Published online May 15, 2024. doi: 10.4239/wjd.v15.i5.898
Peer-review started: January 4, 2024
First decision: January 17, 2024
Revised: January 29, 2024
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: May 15, 2024
Processing time: 126 Days and 20 Hours
The understanding of bile acid (BA) and unsaturated fatty acid (UFA) profiles, as well as their dysregulation, remains elusive in individuals with type 2 diabetes mellitus (T2DM) coexisting with non-alcoholic fatty liver disease (NAFLD). Investigating these metabolites could offer valuable insights into the pathophy-siology of NAFLD in T2DM.
To identify potential metabolite biomarkers capable of distinguishing between NAFLD and T2DM.
A training model was developed involving 399 participants, comprising 113 healthy controls (HCs), 134 individuals with T2DM without NAFLD, and 152 individuals with T2DM and NAFLD. External validation encompassed 172 participants. NAFLD patients were divided based on liver fibrosis scores. The analytical approach employed univariate testing, orthogonal partial least squares-discriminant analysis, logistic regression, receiver operating characteristic curve analysis, and decision curve analysis to pinpoint and assess the diagnostic value of serum biomarkers.
Compared to HCs, both T2DM and NAFLD groups exhibited diminished levels of specific BAs. In UFAs, particular acids exhibited a positive correlation with NAFLD risk in T2DM, while the ω-6:ω-3 UFA ratio demonstrated a negative correlation. Levels of α-linolenic acid and γ-linolenic acid were linked to significant liver fibrosis in NAFLD. The validation cohort substantiated the predictive efficacy of these biomarkers for assessing NAFLD risk in T2DM patients.
This study underscores the connection between altered BA and UFA profiles and the presence of NAFLD in individuals with T2DM, proposing their potential as biomarkers in the pathogenesis of NAFLD.
Core Tip: In the present study, we delineate notable distinctions in serum bile acid (BA) and unsaturated fatty acid (UFA) profiles between individuals with type 2 diabetes mellitus (T2DM) coexisting with non-alcoholic fatty liver disease (NAFLD) and those with T2DM alone. This first large-scale investigation links BA and UFA to NAFLD risk in T2DM patients, with the overarching objective of identifying predictive biomarkers and elucidating their connection to clinically relevant liver fibrosis in the context of NAFLD.
- Citation: Feng SS, Wang SJ, Guo L, Ma PP, Ye XL, Pan ML, Hang B, Mao JH, Snijders AM, Lu YB, Ding DF. Serum bile acid and unsaturated fatty acid profiles of non-alcoholic fatty liver disease in type 2 diabetic patients. World J Diabetes 2024; 15(5): 898-913
- URL: https://www.wjgnet.com/1948-9358/full/v15/i5/898.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i5.898
Non-alcoholic fatty liver disease (NAFLD) is currently the most prevalent chronic liver disease globally[1]. Research indicates that individuals with type 2 diabetes mellitus (T2DM) are twice as likely to experience progression in hepatic diseases compared to non-diabetic NAFLD patients[2]. A comprehensive meta-analysis involving 80 studies revealed a striking 55.48% global prevalence of NAFLD among T2DM individuals[3,4]. Notably, the coexistence of NAFLD and T2DM significantly increases the risks of non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma stemming from fatty liver disease[5]. Additionally, these individuals are more likely to encounter compounded risks of cardiovascular disease, chronic kidney disease, and diabetic retinopathy[6]. Consequently, the identification of metabolite biomarkers linked to T2DM-related complications is critical for the early assessment, prevention, and diagnosis of NAFLD in T2DM patients.
The development of NAFLD is influenced by myriad factors, including the accumulation of adipose tissue, insulin resistance, oxidative stress, mitochondrial dysfunction, genetic predispositions, and gut microbiota perturbations[7,8]. Serum bile acids (BAs) and unsaturated fatty acids (UFAs) are key signaling molecules that link these factors, playing a central role in the pathophysiological processes of NAFLD. BAs, which are amphiphilic cholesterol metabolites, act as essential enteroendocrine hormone-like signaling molecules, regulating glucose, lipid, and energy metabolism through interactions with cell membranes and nuclear receptors[9]. Activating the BA receptor farnesoid x receptor (FXR) has been shown to reduce NAFLD occurrence by inhibiting adipogenesis, reducing liver inflammation and fibrosis[10], and maintaining intestinal barrier integrity[11]. Interestingly, NAFLD patients typically show a higher proportion of serum cholic acid (CA) and chenodeoxycholic acid (CDCA) conjugates, despite not exhibiting a significant difference in total serum BAs[3]. NASH patients, in particular, display total serum BA levels approximately three times higher than healthy controls (HCs)[12], while obese and T2DM individuals exhibit elevated total plasma BAs[13]. Exposure to elevated BAs can induce cytotoxicity and contribute to NAFLD development[14], underscoring the need for comprehensive serum BA analyses in individuals with both NAFLD and T2DM.
UFAs, encompassing polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA), play a critical role in the development of insulin resistance, fat accumulation, and inflammation in NAFLD[15,16]. PUFA, as ligands for G protein-coupled receptors GPR40 and GPR120, enhance insulin secretion through glucagon-like peptide-1 (GLP-1)[17]. However, current findings on UFA in NAFLD present conflicting results, suggesting that alterations in the ω-6 PUFA to ω-3 PUFA ratio may significantly influence NAFLD pathogenesis[18]. While initial studies indicated pro-inflammatory effects of ω-6 PUFAs, a meta-analysis showed that ω-6 PUFA supplementation in NAFLD patients led to liver fat improvements[19]. Moreover, an RCT study involving obese patients demonstrated that supplementation with ω-6 PUFA reduced hepatic steatosis, serum insulin, and inflammatory markers[20] , while another clinical trial reported increased plasma triglyceride (TG) levels following ω-3 PUFAs administration[21]. Evidence suggests ω-3 PUFAs may ameliorate NAFLD by increasing BA synthesis and excretion through upregulating cytochrome P450 7A1 (CYP7A1)[22]. Given these varied findings, the precise impact of UFA and BA, as well as their balance, on diabetic hepatic steatosis remains incompletely understood.
In our hospital-based cross-sectional study, we aimed to: (1) Characterize the serum BA and UFA concentrations in T2DM patients, with and without NAFLD, and explore correlations among BA, UFA, and clinical indicators; (2) assess the risk of NAFLD in T2DM patients based on altered BA and UFA levels; and (3) identify specific BAs and UFA closely associated with NAFLD risk, elucidating their potential roles in the pathogenesis of NAFLD.
