Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1111
Revised: February 29, 2024
Accepted: April 15, 2024
Published online: June 15, 2024
Processing time: 138 Days and 9.7 Hours
Diabetic kidney disease is one of the most severe chronic microvascular complications of diabetes and a primary cause of end-stage renal disease. Clinical studies have shown that renal inflammation is a key factor determining kidney damage during diabetes. With the development of immunological technology, many studies have shown that diabetic nephropathy is an immune complex disease, and that most patients have immune dysfunction. However, the immune response associated with diabetic nephropathy and autoimmune kidney disease, or caused by ischemia or infection with acute renal injury, is different, and has a com-plicated pathological mechanism. In this review, we discuss the pathogenesis of diabetic nephropathy in immune disorders and the intervention mechanism, to provide guidance and advice for early intervention and treatment of diabetic nephropathy.
Core Tip: Diabetic kidney disease (DKD) is a complex immune disease, whose occurrence and development are related to immune inflammatory factors. Currently, the immune process of DKD is mainly regulated by immune cells, inflammatory bodies, immunoglobulins, and complements; thus, interrupting its related regulatory pathways is of great clinical significance.
- Citation: Zhou T, Fang YL, Tian TT, Wang GX. Pathological mechanism of immune disorders in diabetic kidney disease and intervention strategies. World J Diabetes 2024; 15(6): 1111-1121
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1111.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1111
The incidence of diabetes has increased recently. According to statistics, the number of patients with diabetes in China is nearly 130 million, ranking first in the world, with approximately 30% of the patients developing kidney disease. Diabetic kidney disease (DKD) is a chronic kidney disease that is one of the main causes of end-stage renal disease (ESRD)[1-3]. The pathological mechanism of DKD is complex, and most patients have poor treatment outcomes, which seriously affects their quality of life. DKD is mainly related to metabolic disorders, hemodynamic stress, oxidative stress, and genetic factors; however, the specific mechanisms are not fully understood[4-6].
The typical manifestations of DKD are proteinuria and decreased glomerular filtration rate, which are associated with immune regulation. Immune disorder-mediated chronic inflammation is an important mechanisms underlying the development of DKD[7]. The immune system is an important defense system in the body, which mainly includes immune cells, inflammasomes, immunoglobulins, and the complement system. It can resist the invasion of foreign microorganisms, and reduce aging, mutations, deterioration, and apoptosis to maintain the health of the body. Under normal conditions, T lymphocytes, B lymphocytes, macrophages, and other immune cells are maintained at a certain number and proportion to effectively support the body's immune function. When the number and proportion of different cells deviate from their normal ranges, the body undergoes many pathological changes and may produce immune disorders, leading to the occurrence of diseases[8]. Inflammasomes are primarily found in innate immune cells that attack pathogens. When these cells sense exogenous pathogens or endogenous danger signals, they will initiate inflammatory responses and induce pyroptosis. NACHT-leucine-rich repeat and pyrin domain (PYD)-containing protein 3 (NLRP3) inflammasome are involved in the pathogenesis of DKD. Activation of the NLRP3 inflammasome has recently been found in kidneys of patients with diabetes, and loss of NLRP3 or cysteinyl aspartate-specific proteinase (caspase-1) can improve diabetic kidney injury[9]. Immunoglobulins are present in the kidneys, blood, and urine of patients with DKD and are significantly associated with disease progression and prognosis[10]. The complement system is a core component of innate immunity, and several studies have shown that complement activation is involved in the development of DKD[11]. Therefore, this review discusses the pathological mechanism and intervention strategies of immune disorders in DKD and provides novel ideas and strategies for the precise treatment of DKD.
T lymphocytes as the first line of defense against pathogens, occupy a central position in adaptive immune responses. T cell deficiency affects cellular and humoral immune responses, resulting in susceptibility to various pathogenic microorganisms, weakened anti-tumor effects and other pathological phenomena[7,12]. Cellular immune function depends on T cells and the various cytokines they produce. In clinical practice, the total lymphocytes (CD3+), CD4+ and, CD8+T cells, and the CD4+/CD8+ ratio in circulation are often used as sensitive indicators of cellular immune function of the body, and a decrease in the number of these cells is considered an important feature of immune deficiency[7,13]. CD4+ T cells play an important regulatory role in the immune response and can be divided into different subgroups according to their different cell functions, such as Th1, Th2, Th17, and regulatory T cells, which induce different effector functions[13]. Th1 cells mainly produce Interferon-γ (IFN-γ), promote cell-mediated immune response, and play an important role in the occurrence and development of DKD[14]. Th2 cells, defined by the expression of the cytokines interleukin (IL)-4, IL-5, IL-13, and the main transcription factor GATA3, participate in the immune response and inhibit inflammation and fibrosis[15]. Th17 cells participate in the immunity by expressing cytokine IL-17 or IL-22 and main transcription factor RORγt, which can trigger a strong pro-inflammatory response[16]. However, Treg cells expressing Foxp3 maintain the immune balance and prevent excessive immune responses by inhibiting the activity of other immune cells[13]. CD8+ T cells function by directly recognizing and killing cells, unlike CD4+ T cells, which regulate immune responses by secreting cytokines[17]. Most animal and clinical trials have shown reduced numbers of CD3+, CD4+ and CD4+/CD8+ in patients with DKD, indicating that T cell immunity is decreased, which is one of the reasons for the severity of DKD[18,19].
Studies have found that the distribution of T lymphocytes is abnormal in patients with DKD. Therefore, the changes in T lymphocyte subsets may be an important indicator of DKD[20]. However, the clinical treatment of DKD provides a new approach, particularly, inhibiting T lymphocytes and reducing inflammatory reactions. Currently, the most common intervention strategy is to delay or prevent the occurrence of DKD using immunosuppressive agents. In many kidney disease models, T cell antibodies tend to lose T lymphocyte subsets; for example, in Immunoglobulin A nephropathy in a mouse model administration of the CD3+ antibody, significantly decreased the mesangial area IgA deposition, and administration of the anti-glomerular basement membrane nephritis CD4+ monoclonal antibody (mAb), significantly reduced glomerular deposition of macrophages, proteinuria, and crescent formation[21]. In addition, studies have shown that polysaccharides can regulate cytokines and other factors through NF-KB and MAPK signaling pathways. Most naturally derived polysaccharides have significant effects on lowering blood lipid and glucose levels, antithrombosis, and improving body immunity[22]. Drug intervention can restore the balance between CD4+ and CD8+ T lymphocyte subsets, regulate cytokine levels during pathogenesis, and reduce inflammation. Relevant animal experiments have shown that ephedra polysaccharides can improve lung disease injury and inhibit inflammation, apoptosis, and oxidative stress by regulating the level of immune cells such as T helper cells. Codium fragile polysaccharide can induce the activation of mouse dendritic cells in vivo and directly induce natural killer cells to produce IFN-γ in vitro, thereby promoting the apoptosis of target cells and contributing to immune anti-cancer[23-25].
