Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.914
Peer-review started: January 5, 2024
First decision: January 27, 2024
Revised: February 24, 2024
Accepted: March 15, 2024
Article in press: March 15, 2024
Published online: May 15, 2024
Processing time: 126 Days and 3.3 Hours
Gestational diabetes mellitus (GDM) is a special type of diabetes that commonly occurs in women during pregnancy and involves impaired glucose tolerance and abnormal glucose metabolism; GDM is diagnosed for the first time during pregnancy and can affect fetal growth and development.
To investigate the associations of serum D-dimer (D-D) and glycosylated hemoglobin (HbA1c) levels with third-trimester fetal growth restriction (FGR) in GDM patients.
The clinical data of 164 pregnant women who were diagnosed with GDM and delivered at the Obstetrics and Gynecology Hospital of Fudan University from January 2021 to January 2023 were analyzed retrospectively. Among these women, 63 whose fetuses had FGR were included in the FGR group, and 101 women whose fetuses had normal body weights were included in the normal body weight group (normal group). Fasting venous blood samples were collected from the elbow at 28-30 wk gestation and 1-3 d before delivery to measure serum D-D and HbA1c levels for comparative analysis. The diagnostic value of serum D-D and HbA1c levels for FGR was evaluated by receiver operating characteristic analysis, and the influencing factors of third-trimester FGR in GDM patients were analyzed by logistic regression.
Serum fasting blood glucose, fasting insulin, D-D and HbA1c levels were significantly greater in the FGR group than in the normal group, while the homeostasis model assessment of insulin resistance values were lower (P < 0.05). Regarding the diagnosis of FGR based on serum D-D and HbA1c levels, the areas under the curves (AUCs) were 0.826 and 0.848, the cutoff values were 3.04 mg/L and 5.80%, the sensitivities were 81.0% and 79.4%, and the specificities were 88.1% and 87.1%, respectively. The AUC of serum D-D plus HbA1c levels for diagnosing FGR was 0.928, and the sensitivity and specificity were 84.1% and 91.1%, respectively. High D-D and HbA1c levels were risk factors for third-trimester FGR in GDM patients (P < 0.05).
D-D and HbA1c levels can indicate the occurrence of FGR in GDM patients in the third trimester of pregnancy to some extent, and their combination can be used as an important index for the early prediction of FGR.
Core Tip: Pregnancy causes metabolic and immune changes, which may contribute to the development of gestational diabetes mellitus (GDM). Fetal growth restriction (FGR) is a common pregnancy complication, combined with GDM may lead to adverse perinatal outcomes, such as very low birth weight, seriously endangering perinatal life. Some serum markers may be associated with the predicted outcome. This study is to investigate the association of serum D-dimer and glycosylated hemoglobin levels with third-trimester FGR in GDM patients.
- Citation: Zhang Y, Li T, Yue CY, Liu Y. Associations of serum D-dimer and glycosylated hemoglobin levels with third-trimester fetal growth restriction in gestational diabetes mellitus. World J Diabetes 2024; 15(5): 914-922
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/914.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.914
Pregnancy causes metabolic and immune changes, characterized by insulin resistance (IR) and increased immune tolerance to the fetus and placenta. In susceptible women, these physiological changes may contribute to the development of gestational diabetes mellitus (GDM)[1,2]. GDM involves abnormal glucose tolerance of varying degrees that is detected or occurs for the first time during pregnancy as a result of IR and impaired compensatory insulin secretion. GDM has become a growing health problem, affecting approximately 15% of pregnancies worldwide[3,4]. Its occurrence not only increases the risk of short-term complications in mothers and babies (macrosomia, fetal intrauterine growth ab-normalities, maternal predisposition to preeclampsia, etc.) but also affects their long-term health[5,6]. Studies have shown that even though GDM is a transient state in which glycemic regulation usually returns to normal shortly after delivery, it leads to a 40% increase in the risk of developing type 2 diabetes within 10-15 years[7]. Fetal growth restriction (FGR), a common pregnancy complication, is a condition in which the fetus does not develop according to its expected biological potential in the uterus[8]. FGR, in contrast to a small constitution, is a pathological condition (known as placental insufficiency) in which the placenta undersupply nutrients and oxygen to the developing fetus[9]. GDM combined with FGR may lead to adverse perinatal outcomes, such as very low birth weight, seriously endangering perinatal life[10].