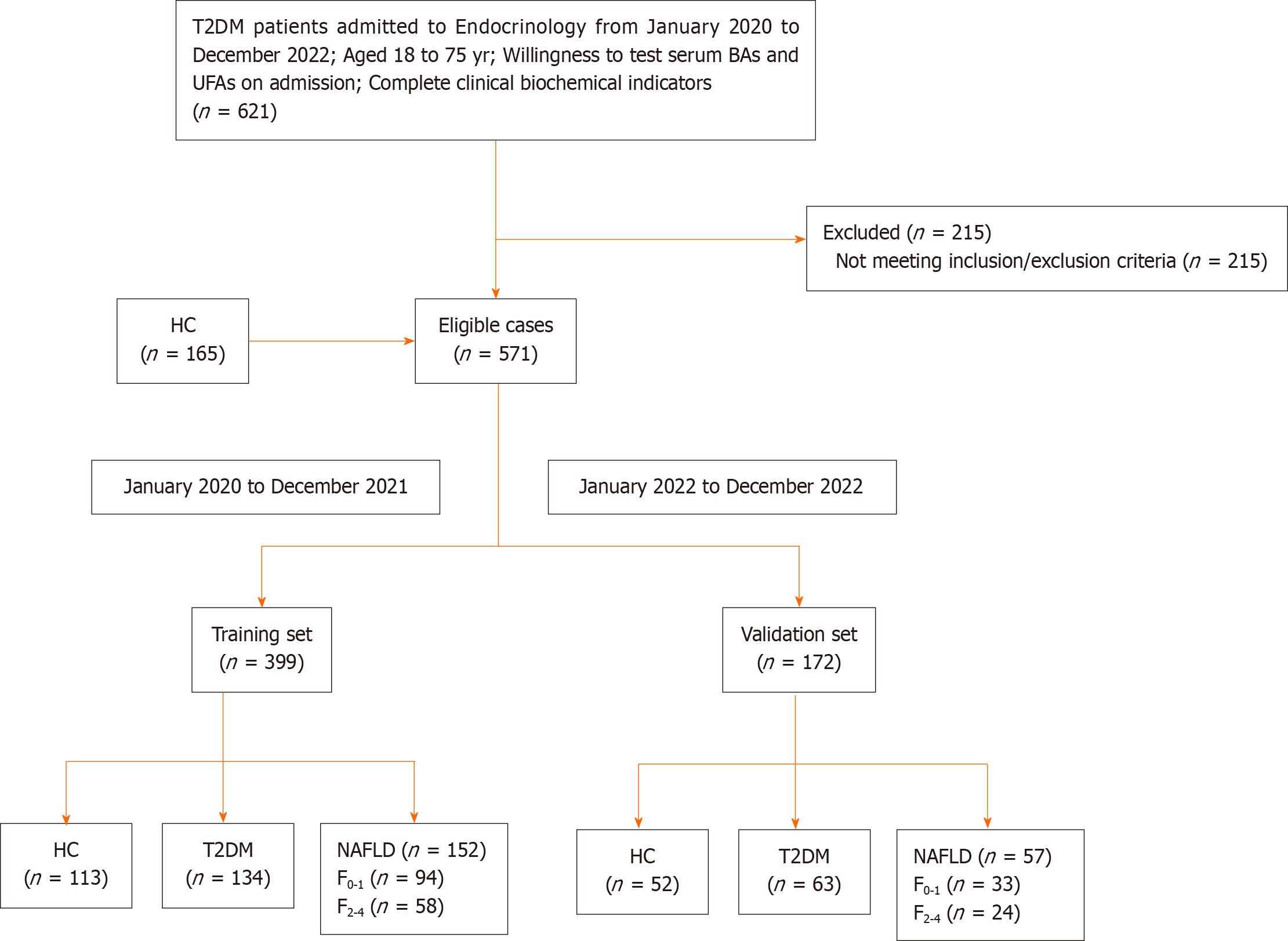
In Figure 1, a total of 406 patients with T2DM were included from the inpatient ward of the Department of Endocrinology at the Second Affiliated Hospital of Nanjing Medical University, along with 165 healthy adult individuals from the physical examination center, spanning the period from January 2020 to December 2022, adhering to predefined inclusion and exclusion criteria. Participants admitted after January 2022 formed an independent validation cohort. Total 571 participants formed two cohorts: Training cohort (HC = 113, T2DM without NAFLD = 134, T2DM with NAFLD = 152); Validation cohort (HC = 52, T2DM without NAFLD = 63, T2DM with NAFLD = 57). Additionally, within the NAFLD group, based on the FIB-4 score, participants were further divided into two subgroups: No or mild Clinically fibrosis with FIB-4 < 1.3 (F0-1) and clinically significant fibrosis with FIB-4 ≥ 1.3 (F2-4)[23].
Inclusion criteria: (1) Participants aged 18 to 75 years; (2) individuals diagnosed with T2DM. T2DM diagnosis based on the 1999 World Health Organization criteria; (3) availability of serum BAs and UFAs measurements; and (4) complete clinical biochemical indicators.
Exclusion criteria for patients with T2DM: (1) Absence of Hepatobiliary and pancreatic ultrasonography; (2) presence of viral hepatitis, drug-induced liver disease, autoimmune liver disease, serious liver dysfunction; (3) alcohol consumption ≥ 70 g per week (women) and ≥ 140 g per week (men); (4) aspartate aminotransferase (AST) ≥ 40U/L or alanine aminotransferase (ALT) ≥ 40 U/L in T2DM without NAFLD; (5) use of hepatoprotective drugs; (6) presence of cholecystitis and pancreatitis; (7) diagnosis of malignancy; (8) occurrence of diabetic ketoacidosis; and (9) pregnancy or breastfeeding.
The diagnostic criteria for NAFLD are as follows[24]: (1) Absence of a history of alcohol consumption or consumption of less than 210 g of alcohol per week in men (less than 140 g per week in women); (2) exclusion for diseases that can lead to NAFLD, including viral hepatitis, drug-induced liver disease, autoimmune liver disease, etc; and (3) consistent liver ultrasound imaging findings, including anterior field echo enhancement (“bright liver”), liver echo greater than kidney, far-field echo attenuation, and unclear display of intrahepatic duct structure.
Information was gathered from the hospital medical record system, including age, sex, duration of diabetes, systolic blood pressure, diastolic blood pressure, body mass index (BMI), and glycosylated hemoglobin (HbA1c). HbA1c levels were determined using a chromatographic technique. Before the collection of blood samples for biochemical tests, subjects were required to fast for a minimum of 8 h overnight. These blood samples were procured in the early morning of the second day post-admission. Biochemical parameters measured in these samples included fasting plasma glucose (FPG), fasting c-peptide (FC-P), TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, ALT, AST, total bilirubin, direct bilirubin, indirect bilirubin, uric acid (UA), blood urea nitrogen, serum creatinine. Time from the first bite of food in the morning, venous blood was drawn 2 h after eating for the determination of C-P 2 h postprandial (2h C-P). Blood specimens, collected by the hospital nurse, were promptly sent to the medical laboratory center by the staff and processed within 2 h. Plasma glucose was determined using a blood glucose biochemical analyzer. Serum C-P was measured using chemiluminescent methods. Routine measurements of biochemical parameters, including serum lipids, liver enzymes, bilirubin, and renal function index, were conducted using enzymatic methods. Additionally, information regarding a patient’s history of hypertension, hypoglycemic agents, and lipid-lowering drugs was obtained by the attending physician upon admission.
BMI was calculated by weight (kg) divided by squared height (m). Triglyceride glucose index (TyG) was calculated by ln [TG (mg/dL) × FPG (mg/dL)]/2[25]. FIB-4 index was calculated by: Age (years) × AST (U/L)/[ALT (U/L)1/2 × platelet count (109/L)].
Following an overnight fasting period of at least eight hours, venous blood samples were collected on the subsequent morning, transported to the Medical Laboratory Center within two hours for serum centrifugation. The serum was then either immediately processed or stored at -80 °C for later analysis. We utilized the BAs Quantitative Assay Kit (Rayborn Medical) and the Derivatization of Various Fatty Acids Quantitative Assay Kit (Jiangsu Haosi Biotechnology Co., Ltd) for the pretreatment of the BAs and UFAs serum samples, respectively. Quantitative analysis was conducted using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS, AB SCIEX JasperTM HPLC-Triple QuadTM 4500MD), employing an Agilent Eclipse Plus C18 column (100.0 mm × 3.0 mm, 3.5 μm) set at 40°. For BAs, the mobile phases involved a 0.05% ammonium formate aqueous solution (Phase A) and 100% acetonitrile (Phase B). For UFAs, the gradient-eluting mobile phases were deionized water (Phase A) and acetonitrile (Phase B). The mass spectrometer was set to electrospray ionization in negative mode. The LC-MS/MS raw data were analyzed using SCIEX Analyst software, which facilitated peak integration, calibration, and quantification for each BA and UFA.
Continuous variables are reported as mean ± SD for normally distributed data and median (upper and lower quartiles) for non-normally distributed data. Categorical variables are presented as frequencies (percentages). Comparisons of continuous variables were made utilizing t-tests, while the nonparametric Wilcoxon test was used for non-normally distributed continuous variables. Comparisons of categorical variables were made using Fisher’s exact test.
Identification of differential BAs and UFAs was based on the Wilcoxon test (P. adj < 0.05, adjusted for false discovery rate using the Benjamini-Hochberg method) and orthogonal partial least squares-discriminant analysis (OPLS-DA), with a cutoff of variable influence on projection (VIP) > 1. Spearman’s correlations were used to assess the relationship between BA and UFA metabolites and their associations with clinical indicators.
Restricted cubic splines (RCS) in logistic regression were utilized to examine non-linear associations between metabolites and NAFLD risk, identifying significant risk variation thresholds for metabolite categorization. These metabolites were then incorporated into regression models as either continuous or categorical variables. Univariate and multivariate logistic regression analyses, accounting for multiple confounding factors, were conducted.
