Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1122
Revised: March 7, 2024
Accepted: May 6, 2024
Published online: June 15, 2024
Processing time: 163 Days and 21.6 Hours
Endothelial function plays a pivotal role in cardiovascular health, and dysfunction in this context diminishes vasorelaxation concomitant with endothelial activity. The nitric oxide-cyclic guanosine monophosphate pathway, prostacyclin-cyclic adenosine monophosphate pathway, inhibition of phosphodiesterase, and the opening of potassium channels, coupled with the reduction of calcium levels in the cell, constitute critical mechanisms governing vasorelaxation. Cardiovascular disease stands as a significant contributor to morbidity and mortality among in
Core Tip: To the best of our knowledge, this study is pioneering, offering a unique perspective that addresses both vasorelaxation and diabetes concerning medicinal plants. The comprehensive collection of medicinal plant references presented in this study is anticipated to serve as a valuable resource, inspiring and guiding future investigations into cardiovascular diseases and diabetes.
- Citation: Demirel S. Vasorelaxant effects of biochemical constituents of various medicinal plants and their benefits in diabetes. World J Diabetes 2024; 15(6): 1122-1141
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1122.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1122
Cardiovascular diseases (CVDs), stemming from disorders affecting the heart and blood vessels, claim tens of millions of lives globally every year[1]. The cardiovascular system comprises the heart and three distinct types of blood vessels[2]. The inner surface of blood vessels is constituted by endothelial cells referred to as the tunica intima layer[3]. Endothelial cells envelop the interior of the vessel and establish interaction with the blood[2]. These cells function as a barrier between the vessel lumen and wall, preventing blood clotting, while mediators released from them exert vasoactive effects[4]. Impaired endothelial function and diminished endothelium-associated vasorelaxation contribute to the de
Hemodynamic forces, such as shear stress, impact endothelial cells, causing unidirectional deformation of endothelial cells[7]. The equilibrium between vasodilator and vasoconstrictor agents regulates vascular tone. Endothelial dysfunction further results in elevated vascular tone, leading to cardiovascular disorders such as hypertension[8]. Vasodilatory agents like endothelium-derived hyperpolarizing factor, nitric oxide (NO), and prostacyclin (PGI2) are produced by the endo
There are studies in the literature about the effects of medicinal plants on either vasorelaxation or diabetes. However, the absence of articles presenting the effects of medicinal plants on both vasorelaxation and diabetes necessitates the inclusion of this review in the literature. Addressing this gap will not only enhance our understanding but also aid in future studies on CVDs, as decreased vasorelaxation is a significant contributor to such conditions[11]. The mechanisms crucial for vasorelaxation are expounded upon in this review, along with accompanying figures. The review encompasses components and aspects of 85 medicinal plants, delineating their effects on vasorelaxation and diabetes in Table 1.
| Plant | Vasorelaxation | Diabetes | 1Ref. | ||||
| Component/extract | Part | Effect | Component/extract | Part | Effect | ||
| Securigera securidaca L. | Hydroalcoholic extract | Seed | Endothelium-dependent vasorelaxation in hyper-cholesterolemic rats | Hydroalcoholic extract | Seed | Anti-diabetic | [61,62] |
| Parkia biglobosa | Aqueous extract | Seed | Smooth muscle vasorelaxation via endothelium due to PGs | Hydromethanolic extract | Stem bark | Anti-diabetic | [63,64] |
| Orthosiphon stamineus | Eupatorin | - | Endothelium-intact aortic ring vasorelaxation on contraction by KCl and endothelium-denuded aortic ring vasorelaxation on contraction by PE | Water extract, methanolic extract | Aerial parts | Anti-diabetic | [65,66] |
| Rosa damascena Mill. | 2-phenyl ethyl alcohol | Spent flower | Vasorelaxation on rat aorta and mesenteric artery without vascular endothelium effect | Methanolic extract | Flower | α-glucosidase inhibitor | [67,68] |
| Eruca sativa Mill. | Crude extract, fractions | - | Endothelium-dependent vasorelaxation on aortic rings of normotensive rats and endothelium-independent vasorelaxation on aortic rings of hypertensive rats | Hexane fraction and its fatty acid-rich fraction | Leaf | Anti-diabetic | [69,70] |
| Echinodorus grandiflorus | Ethanolic extract and its butanol fraction | Leaf | Vasorelaxation on resistance vessels by releasing PGI2 and NO through B2-bradykininergic and endothelial M3- muscarinic receptors and then activating K+ channels in vascular smooth muscle | Ethanolic extract | Leaf | Antiglycation | [52,71] |
| Gynura procumbens | Aqueous extract, methanolic extract | Leaf | Vasorelaxation by activating muscarinic M3 receptors in the existence of endothelium and vasorelaxation on rat thoracic aorta through cholinergic pathway | Leaf extract | Leaf | Anti-diabetic | [52,72] |
| Garcinia cowa | Leaf extract | Leaf | Vasorelaxation by activating KATP and generating prostanoids and NO | Compounds 4 and 8 | Leaf | α-glucosidase inhibitor | [73,74] |
| Bauhinia forficata Link | Ethyl-acetate plus butanol fraction, kaempferitrin, kaempferol | Leaf | Vasorelaxation on the thoracic aorta of hypertensive and normotensive rats | Methanolic extract | Leaf, stem | Hypoglycemic | [39,75] |
| Nelumbo nucifera | Extracts of spornioderm | Spornioderm | Endothelium-dependent vasorelaxation by activating PI3K-eNOS-sGC pathway | Seed extract | Seed | Hypoglycemic | [76,77] |
| Cimicifuga racemosa | Black cohosh extract | Vasorelaxation by way of endothelium-dependent and -independent mechanisms on pre-contracted rat thoracic aortic rings by NE | Extract Ze 450 | Decreasing plasma glucose in ob/ob mice with diabetes | [78,79] | ||
| Crocus sativus L. | Crocetin | Endothelium-dependent vasorelaxation through endothelial NO | Crocins | Stigma | Decreasing levels of glucose and increasing expression of insulin in zebrafish embryo | [80,81] | |
| Morus alba | Root bark extract | Root bark | Endothelium-dependent vasorelaxation partially via NO-cGMP pathway containing TEA sensitive K+ channels activation | Kuwanon H, morin, morusin, oxyresveratrol, kuwanon G | Root bark | α-glucosidase inhibitor | [46,82] |
| Erigeron breviscapus Hand Mazz. | Scutellarin | Endothelium-independent vasorelaxation on thoracic artery rings by blocking the influx of extracellular Ca2+ as independent from VDCCs | Scutellarin | Induces autophagy signal pathway by upregulating autophagy-related factors and blocks apoptotic signal pathway by downregulating apoptosis-related factors, and consequently relief of type 2 DC | [83,84] | ||
| Vernonia amygdalina | Ethanolic extract | Leaf | Vasorelaxation by upregulating NO/cGMP and PGI2 signalization pathways and modulating muscarinic and β2-adrenergic receptor levels, and Ca2+/K+ channels | Leaf extracts | Leaf | α-amylase inhibitor | [54,85] |
| Glycyrrhiza uralensis | 50% ethanolic extract | Vasorelaxation in endothelium-intact aortic rings pre-contracted with PE and KCl | Glycyrrhiza flavonoids | Root | α-glucosidase inhibitor | [86,87] | |
| Salvia miltiorrhiza | S. miltiorrhiza extract | Vasorelaxation of renal, mesenteric, and femoral arteries at low extract concentration and vasorelaxation of coronary arteries at all extract concentrations tested | S. miltiorrhiza extract | Root | Hypoglycemic | [88,89] | |
| Sophora alopecuroides | Oxysophoridine | Vasorelaxation on thoracic aorta rings by being related to KATP and KV channels | Aloperine | Aerial parts | Hypoglycemic | [90,91] | |
| Coriandrum sativum | Coriander crude extract | Vasorelaxation on contracted rabbit aorta with PE and K+ (80 mM) | Aqueous extract | Leaf, stem | α-glucosidase inhibitor | [53,92] | |
| Ligusticum chuanxiong Hort. | Ethanolic extract | Rhizome | Induction of eNOS-derived NO production | Ethanolic extract | Rhizome | Amelioration of diabetic nephropathy | [58,93] |
| Sorbus commixta Hedl. | Methanolic extract | Cortex | Vasorelaxation on vascular smooth muscle through NO-cGMP pathway | Lupenone, lupeol | Stem bark | PTP1B inhibitor | [94,95] |
| Aronia melanocarpa | Conjugated cyanidins, chlorogenic acids | Juice | Inducing endothelial NO production in a coronary artery by getting eNOS phosphorylation due to redox-sensitive activation of the Src/PI3-kinase/Akt pathway | Juice | Hypoglycemic | [96,97] | |
| Annona squamosa | Esquamosan | Leaf | Endothelium-independent vasorelaxation on isolated rat aorta via prevention of intracellular Ca2+ increasing by blocking VDCCs and intracellular storage channels in VSMCs | Hexane extract | Hypoglycemic | [98,99] | |
| Artemisia herba alba | Aqueous extract | Vasorelaxation through endothelial NO production | Aqueous extract | Leaf or bark | Lowering blood glucose levels | [100,101] | |
| Ajuga iva (L.) Schreber (Labiatae) | Aqueous extract | In vitro, NO-mediated and NO-independent vasorelaxation; ex vivo, endothelium-independent vasorelaxation | Lyophilized aqueous extract | Whole plant | Hypoglycemic | [102,103] | |
| Mansoa hirsuta D.C. | Ethanolic extract | Leaf | Endothelium-dependent vasorelaxation | Fraction | α-amylase inhibitor | [104,105] | |
| Mentha longifolia | N-butanol fraction | Aerial parts | Endothelium-independent relaxation owing to increase of cAMP and cGMP levels by blocking diverse PDEs | Anti-diabetic | [40,106] | ||
| Euphorbia humifusa Willd. | Total flavonoids of E. humifusa | Vasorelaxation on rat thoracic aorta with endothelium-dependent NO-cGMP signaling by inducing PI3K/Akt-and Ca2+-eNOS-NO signaling pathway; relaxation of VSMCs by stimulating NO-sGC-cGMP-protein kinase G signaling via L-type Ca2+ channel activity inhibition | Vitexin and astragalin | Whole plant | Anti-diabetic | [42,107] | |
| Sophora flavescens | Ethanolic extract | Root | Relaxation of vascular smooth muscle via the endothelium-dependent NO-sGC-cGMP signaling pathway | Four minor flavonoids (1-4) | Root | α-glucosidase inhibitor | [108,109] |
| Kaempferia parviflora | Ethanolic extract | Rhizome | Vasorelaxation in a dose-dependent manner on aortic rings pre-contracted with PE | Anti-diabetic | [19,110] | ||
| Angelica decursiva | 70% ethanolic extract | Root | Endothelium-independent vasorelaxation via KATP channels as well as blocking of Ca2+ influx through VDCCs and ROCCs | Coumarins 1-6 | α-glucosidase inhibitor, PTP1B inhibitor | [111,112] | |
| Hintonia latiflora | H. latiflora extract, neoflavonoid coutareagenin | Bark | Vasorelaxation on aortic rings pre-contracted with NE | H. latiflora extract, neoflavonoid coutareagenin | Bark | Diminishing blood glucose | [113,114] |
| Kaempferia galanga L. | Ethyl-p-methoxycinnamate | Rhizome | Endothelium-independent but K+ channel-dependent vasorelaxation | Novel K. galanga rhizome essential oil rich in ethyl p-methoxy cinnamate | Rhizome | Anti-diabetic | [115,116] |
| Prunus mume Sieb. et Zucc. | 70% ethanolic extract | Bark | Endothelium-dependent vasorelaxation on isolated rat aortic rings through NO/sGC/cGMP and PGI2 pathway; vasorelaxation partially via KCa, KATP, KV, and Kir channels | 70% ethanolic extract | Leaf | Anti-diabetic | [116,117] |
| Bacopa monnieri | Saponins (bacoside A and bacopaside I), flavonoids (luteolin and apigenin) | Endothelium-intact vasorelaxation and endothelium-denuded vasorelaxation | Bacosine | Antihyperglycemic | [118,119] | ||
| Haloxylon scoparium | Aqueous extract | Vasorelaxation via Ca2+ channels blockade | Decoctate, methanolic extract, macerated methanol, ethyl; acetate extract | Aerial part | A-glucosidase inhibitor, a-amylase inhibitor, ß-asides inhibitor | [56,120] | |
| Swietenia macrophylla King | 50% ethanolic extract | Seed | Inhibiting IP3R, blocking VOCC and activating K+ channels; vasorelaxation via β2-adrenergic pathway and NO/sGC/cGMP signaling pathways | Limonoids | Fruit | Anti-diabetic | [48,121] |
| Eucalyptus globulus | Aqueous extract | Leaf | Dose-dependent vasorelaxation on aortic rings by inducing NO production | Amelioration of hyperglycemia | [122,123] | ||