D-dimer (D-D) is produced through the specific degradation of cross-linked fibrin and is generally considered to be related to hyperfibrinolysis and hypercoagulability in vivo. D-D levels are directly related to the intensity of fibrinolytic activity, which is typically greater in pregnant women than in nonpregnant women and tends to increase in the third trimester as gestation progresses[11]. Although 78% and 99%-100% of pregnant women have D-D levels above the standard cutoff during the second and third trimesters, respectively, severe elevation can lead to disseminated intravascular coagulation and thrombosis; hence, monitoring and prediction are needed for effective prevention[12,13]. Glycosylated hemoglobin (HbA1c), a sensitive indicator of glucose metabolism during pregnancy, is not directly affected by maternal emotions, drugs or diet and is an ideal clinical blood glucose monitoring index[14,15]. Poor glycemic control has been associated with large-for-gestational-age infants among mothers with GDM[16]. Poor blood sugar control during pregnancy may cause complications such as macrosomia, FGR, premature delivery, and spontaneous abortion[17]. Conversely, good glycemic control is the main means to reducing maternal and infant complications and is highly important for improving maternal and infant outcomes in GDM patients[18].
However, the associations of serum D-D and HbA1c levels with third-trimester FGR in GDM patients have rarely been studied. Based on these findings, in this study, we preliminarily investigated the serum D-D and HbA1c levels in GDM patients in the third trimester of pregnancy, analyzed their correlations with neonatal body weight, and determined their diagnostic value for third-trimester FGR to provide a reference for the early diagnosis and treatment of FGR in GDM patients in the third trimester of pregnancy.
This retrospective analysis included 164 pregnant women who were diagnosed with GDM and delivered at the Obstetrics and Gynecology Hospital of Fudan University from January 2021 to January 2023. Sixty-three patients with neonatal body weights meeting the diagnostic criteria for FGR were included in the FGR group (FGR group), and 101 patients with normal neonatal body weights composed the normal body weight group (normal group). The inclusion criteria for pregnant women were as follows: (1) Met the diagnostic criteria for GDM, underwent a 2-h 75-g oral glucose tolerance test after 28 wk of pregnancy and were diagnosed with GDM according to the International Association of Diabetes and Pregnancy Study Groups criteria [a fasting blood glucose (FBG) level ≥ 5.1 mmol/L, 1-h postprandial blood glucose (PBG) level ≥ 10.0 mmol/L, or 2-h PBG level ≥ 8.5 mmol/L)[19]; (2) met the diagnostic criteria for growth restriction: The body weight of the fetus was two standard deviations below the mean weight for the same gestational age or below the 10th percentile of normal weight for the same age[20]; (3) had singleton pregnancies and delivered newborns with no congenital or genetic diseases; (4) had no adverse pregnancy history, such as FGR, fetal death, or stillbirth; (5) had no disease or high-risk factors, such as hyperlipidemia or hypertension, during pregnancy that could cause D-D elevation or abnormal glucose metabolism; and (6) had complete clinical data. The exclusion criteria for pregnant women were as follows: (1) Had GDM or elevated blood sugar levels for other reasons; (2) had a twin pregnancy, a family history of diabetes mellitus, or an HbA1c level ≥ 6.5% in early pregnancy; (3) had other pregnancy complications; and (4) had incomplete clinical data.
The levels of FBG and fasting insulin (FINS) were determined, and the homeostasis model assessment of IR (HOMA-IR) index (HOMA-IR = FBG × FINS/22.5) was calculated. The HOMA-IR model is a mathematical model established to reflect the interaction between glucose and insulin in different organs. The model can assess IR in the body only by using fasting glucose and FINS values. In general, a HOMA-IR value > 2.69 may indicate IR.