Biomarkers were defined as BAs and UFAs that met three criteria: significant significance in the Wilcoxon test (P. adj < 0.05), a VIP score > 1 in OPLS-DA, and statistical significance in multivariate logistic regression (P < 0.05). We formulated three NAFLD prediction models: Model 1 comprising clinical indicators only, model 2 comprising the biomarkers, and model 3 combining both. Their effectiveness for predicting NAFLD risk in T2DM patients was evaluated using receiver operating characteristic (ROC) curves, area under the curve (AUC), integrated discrimination improvement, and net reclassification improvement (NRI). Model performance and accuracy were ascertained through 1000 bootstrap resamples and decision curve analysis. Metabolite levels in two subgroups (F0-1 and F2-4) were analyzed to determine their link to liver fibrosis risk in NAFLD, using logistic regression and ROC curves. Finally, we validated the results using an independent cohort.
All analyses were conducted using R software version 4.1.2 and SIMCA software 14.1.0. A two-tailed P value of < 0.05 was considered to indicate statistical significance.
In the training and validation cohorts (Table 1; Supplementary Table 1), participants with T2DM, both with and without NAFLD, exhibited elevated levels of TyG, TG, FPG, and reduced HDL-C compared to the HC group. Furthermore, patients with NAFLD, when compared to the HC and T2DM-only groups, were characterized by a younger age, higher BMI, TG, AST, ALT, UA, TyG, and reduced HDL-C levels. Relative to the T2DM-only group, the NAFLD group exhibited higher TyG, FC-P, 2h C-P, TC, and UA, and lower HDL-C, alongside a shorter duration of diabetes. Additionally, participants in the F2-4 fibrosis stage group, compared to those in the F0-1 group, demonstrated a longer duration of diabetes and increased AST levels.
| Item | HC | T2DM without NAFLD | T2DM with NAFLD | F0-1 | F2-4 |
| n = 113 | n = 134 | n = 152 | n = 94 | n = 58 | |
| Age in yr | 59.65 ± 14.54 | 60.06 ± 16.60 | 53.61 ± 12.16a,b | 49.44 ± 10.32 | 61.72 ± 10.48c |
| Sex, male, % | 69 (61.06) | 87 (64.93) | 102 (67.11) | 63 (67.02) | 39 (67.24) |
| Duration of diabetes, month | / | 84.00 [27.00, 153.00] | 48.00 [2.00, 120.00]b | 36.00 [7.00, 93.00] | 84.00 [24.00, 144.00]c |
| SBP, mmHg | 128.00 [116.00, 132.00] | 130.00 [120.00, 138.50] | 130.00 [120.00, 140.00] | 130.00 [120.00, 138.00] | 130.00 [120.00, 140.00] |
| DBP, mmHg | 80.00 [74.00, 86.00] | 81.00 [75.00, 89.00] | 82.00 [76.00, 88.00] | 81.00 [80.00, 88.00] | 80.00 [76.00, 90.00] |
| BMI, Kg/m2 | 22.21 [21.54, 23.58] | 23.38 [21.59, 25.98] | 25.96 [23.76, 27.79]a,b | 26.39 [23.98, 29.37] | 25.96 [24.22, 26.70] |
| TyG index | 8.41 [8.21, 9.02] | 9.88 [9.43, 10.40]a | 10.21 [9.69, 10.71]a,b | 10.27 [9.82, 10.66] | 9.98 [9.57, 10.53] |
| FIB-4 index | / | / | / | 0.85 [0.59, 1.02] | 1.79 [1.51, 2.13]c |
| HbA1c, % | / | 9.00 [7.00, 10.50] | 9.10 [7.65, 10.60] | 9.15 [7.92, 10.60] | 8.60 [7.52, 10.47] |
| FPG, mmol/L | 5.21 [4.97, 5.83] | 8.05 [5.99, 11.02]a | 8.13 [6.67, 10.68]a | 8.63 [6.43, 11.32] | 7.86 [6.88, 9.84] |
| FC-P, ng/mL | / | 1.33 [0.61, 2.17] | 1.85 [1.16, 2.79]b | 1.92 [1.16, 3.04] | 1.80 [1.41, 2.48] |
| 2h C-P, ng/mL | / | 2.83 [1.24, 5.00] | 4.13 [2.17, 6.23]b | 4.29 [2.10, 6.39] | 4.31 [2.34, 6.35] |
| TC, mmol/L | 4.44 [3.91, 5.19] | 4.19 [3.63, 4.97] | 4.62 [3.91, 5.44]b | 4.63 [4.01, 5.21] | 4.52 [3.69, 5.47] |
| TG, mmol/L | 1.17 [0.88, 1.79] | 1.35 [1.04, 1.98]a | 1.76 [1.29, 2.99]a,b | 1.95 [1.38, 3.08] | 1.56 [1.21, 2.46] |
| HDL-C, mmol/L | 1.24 [1.11, 1.42] | 1.07 [0.91, 1.28]a | 0.93 [0.82, 1.10]a,b | 0.92 [0.80, 1.08] | 0.98 [0.83, 1.17] |
| LDL-C, mmol/L | 2.70 [2.31, 3.32] | 2.77 [2.27, 3.28] | 2.94 [2.22, 3.67] | 2.94 [2.33, 3.59] | 2.98 [2.07, 3.75] |
| ALT, U/L | 14.10 [10.90, 21.42] | 14.10 [10.10, 20.50] | 25.00 [15.70, 36.30]a,b | 23.40 [15.17, 33.05] | 28.50 [15.90, 41.25] |
