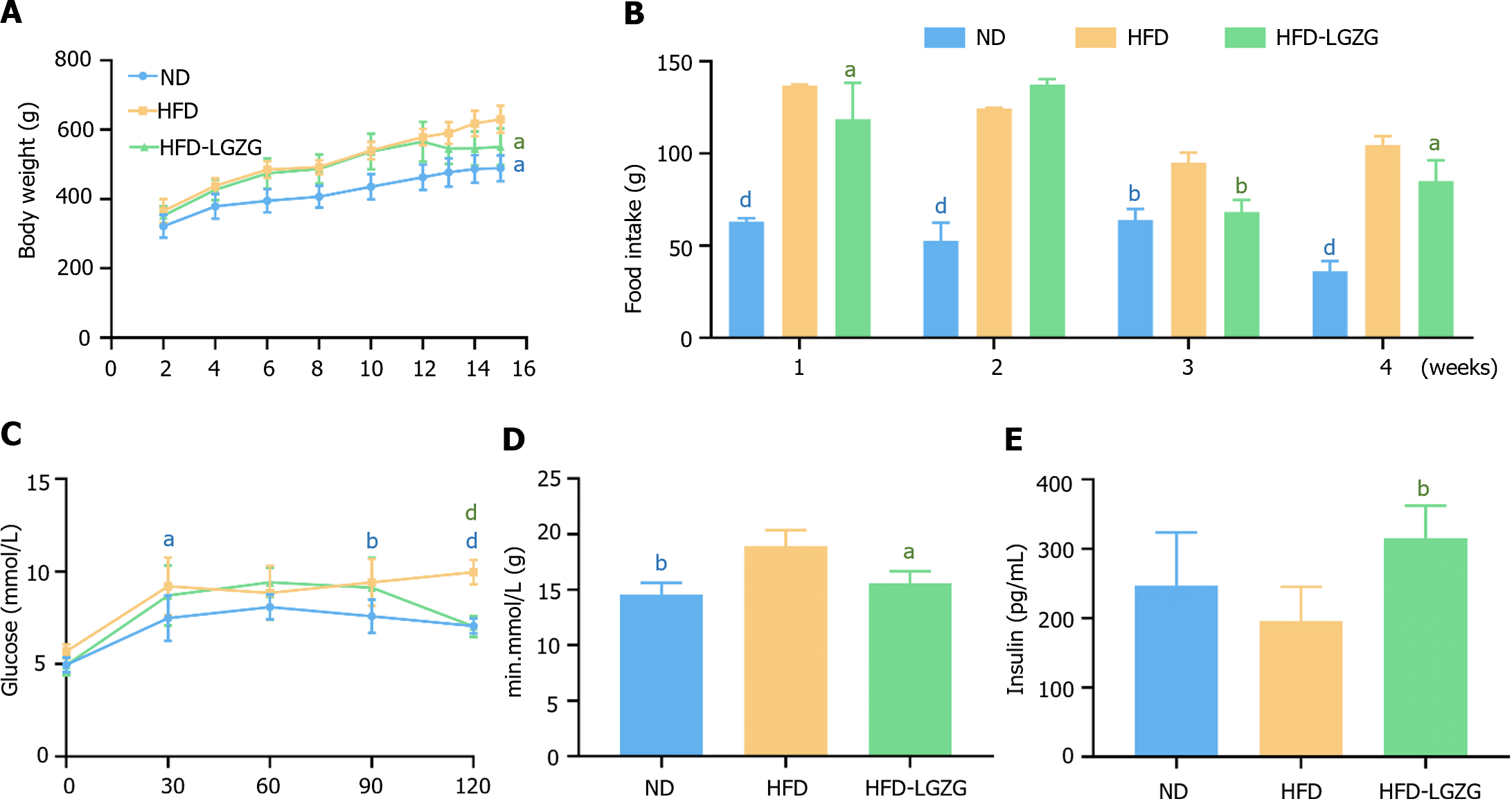
We compared the body weights of the three groups of rats and measured their food intake once a week after drug administration to observe the effect of LGZG decoction on rats with HFD-induced IR. The results indicated that LGZG decoction significantly reduced the body weights of the rats (Figure 1A) and controlled the amount of food consumed by the rats (Figure 1B). The OGTT results demonstrated that blood glucose levels in the HFD-LGZG group were significantly lower at 120 min (Figure 1C) than the HFD group, and the area under the curve was also lower (Figure 1D). These findings collectively indicate that LGZG intervention effectively rescued the rats from the effects of HFD-induced IR and improved insulin sensitivity. Furthermore, the serum insulin concentration was significantly greater in the HFD-LGZG group than in the HFD group. In comparison, rats in the HFD group had markedly lower insulin levels than did those in the ND and HFD-LGZG groups (Figure 1E).