All pregnant women had fasting venous blood samples collected from the elbow at 28-30 wk gestation and 1-3 d before delivery. The plasma D-D level was determined by the ELISA double antibody sandwich method, and its content was calculated using the standard curve obtained by the microplate reader. The HbA1c level was determined by high-performance liquid chromatography with a kit and a hemoglobin analyzer (Lifotronic H-9) provided by Shenzhen Lifotronic Technology Co., Ltd. All the experimental procedures followed the relevant instructions.
The primary outcome measures were serum D-D and HbA1c levels; the secondary outcome measure was the association of serum D-d and HbA1c levels with FGR.
Baseline demographic and clinical characteristics, including maternal age at delivery, parity, and education level (junior middle school, senior middle school, college or above), were considered potential confounders. Besides, weight-related variables, including prepregnancy body mass index (BMI, kg/m2) and prior weight gain (PWG, defined as weight gain before the late second trimester) were the major confounders controlled for. The PWG is obtained by subtracting the self-reported prepregnancy weight from the weight measured during prenatal visits within 23-28 wk of pregnancy, which is closest to the time of oral glucose tolerance testing. We calculated a pregnant woman's preconception BMI based on her self-reported pre-pregnancy weight and height during pregnancy, with the formula being weight (kg) divided by height (m) squared; a BMI of 18.5 kg/m2, 18.5-24.9 kg/m2, 25.0-29.9 kg/m2, and ≥ 30.0 kg/m2 was categorized as underweight, normal weight, overweight, and obese, respectively. None of the women included in this study had obesity, so there was no overweight or obese population.
The statistical software SPSS 22.0 was utilized for the data analyses, with a statistical significance threshold of P < 0.05. The measurement data are presented in the form of the mean ± SD, and a t test was performed for comparative analysis. Receiver operating characteristic (ROC) curves were plotted to visualize the predictive performance of serum D-D and HbA1c levels for FGR. The influencing factors of third-trimester FGR in GDM patients were identified by Logistic regression.
Compared with those in the normal group, the serum FBG, FINS and HOMA-IR in the FGR group were greater (all P < 0.05), but no significant differences were detected in age, weight or gestational age (P > 0.05; Table 1).
| FGR (n = 63) | Normal (n = 101) | t value | P value | |
| Age (yr) | 30.78 ± 4.65 | 30.03 ± 4.58 | 1.012 | 0.313 |
| Ethnicity | ||||
| Ethnic Han | 58 | 90 | 0.385 | 0.535 |
| Ethnic minorities | 5 | 11 | ||
| Weight (kg) | 60.17 ± 3.05 | 60.31 ± 3.37 | 0.259 | 0.796 |
| Parity | 1.3 ± 0.5 | 1.4 ± 0.5 | 1.246 | 0.215 |
| Pre-pregnancy BMI (kg/m2) | 22.83 ± 1.12 | 23.02 ± 1.09 | 1.074 | 0.284 |
| Prior weight gain (kg) | 6.72 ± 1.43 | 6.58 ± 1.35 | 0.631 | 0.528 |
| Education | 0.543 | 0.762 | ||
| Junior middle school | 12 | 23 | ||
| Senior middle school | 24 | 40 | ||
| College and above | 27 | 38 | ||
| Gestational week of delivery | 37.75 ± 1.37 | 37.76 ± 1.62 | 0.067 | 0.947 |
| Serum FBG (mmol/L) | 5.72 ± 1.18 | 5.33 ± 1.16 | 2.094 | 0.038 |
| FINS (pmol/L) | 86.63 ± 28.31 | 76.37 ± 29.93 | 2.181 | 0.031 |
| HOMA-IR | 3.32 ± 1.34 | 4.16 ± 2.28 | 2.651 | 0.008 |
The normal group showed a greater neonatal birth weight than the FGR group (P < 0.05). However, no significant differences in neonatal body length, head circumference, or chest circumference were detected between the groups (P > 0.05; Table 2).