Recently, increasing evidence has shown that B lymphocytes play an important role in disease pathogenesis. Pathological examinations always reveal that lymphocyte infiltration, including B lymphocyte subsets related to islet function, cause insulitis. In animal experiments, after depleting mouse B lymphocytes using a CD20 mAb, the incidence of diabetes was significantly reduced, which proved that B lymphocytes are involved in the pathogenesis of diabetes[26].
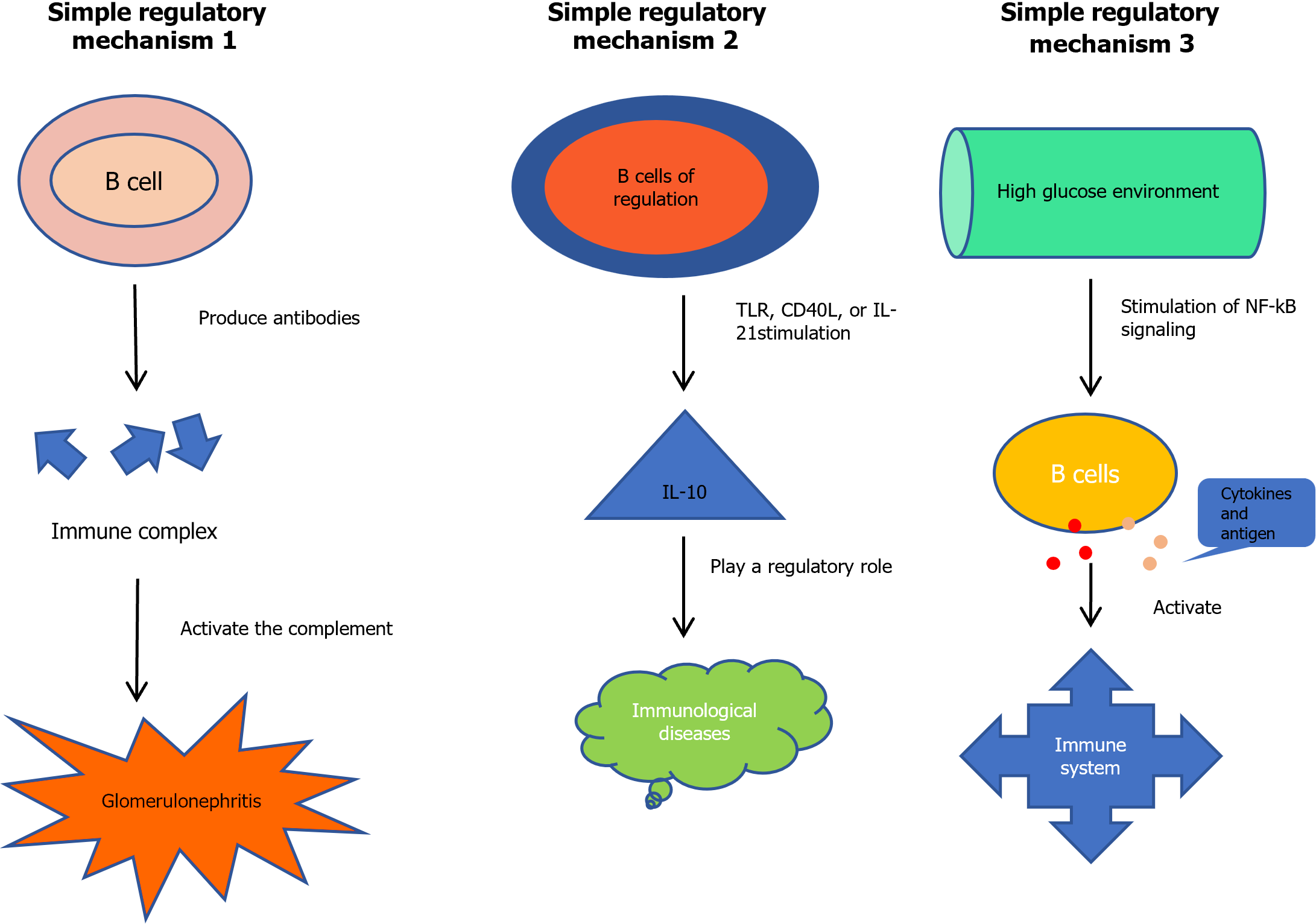
A study on diabetic peripheral blood showed that B cells are associated with DKD deterioration[27]. Its pathological mechanism may have the following three aspects (Figure 1). First, the most likely mechanism is the production of antibodies, leading to the formation of immune complexes in the kidney, and the activation of the complement system causing inflammation and glomerulonephritis. Previous studies have identified immune complexes in the kidneys of patients with DKD[28]. The second mechanism of action of B cells may involve the production of IL-10 by regulatory B cells (Bregs). Under the stimulation of Toll-like receptor (TLR) agonists, CD40L or IL-21, Bregs can express IL-10 and play a regulatory role in immune diseases, such as DKD. It has been shown that the predictive performance of IL-10 levels for proteinuria is high in individuals with long-term DKD[26,29]. Finally, poor blood glucose control is a recognized risk factor of DKD. For example, high blood sugar levels can stimulate the Nuclear Factor k-Light-Chain Enhancer of Activated B Cells (NF-kB) signal transduction, which prompts B lymphocytes to produce cytokines and present antigens to activate the immune system and accelerate the development of DKD[30,31].
B cell-targeted therapy can be used to inhibit the deterioration of immune diseases in clinical practice. The most common method is to remove B cells; however, this reduces the number of antibodies produced by B cells and cause adverse reactions. Recent studies have shown that mAbs can act on B lymphocytes via antigen specificity[32]. For example, Rituxan can induce the production of antibodies by a dependency-mediated cytotoxicity effect, and reduce antigen expression and reactive T cell activation, cracking targeted B cells. The results of a clinical study showed that patients with DKD who received rituximab infusion had a faster recovery of serum levels and required less insulin than those who did not receive rituximab infusion[33]. Other studies have shown that rituximab can inhibit the recovery of pathogenic B cell function and temporarily delay the development of diabetes to a certain extent[34,35]. Rituximab is the only B cell-targeting drug that has been studied in patients with diabetes; however, some therapeutic targets can also function in B cells and participate in cell activation. For example, the second-generation anti-C20 mAb, which increases the binding affinity to the Fc receptor of B cells, has been shown to be superior to rituximab in vivo studies, but has not been confirmed in vitro[36].