| Plumeria rubra | Aqueous-methanolic extract | Leaf | Concentration-dependent vasorelaxation on PE-induced spastic contractions and K+ (80 mM)-induced spastic contractions | Compounds 1-4, 7, 8, and 16 | Flower | α-glucosidase inhibitor, PTP1B inhibitor | [41,124] |
| Prunus persica | P. persica extract | Branch | Endothelium-dependent vasorelaxation via NO-sGC-cGMP, vascular PGI2, and muscarinic receptor transduction pathways; vasorelaxation partially through KATP, BKCa, and KV channels | Anti-diabetic | [19,125] | ||
| Prunus yedoensis Matsum. | Methanolic extract | Bark | Vasorelaxation due to activation of NO production through L-Arg and NO-cGMP pathways; vasorelaxation through blockade of extracellular Ca2+ channels | P. yedoensis extract | Leaf | Antihyperglycemic | [126,127] |
| Xanthoceras sorbifolia Bunge | Ethanolic extract | Leaf | Vasorelaxation on vascular smooth muscle through Akt- and SOCE-eNOS-sGC pathways | Wood | α-glucosidase inhibitor | [128,129] | |
| Passiflora edulis | Hydroethanolic extract | Fruit peel | Vasorelaxation on mesenteric artery rings via activation of K+ channels | Aqueous extract | Fruit peel | Anti-diabetic | [129,130] |
| Apium graveolens L. | Seed extract | Seed | Vasorelaxation through inhibition of ROCCs and VDCCs, the release of EDHF, and activation of Kv channels | Leaf extract | Leaf | Reducing pre-prandial blood glucose levels and post-prandial blood glucose levels in pre-diabetic elderly patients | [60,131] |
| Phyllanthus niruri L. | Methyl brevifolincarboxylate | Leaf | Inhibition of NE-induced vasoconstriction via ROCCs partially mediated by (Ca2+)i decrease | Aqueous extract, ethanolic extract | Aerial part | α-glucosidase inhibitor | [132,133] |
| Marrubium vulgare | Crude extracts | Aerial part | Inhibiting KCl-induced contraction on the rat aorta | Aqueous extract | Anti-diabetic | [134,135] | |
| Psoralea corylifolia L. | P. corylifolia extract, bakuchiol, isobavachalcone, isopsoralen, psoralen | Seed | Endothelium-dependent vasorelaxation through NO-cGMP pathway; attenuating PE-induced vasoconstriction by inhibiting TRPC3 channels in a dose-dependent manner | Compounds 1, 2, 3, 6, 8 | Seed | DGAT1 inhibitor, α-glucosidase inhibitor | [57,136] |
| Ginkgo biloba | Terpenoids (bilobalide, ginkgolides A, B, and C) and flavonoids (quercetin and rutin) | Concentration-dependent vasorelaxation | G. biloba extract | Antihyperglycemic | [137,138] | ||
| Rubus chingii | Ethanolic extract | Dried fruit | Vasorelaxation via Ca2+-eNOS-NO signaling in endothelial cells and later NO-sGC-cGMP-KV channel signaling in VSMCs | Ursane-type triterpenes | Fruit | PTP1B inhibitor | [55,139] |
| Bidens pilosa | Neutral extract | Leaf | Vasorelaxation and behaving as a Ca2+ antagonist | B. pilosa formulation | Anti-diabetic | [140,141] | |
| Allium sativum | L-arginine in aged garlic extract | Endothelium-dependent vasorelaxation on the aorta by inducing NO formation | Silver nanoparticles | Bulb | Anti-diabetic | [142,143] | |
| Petroselinum crispum | Aqueous extract | Aerial part | Vasorelaxation via VOCCs and ROCCs | P. crispum extract | Leaf | Decreasing blood glucose | [144,145] |
| Curcuma longa | Curcubisabolanin A | Rhizome | Partially endothelium-dependent vasorelaxation by regulating NO production in vascular endothelial cells via the PI3K/Akt/eNOS signaling pathway | Enhancing postprandial serum insulin levels with ingestion of 6 g of C. longa | [146,147] | ||
| Allium cepa | A. cepa peel hydroalcoholic extract | Peel | Decreasing aortic contractions probably through depression of Ca2+ influx from extracellular to intracellular, without including endothelium, NO, cGMP, and PGs | Diminishing blood glucose | [148,149] | ||
| Alpinia zerumbet | Essential oil | Leaf | Vasorelaxation by inhibiting both Ca2+ influx and Ca2+ release from intracellular storage; vasorelaxant effect via NOS/sGC pathway | Labdadiene | Rhizome | Antiglycation | [43,150] |
| Paeonia suffruticosa Andr. | 1,2,3,4,6-penta-O-galloyl-beta-d-glucose | Root cortex | Concentration-dependent vasorelaxation on rat aorta pre-contracted with PE | Extract of moutan cortex | Root | Improving inflammation in AGEs-induced mesangial cell dysfunction and high-glucose-fat diet and STZ-induced DN rats | [151,152] |
| Nigella sativa | Seed extract | Seed | Endothelium-independent vasorelaxation on contraction stimulated by PE and KCl via inhibition of extracellular Ca2+ influx, KATP channels, and IP3-mediated receptors | Crude aqueous extract | Seed | In vitro, suppressing electrogenic intestinal absorption of glucose directly; in vivo, ameliorating both body weight and glucose tolerance after chronic oral administration in rats | [153,154] |
| Myrciaria cauliflora Berg | Hydroalcoholic extract | Fruit peel | Endothelium-dependent vasorelaxation via NO/sGC/cGMP pathway | M. cauliflora extract | Lyophilized fruit | Hypoglycemic | [155,156] |
| Morus bombycis Koidzumi | 100% ethanolic extract | Root bark | Vasorelaxation on isolated rat aortic preparations | 2,5-dihydroxy-4,3-di(beta-D-glucopyranosyloxy)-trans-stilbene | Root | Hypoglycemic | [157,158] |
| Humulus lupulus L. | Aqueous hop extract | Vasorelaxation through NOS activation, COX products, and Ca2+ pathways in both male and female rats | Xanthohumol | α-glucosidase inhibitor | [159] | ||
| Sesamum indicum L. | Petroleum ether soluble fraction of root extract | Root | Endothelium-dependent vasorelaxation | Decreasing fasting blood sugar | [160,161] | ||
| Hibiscus sabdariffa | Hibiscus acid | Vasorelaxation by depression of intracellular Ca2+ influx through VDCCs | Ethyl acetate extract, ethanolic extract, aqueous extract | Flower | Anti-diabetic | [162,163] | |
| Jasminum sambac | Hydroalcoholic leaf extract | Leaf | Vasorelaxation completely on endothelium-intact rabbit aorta contracted with PE; vasorelaxation partially on endothelium-intact rabbit aorta contracted with NE | Polyphenol extract | Leaf | Preventing and having a therapeutic effect on DC | [59,164] |
| Hancornia speciosa Gomes | Ethanolic extract | Leaf | NO- and endothelium-dependent vasorelaxation on rat aortic preparations through PI3K activation | Aqueous extract | Latex | Hypoglycemic | [165,166] |
| Pseuderanthemum palatiferum | Water extract | Leaf | Vasorelaxation via partially vascular endothelium not with NO production and muscarinic receptor activation | 80% ethanolic leaf extract | Leaf | Hypoglycemic | [167,168] |
| Terminalia superba | Methylene chloride extract, methylene chloride-methanol extract | Stem bark | Vasorelaxation partially via depression of extracellular Ca2+ influx and/or suppression of intracellular Ca2+ releasing in VSMCs; vasorelaxation via endothelial NO | Methylene chloride-methanol extract | Leaf | Anti-diabetic | [49,169] |
| Guazuma ulmifolia | Procyanidin fraction | Bark | Vasorelaxation through endothelium-related factors, including NO | Aqueous extract | Anti-diabetic | [170,171] | |
| Persea americana Mill. | Aqueous leaf extract | Leaf | Vasorelaxation through endothelial NO production and releasing | Hydroalcoholic extract | Leaf | Anti-diabetic | [172,173] |
| Capparis aphylla | Crude extract | Aerial part | Endothelium-dependent vasorelaxation partially via atropine-sensitive NO pathway; endothelium-independent vasorelaxation partially via the Ca2+ channel blocking activity | Methanolic extract, active fraction | Stem | Decreasing blood glucose levels | [174,175] |
| Rheum undulatum | Piceatannol in rhizome extract | Rhizome | Vasorelaxation through endothelium-dependent NO signaling pathway | E-viniferin, piceatannol, and δ-viniferin in methanolic extract | Rhizome | PTP1B inhibitor | [176,177] |
| Globularia alypum | G. alypum extract | Vasorelaxation due to EDHF via endothelial muscarinic receptor activation | Methanolic extract, water extract | Leaf | Reducing fasting blood glucose | [178,179] | |
| Gmelina arborea | Hexane extract | Leaf | Concentration-dependent vasorelaxation on isolated rat aorta | Aqueous extract | Bark | Antihyperglycemic | [50,180] |
| Coscinium fenestratum | C. fenestratum extract | Endothelium-dependent and -independent vasorelaxation on isolated aortic rings precontracted with PE and KCl | Alcoholic stem extract | Stem | Anti-diabetic | [181,182] | |
| Myrtus communis L. | Crude methanolic extract | Aerial part | Vasorelaxation on isolated rabbit aorta preparations contracted with PE and K+ | Volatile oil | Hypoglycaemic | [183,184] | |
| Thymus linearis Benth. | N-butanolic fraction | Aerial part | Endothelium-independent vasorelaxation due to increase in cAMP and cGMP via inhibition of several PDEs | Ethyl acetate extract, combined extract | Aerial part | Α-amylase inhibitor | [185,186] |
| Vitex agnus-castus | V. agnus-castus extract | Fruit | Endothelium-dependent vasorelaxation via NO/cGMP and PGs production in the aorta | Hydroalcoholic extract | Desiccated fruit | Hypoglycemic | [51,187] |
| Anogeissus leiocarpus | Aqueous extract | Trunk bark | Endothelium-dependent NO-mediated vasorelaxation on porcine coronary arteries via redox-sensitive Src/PI3-kinase/Akt pathway-dependent activation of eNOS | Supernatant fraction, total extract | Root | Anti-diabetic | [188,189] |
| Zanthoxylum armatum DC | Tambulin in methanolic extract | Fruit | Influencing directly vascular smooth muscle through cAMP and/or cGMP-related relaxing pathways | Fruit, bark, and leaf extracts | Fruit, bark, and leaf | Anti-diabetic | [190,191] |
| Cymbopogon martinii | Crude methanolic extract | Leaf | Partial vasorelaxation on isolated rabbit aortic preparations contracted with PE and K+ | Α-glucosidase inhibitor | [192,193] | ||
| Moringa oleifera | M. oleifera leaf extract | Leaf | Endothelium-dependent vasorelaxation through EDHF-mediated hyperpolarization; endothelium-independent vasorelaxation due to inhibition of extracellular Ca2+ influx through VOCCs and ROCCs and suppression of sarcolemmal Ca2+ releasing through IP3R Ca2+ channels | Methanolic extract | Pods | Anti-diabetic | [194,195] |
| Dalbergia odorifera T. Chen | Butein | Vasorelaxation on rat aorta; the novel cAMP-specific PDE inhibitor; vasorelaxant action related intact endothelium | Compounds in ethyl acetate soluble fraction | Heartwood | α-glucosidase inhibitor | [196,197] | |
| Coptis chinensis | Berberine | Decreasing expression of miR-133a; enhancing BH4 levels and production of NO | Polysaccharide | Anti-diabetic | [38,198] | ||
| Angelica keiskei | Xanthoangelol, 4-hydroxyderricin, xanthoangelol E and F in EtOAc-soluble fraction, xanthoangelol B in EtOAc-soluble fraction | Root | Blocking PE-induced vasoconstriction through EDRF/NO synthesis and/or attenuation of PE-induced (Ca2+)i increase; blocking PE-induced vasoconstriction by reducing (Ca2+)i increase and directly inhibiting smooth muscle contraction | Flavonoid-rich ethanolic extract | Leaf | Hypoglycemia | [199,200] |
| Scutellaria baicalensis Georgi | Baicalin | Vasorelaxation on the mesenteric artery by stimulating BKCa channels and blocking VDCCs with endothelium-independent mechanisms, moreover by inducing cGMP/PKG and cAMP/PKA pathways | Root polysaccharide | Root | α-amylase inhibitor, α-glucosidase inhibitor | [201,202] | |
| Ocimum gratissimum | Essential oil | Dose-dependent vasorelaxation on resistance blood vessels of rat mesenteric vascular beds completely via NO; dose-dependent vasorelaxation on rat aorta partially mediated by NO | Chicoric acid in leaf extract | Leaf | Hypoglycemic | [203,204] | |
Several articles investigating the effects of plants on vasorelaxation are outlined below: Luna-Vázquez et al[12] identified 19 compounds isolated from 10 plants used in traditional Mexican medicine that can alter arterial smooth muscle tone. Guerrero et al[13] illustrated that different fractions obtained from two Latin American plants used in Amerindian traditional medicine possess vasorelaxation effects. Luna-Vázquez et al[14] elucidated the mechanism of action of 207 vasorelaxant metabolites. Capettini et al[15] discovered that xanthones derived from Brazilian medicinal plants exhibit vasorelaxant and antioxidant properties. Tang et al[16] highlighted traditional medicinal plants with the potential to prevent and treat hypertension, cardiovascular, and cerebrovascular diseases. Malekmohammad et al[17] reported on metabolites of medicinal plants that stimulate critical vasorelaxation mechanisms.