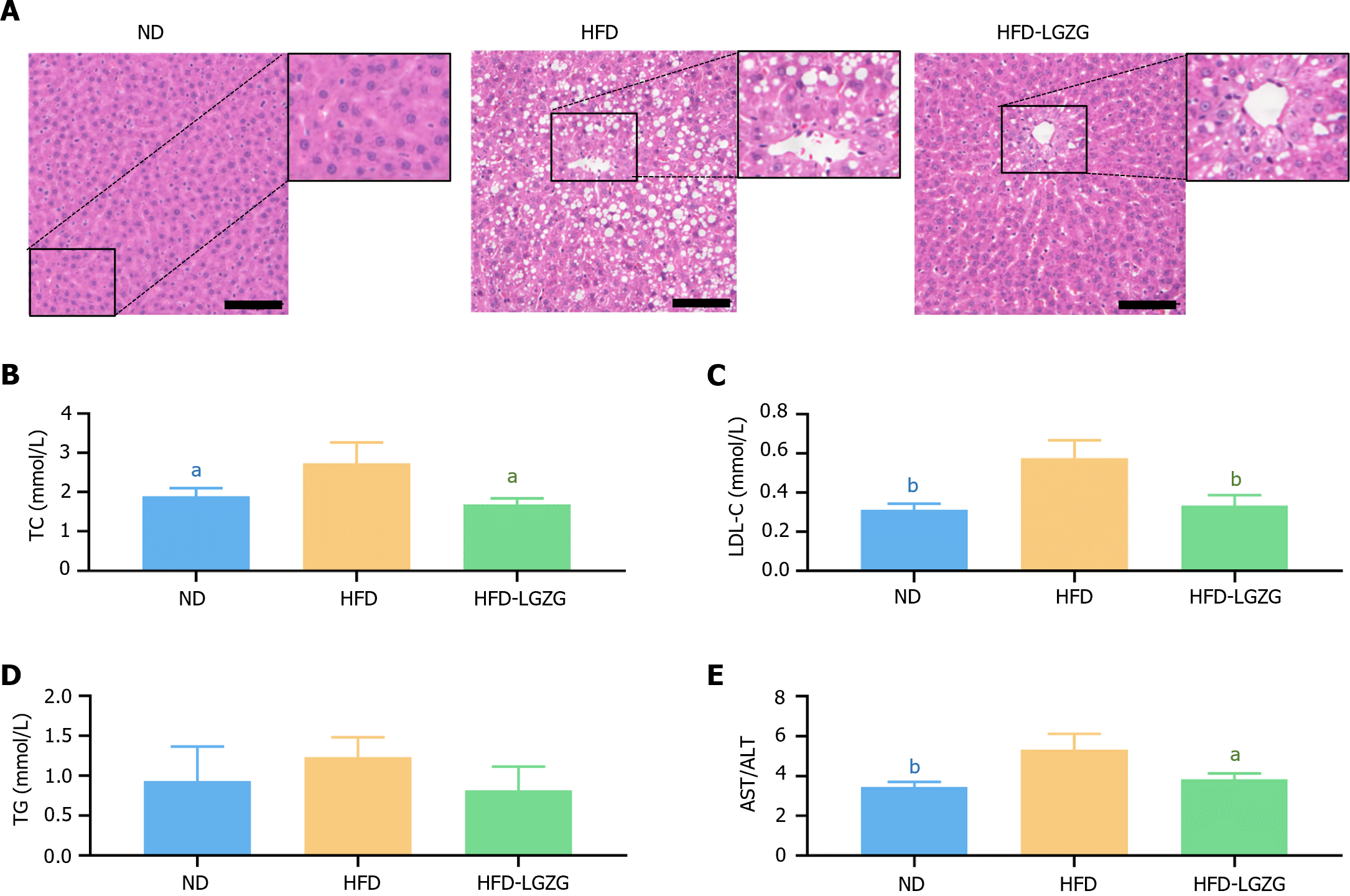
HE staining (Figure 2A) revealed that the livers of HFD-fed rats exhibited marked steatosis characterized by severe cytoplasmic vacuolization, cellular infiltration, and periportal fibrosis of hepatocytes. In contrast, the HFD-LGZG group exhibited a normal central vein, reduced cell vacuolization, and improved hepatocyte conditions. Additionally, the serum TC and LDL-C levels in the HFD-fed rats were significantly greater than those in the ND-fed rats but decreased significantly after LGZG treatment (Figure 2B and C). Notably, the serum TG levels did not significantly change in the HFD-fed rats (Figure 2D). Furthermore, LGZG treatment resulted in significantly lower AST/ALT values than did HFD treatment (Figure 2E). These findings demonstrate the effectiveness of LGZG decoction in reducing hepatic steatosis induced by a HFD in obese rats.