Macrophages are innate immune cells that which play key roles in mediating renal inflammation in diabetes. Macrophages are divided into classically activated macrophages (M1 type) and alternatively activated macrophages (M2 type). M1 macrophages have pro-inflammatory effects, whereas M2 macrophages have anti-inflammatory and immunomodulatory effects. These two successive macrophages can protect the body from pathogens invasion and timeously terminate inflammation and induce tissue repair[37]. In patients with DKD, M1 macrophage polarization and pro-inflammatory cytokine expression are increased, while M2 macrophage polarization and anti-inflammatory cytokine expression are decreased. If the ratio of M1/M2 macrophages is not balanced, it will cause metabolic disorders and adverse reactions can occur in patients with DKD. The mechanism of action of these macrophage types occurs primarily in high sugar environments; they activate NF-KB signaling pathways, which promotes inflammatory cell factor, causing inflammation infiltration, and they promote cell migration, activation and aggregation, causing kidney damage[38,39]. The results of an animal study on renal immune cells showed an increase in resident and infiltrating macrophage subsets in the kidneys of diabetic mice over time, and enhanced expression of pro-inflammatory or anti-inflammatory genes in a subtype-specific manner[40]. Recent immunological studies have further proven that macrophages are also detected in the glomeruli and tubules of patients with DKD, and the number of specific macrophage subsets in DKD is significantly increased compared to that in minimal change disease[41].
In addition, the study found that the degree of kidney damage and fibrosis in DKD is associated with the number of macrophages in the kidney, and macrophages play a major role in the development of fibrosis[42]. Hyperglycemia and glycation end products can induce the expression of cytokines such as intercellular adhesion molecule-1 (ICM-1) and monocyte chemoattractant protein-1 in the kidney, leading to the aggregation of macrophages in the kidney, mediating the release of lysosomes and producing oxygen free radicals, which lead to thickening of the glomerular basement membrane and an increase in interstitial fibrous collagen. Eventually, glomerulosclerosis and interstitial fibrosis develop[43]. In contrast, the use of drugs that can reduce the accumulation of macrophages in the kidneys of patients with DKD, and can reduce the recruitment of macrophages and improve renal function and fibrosis, which may provide significant insight for the treatment of DKD[44]. For example, the use of the ICM-1 antibody in a DKD rat model reduced the infiltration of renal macrophages in DKD and improved renal damage[45]. Related animal models have also shown that the accumulation of macrophages in the kidney is reduced by the depletion or destruction of macrophage recruitment, thereby alleviating kidney injury. Targeted regulation of macrophages may be a new therapeutic strategy for inhibiting DKD progression. Macrophages have the potential to become therapeutic targets or tools to treat human kidney diseases[46-48].
Mast cells are widely distributed around microvessels in the skin and visceral submucosa. They secrete various cytokines and participate in the regulation of cell activation[49,50]. There are three main pathways of mast cell activation: The classical, IgE-FcR cross-linking, and alternative pathways. The primary mechanism of mast cells is to aggravate renal tubulointerstitial fibrosis through the synthesis and release of TGF-β and renin, as well as the release tumor necrosis factor-α, which participates in the pathogenesis of DKD[51,52]. Animal experiments have confirmed the presence of mast cells in the kidney. With the development of DKD, the tubulointerstitial damage to the kidney is aggravated, and the number of mast cells gradually increases[52].
In addition, one study reported that mast cells in the gastrointestinal mucosa of mice responded to ingested antigens. In other words, mice carrying mast cells, as opposed to those with mast cell defects, avoided the ingestion of antigens when given ad libitum access to water with or without antigens, suggesting that mast cells play an important protective role in signaling avoidance behavior. It can prevent or reduce inflammation caused by repeated confrontations between the immune system and antigens[53]. Florsheim et al[53] demonstrated antigen avoidance behavior as a defense strategy that helps an organism minimize the harmful effects of exposure to toxic substances, including allergens[54]. These studies suggest that mast cells, as sensing cells linked to antigen recognition and the induction of avoidance behavior, mediate immune-inflammatory processes. Clinically, mast cell treatment typically takes three forms: Reducing the number of mast cells, regulating the activation and phenotype of mast cells, and changing the secreted mast cell mediators and their downstream effects[55,56].
For example, mAbs and small molecules that specifically inhibit mast cell degranulation through key receptors, block specific signal transduction pathways involved in mast cell activation (e.g., BTK), silence mast cells through inhibitory receptors (e.g., Siglec-8), reduce the number of mast cells, and prevent their differentiation by acting on the mast/stem cell growth factor receptors[57].
The inflammatory corpuscle is a protein complex that includes three parts: Receptor proteins, ASC joint proteins, and proteins downstream of the caspase family, and can be involved in cell apoptosis. Currently, the most widely used inflammatory corpuscle is the NLRP3 inflammatory corpuscle, which contains a sensor protein, NLRP3, ASC joint protein, and pro-CASP1, and has been shown to have antibacterial, antiviral, antifungal, and antiparasitic immune responses[58,59].
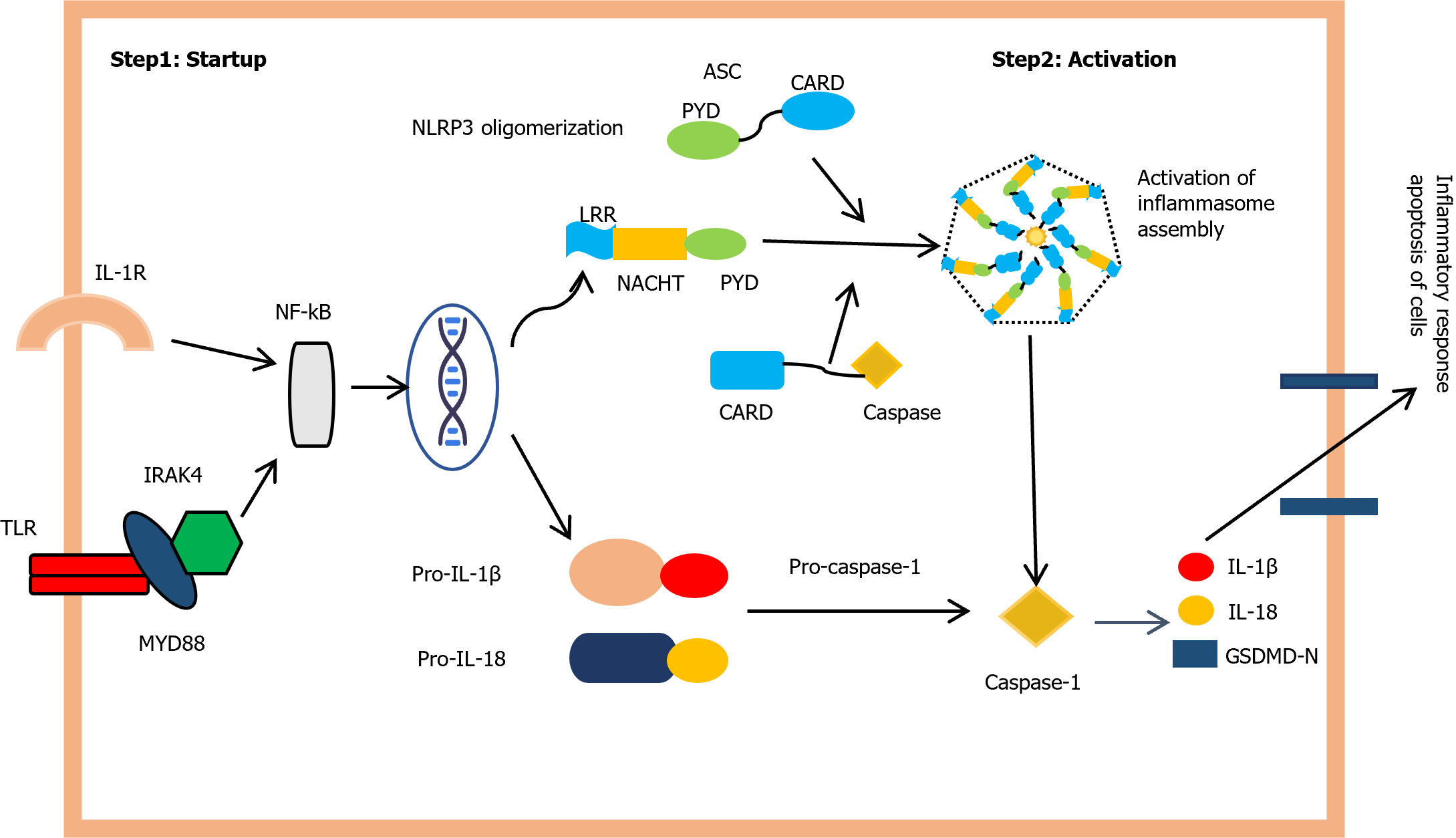