Additionally, numerous articles explore the effects of plants on diabetes: Kadir et al[18] documented an ethnobotanical survey on antidiabetic plants used in traditional Bangladeshi medicine. Salehi et al[19] identified numerous plants and their components effective against diabetes. Trojan-Rodrigues et al[20] identified plant species widely used in diabetes treatment in the state of Rio Grande do Sul in southern Brazil. Garima et al[21] conducted an ethnobotanical survey on anticancer and antidiabetic plants used by local tribes in Mizoram, Northeast India.
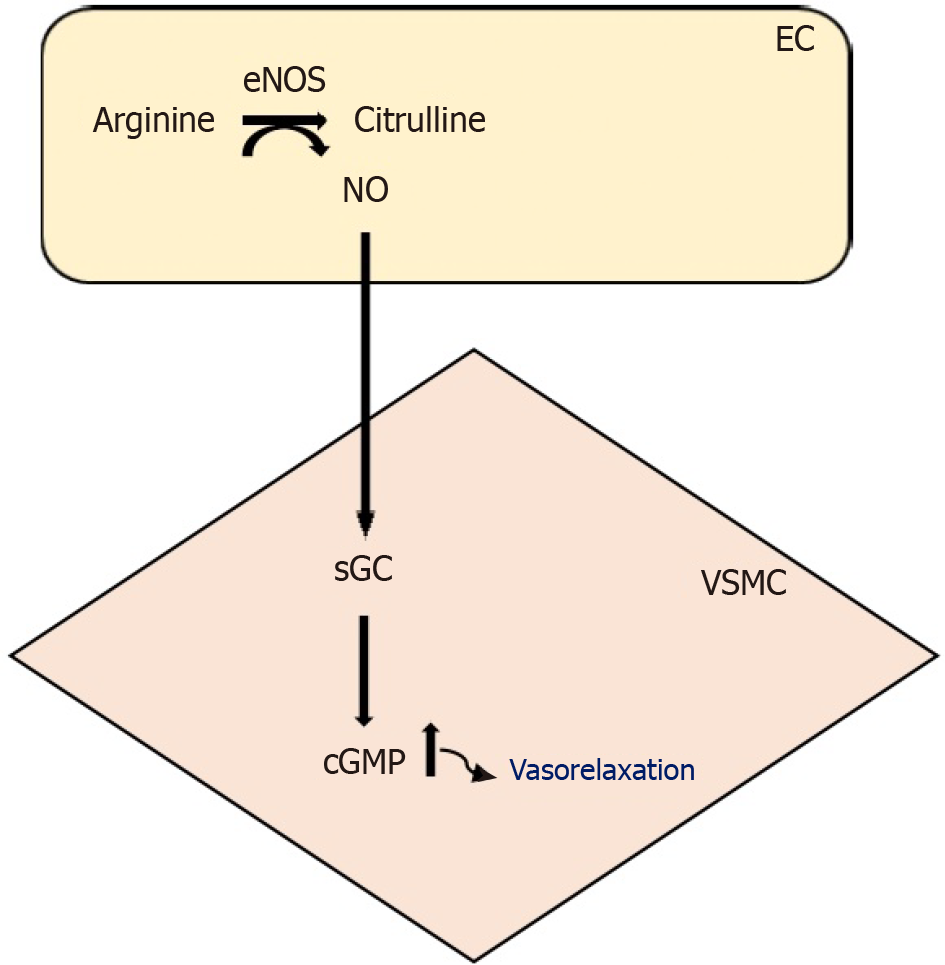
Vascular smooth muscle cell (VSMC) is stimulated by NO that is produced in a catalyzed reaction, formed citrulline amino acid from arginine amino acid, by endothelial nitric oxide synthase (eNOS)[22]. The soluble guanylate cyclase receptor found in adjacent cells is activated by NO[23]. Thus, it is occurred to rise the level of cGMP, which forms vasodilation[10] (Figure 1).
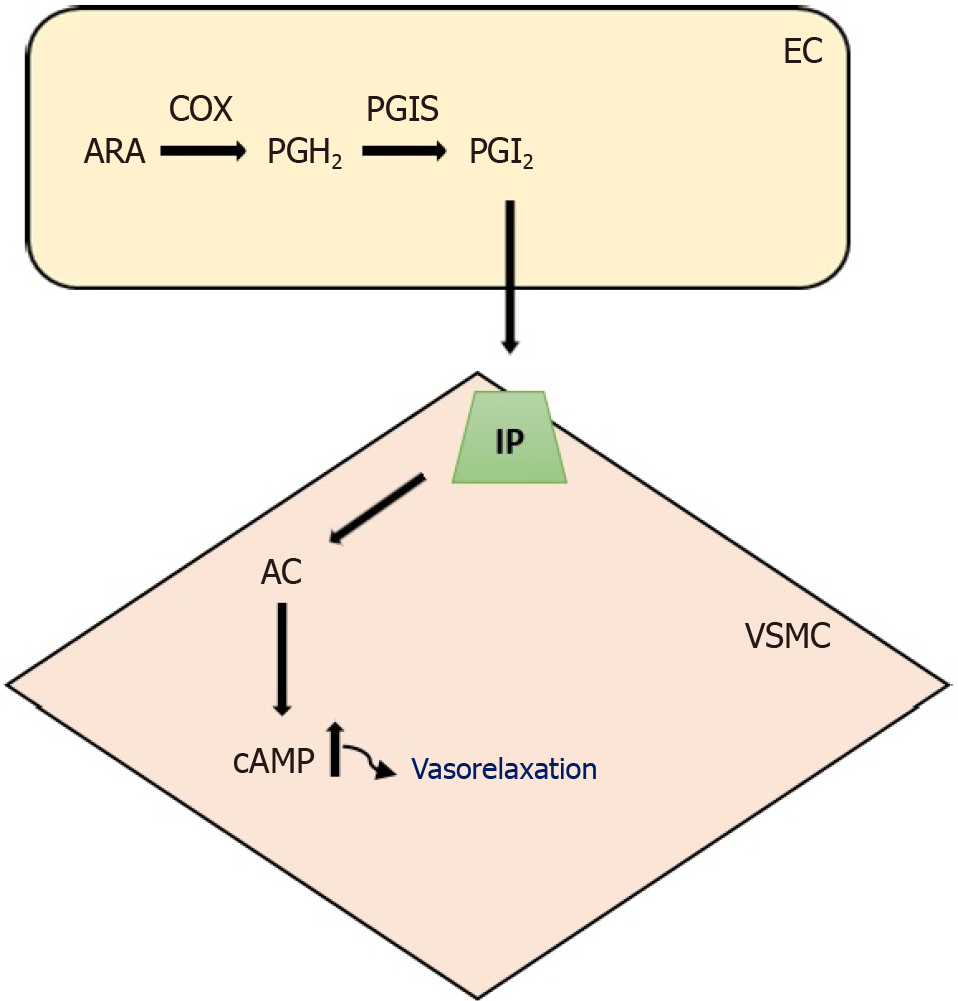
PGI2, which activates the prostacyclin receptor included in the G protein-coupled receptor (GPCR), functions as a vasorelaxant factor[24]. The enzyme cyclooxygenase catalyzes arachidonic acid as a substrate, forming prostaglandin H2, the precursor of PGI2[25]. Additionally, prostacyclin synthase generates PGI2, a lipid, when stimulated by various factors such as shear stress, cytokines, thrombin, and growth factors. The concentration of cAMP increases through the induction of adenylyl cyclase by PGI2[25]. Consequently, this leads to a vasorelaxation impact on VSMCs[26] (Figure 2).
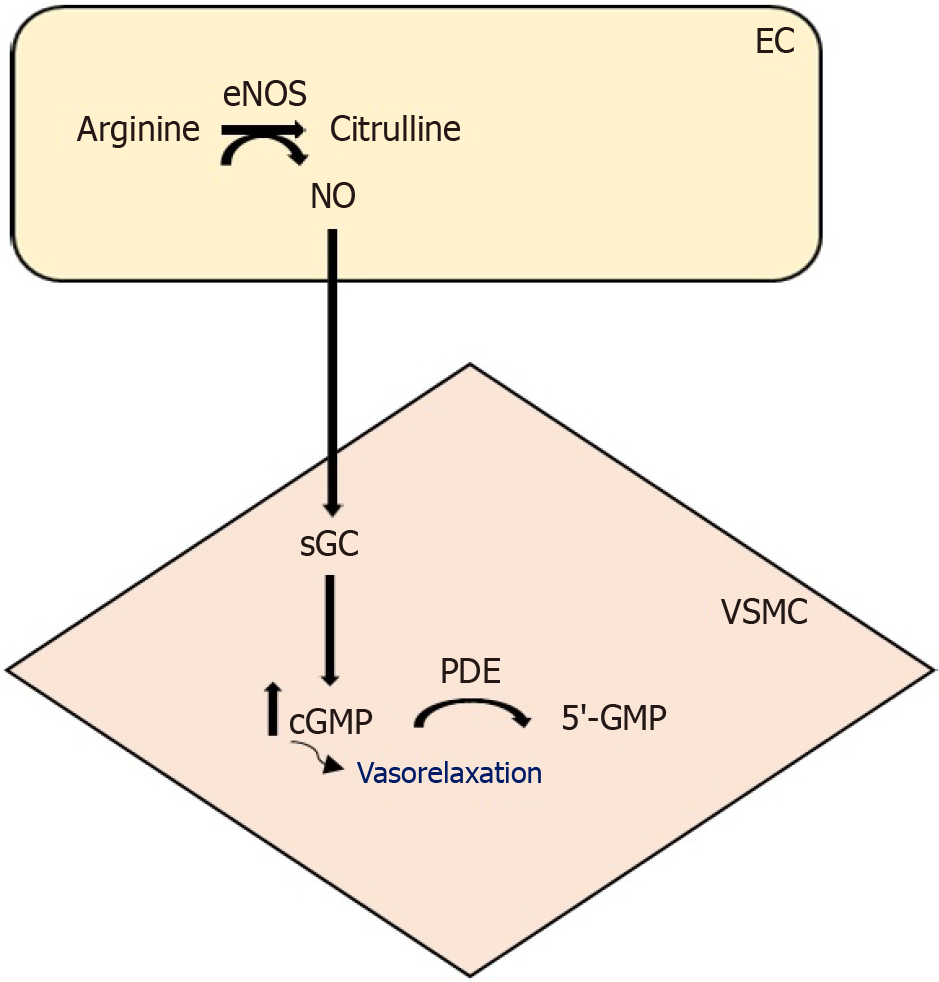
cGMP and cAMP, serving as second messengers in the cell, are hydrolyzed by cyclic nucleotide PDEs[27]. In this manner, PDE enzymes facilitate the breakdown of cAMP into 5’-AMP and cGMP into 5’-GMP. Preventing PDE activation results in heightened concentrations of cyclic nucleotides, such as cAMP and cGMP, promoting vasorelaxation[28] (Figure 3).