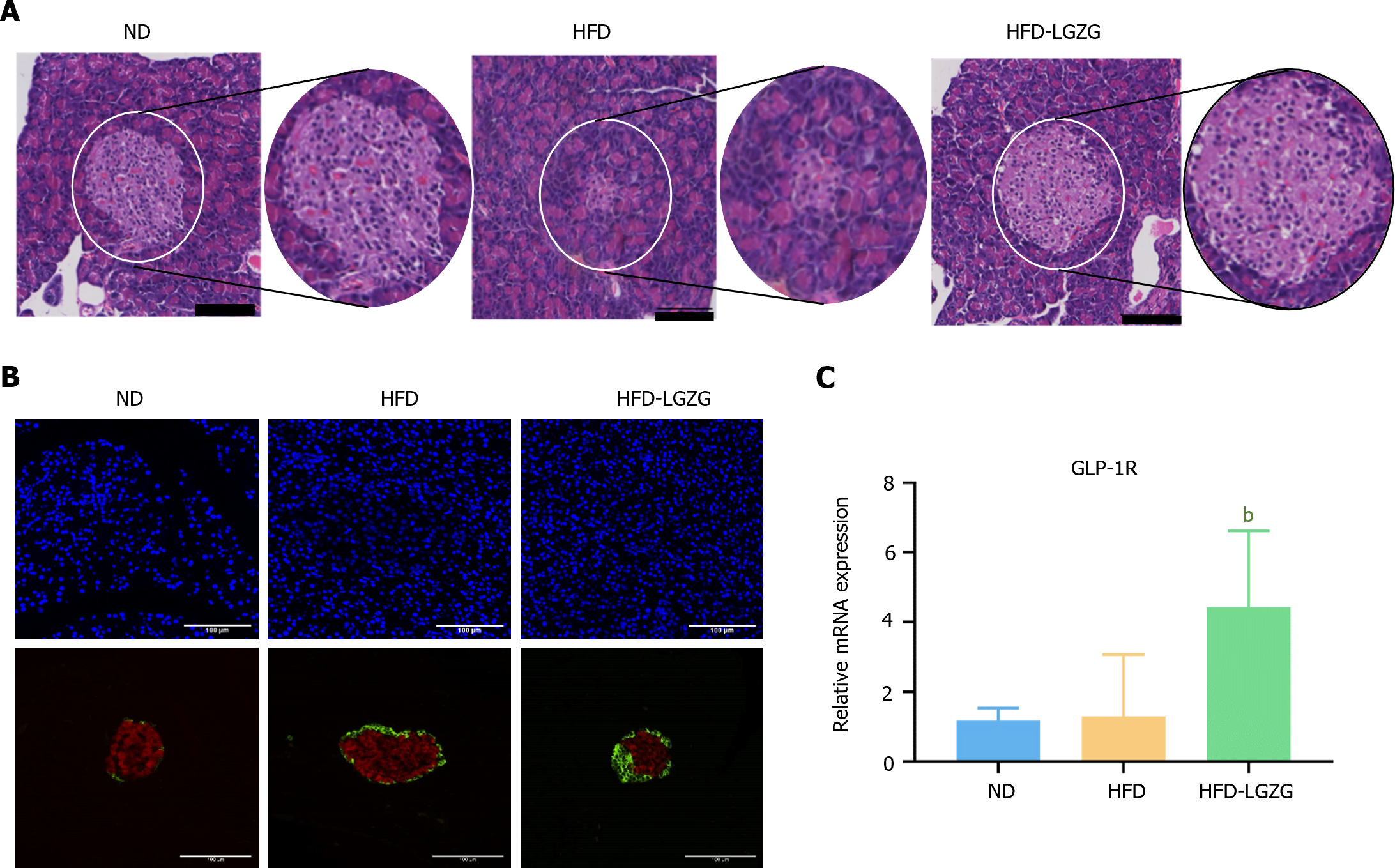
HE staining of the pancreas revealed that, compared with those in the ND group, the rats in the HFD group (Figure 3A) exhibited a decrease in the number of pancreatic islets, which were morphologically atrophied, and were smaller. However, the HFD-LGZG group exhibited preserved islet morphology and a significantly larger area. IF analysis (Figure 3B) demonstrated that the HFD group had higher levels of insulin expression and lower levels of GLP-1R than the ND group. Conversely, compared with those in the HFD group, pancreatic cells of the rats in the HFD-LGZG group exhibited reduced insulin expression and increased GLP-1R expression. qRT-PCR (Figure 3C) results confirmed that the gene expression of GLP-1R was significantly upregulated in the HFD-LGZG group compared to the HFD group, consistent with the findings from IF analysis. These findings collectively suggest that LGZG preserves the morphology of islet cells and enhances the expression of GLP-1R.