The NLRP3 activation process is divided into two steps, as shown in Figure 2. First, the pathogen-associated molecular patterns or damage-associated molecular patterns induces activation of TLR signaling, leading to an NF-KB-dependent activation of NLRP3, pro-IL-1beta (a cytokine precursor), and pro-IL-18. The second step involves multiple cellular mechanisms, such as potassium efflux, calcium influx, mitochondrial dysfunction, and intracellular reactive oxygen species production, which promote NLRP3 complex formation and CASP1 activation. Mitochondrial dysfunction is also a major feature of DKD, and studies have shown that NLRP3 inflammasome activation can lead to apoptosis and mitochondrial dysfunction, thereby aggravating proteinuria in patients with DKD[60,61].
Inflammasomes are involved in various inflammatory diseases; thus, inhibiting their activation can prevent the occurrence and development of inflammatory diseases. Animal experiments have shown that NLRP3 knockout improves renal function, glomerulosclerosis, tubulointerstitial inflammation, and fibrosis[62]. In addition, inhibitors reduce inflammation corpuscle activation; for example, the M-920 cysteine protease inhibitors can reduce renal CASP1, IL-1 beta, IL-18, and the activation of NLRP3 inflammatory corpuscle, and improve proteinuria and reduce the accumulation of extracellular matrix[63,64]. MCC950 NLRP3 inhibitors can reduce active CASP1 membrane cells and the production of IL-beta in mice, effectively improving renal fibrosis and dysfunction[65]. CY-09, another NLRP3 specific inhibitor, can not only block the oligomerization of the NLRP3 inflammasome, but also downregulate blood glucose and insulin levels, improve glucose tolerance and reduce hepatic steatosis in diabetic mice[66,67]. Drugs can also inhibit inflammasome activation. Oridonin, a major component of traditional Chinese medicine, is a specific covalent inhibitor of NLRPS. Oridonin forms a covalent bond with the cysteine 279 of NLRP3 to block the interaction between NLRP3 and NEK7, which inhibits the assembly and activation of the NLRP3 inflammasome and reduces the inflammatory response, thereby reducing kidney injury caused by diabetes[68]. Tranilast is a cell membrane stabilizer that inhibits the expression of the human lymphocyte antigen system-DR on the surface of macrophages, which in turn, inhibits the release of antigen-stimulated IL-2. Additionally, it prevents NLRP3 assembly and mediates immune responses by inhibiting the interaction of NLRP3 molecules with apoptosis-related speck-like proteins containing C-terminal caspase activation and recruitment domains[69,70].
Immunoglobulin is accepted by B cell proliferation differentiation antigen stimulation generated plasma cells produce a glycoprotein that can combine with the corresponding antigen, which is an important effect of mediating humoral immune molecules. The main immune proteins in the human body are IgG, IgA, IgM, IgD, and IgE, which are present in the blood, tissue fluid, and external secretions, and are important indicators of humoral immune function[71,72].
IgG is the most abundant immunoglobulin in the human body, and is commonly found in the B cell re-immune response. It has a good complement activation function and can enhance the ability of phagocytic cells to engulf pathogens. Studies have found that IgG levels are associated with renal prognosis in patients with diabetes, and that the higher the intensity of IgG deposition, the higher the all-cause mortality[10,73]. There are two possible mechanisms underlying this phenomenon. First, changes in the glomerular basement membrane structure leads to changes in the permeability of the filtration membrane, which reduces the permeability of albumin and negatively charged proteins in the plasma, altering the charge on both sides of the basement membrane. In the early stages of diabetes, the proportion of negatively charged IgG cleared by the kidneys increases. Second, IgG is an antibody against collagen IV, which is an important component of the glomerular basement membrane. In most patients with DKD immune dysfunction, the glomerular basement membrane structure stimulates the body to produce IgG, affecting glomerular and renal tubule deposition[74-76].
IgA is the most synthesized human immunoglobulin and its level changes with the risk of cardiovascular and autoimmune diseases, and has a certain predictive effect. IgA plays a role in neutralization, which is damaging the cell to prevent pathogens from entering. IgA also has a regulatory role and can bind to Fc receptors on the surface of monocytes and neutrophils on the mucosal surface to phagocytic pathogens. Studies have found that IgA-positive B cells increase significantly and intestinal secretions increase IgA deposits in the kidney, resulting in hematuria, proteinuria, and renal function changes[77,78].
As an antibody that appears in the body's primary immune response, IgM can be used for the early diagnosis of infection and plays an important role in the early immune defense response. Some studies have found that the incidence of cardiovascular events and renal end points increase when urinary IgM levels increase in patients with diabetes. In addition, albuminuric IgM has higher sensitivity for prognostic judgment[79].
IgD and IgE are the B cells of the first antibody, which are important to the immune system during the development of immune response[80]. IgD can act as a B cell surface receptor that plays an immunomodulatory role, and as a ligand that binds to its receptor to activate downstream signaling pathways[81,82].
Activation of the immune system has a significant influence on the development of DKD. Immunoglobulin, a downstream effector product, is closely related to differences in the clinical manifestations and prognosis of DKD, providing a new target for the treatment of DKD. Clinical studies have shown that specific Immunoglobins react with antigens and generate antigen-antibody complexes, thereby blocking pathogens from harming the body and causing it to lose its pathogenic effects[83].