| Weight (g) | Body length (cm) | Chest circumference (cm) | Head circumference (cm) | |
| FGR (n = 63) | 2401 ± 246 | 50.29 ± 1.49 | 34.36 ± 1.8 | 34.00 ± 1.06 |
| Normal (n = 101) | 2909 ± 499 | 50.62 ± 1.20 | 34.72 ± 1.24 | 34.20 ± 1.09 |
| t value | 7.516 | 1.583 | 1.834 | 1.168 |
| P value | < 0.0001 | 0.116 | 0.069 | 0.245 |
The D-D and HbA1c levels at 1-3 d before delivery were greater than those at 28-30 wk of gestation in both groups, but the differences were not statistically significant (P > 0.05). Intergroup comparisons revealed significant differences in D-D and HbA1c levels at 28-30 wk of gestation and 1-3 d before delivery between the FGR group and normal groups (P < 0.05; Table 3).
| D-D (mg/L) | HbA1c (%) | |||
| 28-30 wk of gestation | 1-3 d before delivery | 28-30 wk of gestation | 1-3 d before delivery | |
| FGR (n = 63) | 4.89 ± 1.73 | 5.22 ± 1.37 | 6.50 ± 1.30 | 6.61 ± 1.22 |
| Normal (n = 101) | 2.66 ± 1.20 | 2.95 ± 1.11 | 5.09 ± 0.70 | 5.07 ± 0.78 |
| t value | 9.736 | 11.610 | 8.999 | 9.862 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
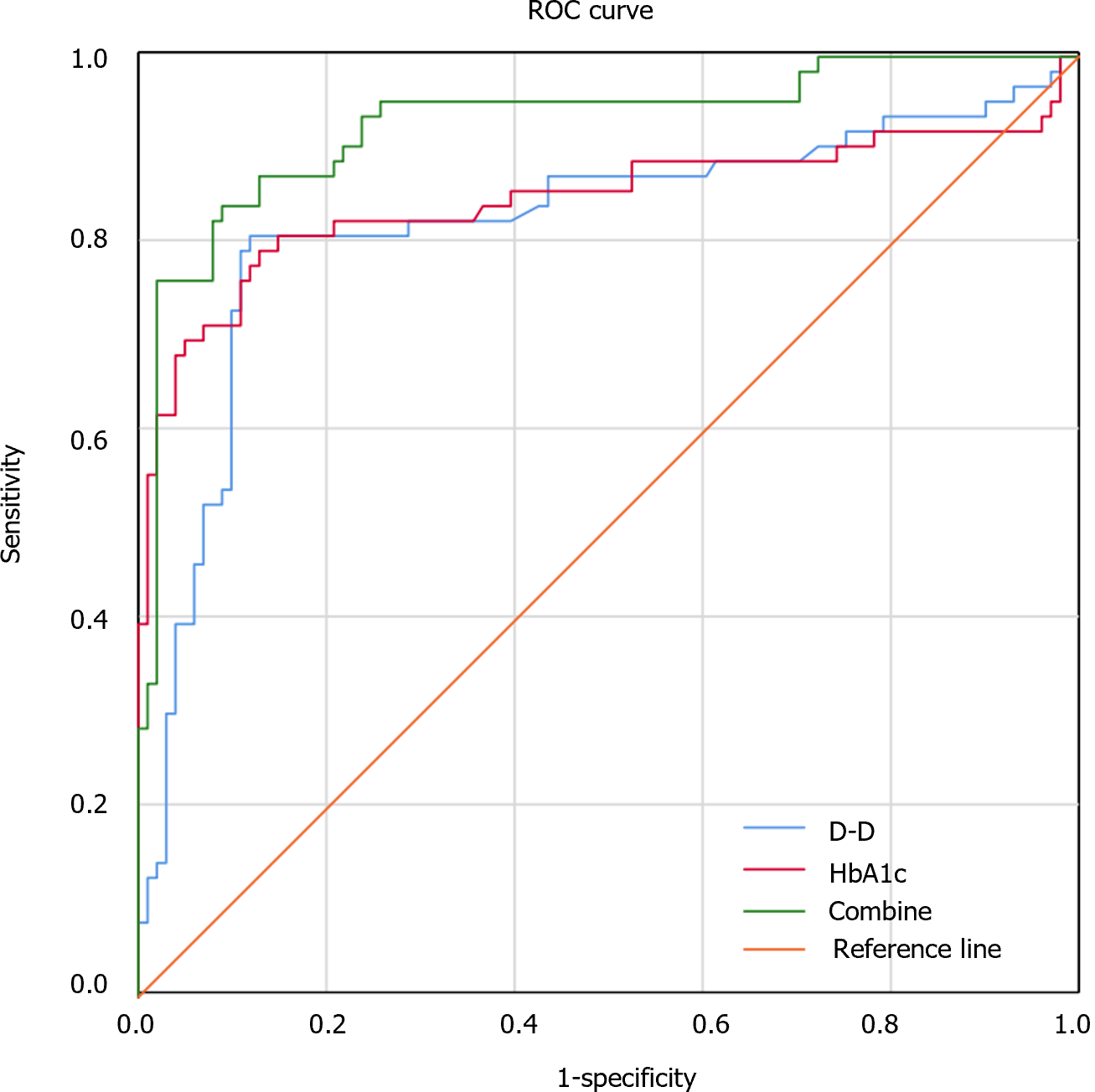
ROC analysis revealed an areas under the curve (AUC), a cutoff, a sensitivity, and a specificity of 0.826 (95%CI: 0.748-0.898), 3.04 mg/L, 81.0%, and 88.1%, respectively, in diagnosing FGR by of the serum D-D level. The AUC, cutoff, sensitivity and specificity of the serum HbA1c level for diagnosing FGR were 0.848 (95%CI: 0.773-0.922), 5.80%, 79.4%, and 87.1%, respectively. The AUC of serum D-D levels combined with HbA1c levels for the diagnosis of FGR was 0.928 (95%CI: 0.884-0.972), with a sensitivity of 84.1% and a specificity of 91.1% (Figure 1 and Table 4).
| AUC | 95%CI | Sensitivity (%) | Specificity (%) | |
| D-D | 0.826 | 0.748-0.898 | 81.0 | 88.1 |