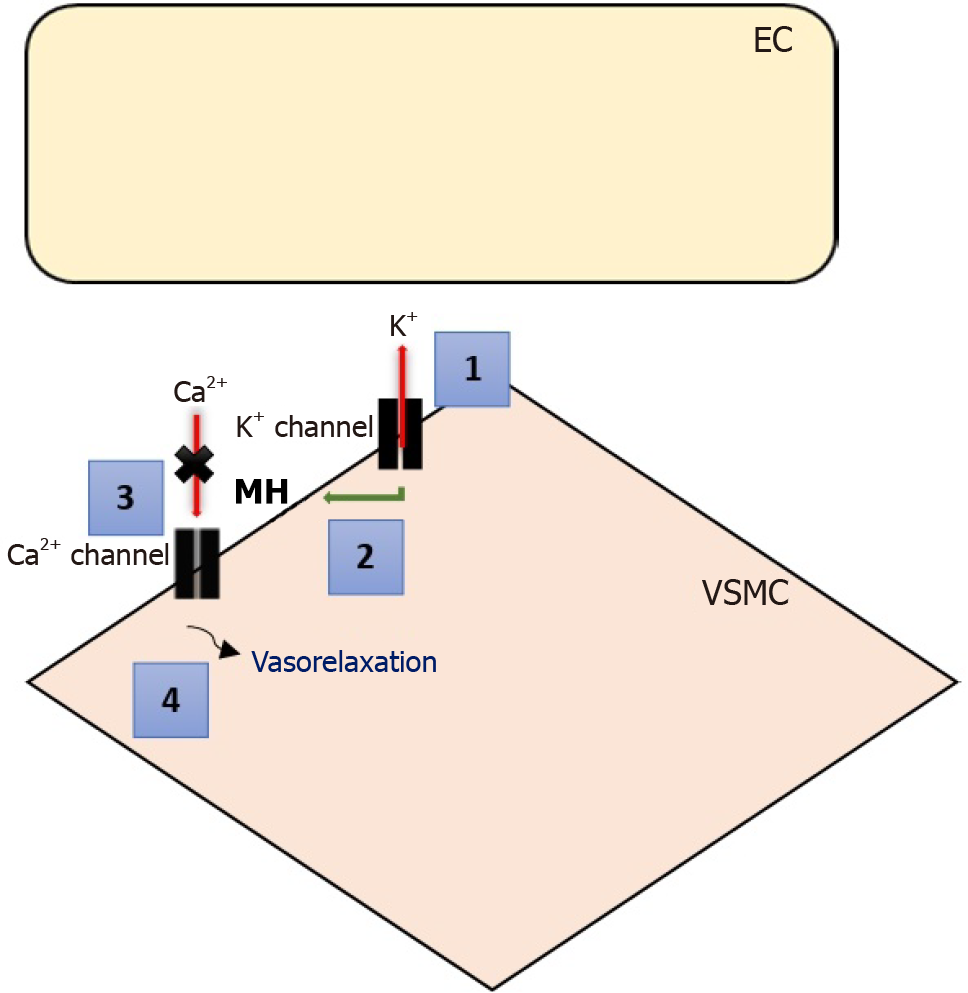
VSMCs harbor different K+ channels, including voltage-sensitive K+ (KV) channels, inward rectifier-type K+ (Kir) channels, ATP-sensitive K+ (KATP) channels, and Ca2+-activated K+ (KCa) channels[29]. Activation of K+ channels induces membrane hyperpolarization, leading to the cessation of voltage-dependent Ca2+ channels’ (VDCCs) activity, blocking the entry of Ca2+ into the cell, and ultimately resulting in vasorelaxation[30]. Additionally, the relaxation of VSMCs occurs when receptor-operated Ca2+ channels or VDCCs, responsible for intracellular calcium ion procurement, are blocked[31].
Diabetes mellitus (DM), a metabolic disease, affected 425 million patients in 2017. The World Health Organization predicts that diabetes will become the seventh leading cause of death by 2030[32]. The major cause of morbidity and mortality in people with diabetes is CVDs. Adults with diabetes face a 2-4 times higher cardiovascular risk compared to those without diabetes[33]. Type 1 DM, characterized by beta cell failure in pancreatic islets and decreased insulin release, is prevalent among teenagers and children[34]. On the other hand, type 2 DM (T2DM), defined by insulin resistance and hyperglycemia, is non-insulin dependent[35]. While T2DM is predominantly observed in adults, there is an increasing incidence among children due to the rising prevalence of obesity[36].
Throughout history, numerous drugs have been derived from the use of medicinal plants. Plants exhibiting effective pharmacological effects with minimal side reactions are preferred for various diseases due to advantages such as economic feasibility and accessibility[37]. This review article highlights medicinal plants’ effectiveness on vasorelaxation and diabetes, emphasizing their potential benefits for CVDs. Given the lack of existing literature on medicinal plants’ impact on vasorelaxation and diabetes, this review aims to address this knowledge gap[38] (Figure 4).
This section focuses on medicinal plants related to vasorelaxation and diabetes, as presented in Table 1. Each herb, identified by its binomial name, categorizes its effects concerning vasorelaxation and diabetes. Formations such as extracts, fractions, compounds, flavonoids, oils, formulations, and polysaccharides obtained from each medicinal plant are detailed in the table. Examples include the methanolic extract from Bauhinia forficata Link[39], n-butanol fraction from Mentha longifolia[40], compounds 1-4, 7, 8, and 16 from Plumeria rubra[41], total flavonoids from Euphorbia humifusa Willd[42], essential oil from Alpinia zerumbet[43], formulation from Bidens Pilosa[44], and polysaccharide from Coptis chinensis[38].
The table indicates whether vasorelaxation is linked to the endothelium or not, and pathways and channels are also highlighted, such as Gynura procumbens[45], Morus alba[46], Prunus mume Sieb. et Zucc[47], Swietenia macrophylla King[48].
Moreover, medicinal plants exhibit diverse specialties in diabetes (Table 1). Examples include anti-diabetic effects with Terminalia superba[49], anti-hyperglycemic effects with Gmelina arborea[50], hypoglycemic effects with Vitex agnus-castus[51], anti-glycation effects with Echinodorus grandifloras[52], α-glucosidase inhibitor activity with Coriandrum sativum[53], α-amylase inhibitor activity with Vernonia amygdalina[54], protein tyrosine phosphatase 1B (PTP1B) inhibition with Rubus chingii[55], ß-galactosidase inhibition with Haloxylon scoparium[56], and diacylglycerol acyltransferase-1 (DGAT1) inhibitory effects with Psoralea corylifolia L[57].
In addition, Table 1 demonstrates that medicinal herbs possess desirable efficacies on diabetic nephropathy, diabetic cardiomyopathy, and prediabetes, exemplified by Ligusticum chuanxiong Hort[58], Jasminum sambac[59], and Apium graveolens L[60], respectively (Table 1[61-204]).
This review article delves into the intersection of vasorelaxation and diabetes within the realm of medicinal plants. Each medicinal herb examined here is intricately connected with both topics, with the overarching aim of providing a promising perspective on cardiovascular disorders. The study reports on various vasorelaxant action mechanisms, encompassing endothelium-dependent and -independent vasorelaxation, observed in various experimental studies in conjunction with medicinal plants.
The review highlights that several medicinal herbs can mitigate the undesirable effects of diabetes, drawing upon extensive literature scans. These herbs exhibit a spectrum of properties, including being anti-diabetic, anti-hyperglycemic, hypoglycemic, promoting insulin expression, anti-glycation, alpha-glucosidase inhibition, α-amylase inhibition, PTP1B inhibition, ß-galactosidase inhibition, and DGAT1 inhibition. Furthermore, the study underscores the influence of medicinal plants on affirmative outcomes in diabetic nephropathy, diabetic cardiomyopathy, and pre-diabetic conditions. In studies focusing on the anti-diabetic activity of medicinal plants, an effectiveness rate of 81% is observed when plant selection is based on ethnobotanical records and traditional folk use. However, this rate decreases to 47% in the case of random plant selection[205]. Most studies investigating the efficacy of medicinal plants on diabetes reveal that total plant extract is more effective than pure secondary metabolites in the extract composition[206].
The reported effects on vasorelaxation and diabetes encompass a wide array of plant components, such as extracts, compounds, fractions, oils, formulations, flavonoids, and polysaccharides, derived from various parts of these plants. To the best of our knowledge, this study is pioneering, offering a unique perspective that addresses both vasorelaxation and diabetes concerning medicinal plants. The comprehensive collection of medicinal plant references presented in this study is anticipated to serve as a valuable resource, inspiring and guiding future investigations into CVDs and diabetes.
In this study, 85 species from 79 genera across 41 plant families were investigated. The majority of the medicinal plants examined belong to families such as Lamiaceae, Fabaceae, Rosaceae, Apiaceae, and Asteraceae, implying a potentially higher therapeutic efficacy in treating and preventing cardiovascular diseases compared to other families. Moreover, employing species from these families in cardiovascular disease studies could result in cost and time savings. The plant species and their respective families are presented in Table 2 for reference.