Complement activation is associated with the occurrence and progression of DKD, and transcriptome analysis has found that the complement system appears to be abnormally expressed in patients with DKD, and various molecular depositions in the kidney, complement, and pathological staging of DKD are significantly correlated[84-86]. Furthermore, complement proteins in the urine of patients with glucose deficiency are significantly associated with the risk of progression of DKD to ESRD and death[87].
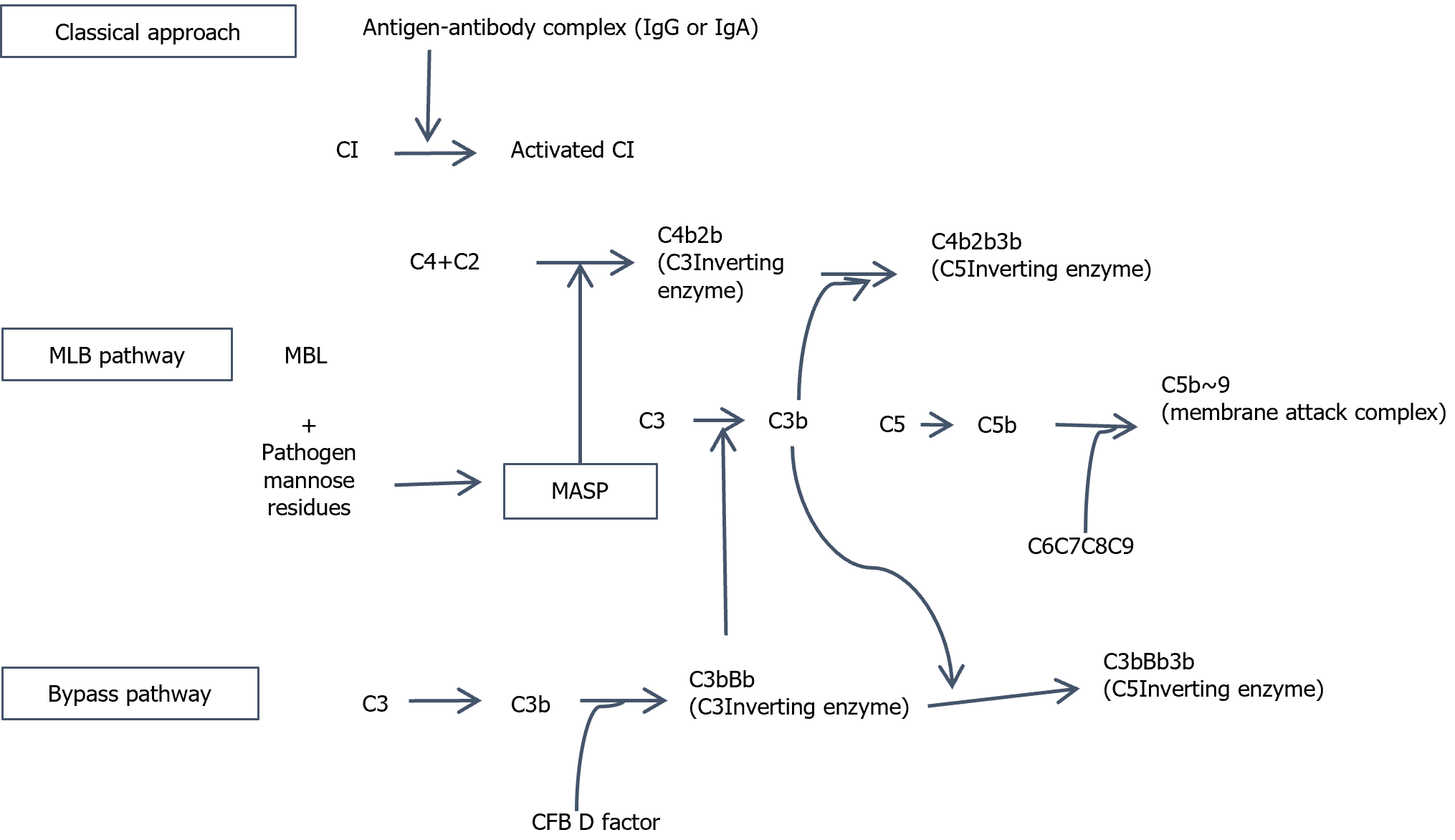
There are three common complement pathways[88,89]: Classical (CP), lectin (LP) and alternative (AP) pathways. Article 3 of the downstream activation pathway is C3 convertase and under the action of C5 invertase, it produces toxic effects, such as C5a- and C3a-mediated tissue injury, and simultaneously forms the membrane attack complex (MAC)-mediated effect of soluble cell (Figure 3). Two key mechanisms are thought to be involved in complement activation in DKD[90,91]. The first mechanism is based on the lectin pathway, which is involved in protein glycosylation. In a high sugar environment with normal tissue protein components produced by glycosylation, lectin binds to mannan activating the complement system; for example, mannan knockout mice administered agglutinin, had improved urinary albumin excretion rate. The second mechanism is hyperglycemia induced glycosylation of complement regulatory proteins. Lys41 and His44 of complement regulatory protein CD59 are easily glycosylated in a high-glucose environment, which renders CD59 unable to prevent the formation of the MAC, leading to tissue and organ damage.
Simultaneously, complement deposition in patients with DKD often results in C5 and C3 level anomalies in the kidney immune system, and the staining intensity is positively associated with kidney disease progression[92,93]. Clinically, C3 and C5 inhibitors can regulate signaling pathways to reduce the inflammatory response[94-96]. For example, application of C3aR inhibitors can improve the disease phenotype of diabetic rats by reducing endothelial cell myofibroblast transition and inflammatory response through downregulating the Wnt/β-catenin, TGF-β/smad3, and IKBα signaling pathways. C3aR inhibitors can also improve mitochondrial damage and oxidative stress in podocytes of diabetic mice. C5aR inhibitor NOX-D21 improved renal tubulointerstitial fibrosis, dyslipidemia, and ectopic lipid deposition in diabetic mice. C5aR1 knockout or application of the C5aR1 inhibitor PMX53 can improve clinical pathological damage in diabetic mice by improving cardiolipin remodeling and normalizing the tricarboxylic acid cycle. Studies have shown that the application of C5aR1 inhibitors can improve pathological damage and the inflammatory response in DKD mice by improving the intestinal flora and increasing the levels of short-chain fatty acids[97,98].
In addition to the aforementioned interventions for immune disorders in patients with DKD, recent studies have provided new therapeutic directions. MicroRNAs (miRNAs) are important mediators of post-transcriptional feedback, and studies have confirmed their involvement in the regulation of metabolism and inflammation, providing a new perspective on the molecules and signaling pathways involved in the pathological mechanism of DKD[99,100]. miRNAs are non-coding and regulate gene expression through epigenetic mechanisms; therefore, drug intervention can be used to prevent DKD before it occurs[101]. Relevant animal studies have confirmed that both miR-192 and miR-21 are related to renal fibrosis, and inhibiting their activation can improve the inflammatory response and restore renal function[102,103]. Therefore, miRNA can be used as a new target for the treatment of DKD; however, relevant clinical trials are not perfect, and miRNAs’ mechanism of action requires further exploration.