| AST, U/L | 15.45 [12.83, 19.25] | 15.20 [12.70, 19.30] | 18.90 [14.35, 25.85]a,b | 16.90 [13.80, 24.70] | 22.50 [16.27, 36.47]c |
| TBIL, μmol/L | 10.75 [7.45, 13.67] | 9.30 [7.60, 12.05] | 11.30 [8.65, 14.30]a,b | 10.95 [8.00, 14.07] | 11.70 [9.50, 14.77] |
| DBIL, μmol/L | 3.90 [2.92, 5.47] | 3.80 [2.95, 4.65] | 4.40 [3.45, 5.55]a,b | 4.25 [3.30, 5.20] | 4.85 [3.70, 6.10]c |
| IBIL, μmol/L | 5.95 [4.08, 8.05] | 5.70 [4.50, 7.45] | 6.90 [4.85, 8.50]a,b | 6.60 [4.45, 8.52] | 6.90 [5.62, 8.55] |
| UA, μmol/L | 277.00 [228.00, 330.00] | 287.00 [234.00, 347.00] | 313.50 [247.25, 374.00]a,b | 315.00 [248.50, 376.25] | 315.50 [247.00, 356.50] |
| SCR, μmol/L | 66.00 [54.12, 75.55] | 67.35 [54.27, 79.55] | 64.20 [52.90, 77.48] | 65.20 [52.25, 80.10] | 65.70 [56.92, 79.12] |
| BUN, mmol/L | 5.42 [4.53, 6.34] | 5.54 [4.75, 6.47] | 5.38 [4.45, 6.06] | 5.41 [4.46, 6.00] | 5.36 [4.48, 6.45] |
| HT, yes, % | / | 72 (53.73) | 73(48.03) | 42 (40.43) | 31 (57.45) |
| Metformin, yes, % | / | 50 (37.31) | 60 (39.47) | 38 (43.63) | 22 (37.93) |
| Acarbose, yes, % | / | 28 (20.90) | 26 (17.11) | 12 (12.77) | 14 (24.14) |
| Sulfonylureas, yes, % | / | 15 (11.19) | 24 (15.79) | 11 (11.70) | 13 (24.41) |
| Glinides, yes, % | / | 3 (2.24) | 10 (6.58) | 7 (7.45) | 3 (5.17) |
| TZDs, yes, % | / | 3 (2.24) | 1 (0.66) | 1 (1.06) | 0 (0.00) |
| DPP-4 inhibitors, yes, % | / | 13 (9.70) | 16 (10.53) | 10 (10.64) | 6 (10.34) |
| SGLT-2 inhibitors, yes, % | / | 16 (11.94) | 14 (9.21) | 9 (9.57) | 5 (8.62) |
| GLP-1 agonists, yes, % | / | 1 (0.75) | 1 (0.66) | 0 (0.00) | 1 (1.72) |
| Insulin, yes, % | / | 35 (26.12) | 27 (17.76) | 16 (17.02) | 11 (18.97) |
| Statins, yes, % | / | 12 (8.96) | 18 (11.84) | 11(11.70) | 7 (12.07) |
In our study, we quantified 15 different BAs and 11 UFAs across groups comprising HC, T2DM, and NAFLD par
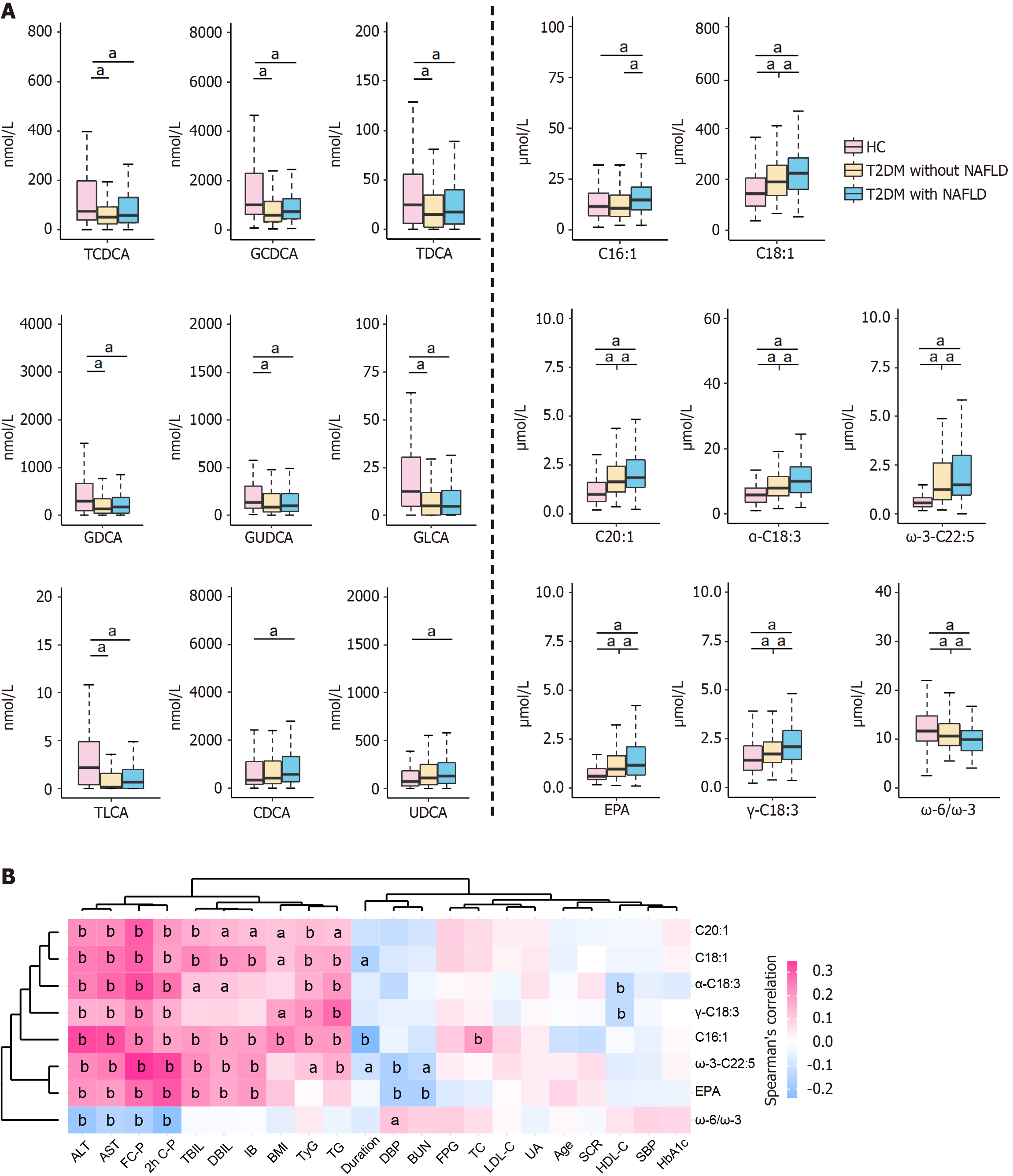
For UFAs, the T2DM and NAFLD groups exhibited elevated levels of 10 out of 11 UFAs, as well as three UFA-related ratios, compared to the HC group (Supplementary Figure 1A). Notably, the NAFLD group demonstrated increased levels of three MUFAs, including octadecenoic acid (C18:1), palmitoleic acid (C16:1), and eicosenoic acid (C20:1), three ω-3 PUFAs, including α-linolenic acid (α-C18:3), eicosapentaenoic acid (EPA), and ω-3 docosapentaenoic acid (ω-3-C22:5), and one ω-6 PUFA (γ-C18:3). Additionally, the NAFLD group displayed a lower ω-6:ω-3 PUFA ratio compared to the HC and T2DM groups (Figure 2A). The T2DM group also exhibited elevated levels of these UFAs relative to the HC group, with the exception of C16:1 (Figure 2A). However, no significant differences in UFA levels were observed between the T2DM and NAFLD groups (Supplementary Figure 1B).
We investigated the correlations between metabolite levels and clinical indicators within the T2DM and NAFLD groups (Figure 2B; Supplementary Figure 1C and D). We observed that both UFAs and BAs demonstrated significant correlations with indices of glycolipid metabolism and liver function. Specifically, four liver function markers, namely ALT, AST, FC-P, and 2h C-P, showed a significant positive correlation with seven distinct UFAs differentially expressed between the T2DM and NAFLD groups. These UFAs include C16:1, C18:1, C20:1, α-C18:3, EPA, ω-3-C22:5, and γ-C18:3 (P < 0.01). Additionally, these liver function markers exhibited a strong negative correlation with the ω-6:ω-3 ratio (Figure 2B). Moreover, TyG and TG were found to have a strong negative correlation with the ratios of GCDCA:CDCA and TCDCA:CDCA. Conversely, TyG and TG showed positive correlations with several UFAs, specifically C16:1, C18:1, C20:1, α-C18:3, ω-3-C22:5, and γ-C18:3. The detailed correlation coefficients and corresponding P values can be found in Supplementary Tables 3 and 4.