| Fabaceae | Lamiaceae | Rosaceae | Brassicaceae | Myrtaceae |
| Securigera securidaca L.; Parkia biglobosa; Bauhinia forficata Link; Dalbergia odorifera T. Chen; Glycyrrhiza uralensis; Sophora alopecuroides; Sophora flavescensi; Psoralea corylifolia L. | Orthosiphon stamineus; Thymus linearis Benth; Gmelina arborea; Vitex agnus-castus; Ocimum gratissimum; Marrubium vulgare; Salvia miltiorrhiza; Mentha longifolia; Scutellaria baicalensis Georgi; Ajuga iva (L.) Schreber | Rosa damascena Mill.; Sorbus commixta Hedl.; Aronia melanocarpa; P. mume Sieb. et Zucc.; Prunus persica; P. yedoensis Matsum.; Rubus chingii | Eruca sativa Mill. | Eucalyptus globulus; Myrciaria cauliflora Berg; Myrtus communis L. |
| Alismataceae | Asteraceae | Nelumbonaceae | Clusiaceae | Apocynaceae |
| Echinodorus grandiflorus | Gynura procumbens; E. breviscapus Hand Mazz.; Vernonia amygdalina; Artemisia herba alba; Bidens pilosa | Nelumbo nucifera | Garcinia cowa | Plumeria rubra; Hancornia speciosa Gomes |
| Iridaceae | Moraceae | Apiaceae | Annonaceae | Sapindaceae |
| Crocus sativus L. | Morus alba; Morus bombycis Koidzumi | Coriandrum sativum; Angelica decursiva; Apium graveolens L.; Petroselinum crispum; L. chuanxiong Hort.; Angelica keiskei | Annona squamosal | Xanthoceras sorbifolia Bunge |
| Poaceae | Bignoniaceae | Euphorbiaceae | Zingiberaceae | Passifloraceae |
| Cymbopogon martinii | Mansoa hirsuta D.C. | E. humifusa Willd. | Kaempferia parviflora; Kaempferia galanga L.; Curcuma longa; Alpinia zerumbet | Passiflora edulis |
| Rubiaceae | Plantaginaceae | Amaranthaceae | Meliaceae | Phyllanthaceae |
| Hintonia latiflora | Bacopa monnieri; Globularia alypum | Haloxylon scoparium | S. macrophylla King | Phyllanthus niruri L. |
| Moringaceae | Ginkgoaceae | Amaryllidaceae | Paeoniaceae | Ranunculaceae |
| Moringa oleifera | Ginkgo biloba | Allium sativum; Allium cepa | P. suffruticosa Andr. | Nigella sativa; Coptis chinensis; Cimicifuga racemosa |
| Cannabaceae | Pedaliaceae | Malvaceae | Oleaceae | Acanthaceae |
| Humulus lupulus L. | Sesamum indicum L. | Hibiscus sabdariffa; Guazuma ulmifolia | Jasminum sambac | P. palatiferum |
| Combretaceae | Lauraceae | Capparaceae | Polygonaceae | Menispermaceae |
| Terminalia superba; Anogeissus leiocarpus | Persea americana Mill. | Capparis aphylla | Rheum undulatum | Coscinium fenestratum |
| Rutaceae | ||||
| Z. armatum DC |
| 1. | Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021;24:213-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Gao Y. Architecture of the Blood Vessels. In: Gao Y, editor. Biology of Vascular Smooth Muscle: Vasoconstriction and Dilatation. Singapore: Springer Nature Singapore, 2022: 3-17. [DOI] [Full Text] |
| 4. | Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 811] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 5. | Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1119] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 6. | Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, Huang L. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. 2023;8:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 384] [Reference Citation Analysis (0)] |
| 7. | Peng Z, Shu B, Zhang Y, Wang M. Endothelial Response to Pathophysiological Stress. Arterioscler Thromb Vasc Biol. 2019;39:e233-e243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 9. | Gao Y. Endothelium-Derived Factors. In: Gao Y, editor. Biology of Vascular Smooth Muscle: Vasoconstriction and Dilatation. Singapore: Springer Nature Singapore, 2022: 131-152. [DOI] [Full Text] |
| 10. | Bartáková A, Nováková M. Secondary Metabolites of Plants as Modulators of Endothelium Functions. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057-1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1007] [Cited by in RCA: 1055] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 12. | Luna-Vázquez FJ, Ibarra-Alvarado C, Camacho-Corona MDR, Rojas-Molina A, Rojas-Molina JI, García A, Bah M. Vasodilator Activity of Compounds Isolated from Plants Used in Mexican Traditional Medicine. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Guerrero EI, Morán-Pinzón JA, Ortíz LG, Olmedo D, del Olmo E, López-Pérez JL, San Feliciano A, Gupta MP. Vasoactive effects of different fractions from two Panamanians plants used in Amerindian traditional medicine. J Ethnopharmacol. 2010;131:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Luna-Vázquez FJ, Ibarra-Alvarado C, Rojas-Molina A, Rojas-Molina I, Zavala-Sánchez MA. Vasodilator compounds derived from plants and their mechanisms of action. Molecules. 2013;18:5814-5857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Capettini LS, Campos LV, Dos Santos MH, Nagem TJ, Lemos VS, Cortes SF. Vasodilator and antioxidant effect of xanthones isolated from Brazilian medicinal plants. Planta Med. 2009;75:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Tang F, Yan HL, Wang LX, Xu JF, Peng C, Ao H, Tan YZ. Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front Pharmacol. 2021;12:627458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Malekmohammad K, Sewell RDE, Rafieian-Kopaei M. Mechanisms of Medicinal Plant Activity on Nitric Oxide (NO) Bioavailability as Prospective Treatments for Atherosclerosis. Curr Pharm Des. 2020;26:2591-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Kadir MF, Bin Sayeed MS, Shams T, Mia MM. Ethnobotanical survey of medicinal plants used by Bangladeshi traditional health practitioners in the management of diabetes mellitus. J Ethnopharmacol. 2012;144:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Salehi B, Ata A, V Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Tsouh Fokou PV, Kobarfard F, Amiruddin Zakaria Z, Iriti M, Taheri Y, Martorell M, Sureda A, Setzer WN, Durazzo A, Lucarini M, Santini A, Capasso R, Ostrander EA; Atta-ur-Rahman, Choudhary MI, Cho WC, Sharifi-Rad J. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 20. | Trojan-Rodrigues M, Alves TL, Soares GL, Ritter MR. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J Ethnopharmacol. 2012;139:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Garima S, Ajit Kumar P, Marcy DM, Sakthivel R, Bhim Pratap S, Nachimuthu Senthil K. Ethnobotanical survey of medicinal plants used in the management of cancer and diabetes. J Tradit Chin Med. 2020;40:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci. 2015;129:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 23. | Montfort WR, Wales JA, Weichsel A. Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor. Antioxid Redox Signal. 2017;26:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Zeng C, Liu J, Zheng X, Hu X, He Y. Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension. Respir Res. 2023;24:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 25. | Korbecki J, Rębacz-Maron E, Kupnicka P, Chlubek D, Baranowska-Bosiacka I. Synthesis and Significance of Arachidonic Acid, a Substrate for Cyclooxygenases, Lipoxygenases, and Cytochrome P450 Pathways in the Tumorigenesis of Glioblastoma Multiforme, Including a Pan-Cancer Comparative Analysis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 26. | Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114:529-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 499] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 27. | Muthal AP, Kulkarni R, Dileep K, Mukherjee-Kandhare CB, Kandhare AD, Ambavade SD, Wagh V, Bodhankar SL. Cyclic adenosine monophosphate: Recent and future perspectives on various diseases. JAPS. 2022;12:1-15. [DOI] [Full Text] |
| 28. | Calamera G, Moltzau LR, Levy FO, Andressen KW. Phosphodiesterases and Compartmentation of cAMP and cGMP Signaling in Regulation of Cardiac Contractility in Normal and Failing Hearts. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Checchetto V, Leanza L, De Stefani D, Rizzuto R, Gulbins E, Szabo I. Mitochondrial K(+) channels and their implications for disease mechanisms. Pharmacol Ther. 2021;227:107874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Gao Y. Intracellular Ca2+ Regulation. In: Gao Y, editor. Biology of Vascular Smooth Muscle: Vasoconstriction and Dilatation. Singapore: Springer Nature Singapore, 2022: 191-211. [DOI] [Full Text] |
| 31. | Dhoble S, Patravale V, Weaver E, Lamprou DA, Patravale T. Comprehensive review on novel targets and emerging therapeutic modalities for pulmonary arterial Hypertension. Int J Pharm. 2022;621:121792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 32. | Fan W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc Endocrinol. 2017;6:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 33. | Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JW. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 607] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 34. | Chetan MR, Thrower SL, Narendran P. What is type 1 diabetes? Medicine. 2019;47:5-9. [DOI] [Full Text] |
| 35. | Rachdaoui N. Insulin: The Friend and the Foe in the Development of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 36. | Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12:344-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (12)] |
| 37. | Aware CB, Patil DN, Suryawanshi SS, Mali PR, Rane MR, Gurav RG, Jadhav JP. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S Afr J Bot. 2022;151:512-28. [DOI] [Full Text] |
| 38. | Cui L, Liu M, Chang X, Sun K. The inhibiting effect of the Coptis chinensis polysaccharide on the type II diabetic mice. Biomed Pharmacother. 2016;81:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Chávez-Bustos EA, Morales-González A, Anguiano-Robledo L, Madrigal-Santillán EO, Valadez-Vega C, Lugo-Magaña O, Mendoza-Pérez JA, Fregoso-Aguilar TA. Bauhinia forficata Link, Antioxidant, Genoprotective, and Hypoglycemic Activity in a Murine Model. Plants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Alamgeer, Asif H, Chohan TA, Irfan HM, Asim MH, Bukhari SNA, Younis W, Althobaiti YS, Ullah A, Khan AQ, Hakami AY. Ex vivo, in vitro, and in silico approaches to unveil the mechanisms underlying vasorelaxation effect of Mentha Longifolia (L.) in porcine coronary artery. Biomed Pharmacother. 2022;153:113298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Zhang SN, Song HZ, Ma RJ, Liang CQ, Wang HS, Tan QG. Potential anti-diabetic isoprenoids and a long-chain δ-lactone from frangipani (Plumeria rubra). Fitoterapia. 2020;146:104684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Wang TT, Zhou ZQ, Wang S, Ji XW, Wu B, Sun LY, Wen JF, Kang DG, Lee HS, Cho KW, Jin SN. Mechanisms of vasorelaxation induced by total flavonoids of Euphorbia humifusa in rat aorta. J Physiol Pharmacol. 2017;68:619-628. [PubMed] |
| 43. | Rocha DG, Holanda TM, Braz HLB, de Moraes JAS, Marinho AD, Maia PHF, de Moraes MEA, Fechine-Jamacaru FV, de Moraes Filho MO. Vasorelaxant effect of Alpinia zerumbet's essential oil on rat resistance artery involves blocking of calcium mobilization. Fitoterapia. 2023;169:105623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 44. | Lai BY, Chen TY, Huang SH, Kuo TF, Chang TH, Chiang CK, Yang MT, Chang CL. Bidens pilosa Formulation Improves Blood Homeostasis and β -Cell Function in Men: A Pilot Study. Evid Based Complement Alternat Med. 2015;2015:832314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Shahlehi S, Petalcorin MIR. Activation of cholinergic pathway induced vasodilation in rat aorta using aqueous and methanolic leaf extracts of Gynura procumbens. Biomed Pharmacother. 2021;143:112066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Panth N, Paudel KR, Gong DS, Oak MH. Vascular Protection by Ethanol Extract of Morus alba Root Bark: Endothelium-Dependent Relaxation of Rat Aorta and Decrease of Smooth Muscle Cell Migration and Proliferation. Evid Based Complement Alternat Med. 2018;2018:7905763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Jo C, Kim B, Lee K, Choi HY. Vascular Relaxation and Blood Pressure Lowering Effects of Prunus mume in Rats. Bioengineering (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 48. | Ch'ng YS, Loh YC, Tan CS, Ahmad M, Asmawi MZ, Wan Omar WM, Yam MF. Vasodilation and Antihypertensive Activities of Swietenia macrophylla (Mahogany) Seed Extract. J Med Food. 2018;21:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Kamtchouing P, Kahpui SM, Dzeufiet PD, Tédong L, Asongalem EA, Dimo T. Anti-diabetic activity of methanol/methylene chloride stem bark extracts of Terminalia superba and Canarium schweinfurthii on streptozotocin-induced diabetic rats. J Ethnopharmacol. 