With the development of islet pathology, immunogenetics, islet autoantibodies, and immunotherapy, a theory for the autoimmune etiology of diabetes has been established. Since the 1990s, the inflammatory immune pathogenesis of diabetes has been gradually revealed. There are many links between diabetes and immune dysfunction. Exploring the mechanism of immune regulation and intervention strategies can help improve the treatment outcomes and prognosis of patients with DKD. If these findings can be translated into clinical practice, they may promote continuous developments in the field of molecular biology with important clinical significance. Clinical studies have shown that various pro-inflammatory pathways involving immune cells, inflammasomes, immunoglobulins, complement system, and miRNAs are related to the occurrence and development of diabetes. Intervention through related regulatory pathways can reduce cell damage and inflammatory responses to a certain extent. This review provides novel ideas and strategies for mechanism research and the potential treatment of DKD, especially regarding the exploration of disease-related mechanisms and intervention strategies from the aspects of inflammatory factors and disease targets. However, owing to the specificity of human autoimmune cells and the limitations of related clinical trials, many challenges remain in disease immunotherapy. With the continuous progress in technology and research, major breakthroughs will likely be made in this field in the future.
We thank all the working partners who participated in this project.
| 1. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 602] [Article Influence: 120.4] [Reference Citation Analysis (1)] |
| 2. | Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic Kidney Disease. Prim Care. 2020;47:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 3. | Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14:291-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 467] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 4. | Jiang Y, Xie F, Lv X, Wang S, Liao X, Yu Y, Dai Q, Zhang Y, Meng J, Hu G, Peng Z, Tao L. Mefunidone ameliorates diabetic kidney disease in STZ and db/db mice. FASEB J. 2021;35:e21198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Chen SJ, Lv LL, Liu BC, Tang RN. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 2020;53:e12763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Zhong Y, Lee K, Deng Y, Ma Y, Chen Y, Li X, Wei C, Yang S, Wang T, Wong NJ, Muwonge AN, Azeloglu EU, Zhang W, Das B, He JC, Liu R. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat Commun. 2019;10:4523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 7. | Chen J, Liu Q, He J, Li Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front Immunol. 2022;13:958790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 8. | Vieira E, Mirizio GG, Barin GR, de Andrade RV, Nimer NFS, La Sala L. Clock Genes, Inflammation and the Immune System-Implications for Diabetes, Obesity and Neurodegenerative Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Yang M, Wang X, Han Y, Li C, Wei L, Yang J, Chen W, Zhu X, Sun L. Targeting the NLRP3 Inflammasome in Diabetic Nephropathy. Curr Med Chem. 2021;28:8810-8824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Tang X, Wan F, Zhu Q, Ye T, Jiang X, Yang H. IgG subclass deposition in diabetic nephropathy. Eur J Med Res. 2022;27:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 12. | Lu K, Wang L, Fu Y, Li G, Zhang X, Cao M. Bioinformatics analysis identifies immune-related gene signatures and subtypes in diabetic nephropathy. Front Endocrinol (Lausanne). 2022;13:1048139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Liu J, Zhang Y, Sheng H, Liang C, Liu H, Moran Guerrero JA, Lu Z, Mao W, Dai Z, Liu X, Zhang L. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice With Type 2 Diabetes Mellitus. Front Immunol. 2021;12:733808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 352] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 386] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 17. | Zhang F, Wang C, Wen X, Chen Y, Mao R, Cui D, Li L, Liu J, Cheng J, Lu Y. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103(+) DCs-mediated CD8(+) T cell responses. J Cell Mol Med. 2020;24:5817-5831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 285] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Zheng Z, Zheng F. Immune Cells and Inflammation in Diabetic Nephropathy. J Diabetes Res. 2016;2016:1841690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Lv Y, Zhang T, Huang T, Lang Y, Sheng Q, Liu Y, Kong Z, Gao Y, Lu S, Yang M, Luan Y, Wang X, Lv Z. T cells and their products in diabetic kidney disease. Front Immunol. 2023;14:1084448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 21. | Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, Guerrero-Hue M, García-Caballero C, Vázquez-Carballo C, Mas S, Sanz AB, Herencia C, Mezzano S, Gómez-Guerrero C, Moreno JA, Egido J. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 22. | Xu L, Kwak M, Zhang W, Zeng L, Lee PC, Jin JO. Rehmannia glutinosa polysaccharide induces toll-like receptor 4 dependent spleen dendritic cell maturation and anti-cancer immunity. Oncoimmunology. 2017;6:e1325981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Niu Y, Xue Q, Fu Y. Natural Glycan Derived Biomaterials for Inflammation Targeted Drug Delivery. Macromol Biosci. 2021;21:e2100162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Zhang B, Zeng M, Zhang Q, Wang R, Jia J, Cao B, Liu M, Guo P, Zhang Y, Zheng X, Feng W. Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol Immunol. 2022;152:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 25. | Park HB, Hwang J, Zhang W, Go S, Kim J, Choi I, You S, Jin JO. Polysaccharide from Codium fragile Induces Anti-Cancer Immunity by Activating Natural Killer Cells. Mar Drugs. 2020;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Xiao X, Ma B, Dong B, Zhao P, Tai N, Chen L, Wong FS, Wen L. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J Autoimmun. 2009;32:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, Bassi R, D'Addio F, Schatton T, Abdi R, Atkinson M, Sayegh MH, Wen L, Wasserfall CH, O'Connor KC, Fiorina P. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes. 2015;64:158-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Yang X, Mou S. Role of Immune Cells in Diabetic Kidney Disease. Curr Gene Ther. 2017;17:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Rawlings DJ, Metzler G, Wray-Dutra M, Jackson SW. Altered B cell signalling in autoimmunity. Nat Rev Immunol. 2017;17:421-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 31. | Myśliwska J, Zorena K, Semetkowska-Jurkiewicz E, Rachoń D, Suchanek H, Myśliwski A. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur Cytokine Netw. 2005;16:117-122. [PubMed] |