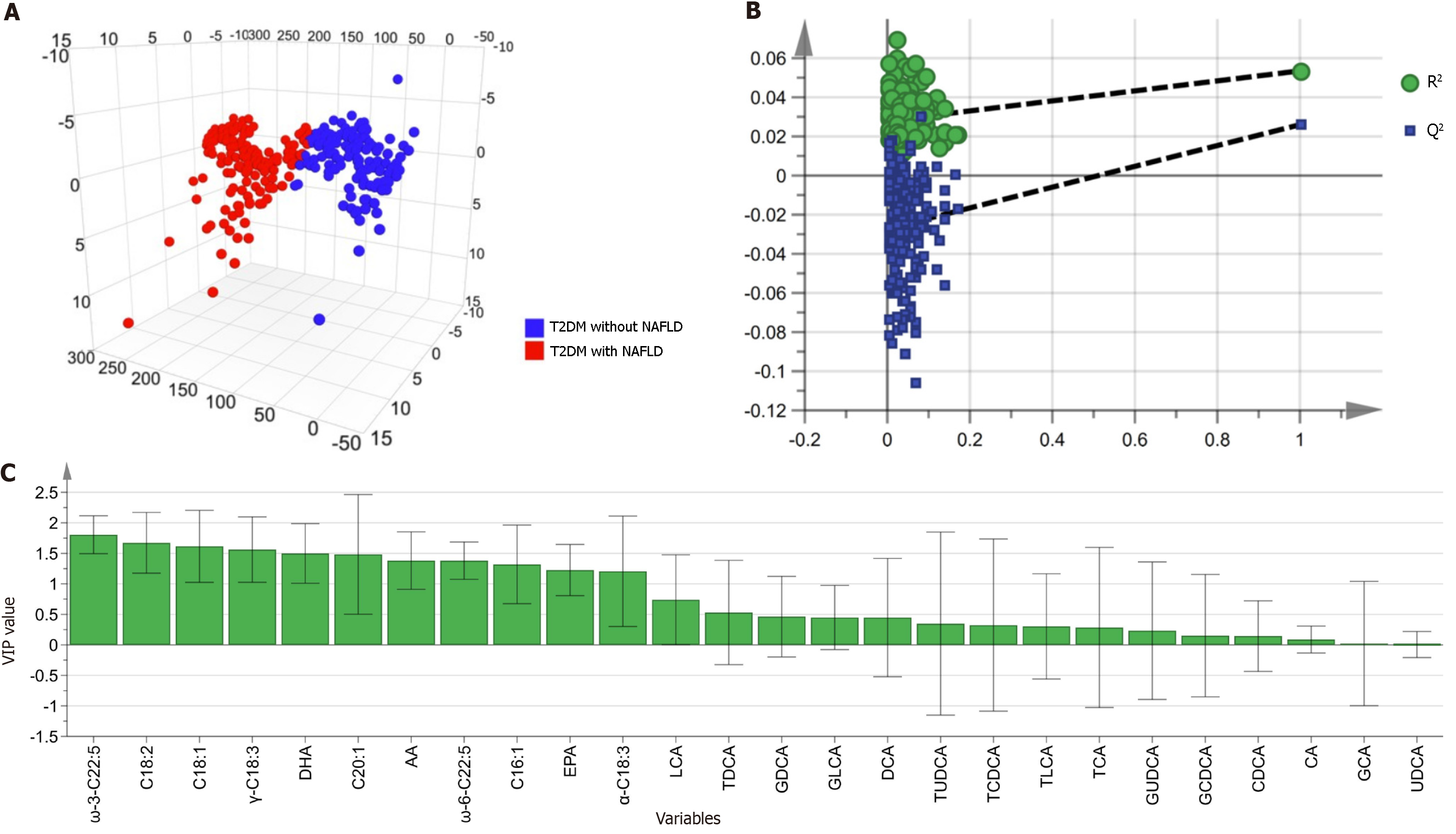
To identify biomarkers capable of differentiating between T2DM patients with and without NAFLD, we employed a multivariate analysis approach. This analysis was conducted on a comprehensive panel comprising 15 BAs and 11 UFAs (Figure 3). Utilizing SIMCA software, the data were standardized through the unit variance scaling method to ensure comparability. The OPLS-DA model, depicted in Figure 3A, successfully delineated the T2DM cohorts (± NAFLD), indicating clear group separation. To verify the reliability of our OPLS-DA model and rule out overfitting, we imple-mented a 200-permutation test, which confirmed the validity of our model (Figure 3B). The significance of these metabolites in differentiating between the groups was evaluated based on their VIP scores (Figure 3C; Supplementary Table 5). All UFAs demonstrated VIP values exceeding the threshold of 1, highlighting their potential as discriminative biomarkers. In contrast, none of the BAs achieved a VIP score above this threshold. We then selected UFAs and BAs that exhibited both statistical significance (P < 0.05 in the Wilcoxon test) and a VIP score > 1, when comparing the T2DM and NAFLD groups. These metabolites were deemed differential and were subsequently used for further logistic regression analysis (Table 2).
| Characteristics | Univariate | Multivariable adjusted | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| MUFAs | ||||||
| C16:1 (≥ 12.90 μmol/L) | 2.289 | 1.429-3.696 | 0.001a | 1.969 | 1.135-3.439 | 0.016a |
| C18:1 | 1.002 | 1.000-1.004 | 0.032a | 1.002 | 1.000-1.004 | 0.100 |
| C20:1 | 1.270 | 1.071-1.543 | 0.011a | 1.224 | 1.006-1.516 | 0.055 |
| ω-3 PUFAs | ||||||
| α-C18:3 (≥ 9.21 μmol/L) | 2.093 | 1.309-3.371 | 0.002a | 1.795 | 1.040-3.113 | 0.036a |
| EPA | 1.187 | 1.044-1.376 | 0.014a | 1.208 | 1.234-1.448 | 0.006a |
| ω-3-C22:5 | 1.239 | 1.066-1.457 | 0.007a | 1.262 | 1.051-1.531 | 0.015a |
| ω-6 PUFA | ||||||
| γ-C18:3 | 1.361 | 1.126-1.694 | 0.003a | 1.381 | 1.106-1.783 | 0.008a |
| MUFAs ratio | ||||||
| ω-6/ω-3 | 0.904 | 0.838-0.969 | 0.006a | 0.908 | 0.832-0.986 | 0.026a |
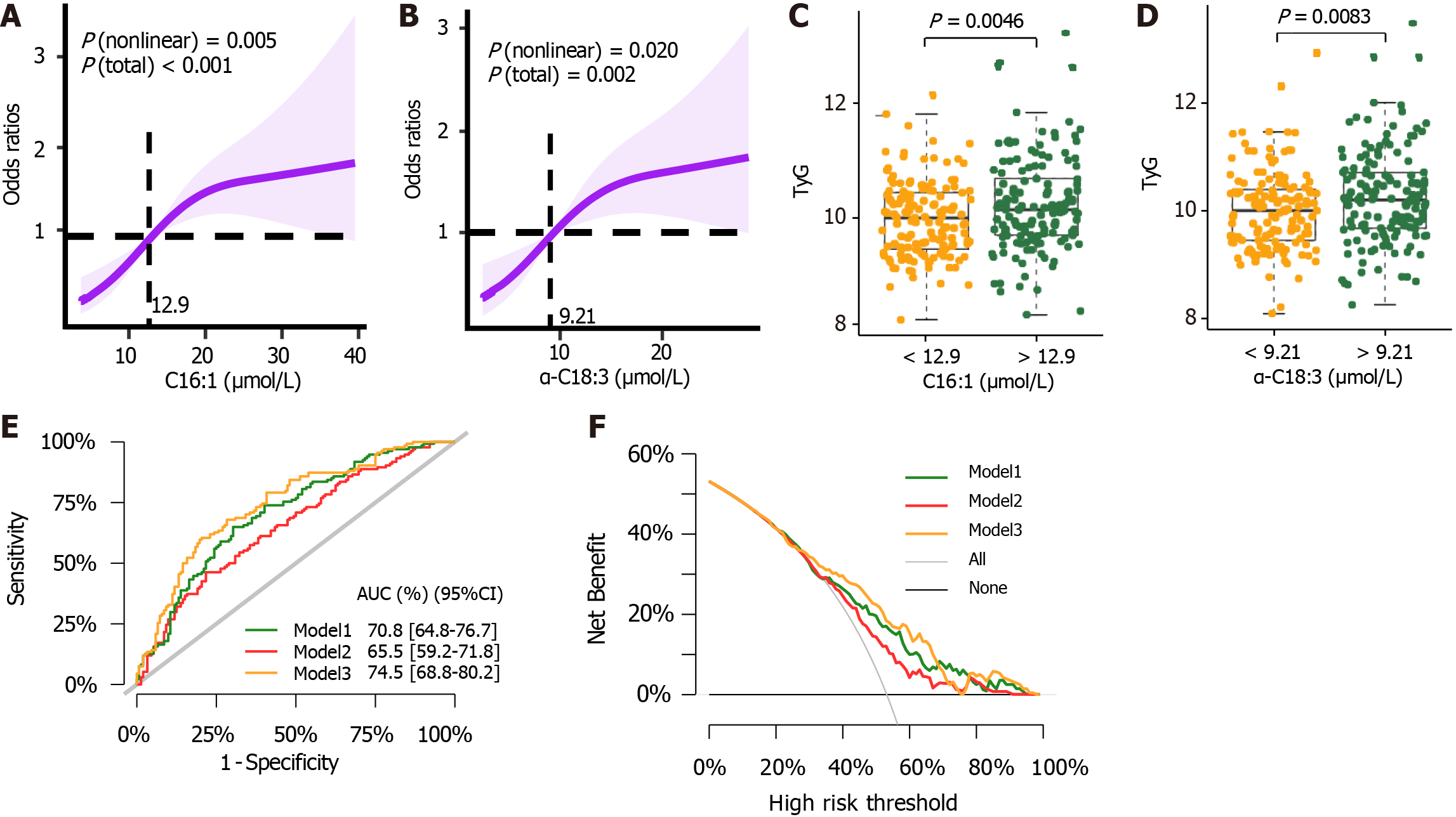
In our logistic regression analysis, RCS was utilized to examine potential non-linear relationships between metabolite levels and the risk of NAFLD in patients with T2DM. Through RCS, we identified critical thresholds where significant increases or decreases in NAFLD risk were evident. Metabolites were then categorized based on these thresholds and included in the regression model as continuous variables, with increments corresponding to their SD. Figure 4A and B illustrates the threshold effects for C16:1 (≥ 12.90 μmol/L) and α-C18:3 (≥ 9.21 μmol/L). These figures show that levels exceeding these cutoff points are associated with an increased risk of developing NAFLD in T2DM patients. Additionally, Figure 4C and D explores the relationship between α-C18:3 and C16:1 with TyG, a marker of β-cell function, using threshold analysis. Our multivariate logistic regression, adjusted for numerous confounding factors, demonstrated positive correlations between the risk of NAFLD in T2DM and several UFAs: C16:1, α-C18:3, EPA, ω-3-C22:5, and γ-C18:3. Conversely, negative correlation was observed with the ω-6:ω-3 PUFA ratio (Table 2).