2006;104:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Kulkarni YA, Veeranjaneyulu A. Effects of Gmelina arborea extract on experimentally induced diabetes. Asian Pac J Trop Med. 2013;6:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Ahangarpour A, Oroojan AA, Khorsandi L, Najimi SA. Pancreatic protective and hypoglycemic effects of Vitex agnus-castus L. fruit hydroalcoholic extract in D-galactose-induced aging mouse model. Res Pharm Sci. 2017;12:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Franco RR, da Silva Carvalho D, de Moura FBR, Justino AB, Silva HCG, Peixoto LG, Espindola FS. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J Ethnopharmacol. 2018;215:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Brindis F, González-Andrade M, González-Trujano ME, Estrada-Soto S, Villalobos-Molina R. Postprandial glycaemia and inhibition of α-glucosidase activity by aqueous extract from Coriandrum sativum. Nat Prod Res. 2014;28:2021-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Patathananone S, Pothiwan M, Uapipatanakul B, Kunu W. Inhibitory Effects of Vernonia amygdalina Leaf Extracts on Free Radical Scavenging, Tyrosinase, and Amylase Activities. Prev Nutr Food Sci. 2023;28:302-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Zhang XY, Li W, Wang J, Li N, Cheng MS, Koike K. Protein tyrosine phosphatase 1B inhibitory activities of ursane-type triterpenes from Chinese raspberry, fruits of Rubus chingii. Chin J Nat Med. 2019;17:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Lachkar N, Lamchouri F, Bouabid K, Boulfia M, Senhaji S, Stitou M, Toufik H. Mineral Composition, Phenolic Content, and In Vitro Antidiabetic and Antioxidant Properties of Aqueous and Organic Extracts of Haloxylon scoparium Aerial Parts. Evid Based Complement Alternat Med. 2021;2021:9011168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Zhu G, Luo Y, Xu X, Zhang H, Zhu M. Anti-diabetic compounds from the seeds of Psoralea corylifolia. Fitoterapia. 2019;139:104373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Yang WJ, Li YR, Gao H, Wu XY, Wang XL, Wang XN, Xiang L, Ren DM, Lou HX, Shen T. Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin-induced diabetic nephropathy in mice. J Ethnopharmacol. 2018;227:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Umar U, Ahmed S, Iftikhar A, Iftikhar M, Majeed W, Liaqat A, Shahzad S, Abbas M, Mehmood T, Anwar F. Phenolics Extracted from Jasminum sambac Mitigates Diabetic Cardiomyopathy by Modulating Oxidative Stress, Apoptotic Mediators and the Nfr-2/HO-1 Pathway in Alloxan-Induced Diabetic Rats. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 60. | Yusni Y, Zufry H, Meutia F, Sucipto KW. The effects of celery leaf (apium graveolens L.) treatment on blood glucose and insulin levels in elderly pre-diabetics. Saudi Med J. 2018;39:154-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Garjani A, Fathiazad F, Zakheri A, Akbari NA, Azarmie Y, Fakhrjoo A, Andalib S, Maleki-Dizaji N. The effect of total extract of Securigera securidaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol. 2009;126:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Alizadeh-Fanalou S, Babaei M, Hosseini A, Azadi N, Nazarizadeh A, Shojaii A, Borji M, Malekinejad H, Bahreini E. Effects of Securigera Securidaca seed extract in combination with glibenclamide on antioxidant capacity, fibroblast growth factor 21 and insulin resistance in hyperglycemic rats. J Ethnopharmacol. 2020;248:112331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Ouédraogo S, Somé N, Ouattara S, Kini FB, Traore A, Bucher B, Guissou IP. Acute toxicity and vascular properties of seed of Parkia biglobosa (JACQ) R. Br Gift (Mimosaceae) on rat aorta. Afr J Tradit Complement Altern Med. 2012;9:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Oyedemi SO, Eze K, Aiyegoro OA, Ibeh RC, Ikechukwu GC, Swain SS, Ejiofor E, Oyedemi BO. Computational, chemical profiling and biochemical evaluation of antidiabetic potential of Parkia biglobosa stem bark extract in type 2 model of rats. J Biomol Struct Dyn. 2022;40:9948-9961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Yam MF, Tan CS, Ahmad M, Shibao R. Mechanism of vasorelaxation induced by eupatorin in the rats aortic ring. Eur J Pharmacol. 2016;789:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Bassalat N, Kadan S, Melamed S, Yaron T, Tietel Z, Karam D, Kmail A, Masalha M, Zaid H. In Vivo and In Vitro Antidiabetic Efficacy of Aqueous and Methanolic Extracts of Orthosiphon Stamineus Benth. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 67. | Singh MK, Savita K, Singh S, Mishra D, Rani P, Chanda D, Verma RS. Vasorelaxant property of 2-phenyl ethyl alcohol isolated from the spent floral distillate of damask rose (Rosa damascena Mill.) and its possible mechanism. J Ethnopharmacol. 2023;313:116603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 68. | Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on alpha-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Salma U, Khan T, Shah AJ. Antihypertensive effect of the methanolic extract from Eruca sativa Mill., (Brassicaceae) in rats: Muscarinic receptor-linked vasorelaxant and cardiotonic effects. J Ethnopharmacol. 2018;224:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Hetta MH, Owis AI, Haddad PS, Eid HM. The fatty acid-rich fraction of Eruca sativa (rocket salad) leaf extract exerts antidiabetic effects in cultured skeletal muscle, adipocytes and liver cells. Pharm Biol. 2017;55:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | de Carvalho ES, Tirloni CAS, Palozi RAC, Schaedler MI, Guarnier LP, Silva AO, Mota JDS, Cardoso CAL, de Barros ME, Gasparotto Junior A. Endothelium-Dependent Effects of Echinodorus grandiflorus (Cham. & Schltdl.) Micheli Mediated by M3-Muscarinic and B2-Bradykininergic Receptors on Peripheral Vascular Resistance and Its Modulatory Effects on K+ Channels in Mesenteric Vascular Beds. Evid Based Complement Alternat Med. 2019;2019:4109810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 72. | Tahsin MR, Tithi TI, Mim SR, Haque E, Sultana A, Bahar NB, Ahmed R, Chowdhury JA, Chowdhury AA, Kabir S, Aktar F, Uddin MS, Amran MS. In Vivo and In Silico Assessment of Diabetes Ameliorating Potentiality and Safety Profile of Gynura procumbens Leaves. Evid Based Complement Alternat Med. 2022;2022:9095504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Yorsin S, Sriwiriyajan S, Chongsa W. Vasorelaxing effect of Garcinia cowa leaf extract in rat thoracic aorta and its underlying mechanisms. J Tradit Complement Med. 2023;13:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 74. | Raksat A, Phukhatmuen P, Yang J, Maneerat W, Charoensup R, Andersen RJ, Wang YA, Pyne SG, Laphookhieo S. Phloroglucinol Benzophenones and Xanthones from the Leaves of Garcinia cowa and Their Nitric Oxide Production and α-Glucosidase Inhibitory Activities. J Nat Prod. 2020;83:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Cechinel-Zanchett CC, da Silva RCMVAF, Tenfen A, Siebert DA, Micke G, Vitali L, Cechinel-Filho V, Faloni de Andrade S, de Souza P. Bauhinia forficata link, a Brazilian medicinal plant traditionally used to treat cardiovascular disorders, exerts endothelium-dependent and independent vasorelaxation in thoracic aorta of normotensive and hypertensive rats. J Ethnopharmacol. 2019;243:112118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Gong DS, Kang SW, Sharma K, Kim DW, Oak MH. The Vasorelaxatory Effect of Nelumbo nucifera Spornioderm on Porcine Coronary Artery. J Nanosci Nanotechnol. 2019;19:1176-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 77. | Pipil N, Gupta PP, Soni S, Chopra D, Lhamo Y, Singh N, Shree B. Hypoglycemic Effect of Nelumbo Nucifera Seed Extract on GLUT-4 mRNA and GLUT-4 Protein in Streptozotocin-Induced Diabetic Rats. J Pharm Bioallied Sci. 2023;15:S1059-S1061. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | Kim EY, Lee YJ, Rhyu MR. Black cohosh (Cimicifuga racemosa) relaxes the isolated rat thoracic aorta through endothelium-dependent and -independent mechanisms. J Ethnopharmacol. 2011;138:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Moser C, Vickers SP, Brammer R, Cheetham SC, Drewe J. Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine. 2014;21:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Mancini A, Serrano-Díaz J, Nava E, D'Alessandro AM, Alonso GL, Carmona M, Llorens S. Crocetin, a carotenoid derived from saffron (Crocus sativus L.), improves acetylcholine-induced vascular relaxation in hypertension. J Vasc Res. 2014;51:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Kakouri E, Agalou A, Kanakis C, Beis D, Tarantilis PA. Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Hsu JH, Yang CS, Chen JJ. Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Pan Z, Feng T, Shan L, Cai B, Chu W, Niu H, Lu Y, Yang B. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res. 2008;22:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Su Y, Fan X, Li S, Li Z, Tian M. Scutellarin Improves Type 2 Diabetic Cardiomyopathy by Regulating Cardiomyocyte Autophagy and Apoptosis. Dis Markers. 2022;2022:3058354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 85. | Ch'ng YS, Loh YC, Tan CS, Ahmad M, Asmawi MZ, Wan Omar WM, Yam MF. Vasorelaxant properties of Vernonia amygdalina ethanol extract and its possible mechanism. Pharm Biol. 2017;55:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Tan CS, Ch'ng YS, Loh YC, Zaini Asmawi M, Ahmad M, Yam MF. Vasorelaxation effect of Glycyrrhizae uralensis through the endothelium-dependent Pathway. J Ethnopharmacol. 2017;199:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Gou SH, Liu J, He M, Qiang Y, Ni JM. Quantification and bio-assay of α-glucosidase inhibitors from the roots of Glycyrrhiza uralensis Fisch. Nat Prod Res. 2016;30:2130-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Lei XL, Chiou GC. Cardiovascular pharmacology of Panax notoginseng (Burk) F.H. Chen and Salvia miltiorrhiza. Am J Chin Med. 1986;14:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Carai MA, Colombo G, Loi B, Zaru A, Riva A, Cabri W, Morazzoni P. Hypoglycemic Effects of a Standardized Extract of Salvia miltiorrhiza Roots in Rats. Pharmacogn Mag. 2015;11:S545-S549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Li N, Chen Y, Pei Y, Han L, Ren J, Zhou W, Zhou R. Vasorelaxation effect of oxysophoridine on isolated thoracicc aorta rings of rats. Chin J Physiol. 2021;64:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Song G, Huang Y, Xiong M, Yang Z, Liu Q, Shen J, Zhao P, Yang X. Aloperine Relieves Type 2 Diabetes Mellitus via Enhancing GLUT4 Expression and Translocation. Front Pharmacol. 2020;11:561956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Jabeen Q, Bashir S, Lyoussi B, Gilani AH. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J Ethnopharmacol. 2009;122:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Li CM, Guo YQ, Dong XL, Li H, Wang B, Wu JH, Wong MS, Chan SW. Ethanolic extract of rhizome of Ligusticum chuanxiong Hort. (chuanxiong) enhances endothelium-dependent vascular reactivity in ovariectomized rats fed with high-fat diet. Food Funct. 2014;5:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Kang DG, Lee JK, Choi DH, Sohn EJ, Moon MK, Lee HS. Vascular relaxation by the methanol extract of Sorbus cortex via NO-cGMP pathway. Biol Pharm Bull. 2005;28:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Na M, Kim BY, Osada H, Ahn JS. Inhibition of protein tyrosine phosphatase 1B by lupeol and lupenone isolated from Sorbus commixta. J Enzyme Inhib Med Chem. 2009;24:1056-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Kim JH, Auger C, Kurita I, Anselm E, Rivoarilala LO, Lee HJ, Lee KW, Schini-Kerth VB. Aronia melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitric Oxide. 2013;35:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Simeonov SB, Botushanov NP, Karahanian EB, Pavlova MB, Husianitis HK, Troev DM. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med (Plovdiv). 2002;44:20-23. [PubMed] |
| 98. | Di Giulio C, Gonzalez Guzman JM, Dutra Gomes JV, Choi YH, Magalhães PO, Fonseca-Bazzo YM, Silveira D, Estrada O. A New Lignan from Annona squamosa L. (Annonaceae) Demonstrates Vasorelaxant Effects In Vitro. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 99. | Ranjana, Tripathi YB. Insulin secreting and alpha-glucosidase inhibitory activity of hexane extract of Annona squamosa Linn. in streptozotocin (STZ) induced diabetic rats. Indian J Exp Biol. 2014;52:623-629. [PubMed] |