| 32. | Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 34. | Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, Rodriguez H, Schatz DA, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 35. | Pavlasova G, Mraz M. The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica. 2020;105:1494-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 36. | Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford). 2017;56:1227-1237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 37. | Li HD, You YK, Shao BY, Wu WF, Wang YF, Guo JB, Meng XM, Chen H. Roles and crosstalks of macrophages in diabetic nephropathy. Front Immunol. 2022;13:1015142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 38. | Wei J, Xu Z, Yan X. The role of the macrophage-to-myofibroblast transition in renal fibrosis. Front Immunol. 2022;13:934377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Fu J, Sun Z, Wang X, Zhang T, Yuan W, Salem F, Yu SM, Zhang W, Lee K, He JC. The single-cell landscape of kidney immune cells reveals transcriptional heterogeneity in early diabetic kidney disease. Kidney Int. 2022;102:1291-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 40. | Nordlohne J, Hulsmann I, Schwafertz S, Zgrajek J, Grundmann M, von Vietinghoff S, Eitner F, Becker MS. A flow cytometry approach reveals heterogeneity in conventional subsets of murine renal mononuclear phagocytes. Sci Rep. 2021;11:13251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, Feng R, Zang DD, Meng XM, Huang C, Li J, Ma TT. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics. 2022;12:324-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 42. | Rao J, Wang H, Ni M, Wang Z, Wei S, Liu M, Wang P, Qiu J, Zhang L, Wu C, Shen H, Wang X, Cheng F, Lu L. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut. 2022;71:2539-2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 43. | do Valle Duraes F, Lafont A, Beibel M, Martin K, Darribat K, Cuttat R, Waldt A, Naumann U, Wieczorek G, Gaulis S, Pfister S, Mertz KD, Li J, Roma G, Warncke M. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 44. | Yang H, Xie T, Li D, Du X, Wang T, Li C, Song X, Xu L, Yi F, Liang X, Gao L, Yang X, Ma C. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway. Mol Metab. 2019;23:24-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 45. | Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka HU, Nishishita S, Sato C, Funakoshi A, Nishimori H, Uchida HA, Ogawa D, Makino H. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage: anti-inflammatory effect of cholecystokinin. Diabetes. 2012;61:897-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, IJpelaar DHT. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2017;32:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F762-F768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Zitman-Gal T, Einbinder Y, Ohana M, Katzav A, Kartawy A, Benchetrit S. Effect of liraglutide on the Janus kinase/signal transducer and transcription activator (JAK/STAT) pathway in diabetic kidney disease in db/db mice and in cultured endothelial cells. J Diabetes. 2019;11:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Jackson CW, Pratt CM, Rupprecht CP, Pattanaik D, Krishnaswamy G. Mastocytosis and Mast Cell Activation Disorders: Clearing the Air. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Aponte-López A, Muñoz-Cruz S. Mast Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 51. | Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015;373:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 52. | Galli SJ, Gaudenzio N, Tsai M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu Rev Immunol. 2020;38:49-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 53. | Florsheim EB, Bachtel ND, Cullen JL, Lima BGC, Godazgar M, Carvalho F, Chatain CP, Zimmer MR, Zhang C, Gautier G, Launay P, Wang A, Dietrich MO, Medzhitov R. Immune sensing of food allergens promotes avoidance behaviour. Nature. 2023;620:643-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 54. | Plum T, Binzberger R, Thiele R, Shang F, Postrach D, Fung C, Fortea M, Stakenborg N, Wang Z, Tappe-Theodor A, Poth T, MacLaren DAA, Boeckxstaens G, Kuner R, Pitzer C, Monyer H, Xin C, Bonventre JV, Tanaka S, Voehringer D, Vanden Berghe P, Strid J, Feyerabend TB, Rodewald HR. Mast cells link immune sensing to antigen-avoidance behaviour. Nature. 2023;620:634-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 55. | Arthur GK, Cruse G. Regulation of Trafficking and Signaling of the High Affinity IgE Receptor by FcεRIβ and the Potential Impact of FcεRIβ Splicing in Allergic Inflammation. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Duan S, Arlian BM, Nycholat CM, Wei Y, Tateno H, Smith SA, Macauley MS, Zhu Z, Bochner BS, Paulson JC. Nanoparticles Displaying Allergen and Siglec-8 Ligands Suppress IgE-FcεRI-Mediated Anaphylaxis and Desensitize Mast Cells to Subsequent Antigen Challenge. J Immunol. 2021;206:2290-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 57. | Kolkhir P, Elieh-Ali-Komi D, Metz M, Siebenhaar F, Maurer M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat Rev Immunol. 2022;22:294-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 58. | Wu M, Yang Z, Zhang C, Shi Y, Han W, Song S, Mu L, Du C. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism. 2021;118:154748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 59. | Olona A, Leishman S, Anand PK. The NLRP3 inflammasome: regulation by metabolic signals. Trends Immunol. 2022;43:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 60. | Williams BM, Cliff CL, Lee K, Squires PE, Hills CE. The Role of the NLRP3 Inflammasome in Mediating Glomerular and Tubular Injury in Diabetic Nephropathy. Front Physiol. 2022;13:907504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 61. | Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front Cell Infect Microbiol. 2019;9:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 536] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 62. | Qiu YY, Tang LQ. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol Res. 2016;114:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 63. | Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 1075] [Article Influence: 215.0] [Reference Citation Analysis (0)] |
| 64. | Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, Broz P. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 65. | Zhao W, Zhou L, Novák P, Shi X, Lin CB, Zhu X, Yin K. Metabolic Dysfunction in the Regulation of the NLRP3 Inflammasome Activation: A Potential Target for Diabetic Nephropathy. J Diabetes Res. 2022;2022:2193768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219-3238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 619] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 67. | Vong CT, Tseng HHL, Yao P, Yu H, Wang S, Zhong Z, Wang Y. Specific NLRP3 inflammasome inhibitors: promising therapeutic agents for inflammatory diseases. Drug Discov Today. 2021;26:1394-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 68. | Li J, Bao L, Zha D, Zhang L, Gao P, Zhang J, Wu X. Oridonin protects against the inflammatory response in diabetic nephropathy by inhibiting the TLR4/p38-MAPK and TLR4/NF-κB signaling pathways. Int Immunopharmacol. 2018;55:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res. 2015;91:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 70. | Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, Deng X, Liang G, Zhang H, Jiang W, Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 71. | Tesch GH. Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond). 2017;131:2183-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 72. | Liu J, Li Y, Dai J, Lin B, Xiao C, Zhang X, Luo L, Wang T, Li X, Yu Y, Chen S, Wu L, Liu Y, Yu X, Qin X. Comprehensive Analyses of the Immunoglobulin Proteome for the Classification of Glomerular Diseases. J Proteome Res. 2020;19:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Chadha GS, Morris ME. Effect of Type 2 Diabetes Mellitus and Diabetic Nephropathy on IgG Pharmacokinetics and Subcutaneous Bioavailability in the Rat. AAPS J. 2015;17:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Kojima C, Honda K, Shimizu A, Moriyama T, Sugiura H, Itabashi M, Takei T, Taneda S, Ehara T, Nitta K. Combined IgG4κ and IgG1λ deposition in the glomerular and tubular basement membrane accompanied by autoimmune neutropenia (AIN) and immune thrombocytopenia (ITP). CEN Case Rep. 2015;4:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 75. | Xu XM, Zhu SS, Wang XH, Shao XF, Li B, Zhang Y, Liu Q, Li JM, Wang HL, Li YQ, Zou HQ. [Clinical and pathological features in IgA nephropathy with IgG deposition in the glomerular mesangial area]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:308-311. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Kuroda N, Nao T, Fukuhara H, Karashima T, Inoue K, Taniguchi Y, Takeuchi M, Zen Y, Sato Y, Notohara K, Yoshino T. IgG4-related renal disease: clinical and pathological characteristics. Int J Clin Exp Pathol. 2014;7:6379-6385. [PubMed] |