Taking into account the VIP scores, results from the Wilcoxon test, and logistic regression findings, we identified these five UFA species as significant biomarkers: C16:1, α-C18:3, EPA, ω-3-C22:5, and γ-C18:3. The incremental effects and ROC of NAFLD risk prediction models are shown in Figure 4E and Supplementary Table 6. These demonstrate that the inclusion of these five biomarkers significantly enhances the clinical diagnosis of NAFLD. Moreover, decision curve analysis revealed a clear net benefit of incorporating these potential biomarkers alongside clinical indicators, underscoring their practical utility in clinical settings (Figure 4F).
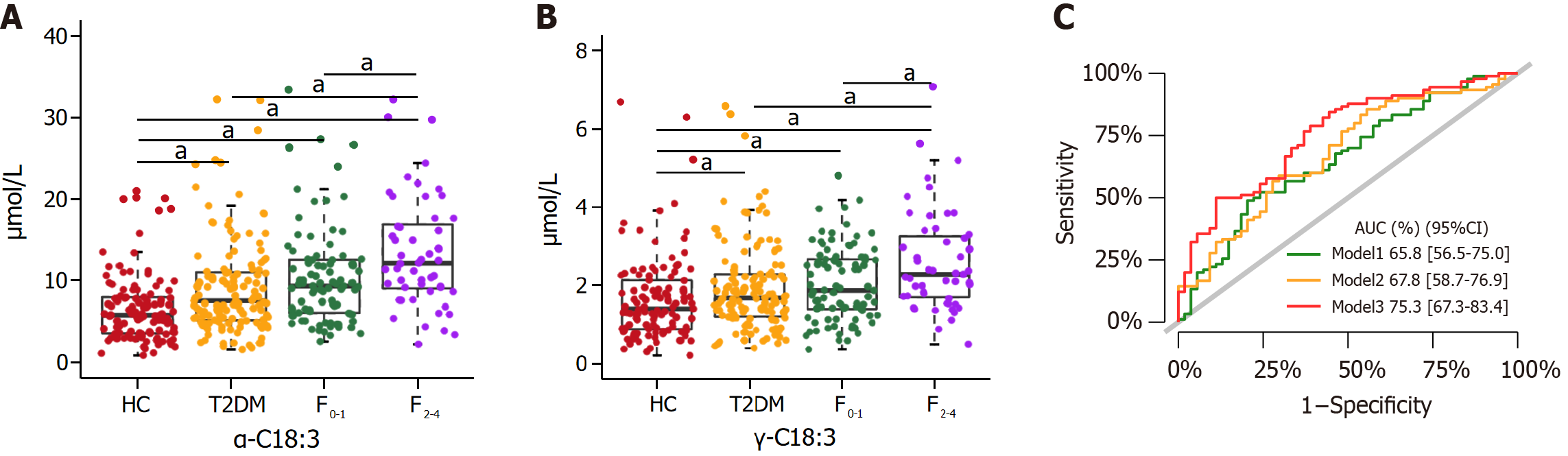
We further delved into the potential of certain metabolites, previously associated with the risk of NAFLD, to also serve as indicators for clinically significant liver fibrosis. Our observations revealed that α-C18:3 and γ-C18:3 exhibited significant differences between the NAFLD fibrosis stages F0-1 and F2-4 (Figure 5A and B; Supplementary Table 7). Further analysis through multivariate logistic regression established α-C18:3 and γ-C18:3 as predictive biomarkers for clinically significant fibrosis in the context of NAFLD (Table 3). The AUC values for predicting clinically significant fibrosis in NAFLD were 0.654 for α-C18:3 and 0.639 for γ-C18:3, indicating a moderate predictive ability (Supplementary Figure 2). Expanding our analysis, we constructed three risk prediction models (model 1, model 2, and model 3), specifically tailored for clinically significant fibrosis in NAFLD (Figure 5C). A comparative analysis of these models, focusing on their incremental effects, showed that the integration of α-C18:3 and γ-C18:3 into the models markedly enhanced the diagnostic accuracy for clinically significant liver fibrosis in NAFLD patients (Supplementary Table 8).
The performance of the biomarker panel model, designed to distinguish between NAFLD and non-NAFLD states, was evaluated using an independent validation cohort. Supplementary Table 9 and Figure 3A highlight significant differences in four UFAs, specifically C16:1, γ-C18:3, α-C18:3, and ω-3-C22:5, among the three training cohort groups. Supplementary Figure 3 shows a significant positive correlation between liver function markers (ALT, AST, FC-P, 2h C-P, TyG, TG, and UA), and these four differential UFAs in the T2DM and NAFLD groups (P < 0.05). The OPLS-DA model clustering, presented in Supplementary Figure 4, effectively differentiated between T2DM groups with and without NAFLD. The VIP scores of C16:1, γ-C18:3, α-C18:3, and ω-3-C22:5 was all equal to or greater than 1, signifying their relevance in the model (Supplementary Table 5; Figure 5C). The AUC values for model 1, model 2, and model 3 were presented in Supplementary Figure 5A. The comparative assessment between model 3 and model 2 in the validation cohort parallelled the observations from the training cohort (Supplementary Table 6; Figure 5). However, it is noteworthy that the multivariate logistic regression results for C16:1, γ-C18:3, α-C18:3, and ω-3-C22:5 (Supplementary Table 10) as well as the differences in γ-C18:3 and α-C18:3 levels between the two NAFLD subgroups, did not reach statistical significance in the validation cohort.
This study highlights the association between altered serum BA and UFA metabolism and NAFLD or its clinically significant fibrosis. Notably, we observed decreased levels of seven out of ten conjugated BAs in both T2DM and NAFLD groups compared to the HC group. In the T2DM group, after adjusting for confounders, an association was identified between the presence of NAFLD and increased levels of one MUFA (C16:1), one ω-6 PUFA (γ-C18:3), three ω-3 PUFAs (α-C18:3, EPA, and ω-3-C22:5), as well as a decreased ω-6:ω-3 PUFA ratio. Furthermore, α-C18:3 and γ-C18:3 emerged as potential biomarkers for clinically significant liver fibrosis in NAFLD. These findings support the association between alterations in serum BA and UFA metabolism and NAFLD or its clinically significant fibrosis. However, the exact causal relationship remains uncertain, laying a foundation for further research into the intricate role played by BAs and UFAs in the pathogenesis and progression of NAFLD.
CDCA serves as the primary BA, functioning as a natural ligand for FXR and G protein-coupled BA receptor 1 (TGR5), both crucial in lipid metabolism, insulin sensitivity, and weight regulation[9]. Our study revealed elevated levels of CDCA in the T2DM and NAFLD groups, aligning with previous observations linking CDCA to parameters such as BMI, TG, and HDL-C[3,25-27], indicators of NAFLD occurrence and alteration. Additionally, the hydrophobic nature of CDCA has been shown to induce hepatocyte injury[28]. Conjugated BAs, synthesized in the liver, undergo biotransformation and degradation by intestinal bacteria, forming unconjugated BAs that are reabsorbed through enterohepatic circulation[29]. In our T2DM or NAFLD patients, decreased levels of glycine- and taurine-conjugated BAs were observed. The causes of this decrease, particularly whether disruptions in the structural composition of intestinal flora contribute to this phenomenon, remain unclear. Furthermore, conjugated deoxycholic acid (DCA; including GDCA and TDCA) and conjugated lithocholic acid (LCA; including TLCA and GLCA) were found to be negatively associated with an increased risk of fatty liver or obesity[3,30]. The underlying mechanism for this association may involve the BA-TGR5 signaling pathway. In this pathway, DCA and LCA function as potent agonists of TGR5, which are known to exert anti-inflammatory effects and ameliorate metabolic disorders induced by a high-fat diet[31].