| 100. | Naoufel Z, Hebi M, Ajebli M, Michel JB, Eddouks M. In vitro Vasorelaxant Effect of Artemisia herba alba Asso. in Spontaneously Hypertensive Rats. Cardiovasc Hematol Agents Med Chem. 2017;14:190-196. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 101. | al-Khazraji SM, al-Shamaony LA, Twaij HA. Hypoglycaemic effect of Artemisia herba alba. I. Effect of different parts and influence of the solvent on hypoglycaemic activity. J Ethnopharmacol. 1993;40:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | El-Hilaly J, Lyoussi B, Wibo M, Morel N. Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J Ethnopharmacol. 2004;93:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | El Hilaly J, Lyoussi B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2002;80:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Campana PR, Braga FC, Cortes SF. Endothelium-dependent vasorelaxation in rat thoracic aorta by Mansoa hirsuta D.C. Phytomedicine. 2009;16:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Pereira JR, Queiroz RF, Siqueira EA, Brasileiro-Vidal AC, Sant'ana AEG, Silva DM, Affonso PRAM. Evaluation of cytogenotoxicity, antioxidant and hypoglycemiant activities of isolate compounds from Mansoa hirsuta D.C. (Bignoniaceae). An Acad Bras Cienc. 2017;89:317-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Bai X, Aimila A, Aidarhan N, Duan X, Maiwulanjiang M. Chemical constituents and biological activities of essential oil from Mentha longifolia: effects of different extraction methods. Int J Food Prop. 2020;23:1951-1960. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | Rakotondrabe TF, Fan M, Guo M. Exploring potential antidiabetic and anti-inflammatory flavonoids from Euphorbia humifusa with an integrated strategy. Front Pharmacol. 2022;13:980945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 108. | Jin SN, Wen JF, Li X, Kang DG, Lee HS, Cho KW. The mechanism of vasorelaxation induced by ethanol extract of Sophora flavescens in rat aorta. J Ethnopharmacol. 2011;137:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Kim JH, Cho CW, Kim HY, Kim KT, Choi GS, Kim HH, Cho IS, Kwon SJ, Choi SK, Yoon JY, Yang SY, Kang JS, Kim YH. α-Glucosidase inhibition by prenylated and lavandulyl compounds from Sophora flavescens roots and in silico analysis. Int J Biol Macromol. 2017;102:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Chansuvanich N. Vasorelaxation and antispasmodic effects of Kaempferia parviflora ethanolic extract in isolated rat organ studies. Fitoterapia. 2008;79:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Kim B, Kwon Y, Lee S, Lee K, Ham I, Choi HY. Vasorelaxant effects of Angelica decursiva root on isolated rat aortic rings. BMC Complement Altern Med. 2017;17:474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Ali MY, Jannat S, Jung HA, Jeong HO, Chung HY, Choi JS. Coumarins from Angelica decursiva inhibit α-glucosidase activity and protein tyrosine phosphatase 1B. Chem Biol Interact. 2016;252:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Vierling C, Baumgartner CM, Bollerhey M, Erhardt WD, Stampfl A, Vierling W. The vasodilating effect of a Hintonia latiflora extract with antidiabetic action. Phytomedicine. 2014;21:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Korecova M, Hladikova M. Treatment of mild and moderate type-2 diabetes: open prospective trial with Hintonia latiflora extract. Eur J Med Res. 2014;19:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 115. | Srivastava N, Mishra S, Iqbal H, Chanda D, Shanker K. Standardization of Kaempferia galanga L. rhizome and vasorelaxation effect of its key metabolite ethyl p-methoxycinnamate. J Ethnopharmacol. 2021;271:113911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Begum T, Gogoi R, Sarma N, Pandey SK, Lal M. Novel ethyl p-methoxy cinnamate rich Kaempferia galanga (L.) essential oil and its pharmacological applications: special emphasis on anticholinesterase, anti-tyrosinase, α-amylase inhibitory, and genotoxic efficiencies. PeerJ. 2023;11:e14606. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 117. | Lee MW, Kwon JE, Lee YJ, Jeong YJ, Kim I, Cho YM, Kim YM, Kang SC. Prunus mume leaf extract lowers blood glucose level in diabetic mice. Pharm Biol. 2016;54:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 118. | Kamkaew N, Paracha TU, Ingkaninan K, Waranuch N, Chootip K. Vasodilatory Effects and Mechanisms of Action of Bacopa monnieri Active Compounds on Rat Mesenteric Arteries. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Ghosh T, Maity TK, Singh J. Antihyperglycemic activity of bacosine, a triterpene from Bacopa monnieri, in alloxan-induced diabetic rats. Planta Med. 2011;77:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Amtaghri S, Eddouks M. Study of the Antihypertensive and Vasorelaxant Activities of Haloxylon scoparium in Rats. Cardiovasc Hematol Agents Med Chem. 2023;21:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 121. | Duan JY, Wang YJ, Chen W, Zhao YQ, Bai ZH, He LL, Zhang CP. Limonoids isolated from fruits of Swietenia macrophylla king enhance glucose consumption in insulin-resistant HepG2 cells via activating PPARγ. J Food Biochem. 2021;45:e13668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Ajebli M, Eddouks M. Eucalyptus globulus possesses antihypertensive activity in L-NAME-induced hypertensive rats and relaxes isolated rat thoracic aorta through nitric oxide pathway. Nat Prod Res. 2021;35:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Bokaeian M, Nakhaee A, Moodi B, Ali Khazaei H. Eucalyptus globulus (eucalyptus) treatment of candidiasis in normal and diabetic rats. Iran Biomed J. 2010;14:121-126. [PubMed] |
| 124. | Khan IA, Hussain M, Syed SK, Saadullah M, Alqahtani AM, Alqahtani T, Aldahish AA, Asiri S, Zeng LH. Pharmacological Justification for the Medicinal Use of Plumeria rubra Linn. in Cardiovascular Disorders. Molecules. 2021;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 125. | Kim B, Kim KW, Lee S, Jo C, Lee K, Ham I, Choi HY. Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Lee K, Ham I, Yang G, Lee M, Bu Y, Kim H, Choi HY. Vasorelaxant effect of Prunus yedoensis bark. BMC Complement Altern Med. 2013;13:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 127. | Jo K, Lee SE, Lee SW, Hwang JK. Prunus yedoensis Matsum. stimulates glucose uptake in L6 rat skeletal muscle cells by activating AMP-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways. Nat Prod Res. 2012;26:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 128. | Jin SN, Wen JF, Kim HY, Kang DG, Lee HS, Cho KW. Vascular relaxation by ethanol extract of Xanthoceras sorbifolia via Akt- and SOCE-eNOS-cGMP pathways. J Ethnopharmacol. 2010;132:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Zhang Y, Ma JN, Ma CL, Qi Z, Ma CM. Simultaneous quantification of ten constituents of Xanthoceras sorbifolia Bunge using UHPLC-MS methods and evaluation of their radical scavenging, DNA scission protective, and α-glucosidase inhibitory activities. Chin J Nat Med. 2015;13:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Cabral B, Bortolin RH, Gonçalves TAF, Maciel PMP, de Arruda AV, de Carvalho TG, Abboud KY, Alves JSF, Cordeiro LMC, de Medeiros IA, de Rezende AA, Zucolotto SM. Hypoglycemic and Vasorelaxant Effect of Passiflora edulis Fruit Peel By-Product. Plant Foods Hum Nutr. 2021;76:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Sohrabi F, Niazmand S, Mahmoudabady M, Niazmand MJ. The vasodilatory effect of Apium graveolens L (celery) seed in isolated rat aorta: The roles of endothelium, calcium and potassium channels. Avicenna J Phytomed. 2021;11:44-53. [PubMed] |
| 132. | Iizuka T, Moriyama H, Nagai M. Vasorelaxant effects of methyl brevifolincarboxylate from the leaves of Phyllanthus niruri. Biol Pharm Bull. 2006;29:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 133. | Najari Beidokhti M, Andersen MV, Eid HM, Sanchez Villavicencio ML, Staerk D, Haddad PS, Jäger AK. Investigation of antidiabetic potential of Phyllanthus niruri L. using assays for α-glucosidase, muscle glucose transport, liver glucose production, and adipogenesis. Biochem Biophys Res Commun. 2017;493:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 134. | El Bardai S, Morel N, Wibo M, Fabre N, Llabres G, Lyoussi B, Quetin-Leclercq J. The vasorelaxant activity of marrubenol and marrubiin from Marrubium vulgare. Planta Med. 2003;69:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Boudjelal A, Henchiri C, Siracusa L, Sari M, Ruberto G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia. 2012;83:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 136. | Kassahun Gebremeskel A, Wijerathne TD, Kim JH, Kim MJ, Seo CS, Shin HK, Lee KP. Psoralea corylifolia extract induces vasodilation in rat arteries through both endothelium-dependent and -independent mechanisms involving inhibition of TRPC3 channel activity and elaboration of prostaglandin. Pharm Biol. 2017;55:2136-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Nishida S, Satoh H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin Chim Acta. 2004;339:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int. 2013;2013:162724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 139. | Su XH, Duan R, Sun YY, Wen JF, Kang DG, Lee HS, Cho KW, Jin SN. Cardiovascular effects of ethanol extract of Rubus chingii Hu (Rosaceae) in rats: an in vivo and in vitro approach. J Physiol Pharmacol. 2014;65:417-424. [PubMed] |
| 140. | Nguelefack TB, Dimo T, Mbuyo EP, Tan PV, Rakotonirina SV, Kamanyi A. Relaxant effects of the neutral extract of the leaves of Bidens pilosa Linn on isolated rat vascular smooth muscle. Phytother Res. 2005;19:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 141. | Xuan TD, Khanh TD. Chemistry and pharmacology of Bidens pilosa: an overview. J Pharm Investig. 2016;46:91-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 142. | Takashima M, Kanamori Y, Kodera Y, Morihara N, Tamura K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine. 2017;24:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 143. | Jini D, Sharmila S, Anitha A, Pandian M, Rajapaksha RMH. In vitro and in silico studies of silver nanoparticles (AgNPs) from Allium sativum against diabetes. Sci Rep. 2022;12:22109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 144. | Ajebli M, Eddouks M. Antihypertensive activity of Petroselinum crispum through inhibition of vascular calcium channels in rats. J Ethnopharmacol. 2019;242:112039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 145. | Yanardağ R, Bolkent S, Tabakoğlu-Oğuz A, Ozsoy-Saçan O. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Biol Pharm Bull. 2003;26:1206-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 146. | Chen JF, Liu F, Qiao MM, Shu HZ, Li XC, Peng C, Xiong L. Vasorelaxant effect of curcubisabolanin A isolated from Curcuma longa through the PI3K/Akt/eNOS signaling pathway. J Ethnopharmacol. 2022;294:115332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 147. | Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 148. | Naseri MK, Arabian M, Badavi M, Ahangarpour A. Vasorelaxant and hypotensive effects of Allium cepa peel hydroalcoholic extract in rat. Pak J Biol Sci. 2008;11:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Bang MA, Kim HA, Cho YJ. Alterations in the blood glucose, serum lipids and renal oxidative stress in diabetic rats by supplementation of onion (Allium cepa. Linn). Nutr Res Pract. 2009;3:242-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 150. | Chompoo J, Upadhyay A, Kishimoto W, Makise T, Tawata S. Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011;129:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Kang DG, Moon MK, Choi DH, Lee JK, Kwon TO, Lee HS. Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway. Eur J Pharmacol. 2005;524:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Zhang MH, Feng L, Zhu MM, Gu JF, Jiang J, Cheng XD, Ding SM, Wu C, Jia XB. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol. 2014;151:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 153. | Niazmand S, Fereidouni E, Mahmoudabady M, Mousavi SM. Endothelium-independent vasorelaxant effects of hydroalcoholic extract from Nigella sativa seed in rat aorta: the roles of Ca2+ and K+ channels. Biomed Res Int. 2014;2014:247054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 154. | Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, Martineau LC, Cherrah Y, Haddad PS. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 155. | Lobo de Andrade DM, Reis Cde F, Castro PF, Borges LL, Amaral NO, Torres IM, Rezende SG, Gil Ede S, Cardoso da Conceição E, Pedrino GR, Lavorenti Rocha M. Vasorelaxant and Hypotensive Effects of Jaboticaba Fruit (Myrciaria cauliflora) Extract in Rats. Evid Based Complement Alternat Med. 2015;2015:696135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | Hsu JD, Wu CC, Hung CN, Wang CJ, Huang HP. Myrciaria cauliflora extract improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-nicotinamide mice. J Food Drug Anal. 2016;24:730-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 157. | Oh KS, Han W, Wang MH, Lee BH. The effects of chronic treatment with Morus bombycis KOIDZUMI in spontaneously hypertensive rats. Biol Pharm Bull. 2007;30:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Heo SI, Jin YS, Jung MJ, Wang MH. Antidiabetic properties of 2,5-dihydroxy-4,3'-di(beta-D-glucopyranosyloxy)-trans-stilbene from mulberry (Morus bombycis koidzumi) root in streptozotocin-induced diabetic rats. J Med Food. 2007;10:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | Figard H, Girard C, Mougin F, Demougeot C, Berthelot A. Effects of aqueous hop (Humulus Lupulus L.) extract on vascular reactivity in rats: mechanisms and influence of gender and hormonal status. Phytomedicine. 2008;15:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 160. | Liu M, Yin H, Liu G, Dong J, Qian Z, Miao J. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J Agric Food Chem. 2014;62:5548-5554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 161. | Suresh Kumar P, Patel JS, Saraf MN. Mechanism of vasorelaxant activity of a fraction of root extract of Sesamum indicum Linn. Indian J Exp Biol. 2008;46:457-464. [PubMed] |