| HbA1c | 0.848 | 0.773-0.922 | 79.4 | 87.1 |
| Combined detection | 0.928 | 0.884-0.972 | 84.1 | 91.1 |
Logistic regression analysis was conducted with D-D, HbA1c, FBG, FINS and HOMA-IR values as independent variables and FGR (occurrence =1, nonoccurrence =0) as the dependent variable. The analysis identified high D-D and HbA1c levels as risk factors for third-trimester FGR in GDM patients (P < 0.05; Table 5).
| β | SE | Wald | P value | OR | 95%CI | |
| D-D | 0.916 | 0.161 | 32.417 | 0.000 | 2.499 | 1.823-3.424 |
| HbA1c | 1.450 | 0.294 | 24.301 | 0.000 | 4.264 | 2.396-7.590 |
| FBG | 0.381 | 0.430 | 0.783 | 0.376 | 1.463 | 0.630-3.399 |
| FINS | 0.008 | 0.026 | 0.088 | 0.767 | 1.008 | 0.957-1.061 |
| HOMA-IR | 0.042 | 0.103 | 0.162 | 0.687 | 1.043 | 0.851-1.277 |
GDM is a common maternal complication with a global standardized total prevalence of 14.0%[21]. Zhu et al[22] reported that the incidence of GDM among generally screened urban women in China increased significantly from 4% in 2010 to 21% in 2020. Although the increase in the prevalence of GDM has slowed since the implementation of China's universal two-child policy, it remains high. GDM can cause female reproductive tract infection, pregnancy-induced hypertension, preterm birth, fetal distress, FGR and other adverse outcomes, while FGR can lead to perinatal death or metabolic syndrome, mental retardation and short stature, seriously threatening the health of infants[23]. However, the occurrence of FGR is influenced by many factors, such as fetal, maternal, and placental factors, with no effective clinical predictors at present[24]. Therefore, exploring biological targets that can be used to effectively diagnose and treat FGR and improve maternal and infant quality of life is highly important.