The observed elevated UFAs in T2DM and NAFLD patients are likely linked to insulin resistance and adipose tissue lipolysis[32]. NAFLD in T2DM exacerbates insulin resistance, promoting lipid mobilization to the liver[33]. High serum UFAs in these patients, alongside elevated TyG index and C-P levels, may indicate β cell dysfunction.
γ-C18:3, a PUFA derived from essential fatty acid C18:2, involves D6 desaturase (D6D) in its conversion, with heightened D6D activity linked to increased insulin resistance and T2DM risk[34]. Elevated γ-C18:3 is positively associated with susceptibility to T2DM and NAFLD[35,36], aligning with our findings. α-C18:3, an ω-3 PUFA, can’t be synthesized endogenously and showed elevated levels in our T2DM and NAFLD patients[37]. Studies highlight its pharmacological benefits in metabolic syndrome, yet note potential inflammatory and oxidative stress concerns at varying concentrations[38,39]. Elevated α-C18:3 levels have been correlated with impaired insulin signaling, mito-chondrial function, and increased reactive oxygen species production observed in obese Zucker rats[40]. α-C18:3 converts to EPA via delta 6-desaturase and delta 5-desaturase[41]. EPA, extensively studied for its cardiovascular benefits, presents potential adverse effects, including oxidative stress and increased bleeding risk[42]. Another study indicated higher EPA intake associated with elevated T2DM risk[43]. Further research is necessary to understand elevated EPA levels in our patients. C16:1, a primary MUFA, plays a pivotal role in metabolic regulation[44]. Human studies link elevated C16:1 to NAFLD, inflammatory markers, obesity, insulin resistance, and heightened diabetes risk[45]. Palmitate, its precursor, triggers β cell and endothelial cell apoptosis, contributing to insulin resistance and atherosclerosis[46]. Recent research correlates C16:1 with metabolic risk factors in polycystic ovary syndrome[47]. Non-metabolic conditions such as asthma and breast cancer have also been notably associated with C16:1[48,49]. However, despite this evidence, there is a need for further investigation to elucidate the reasons behind elevated levels of C16:1 in T2DM and NAFLD patients and to understand the underlying pathophysiology in these metabolic diseases.
In our study, we observed linear correlations between EPA, ω-3-C22:5, γ-C18:3, and the risk of NAFLD in T2DM. RCS analysis revealed non-linear correlations for α-C18:3 and C16:1 PUFAs. A meta-analysis has indicated a significant non-linear association between specific PUFAs and T2DM[43]. However, the relationship between PUFA levels and NAFLD in T2DM patients remains to be fully elucidated. Investigating the precise threshold effects of α-C18:3 and C16:1 PUFAs is crucial for understanding their potential benefits at lower concentrations.
Several studies highlight the metabolic benefits of ω-3 PUFAs. However, total dietary fat intake exceeding 37% of energy has been associated with an increased risk of insulin resistance, irrespective of the type of fat. A balanced ω-6:ω-3 ratio is crucial due to the competition between enzymes during their biosynthesis. Our findings align with Luo et al[50], showing a decreasing trend in the ω-6:ω-3 ratio from HC to T2DM to NAFLD groups. An imbalanced ratio may affect postprandial blood glucose through alternative pathways, potentially impacting carbohydrate-regulating proteins[51]. We observed a greater increase in ω-3 PUFA levels compared to ω-6 PUFAs, aligning with studies suggesting that elevated ω-3 PUFA intake is associated with an increased risk of T2DM[43], likely due to heightened blood glucose levels and reduced insulin sensitivity[52]. This finding is consistent with the well-established link between insulin resistance and the risk of NAFLD associated with T2DM[52]. These insights may provide valuable UFA-centric perspectives on the etiology of diabetes and NAFLD. Further research is needed to determine the impact of an imbalanced ω-6:ω-3 PUFA ratio on glucose and lipid metabolism.
The present study has some limitations. Firstly, its retrospective nature necessitates more prospective studies to determine whether changes in BA and UFA levels are causative or consequential in relation to NAFLD. Secondly, lack of data on physical activity and diet, factors closely associated with BMI, is a limitation. Nevertheless, we adjusted for BMI in our multifactorial logistic regression analysis, and certain associations between UFA and NAFLD remained statistically significant. Thirdly, while ultrasonography is a clinically practical diagnostic tool, it may not be accurate as liver biopsy in diagnosing fatty liver.
This study represents the first large-scale investigation to establish a link between BAs and UFAs with the risk of NAFLD in T2DM patients. Our aim was to identify predictive biomarkers and to enhance the understanding of the complex relationships between these metabolites and NAFLD. While we have made significant strides, the underlying mechanisms remain to be elucidated. Future research should focus on expanding the sample size and conducting prospective studies or animal experiments to explore the complex interplay between NAFLD and T2DM. Such studies are essential to deepen our understanding of the pathophysiological processes influencing BA and UFA metabolism in the context of T2DM. In the realm of clinical practice, our findings may serve as a valuable reference for monitoring these biomarkers, thereby aiding in the assessment of NAFLD risk in patients with T2DM.
Previous studies revealed that impaired metabolism of serum bile acid (BA) and unsaturated fatty acid (UFA) are associated with the onset and progression of type 2 diabetes mellitus (T2DM). However, the BA and UFA profiles and their alterations associated with the risk of developing Non-alcoholic fatty liver disease (NAFLD) remain unknown.
This study aims to delineate the differences in BA and UFA profiles between T2DM patients with and without NAFLD, seeking to elucidate the underlying pathogenic mechanisms of NAFLD in the context of T2DM.
To identify distinct metabolite biomarkers within BA and UFA profiles that are associated with NAFLD risk in individuals with T2DM.
A training model, consisting of 399 participants (113 healthy controls, 134 T2DM without NAFLD, and 152 T2DM with NAFLD), was established alongside an external validation model comprising 172 participants. NAFLD patients were stratified based on liver fibrosis scores. Fasting venous blood samples were collected from all subjects for the analysis of BA and UFA profiles, utilizing liquid chromatography coupled with tandem mass spectrometry.
Both T2DM and NAFLD groups exhibited lower levels of certain BAs compared to healthy controls. Certain UFAs demonstrated a positive correlation with NAFLD risk in T2DM. Levels of α-linolenic acid and γ-linolenic acid were associated with significant liver fibrosis in NAFLD, and validation confirmed their predictive power for NAFLD risk in T2DM patients.
Our findings reveal significant differences in serum BA and UFA profiles in T2DM patients with NAFLD compared to those without, suggesting a potential role in the pathogenesis of NAFLD.
To unravel the intricate interplay between NAFLD and T2DM, future research endeavors should encompass larger sample sizes and incorporate prospective studies or animal experiments, particularly focusing on the pathophysiological conditions influencing BA and UFA metabolism.