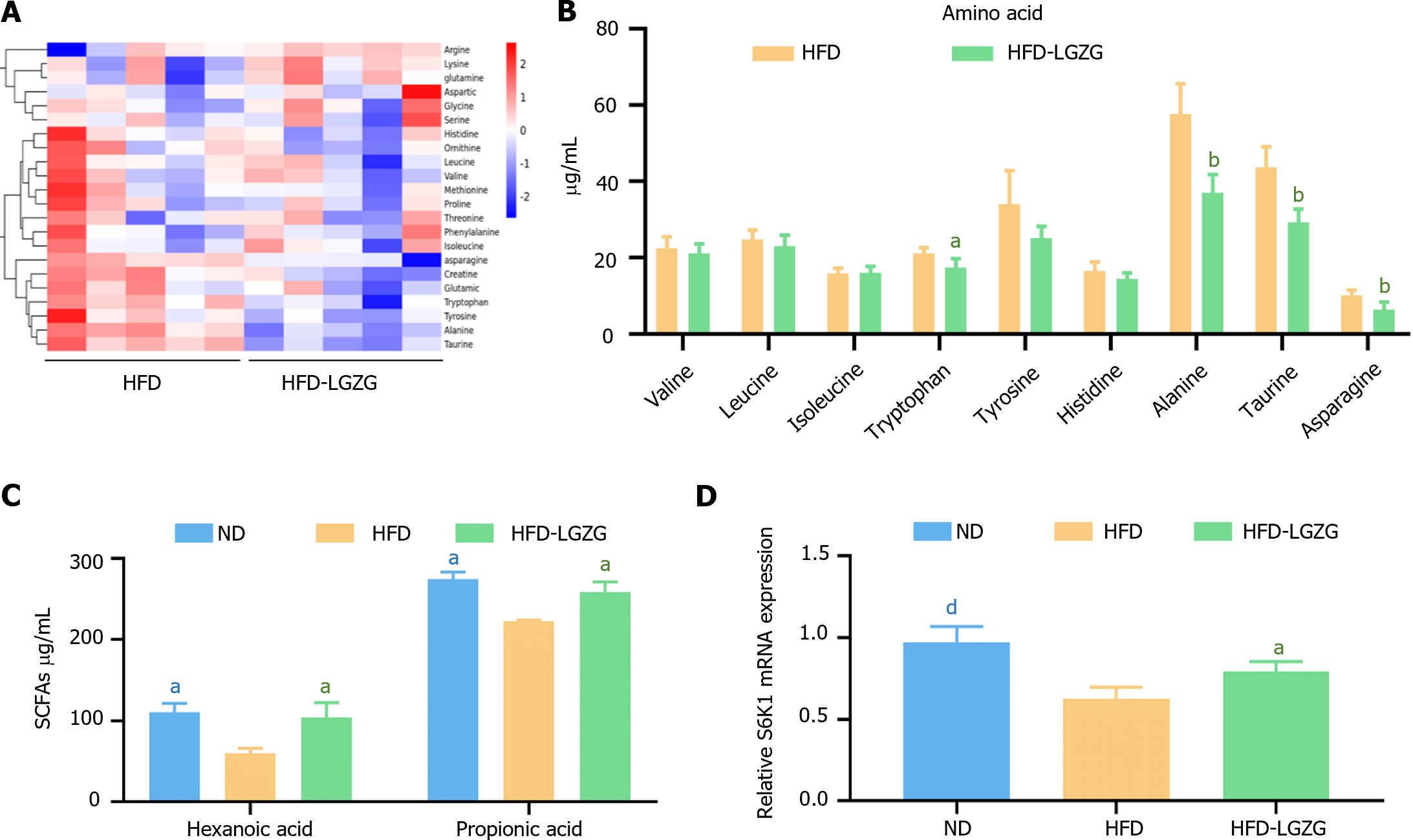
Elevated levels of circulating fasting BCAAs have been observed under conditions of increased IR[11]. Experimental research has shown that changes in the microbiota can affect obesity and energy metabolism, including insulin sensitivity. The reduction in glucose levels is also related to SCFAs, with propionic acid playing a significant role[12]. In this study, serum-targeted metabolomics analysis was conducted to assess the modulation of IR by LGZG decoction. The results (Figure 4A and B) indicate that the levels of amino acids, such as tryptophan, alanine, taurine, and asparagine, were significantly lower in the HFD-LGZG group than in the HFD group. Moreover, among the SCFAs (Figure 4C), the serum levels of hexanoic acid and propionic acid were significantly greater in the HFD-LGZG group than in the HFD group.
Previous studies have revealed that amino acids do not directly signal through the phosphoinositide 3-kinase (PI3K) pathway[13]. Instead, they first activate S6K1, inhibiting PI3K activation. These findings indicate that S6K1 serves as an amino acid activator[13]. Therefore, we investigated the expression of S6K1 in liver tissue (Figure 4D). Similarly, the expression of S6K1 was downregulated in the HFD group and upregulated in the HFD-LGZG group compared to that in the ND group. These results support the involvement of S6K1 in the development of IR.
A HFD can cause biological health damage, such as obesity or IR[14]. In experiments studying HFD-induced IR, we discovered that LGZG controlled the adverse effects of IR, including overweight, food intake, decreased glucose tolerance, hepatic steatosis, and maintenance of pancreatic morphology. First, LGZG positively affected obese rats, resulting in decreased body weight and reduced food intake. This could be attributed to the metabolic regulation of the spleen by LGZG, as the relationship between body weight and glycolipid metabolism is highly interconnected[15]. Under normal conditions, the serum insulin levels of the rats in the HFD group were greater than those in the ND group, while the rats in the HFD-LGZG group had slightly greater serum insulin levels than did those in the ND group. Interestingly, rats