As a specific thrombus marker, D-D levels can be used to detect hypercoagulability in vivo. The blood of pregnant women is usually physiologically hypercoagulable. During pregnancy, D-D levels tend to increase during endometrial growth along with trophoblasts, which in turn erode venous sinuses and spiral arteries, leading to the constant activation of the coagulation and fibrinolysis system to remove thrombi[25]. The results of this study revealed a significant di-fference in D-D expression between the FGR and normal groups at 28-30 wk of pregnancy and 1-3 d before delivery. It is suggested that sustained hyperglycemia in patients with FGR may damage vascular endothelial cells, disrupting the balance among fibrinolysis, coagulation and anticoagulation. With increasing gestational age, vascular endothelial damage and placental villus necrosis worsen, leading to the release of a large amount of tissue thromboplastin and causing widespread microthrombosis, further affecting the placental blood supply and causing FGR and increased obstetric infections that exacerbate the impact on the fetus. In addition, several studies have shown that the endothelial decompensation severity determines the formation of a local "catastrophe" in the fetal-placental unit in the form of FGR[26].
Clinically, the traditional evaluation of glycemic control is usually achieved by blood sugar monitoring, but blood sugar is often affected by many interfering factors and great fluctuations. The concentration of HbA1c, formed by hemoglobin and glucose in red blood cells in human blood, is directly proportional to the blood sugar concentration and is maintained for approximately 120 d, which means that the blood sugar concentration before 120 d can be measured by detecting HbA1c[27]. The characteristics of HbA1c are highly important for diabetes monitoring. The higher the blood sugar level is, the greater the HbA1c level is. Due to its slow formation, HbA1c is quite stable and not easily decomposed. Therefore, although it cannot reflect short-term blood sugar fluctuations, it can well reflect the degree of glycemic control over a longer period of time[28]. Related studies have shown that good control of HbA1c levels can greatly reduce the possibility of microvascular complications in diabetic patients[29]. In this study, HbA1c levels were significantly different between the FGR group and the normal group at 28-30 wk of pregnancy and 1-3 d before delivery. It was also found that the mean HbA1c level in the FGR group was greater than 6%, compared with that of 4%-6% in the general population[30]. Therefore, controlling HbA1c to below 6% can reduce the incidence of FDR in GDM patients.
The AUCs of the serum D-D and HbA1c levels for the diagnosis of FGR were determined to be 0.826 and 0.848, respectively. When the serum D-D level was greater than 3.04 mg/L or the HbA1c level was above 5.80%, the probability of developing third-trimester FGR was increased in pregnant women with GDM, suggesting that D-D and HbA1c levels have certain diagnostic value for FGR. The AUC of the diagnosis of FGR based on the combination of serum D-D and HbA1c levels was 0.928, and the sensitivity and specificity were 84.1% and 91.1%, respectively. It is suggested that the combination of D-D and HbA1c levels can effectively improve the specificity and diagnostic efficiency for FGR, which is helpful for predicting the occurrence of third-trimester FGR in pregnant women with GDM. Further study revealed that high D-D and HbA1c levels were risk factors for third-trimester FGR in GDM patients, suggesting that increased serum D-D and HbA1c levels may increase the risk of FGR. Timely monitoring of D-D and HbA1c levels is therefore conducive to the early diagnosis of FGR and the control of disease progression. We believe that the relationship between D-D and HbA1c levels and FGR lies in the fact that high levels of D-D and HbA1c can predict the occurrence of FGR to a certain extent and that HbA1c is not easily decomposed, has fewer interfering factors, has a high degree of stability, and can be tested instantly at any time without the need for fasting blood sampling; these characteristics enable us to more accurately assess the overall blood glucose level at certain times. A high HbA1c level indicates that a patient is hyperglycemic, and persistent hyperglycemia leads to damage to vascular endothelial cells, worsens the necrosis of placental villi, leads to the release of a large amount of tissue thromboplastin, and causes extensive microthrombosis, which in turn affects the blood supply of the placenta. D-D levels can indicate a hypercoagulable state in vivo. Therefore, we believe that the com-bination of the two parameters can better predict the occurrence of FGR.