| 162. | Zheoat AM, Gray AI, Igoli JO, Ferro VA, Drummond RM. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia. 2019;134:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 163. | Mohamed AI, Salau VF, Erukainure OL, Islam MS. Hibiscus sabdariffa L. polyphenolic-rich extract promotes muscle glucose uptake and inhibits intestinal glucose absorption with concomitant amelioration of Fe(2+) -induced hepatic oxidative injury. J Food Biochem. 2022;46:e14399. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 164. | Khan IA, Hussain M, Munawar SH, Iqbal MO, Arshad S, Manzoor A, Shah MA, Abbas K, Shakeel W, Syed SK. Jasminum sambac: A Potential Candidate for Drug Development to Cure Cardiovascular Ailments. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 165. | Ferreira HC, Serra CP, Lemos VS, Braga FC, Cortes SF. Nitric oxide-dependent vasodilatation by ethanolic extract of Hancornia speciosa via phosphatidyl-inositol 3-kinase. J Ethnopharmacol. 2007;109:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 166. | Tomazi R, Figueira ÂC, Ferreira AM, Ferreira DQ, de Souza GC, de Souza Pinheiro WB, Pinheiro Neto JR, da Silva GA, de Lima HB, da Silva Hage-Melim LI, Pereira ACM, Carvalho JCT, da Silva de Almeida SSM. Hypoglycemic Activity of Aqueous Extract of Latex from Hancornia speciosa Gomes: A Study in Zebrafish and In Silico. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 167. | Khonsung P, Panthong A, Chiranthanut N, Intahphuak S. Hypotensive effect of the water extract of the leaves of Pseuderanthemum palatiferum. J Nat Med. 2011;65:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Padee P, Nualkaew S, Talubmook C, Sakuljaitrong S. Hypoglycemic effect of a leaf extract of Pseuderanthemum palatiferum (Nees) Radlk. in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;132:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 169. | Tom EN, Girard C, Dimo T, Mbafor JT, Berthelot A, Demougeot C. Vasorelaxant effects of extracts of the stem bark of Terminalia superba Engler & Diels (Combretaceae). J Ethnopharmacol. 2010;127:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Magos GA, Mateos JC, Páez E, Fernández G, Lobato C, Márquez C, Enríquez RG. Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J Ethnopharmacol. 2008;117:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 171. | Alonso-Castro AJ, Salazar-Olivo LA. The anti-diabetic properties of Guazuma ulmifolia Lam are mediated by the stimulation of glucose uptake in normal and diabetic adipocytes without inducing adipogenesis. J Ethnopharmacol. 2008;118:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 172. | Ojewole JA, Kamadyaapa DR, Gondwe MM, Moodley K, Musabayane CT. Cardiovascular effects of Persea americana Mill (Lauraceae) (avocado) aqueous leaf extract in experimental animals. Cardiovasc J Afr. 2007;18:69-76. [PubMed] |
| 173. | Lima CR, Vasconcelos CF, Costa-Silva JH, Maranhão CA, Costa J, Batista TM, Carneiro EM, Soares LA, Ferreira F, Wanderley AG. Anti-diabetic activity of extract from Persea americana Mill. leaf via the activation of protein kinase B (PKB/Akt) in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2012;141:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 174. | Shah AJ, Gilani AH. Blood pressure lowering effect of the extract of aerial parts of Capparis aphylla is mediated through endothelium-dependent and independent mechanisms. Clin Exp Hypertens. 2011;33:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 175. | Dangi KS, Mishra SN. Antihyperglycemic, antioxidant and hypolipidemic effect of Capparis aphylla stem extract in streptozotocin induced diabetic rats. Biology and Medicine. 2010;2:35-44. |
| 176. | Yoo MY, Oh KS, Lee JW, Seo HW, Yon GH, Kwon DY, Kim YS, Ryu SY, Lee BH. Vasorelaxant effect of stilbenes from rhizome extract of rhubarb (Rheum undulatum) on the contractility of rat aorta. Phytother Res. 2007;21:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 177. | Ha MT, Park DH, Shrestha S, Kim M, Kim JA, Woo MH, Choi JS, Min BS. PTP1B inhibitory activity and molecular docking analysis of stilbene derivatives from the rhizomes of Rheum undulatum L. Fitoterapia. 2018;131:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 178. | Chokri A, El Abida K, Zegzouti YF, Ben Cheikh R. Endothelium-dependent vascular relaxation induced by Globularia alypum extract is mediated by EDHF in perfused rat mesenteric arterial bed. Can J Physiol Pharmacol. 2012;90:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 179. | Tiss M, Hamden K. Globularia alypum Extracts Attenuate Hyperglycemia and Protect against Various Organ Toxicities in Alloxan-Induced Experimental Diabetic Rats. Evid Based Complement Alternat Med. 2022;2022:6816942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 180. | Wansi SL, Nyadjeu P, Nguelefack TB, Fodouop SF, Donatien AA, Kamanyi A. In vivo antioxidant and vasodilating activities of Gmelina arborea (Verberaceae) leaves hexane extract. J Complement Integr Med. 2012;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 181. | Wongcome T, Panthong A, Jesadanont S, Kanjanapothi D, Taesotikul T, Lertprasertsuke N. Hypotensive effect and toxicology of the extract from Coscinium fenestratum (Gaertn.) Colebr. J Ethnopharmacol. 2007;111:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 182. | Punitha IS, Rajendran K, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 183. | Janbaz KH, Nisa M, Saqib F, Imran I, Zia-Ul-Haq M, De Feo V. Bronchodilator, vasodilator and spasmolytic activities of methanolic extract of Myrtus communis L. J Physiol Pharmacol. 2013;64:479-484. [PubMed] |
| 184. | Sepici A, Gürbüz I, Cevik C, Yesilada E. Hypoglycaemic effects of myrtle oil in normal and alloxan-diabetic rabbits. J Ethnopharmacol. 2004;93:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 185. | Alamgeer, Auger C, Chabert P, Lugnier C, Mushtaq MN, Schini-Kerth VB. Mechanisms underlying vasorelaxation induced in the porcine coronary arteries by Thymus linearis, Benth. J Ethnopharmacol. 2018;225:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Younatan Y, Majid M, Phull AR, Baig MW, Irshad N, Fatima H, Nasir B, Zafar A, Majid A, Parveen A, Haq IU. Thymus linearis Extracts Ameliorate Indices of Metabolic Syndrome in Sprague Dawley Rats. Oxid Med Cell Longev. 2023;2023:5648837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 187. | Thaçi S, Krasniqi B, Dërmaku-Sopjani M, Rifati-Nixha A, Abazi S, Sopjani M. Vasorelaxant Effects of the Vitex Agnus-Castus Extract. Evid Based Complement Alternat Med. 2022;2022:7708781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 188. | Belemnaba L, Ouédraogo S, Nitiéma M, Chataigneau T, Guissou IP, Schini-Kerth VB, Bucher B, Auger C. An aqueous extract of the Anogeissus leiocarpus bark (AEAL) induces the endothelium-dependent relaxation of porcine coronary artery rings involving predominantly nitric oxide. J Basic Clin Physiol Pharmacol. 2018;29:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 189. | Motto AE, Lawson-Evi P, Eklu-Gadegbeku K. Antidiabetic and antioxidant potential of total extract and supernatant fraction of the roots of Anogeissus leiocarpus in HFD-fed and Streptozocin -induced diabetic rats. Biomed Pharmacother. 2022;154:113578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 190. | Mushtaq MN, Ghimire S, Alamgeer, Akhtar MS, Adhikari A, Auger C, Schini-Kerth VB. Tambulin is a major active compound of a methanolic extract of fruits of Zanthoxylum armatum DC causing endothelium-independent relaxations in porcine coronary artery rings via the cyclic AMP and cyclic GMP relaxing pathways. Phytomedicine. 2019;53:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 191. | Alam F, Saqib QNU, Ashraf M. Zanthoxylum armatum DC extracts from fruit, bark and leaf induce hypolipidemic and hypoglycemic effects in mice- in vivo and in vitro study. BMC Complement Altern Med. 2018;18:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 192. | Janbaz KH, Qayyum A, Saqib F, Imran I, Zia-Ul-Haq M, de Feo V. Bronchodilator, vasodilator and spasmolytic activities of Cymbopogon martinii. J Physiol Pharmacol. 2014;65:859-866. [PubMed] |
| 193. | Ghadyale V, Takalikar S, Haldavnekar V, Arvindekar A. Effective Control of Postprandial Glucose Level through Inhibition of Intestinal Alpha Glucosidase by Cymbopogon martinii (Roxb.). Evid Based Complement Alternat Med. 2012;2012:372909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 194. | Aekthammarat D, Pannangpetch P, Tangsucharit P. Moringa oleifera leaf extract induces vasorelaxation via endothelium-dependent hyperpolarization and calcium channel blockade in mesenteric arterial beds isolated from L-NAME hypertensive rats. Clin Exp Hypertens. 2020;42:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 195. | Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 196. | Yu SM, Cheng ZJ, Kuo SC. Endothelium-dependent relaxation of rat aorta by butein, a novel cyclic AMP-specific phosphodiesterase inhibitor. Eur J Pharmacol. 1995;280:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 197. | Zhao C, Liu Y, Cong D, Zhang H, Yu J, Jiang Y, Cui X, Sun J. Screening and determination for potential α-glucosidase inhibitory constituents from Dalbergia odorifera T. Chen using ultrafiltration-LC/ESI-MS(n). Biomed Chromatogr. 2013;27:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 198. | Yin S, Bai W, Li P, Jian X, Shan T, Tang Z, Jing X, Ping S, Li Q, Miao Z, Wang S, Ou W, Fei J, Guo T. Berberine suppresses the ectopic expression of miR-133a in endothelial cells to improve vascular dementia in diabetic rats. Clin Exp Hypertens. 2019;41:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 199. | Matsuura M, Kimura Y, Nakata K, Baba K, Okuda H. Artery relaxation by chalcones isolated from the roots of Angelica keiskei. Planta Med. 2001;67:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 200. | Zhang W, Jin Q, Luo J, Wu J, Wang Z. Phytonutrient and anti-diabetic functional properties of flavonoid-rich ethanol extract from Angelica Keiskei leaves. J Food Sci Technol. 2018;55:4406-4412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 201. | Lin YL, Dai ZK, Lin RJ, Chu KS, Chen IJ, Wu JR, Wu BN. Baicalin, a flavonoid from Scutellaria baicalensis Georgi, activates large-conductance Ca2+-activated K+ channels via cyclic nucleotide-dependent protein kinases in mesenteric artery. Phytomedicine. 2010;17:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 202. | Yun C, Ji X, Chen Y, Zhao Z, Gao Y, Gu L, She D, Ri I, Wang W, Wang H. Ultrasound-assisted enzymatic extraction of Scutellaria baicalensis root polysaccharide and its hypoglycemic and immunomodulatory activities. Int J Biol Macromol. 2023;227:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 203. | Pires AF, Madeira SV, Soares PM, Montenegro CM, Souza EP, Resende AC, Soares de Moura R, Assreuy AM, Criddle DN. The role of endothelium in the vasorelaxant effects of the essential oil of Ocimum gratissimum in aorta and mesenteric vascular bed of rats. Can J Physiol Pharmacol. 2012;90:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 204. | Casanova LM, da Silva D, Sola-Penna M, Camargo LM, Celestrini Dde M, Tinoco LW, Costa SS. Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study. Fitoterapia. 2014;93:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 205. | Arituluk Z, Ozkul Kocak C, Renda G, Ekizoglu M, Ezer N. Antimicrobial activity of three Scutellaria L. species from Turkey. J Res Pharm. 2019;23:552-558. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 206. | Karaman Ö, Cebe G. Diabetes and antidiabetic plants used in Turkey. J Fac Pharm of Ankara Univ. 2016;40:47-61. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/