in the HFD group had lower serum insulin levels, while those in the HFD-LGZG group had higher serum insulin levels than did those in the ND group. Previous studies have demonstrated that GLP-1R significantly positively affects glycemic control, dietary suppression, and weight loss[16]. In pancreatic islet cells, GLP-1R prominently promotes the proliferation of pancreatic islet β-cells and increases insulin synthesis and release[17]. Therefore, LGZG may stimulate insulin production by activating GLP-1R, leading to increased secretion of insulin by pancreatic islet cells into the bloodstream. On the other hand, the HFD group exhibited suppressed GLP-1R expression and pancreatic atrophy, which could explain the decreased serum insulin levels observed in these rats. Additionally, LGZG was found to increase serum insulin levels[6]. Hence, these findings reasonably explain the observed differences in serum insulin levels between the HFD-LGZG and HFD groups.
To further investigate the mechanism of action of IR therapy, an experiment was conducted to evaluate targeted metabolomics. In mammals, BCAAs contribute to IR by enhancing rapamycin complex 1 (mTORC1) expression and inhibiting insulin signaling[18]. Numerous studies have indicated that elevated blood levels of BCAAs can be biomarkers for IR[15]. The results of our study revealed increased blood BCAA levels in the HFD group, which decreased after LGZG intervention. Notably, the HFD-LGZG group exhibited a significant decrease in the amino acid levels of tyrosine, taurine, aspartic acid, and asparagine. Previous research has suggested that these changes in amino acid concentrations are associated with IR[19]. Therefore, it is crucial to understand how alterations in amino acid levels affect the IR signaling pathway. According to previous reports, the presence of amino acids activates S6K1, which inhibits the activation of PI3K in insulin signaling and leads to IR[13]. Through qRT-PCR analysis of liver tissues, we observed significant upregulation of S6K1 expression after LGZG intervention. Previous studies have demonstrated that the activation of S6K1 positively impacts hepatic IR[20], and our experimental results align with these findings.