Several limitations of our study should be acknowledged. Our results should be considered in light of certain limitations of our design. First, the limited samples included and the fact that maternal serum biomarkers were measured only during the late third trimester but not earlier trimesters may lead to discrepancies compared to other research. Second, although the study design revealed that D-D and HbA1c levels could be somewhat suggestive of the development of FGR in patients with GDM in late pregnancy, it was not possible to draw conclusions about the potential dynamics of changes throughout pregnancy, as relevant indicators were quantified only during late pregnancy. Another limitation of the study was the use of self-reported prepregnancy BMI data. In our area, all women trying to conceive utilize community health services and learn how to measure their weight and height. These women only visit obstetric clinics after becoming pregnant. Although we were unable to personally check the prenatal BMI of the participants, the self-reported data under the supervision of community caregivers were generally reliable. In addition, we were not able to test this hypothesis across BMI categories because of the small sample size. Additionally, there were inevitably some unmeasured confounders in this study, including household income and smoking status, as they were not captured in the electronic medical records system. Thus, the inclusion of large sample sizes and large amounts of data for these categories to replicate our findings is essential.
Taken together, D-D and HbA1c levels are closely associated with the occurrence of FGR in GDM patients in the third trimester of pregnancy. The combined detection of the two parameters in GDM patients can be used as an important index for the early prediction of FGR.
Gestational diabetes mellitus (GDM) is a common type of special diabetes that occurs before/during pregnancy in women with impaired glucose tolerance and abnormal glucose metabolism and is diagnosed for the first time during pregnancy, which can affect fetal growth and development.
The association of serum levels of D-dimer (D-D) and glycosylated hemoglobin (HbA1c) with third-trimester fetal growth restriction (FGR) in GDM patients has rarely been studied. Based on this, this study preliminarily observed serum D-D and HbA1c expression in GDM patients in the third trimester of pregnancy.
Preliminarily observed serum D-D and HbA1c expression in GDM patients in the third trimester of pregnancy, analyzed their correlations with neonatal body weight, and discussed their diagnostic value for third-trimester FGR.
One hundred and sixty-four pregnant women who were diagnosed as GDM and delivered in Obstetrics and Gynecology Hospital of Fudan University from January 2021 to January 2023 were included. Among them, 63 cases of neonatal body weight meeting the diagnostic criteria for FGR were regarded as the FGR group (FGR group), and 101 cases of normal neonatal body weight were set as the normal body weight group (normal group). Fasting elbow venous blood was collected at 28-30 wk' gestation and 1-3 d before delivery to measure serum D-D and HbA1c levels for comparative analysis. The diagnostic value of serum D-D and HbA1c for FGR was evaluated.
The FGR group had significant differences in HbA1c levels at 28-30 wk of pregnancy and 1-3 d before delivery compared with the normal group. The areas under the curves (AUCs) of serum D-D and HbA1c levels for FGR diagnosis were determined to be 0.826 and 0.848, respectively. D-D and HbA1c were risk factors for third-trimester FGR in GDM patients.
D-D and HbA1c levels are closely associated with the occurrence of FGR in GDM patients in the third trimester of pregnancy. The combined detection of the two in GDM patients can be used as an important index for early prediction of FGR.
In order to provide reference for early diagnosis and treatment of FGR in GDM patients in the third trimester of pregnancy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dagklis T, Greece; Maranta F, Italy S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Crusell MKW, Hansen TH, Nielsen T, Allin KH, Rühlemann MC, Damm P, Vestergaard H, Rørbye C, Jørgensen NR, Christiansen OB, Heinsen FA, Franke A, Hansen T, Lauenborg J, Pedersen O. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome. 2018;6:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 2. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (1)] |
| 3. | Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, Crowther CA. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2020;6:CD012394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 5736] [Article Influence: 1434.0] [Reference Citation Analysis (37)] |