| 77. | Anders HJ, Peired AJ, Romagnani P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, 'diabetic nephropathy', IgA nephropathy and podocytopathies with FSGS lesions. Nephrol Dial Transplant. 2022;37:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Lyngdoh BS, Baishya P, Mishra J, Marbaniang E, Dey B. IgA nephropathy superimposed on diabetic nephropathy: A case report with review of literature in eight Indian studies. J Family Med Prim Care. 2021;10:2419-2422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 79. | Tang X, Li H, Li L, Zhang J, Xu H, Liu F. The Clinical Impact of Glomerular Immunoglobulin M Deposition in Patients with Type 2 Diabetic Nephropathy. Am J Med Sci. 2018;356:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Dai X, Wu YJ, Jia XY, Chang Y, Wu HX, Wang C, Wei W. Immunoglobulin D (IgD) and IgD receptor expression in diffuse large B-cell lymphoma. Hematology. 2019;24:544-551. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Gutzeit C, Chen K, Cerutti A. The enigmatic function of IgD: some answers at last. Eur J Immunol. 2018;48:1101-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 82. | Londregan J, Maslanka J, Goldman N, Somerville J, Riggs JE. IgD ligation allows peritoneal cavity B cell proliferation. Immunobiology. 2022;227:152181. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Zhang J, Zhang J, Zhang R, Wang Y, Liang Y, Yang Z, Wang T, Xu X, Liu F. Implications of immunoglobulin G deposit in glomeruli in Chinese patients with diabetic nephropathy. J Diabetes. 2020;12:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Budge K, Dellepiane S, Yu SM, Cravedi P. Complement, a Therapeutic Target in Diabetic Kidney Disease. Front Med (Lausanne). 2020;7:599236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Chang DY, Li XQ, Chen M, Zhao MH. Dapagliflozin Ameliorates Diabetic Kidney Disease via Upregulating Crry and Alleviating Complement Over-activation in db/db Mice. Front Pharmacol. 2021;12:729334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Frühbeck G, Catalán V, Ramírez B, Valentí V, Becerril S, Rodríguez A, Moncada R, Baixauli J, Silva C, Escalada J, Gómez-Ambrosi J. Serum Levels of IL-1 RA Increase with Obesity and Type 2 Diabetes in Relation to Adipose Tissue Dysfunction and are Reduced After Bariatric Surgery in Parallel to Adiposity. J Inflamm Res. 2022;15:1331-1345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Bus P, Chua JS, Klessens CQF, Zandbergen M, Wolterbeek R, van Kooten C, Trouw LA, Bruijn JA, Baelde HJ. Complement Activation in Patients With Diabetic Nephropathy. Kidney Int Rep. 2018;3:302-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 88. | Bordron A, Bagacean C, Tempescul A, Berthou C, Bettacchioli E, Hillion S, Renaudineau Y. Complement System: a Neglected Pathway in Immunotherapy. Clin Rev Allergy Immunol. 2020;58:155-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (9)] |
| 89. | So BYF, Chan GCW, Yap DYH, Chan TM. The role of the complement system in primary membranous nephropathy: A narrative review in the era of new therapeutic targets. Front Immunol. 2022;13:1009864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 90. | Jiao Y, Jiang S, Wang Y, Yu T, Zou G, Zhuo L, Li W. Activation of complement C1q and C3 in glomeruli might accelerate the progression of diabetic nephropathy: Evidence from transcriptomic data and renal histopathology. J Diabetes Investig. 2022;13:839-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Sun ZJ, Li XQ, Chang DY, Wang SX, Liu G, Chen M, Zhao MH. Complement deposition on renal histopathology of patients with diabetic nephropathy. Diabetes Metab. 2019;45:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Kenawy HI, Boral I, Bevington A. Complement-Coagulation Cross-Talk: A Potential Mediator of the Physiological Activation of Complement by Low pH. Front Immunol. 2015;6:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Gao S, Cui Z, Zhao MH. Complement C3a and C3a Receptor Activation Mediates Podocyte Injuries in the Mechanism of Primary Membranous Nephropathy. J Am Soc Nephrol. 2022;33:1742-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 94. | Jiang S, Jiao Y, Zou G, Gao H, Zhuo L, Li W. Activation of Complement Pathways in Kidney Tissue May Mediate Tubulointerstitial Injury in Diabetic Nephropathy. Front Med (Lausanne). 2022;9:845679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 95. | Zheng JM, Wang SS, Tian X, Che DJ. Sustained activation of C3aR in a human podocyte line impairs the morphological maturation of the cells. Mol Med Rep. 2020;22:5326-5338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Zhang L, Li W, Gong M, Zhang Z, Xue X, Mao J, Zhang H, Li S, Liu X, Wu F, Shi J, Fu G. C-reactive protein inhibits C3a/C3aR-dependent podocyte autophagy in favor of diabetic kidney disease. FASEB J. 2022;36:e22332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Zhang MF, Huang J, Zhang YM, Qu Z, Wang X, Wang F, Meng LQ, Cheng XY, Cui Z, Liu G, Zhao MH. Complement activation products in the circulation and urine of primary membranous nephropathy. BMC Nephrol. 2019;20:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 98. | Tan SM, Snelson M, Østergaard JA, Coughlan MT. The Complement Pathway: New Insights into Immunometabolic Signaling in Diabetic Kidney Disease. Antioxid Redox Signal. 2022;37:781-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 99. | Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci. 2015;1353:72-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 100. | Zhou H, Ni WJ, Meng XM, Tang LQ. MicroRNAs as Regulators of Immune and Inflammatory Responses: Potential Therapeutic Targets in Diabetic Nephropathy. Front Cell Dev Biol. 2020;8:618536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 101. | Tang J, Yao D, Yan H, Chen X, Wang L, Zhan H. The Role of MicroRNAs in the Pathogenesis of Diabetic Nephropathy. Int J Endocrinol. 2019;2019:8719060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 102. | Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys. 2013;67:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 103. | Wang LP, Geng JN, Sun B, Sun CB, Shi Y, Yu XY. MiR-92b-3p is Induced by Advanced Glycation End Products and Involved in the Pathogenesis of Diabetic Nephropathy. Evid Based Complement Alternat Med. 2020;2020:6050874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: Https://creativecommons.org/licenses/by-nc/4.0/