The serum levels of SCFAs, particularly hexanoic and propionic acid, increased after LGZG treatment. Notably, an increase in SCFAs has been associated with improved glucose homeostasis and insulin sensitivity[21]; however, most studies have focused on investigating the gut microbiota as the main contributing factor.
LGZG decoction has the potential to improve IR and alleviate the adverse effects associated with weight, glucose tolerance, and liver steatosis. Our findings suggest that the efficacy of LGZG in ameliorating IR may be attributed to its modulation of the S6K1 metabolic pathway.
| 1. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4614] [Article Influence: 219.7] [Reference Citation Analysis (0)] |
| 2. | Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Casanova P, Monleon D. Role of selenium in type 2 diabetes, insulin resistance and insulin secretion. World J Diabetes. 2023;14:147-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 4. | Yang W, Jiang W, Guo S. Regulation of Macronutrients in Insulin Resistance and Glucose Homeostasis during Type 2 Diabetes Mellitus. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Matthaei S, Stumvoll M, Kellerer M, Häring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. 2000;21:585-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Yao L, Wei J, Shi S, Guo K, Wang X, Wang Q, Chen D, Li W. Modified lingguizhugan decoction incorporated with dietary restriction and exercise ameliorates hyperglycemia, hyperlipidemia and hypertension in a rat model of the metabolic syndrome. BMC Complement Altern Med. 2017;17:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Dai L, Xu J, Liu B, Dang Y, Wang R, Zhuang L, Li D, Jiao L, Wang J, Zhang L, Zhong LLD, Zhou W, Ji G. Lingguizhugan Decoction, a Chinese herbal formula, improves insulin resistance in overweight/obese subjects with non-alcoholic fatty liver disease: a translational approach. Front Med. 2022;16:745-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (9)] |
| 8. | White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol Metab. 2021;52:101261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 9. | Choi BS, Daniel N, Houde VP, Ouellette A, Marcotte B, Varin TV, Vors C, Feutry P, Ilkayeva O, Ståhlman M, St-Pierre P, Bäckhed F, Tremblay A, White PJ, Marette A. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Ning Y, Gong Y, Zheng T, Xie Y, Yuan S, Ding W. Lingguizhugan Decoction Targets Intestinal Microbiota and Metabolites to Reduce Insulin Resistance in High-Fat Diet Rats. Diabetes Metab Syndr Obes. 2022;15:2427-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jørgensen ME, Grarup N, Pedersen O, Kilpeläinen TO, Hansen T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017;60:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19:654-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 483] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 13. | Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 551] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 14. | Kumar A, Sundaram K, Teng Y, Mu J, Sriwastva MK, Zhang L, Hood JL, Yan J, Zhang X, Park JW, Merchant ML, Zhang HG. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics. 2022;12:1388-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Wu R, Zhao D, An R, Wang Z, Li Y, Shi B, Ni Q. Linggui Zhugan Formula Improves Glucose and Lipid Levels and Alters Gut Microbiota in High-Fat Diet-Induced Diabetic Mice. Front Physiol. 2019;10:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, Xue H, Liu Y, Zhang Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol (Lausanne). 2021;12:721135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 17. | El K, Douros JD, Willard FS, Novikoff A, Sargsyan A, Perez-Tilve D, Wainscott DB, Yang B, Chen A, Wothe D, Coupland C, Tschöp MH, Finan B, D'Alessio DA, Sloop KW, Müller TD, Campbell JE. The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets. Nat Metab. 2023;5:945-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 18. | Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, Chen M, Wynn RM, Wang J, Gui WJ, Qi X, Lusis AJ, Li Z, Wang W, Ning G, Yang X, Chuang DT, Wang Y, Sun H. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes. 2019;68:1730-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 19. | Gaggini M, Carli F, Rosso C, Buzzigoli E, Marietti M, Della Latta V, Ciociaro D, Abate ML, Gambino R, Cassader M, Bugianesi E, Gastaldelli A. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 20. | Zhang Q, Yu J, Liu B, Lv Z, Xia T, Xiao F, Chen S, Guo F. Central activating transcription factor 4 (ATF4) regulates hepatic insulin resistance in mice via S6K1 signaling and the vagus nerve. Diabetes. 2013;62:2230-2239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1600] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0