The authors thank the research volunteers for their participation in this study.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7965] [Article Influence: 796.5] [Reference Citation Analysis (8)] |
| 2. | Simeone JC, Bae JP, Hoogwerf BJ, Li Q, Haupt A, Ali AK; Boardman MK; Nordstrom BL. Clinical course of nonalcoholic fatty liver disease: an assessment of severity, progression, and outcomes. Clin Epidemiol. 2017;9:679-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Caussy C, Hsu C, Singh S, Bassirian S, Kolar J, Faulkner C, Sinha N, Bettencourt R, Gara N, Valasek MA, Schnabl B, Richards L, Brenner DA, Hofmann AF, Loomba R. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. 2019;49:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 358] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 5. | Qi Y, Fan L, Ran D, Xu J, Wang Y, Wu J, Zhang Z. Main Risk Factors of Type 2 Diabetes Mellitus with Nonalcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J Oncol. 2021;2021:7764817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Kaya E, Yilmaz Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J Clin Transl Hepatol. 2022;10:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 8. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1953] [Article Influence: 390.6] [Reference Citation Analysis (33)] |
| 9. | Perino A, Schoonjans K. Metabolic Messengers: bile acids. Nat Metab. 2022;4:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 10. | Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, Windmolders P, Farre R, Wenes M, Mazzone M, Nevens F, van Grunsven LA, Trebicka J, Laleman W. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 920] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 12. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 566] [Article Influence: 70.8] [Reference Citation Analysis (1)] |
| 13. | Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsbøll T, Knop FK. Postprandial Plasma Concentrations of Individual Bile Acids and FGF-19 in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:3002-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 14. | Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AS 4th. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 15. | Hodson L, Rosqvist F, Parry SA. The influence of dietary fatty acids on liver fat content and metabolism. Proc Nutr Soc. 2020;79:30-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Mocciaro G, Allison M, Jenkins B, Azzu V, Huang-Doran I, Herrera-Marcos LV, Hall Z, Murgia A, Susan D, Frontini M, Vidal-Puig A, Koulman A, Griffin JL, Vacca M. Non-alcoholic fatty liver disease is characterised by a reduced polyunsaturated fatty acid transport via free fatty acids and high-density lipoproteins (HDL). Mol Metab. 2023;73:101728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Coelho OGL, da Silva BP, Rocha DMUP, Lopes LL, Alfenas RCG. Polyunsaturated fatty acids and type 2 diabetes: Impact on the glycemic control mechanism. Crit Rev Food Sci Nutr. 2017;57:3614-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Molendi-Coste O, Legry V, Leclercq IA. Why and How Meet n-3 PUFA Dietary Recommendations? Gastroenterol Res Pract. 2011;2011:364040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Lee CH, Fu Y, Yang SJ, Chi CC. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlström H, Risérus U. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 21. | Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulangé A, Vidal H, Slama G, Clément K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Thérien A, Cieślak A, Verreault M, Perreault M, Trottier J, Gobeil S, Vohl MC, Barbier O. Omega-3 Polyunsaturated Fatty Acid: A Pharmaco-Nutraceutical Approach to Improve the Responsiveness to Ursodeoxycholic Acid. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Wiesmann UN, DiDonato S, Herschkowitz NN. Effect of chloroquine on cultured fibroblasts: release of lysosomal hydrolases and inhibition of their uptake. Biochem Biophys Res Commun. 1975;66:1338-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Fan JG, Wei L, Zhuang H; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 26. | Yu H, Ni Y, Bao Y, Zhang P, Zhao A, Chen T, Xie G, Tu Y, Zhang L, Su M, Wei L, Jia W. Chenodeoxycholic Acid as a Potential Prognostic Marker for Roux-en-Y Gastric Bypass in Chinese Obese Patients. J Clin Endocrinol Metab. 2015;100:4222-4230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Wu T, Yang M, Xu H, Wang L, Wei H, Ji G. Serum Bile Acid Profiles Improve Clinical Prediction of Nonalcoholic Fatty Liver in T2DM patients. J Proteome Res. 2021;20:3814-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun Rev. 2017;16:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Fall T, Salihovic S, Brandmaier S, Nowak C, Ganna A, Gustafsson S, Broeckling CD, Prenni JE, Kastenmüller G, Peters A, Magnusson PK, Wang-Sattler R, Giedraitis V, Berne C, Gieger C, Pedersen NL, Ingelsson E, Lind L. Non-targeted metabolomics combined with genetic analyses identifies bile acid synthesis and phospholipid metabolism as being associated with incident type 2 diabetes. Diabetologia. 2016;59:2114-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Li W, Liu R, Li X, Tao B, Zhai N, Wang X, Li Q, Zhang Y, Gu W, Wang W, Ning G. Saxagliptin alters bile acid profiles and yields metabolic benefits in drug-naïve overweight or obese type 2 diabetes patient. J Diabetes. 2019;11:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Chiappini F, Coilly A, Kadar H, Gual P, Tran A, Desterke C, Samuel D, Duclos-Vallée JC, Touboul D, Bertrand-Michel J, Brunelle A, Guettier C, Le Naour F. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci Rep. 2017;7:46658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (1)] |
| 33. | Mu W, Cheng XF, Liu Y, Lv QZ, Liu GL, Zhang JG, Li XY. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front Pharmacol. 2018;9:1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 34. | Murff HJ, Edwards TL. Endogenous Production of Long-Chain Polyunsaturated Fatty Acids and Metabolic Disease Risk. Curr Cardiovasc Risk Rep. 2014;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Mäkelä TNK, Tuomainen TP, Hantunen S, Virtanen JK. Associations of serum n-3 and n-6 polyunsaturated fatty acids with prevalence and incidence of nonalcoholic fatty liver disease. Am J Clin Nutr. 2022;116:759-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Prada M, Eichelmann F, Wittenbecher C, Kuxhaus O, Schulze MB. Plasma Lipidomic n-6 Polyunsaturated Fatty Acids and Type 2 Diabetes Risk in the EPIC-Potsdam Prospective Cohort Study. Diabetes Care. 2023;46:836-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 37. | Edel AL, Patenaude AF, Richard MN, Dibrov E, Austria JA, Aukema HM, Pierce GN, Aliani M. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults. Eur J Nutr. 2016;55:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 38. | Heskey CE, Jaceldo-Siegl K, Sabaté J, Fraser G, Rajaram S. Adipose tissue α-linolenic acid is inversely associated with insulin resistance in adults. Am J Clin Nutr. 2016;103:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Shen Y, Chen G, Xiao A, Xie Y, Liu L, Cao Y. In vitro effect of flaxseed oil and α-linolenic acid against the toxicity of lipopolysaccharide (LPS) to human umbilical vein endothelial cells. Inflammopharmacology. 2018;26:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Wen J, Khan I, Li A, Chen X, Yang P, Song P, Jing Y, Wei J, Che T, Zhang C. Alpha-linolenic acid given as an anti-inflammatory agent in a mouse model of colonic inflammation. Food Sci Nutr. 2019;7:3873-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Monaco CMF, Proudfoot R, Miotto PM, Herbst EAF, MacPherson REK, Holloway GP. α-linolenic acid supplementation prevents exercise-induced improvements in white adipose tissue mitochondrial bioenergetics and whole-body glucose homeostasis in obese Zucker rats. Diabetologia. 2018;61:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Jairoun AA, Shahwan M, Zyoud SH. Fish oil supplements, oxidative status, and compliance behaviour: Regulatory challenges and opportunities. PLoS One. 2020;15:e0244688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Hu M, Fang Z, Zhang T, Chen Y. Polyunsaturated fatty acid intake and incidence of type 2 diabetes in adults: a dose response meta-analysis of cohort studies. Diabetol Metab Syndr. 2022;14:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 876] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 45. | Miyake T, Furukawa S, Matsuura B, Yoshida O, Miyazaki M, Shiomi A, Kanzaki S, Nakaguchi H, Sunago K, Nakamura Y, Imai Y, Watanabe T, Yamamoto Y, Koizumi Y, Tokumoto Y, Hirooka M, Kumagi T, Abe M, Hiasa Y. Plasma Fatty Acid Composition Is Associated with Histological Findings of Nonalcoholic Steatohepatitis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 46. | Maris M, Robert S, Waelkens E, Derua R, Hernangomez MH, D’Hertog W, Cnop M, Mathieu C, Overbergh L. Role of the saturated nonesterified fatty acid palmitate in beta cell dysfunction. J Proteome Res. 2013;12:347-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Tian Y, Zhang J, Li M, Shang J, Bai X, Zhang H, Wang Y, Chen H, Song X. Serum fatty acid profiles associated with metabolic risk in women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2023;14:1077590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 48. | Tashima N, Matsumoto H, Nishi K, Terada S, Kogo M, Nomura N, Morimoto C, Sunadome H, Nagasaki T, Oguma T, Nakatsuka Y, Murase K, Kawaguchi T, Tabara Y, Chin K, Sonomura K, Matsuda F, Hirai T. Evaluation of elevated plasma fatty acids as relevant factors for adult-onset asthma: The Nagahama Study. Allergol Int. 2024;73:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 49. | Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nälsén C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH; KANWU Study. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 733] [Article Influence: 29.3] [Reference Citation Analysis (14)] |
| 50. | Luo HH, Zhao MD, Feng XF, Gao XQ, Hong M, Liu ML, Li YP, Liu WQ, Liu YM, Yu CC, Cao YF, Yang XL, Fang ZZ, Zhang P. Decreased plasma n6 : n3 polyunsaturated fatty acids ratio interacting with high C-peptide promotes non-alcoholic fatty liver disease in type 2 diabetes patients. J Diabetes Investig. 2021;12:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Zhang Y, Zhuang P, Mao L, Chen X, Wang J, Cheng L, Ding G, Jiao J. Current level of fish and omega-3 fatty acid intakes and risk of Type 2 diabetes in China. J Nutr Biochem. 2019;74:108249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsaidan A, Saudi Arabia; Horowitz M, Australia S-Editor: Chen YL L-Editor: A P-Editor: Guo X