| 5. | Moon JH, Jang HC. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022;46:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 6. | Lara-Barea A, Sánchez-Lechuga B, Campos-Caro A, Córdoba-Doña JA, de la Varga-Martínez R, Arroba AI, Bugatto F, Aguilar-Diosdado M, López-Tinoco C. Angiogenic Imbalance and Inflammatory Biomarkers in the Prediction of Hypertension as Well as Obstetric and Perinatal Complications in Women with Gestational Diabetes Mellitus. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol Metab. 2018;29:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 564] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 8. | Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB, Araujo Júnior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295:1061-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 397] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 9. | Lees CC, Romero R, Stampalija T, Dall'Asta A, DeVore GA, Prefumo F, Frusca T, Visser GHA, Hobbins JC, Baschat AA, Bilardo CM, Galan HL, Campbell S, Maulik D, Figueras F, Lee W, Unterscheider J, Valensise H, Da Silva Costa F, Salomon LJ, Poon LC, Ferrazzi E, Mari G, Rizzo G, Kingdom JC, Kiserud T, Hecher K. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022;226:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 10. | Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Baboolall U, Zha Y, Gong X, Deng DR, Qiao F, Liu H. Variations of plasma D-dimer level at various points of normal pregnancy and its trends in complicated pregnancies: A retrospective observational cohort study. Medicine (Baltimore). 2019;98:e15903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Kline JA, Williams GW, Hernandez-Nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005;51:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Kovac M, Mikovic Z, Rakicevic L, Srzentic S, Mandic V, Djordjevic V, Radojkovic D, Elezovic I. The use of D-dimer with new cutoff can be useful in diagnosis of venous thromboembolism in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2010;148:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Hughes RC, Rowan J, Florkowski CM. Is There a Role for HbA1c in Pregnancy? Curr Diab Rep. 2016;16:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Claire B, Sharon H. Should HbA1C be used to screen pregnant women for undiagnosed diabetes in the first trimester? A review of the evidence. J Public Health (Oxf). 2020;42:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Kiefer MK, Finneran MM, Ware CA, Fareed N, Joseph J, Thung SF, Costantine MM, Landon MB, Gabbe SG, Venkatesh KK. Association of change in haemoglobin A1c with adverse perinatal outcomes in women with pregestational diabetes. Diabet Med. 2022;39:e14822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Finneran MM, Kiefer MK, Ware CA, Buschur EO, Thung SF, Landon MB, Gabbe SG. The use of longitudinal hemoglobin A1c values to predict adverse obstetric and neonatal outcomes in pregnancies complicated by pregestational diabetes. Am J Obstet Gynecol MFM. 2020;2:100069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Davidson AJF, Park AL, Berger H, Aoyama K, Harel Z, Cohen E, Cook JL, Ray JG. Association of Improved Periconception Hemoglobin A1c With Pregnancy Outcomes in Women With Diabetes. JAMA Netw Open. 2020;3:e2030207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Gopalakrishnan V, Singh R, Pradeep Y, Kapoor D, Rani AK, Pradhan S, Bhatia E, Yadav SB. Evaluation of the prevalence of gestational diabetes mellitus in North Indians using the International Association of Diabetes and Pregnancy Study groups (IADPSG) criteria. J Postgrad Med. 2015;61:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Combs CA, Castillo R, Webb GW, Del Rosario A. Impact of adding abdominal circumference to the definition of fetal growth restriction. Am J Obstet Gynecol MFM. 2021;3:100382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 688] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 22. | Zhu H, Zhao Z, Xu J, Chen Y, Zhu Q, Zhou L, Cai J, Ji L. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol (Lausanne). 2022;13:960877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Natamba BK, Namara AA, Nyirenda MJ. Burden, risk factors and maternal and offspring outcomes of gestational diabetes mellitus (GDM) in sub-Saharan Africa (SSA): a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Maulik D, Frances Evans J, Ragolia L. Fetal growth restriction: pathogenic mechanisms. Clin Obstet Gynecol. 2006;49:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Siennicka A, Kłysz M, Chełstowski K, Tabaczniuk A, Marcinowska Z, Tarnowska P, Kulesza J, Torbe A, Jastrzębska M. Reference Values of D-Dimers and Fibrinogen in the Course of Physiological Pregnancy: the Potential Impact of Selected Risk Factors-A Pilot Study. Biomed Res Int. 2020;2020:3192350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Palieva NV, Botasheva TL, Petrov YA, Pogorelova TN, Drukker NA, Levkovich MA, Gun’ko VO. Carbohydrate metabolism and hemostatic system in women with gestational diabetes mellitus, preeclampsia, and fetal growth restriction. Obstet Gynecol. 2021;69-76. [DOI] [Full Text] |
| 27. | Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther. 2019;10:853-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-to-Visit HbA(1c) Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care. 2020;43:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Nitin S. HbA1c and factors other than diabetes mellitus affecting it. Singapore Med J. 2010;51:616-622. [PubMed] |