Published online Jul 15, 2023. doi: 10.4239/wjd.v14.i7.1103
Peer-review started: March 28, 2023
First decision: April 10, 2023
Revised: April 23, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: July 15, 2023
Processing time: 106 Days and 22.5 Hours
Retinopathy is the most common microvascular disease of type 2 diabetes, and seriously threatens the life, health and quality of life of patients. It is worth noting that the development of diabetic retinopathy (DR) can be hidden, with few symptoms. Therefore, the preliminary screening of diabetic patients should identify DR as soon as possible, delay disease progression, and play a vital role in its diagnosis and treatment.
To investigate the correlation between glycated hemoglobin A1c (HbA1c), urinary microalbumin (U-mALB), urinary creatinine (U-CR), mALB/U-CR ratio, β2 microglobulin (β2MG), retinol binding protein (RBP) and DR.
A total of 180 patients with type 2 diabetes mellitus attending the Second People’s Hospital of Hefei from January 2022 to August 2022 were retrospectively enrolled by ophthalmologists. Based on whether they had combined retinopathy and its degree, 68 patients with diabetes mellitus without retinopathy (NDR) were assigned to the NDR group, 54 patients with non-proliferative DR (NPDR) to the NPDR group, and 58 patients with proliferative DR to the PDR group. General data, and HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR results were collected from the patients and compared among the groups. Pearson's correlation method was used to analyze the correlation between HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR indices, and multiple linear regression was applied to identify the risk factors for DR. Receiver operator characteristic (ROC) curves were also drawn.
The differences in age, gender, systolic and diastolic blood pressure between the groups were not statistically significantly (P > 0.05), but the difference in disease duration was statistically significant (P < 0.05). The differences in fasting blood glucose, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, and triglyceride between the groups were not statistically significant (P > 0.05). HbA1c in the PDR group was higher than that in the NPDR and NDR groups (P < 0.05). The levels of mALB, β2MG, RBP, mALB/U-CR and U-CR in the PDR group were higher than those in the NPDR and NDR groups (P < 0.05). Multiple linear regression analysis showed that disease duration, HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR were risk factors for the development of DR. The ROC curve showed that the area under the curve (AUC) for the combination of indices (HbA1c + mALB + mALB/U-CR + U-CR + β2MG + RBP) was 0.958, with a sensitivity of 94.83% and specificity of 96.72%, which was higher than the AUC for single index prediction (P < 0.05).
HbA1c, mALB, mALB/U-CR, U-CR, β2MG and RBP can reflect the development of DR and are risk factors affecting PDR, and the combination of these six indices has predictive value for PDR.
Core Tip: Diabetes retinopathy (DR) is a common complication of diabetes, which can eventually lead to blindness in diabetic patients and seriously affect the quality of life of patients. The identification of risk factors for DR is significant for early intervention. Here we retrospectively analyzed 180 patients with type 2 diabetes mellitus to examine the correlation between glycated hemoglobin A1c, microalbumin (mALB), mALB/urinary creatinine (U-CR), U-CR, ββ2 microglobulin, retinol binding protein and DR in diabetic patients in order to provide a scientific basis and guidance for clinical application.
- Citation: Song JJ, Han XF, Chen JF, Liu KM. Correlation between glycated hemoglobin A1c, urinary microalbumin, urinary creatinine, β2 microglobulin, retinol binding protein and diabetic retinopathy. World J Diabetes 2023; 14(7): 1103-1111
- URL: https://www.wjgnet.com/1948-9358/full/v14/i7/1103.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i7.1103
Diabetic retinopathy (DR) is an irreversible blindness-causing disease[1]. The prevalence of diabetes in China accounts for 26.2% of the global diabetic population, and the prevalence of DR is approximately 35%-50%[2]. The prevalence of DR in Singapore and the United States is 20.1% and 25.7%, respectively[3]. The disease progresses rapidly and if not diagnosed and treated early, it will seriously affect the visual field and vision. In severe cases, patients may even lose their sight, which causes many inconveniences to their life and work and hinders their normal life. Therefore, early clinical diagnosis is important for the subsequent treatment of DR patients[3]. Currently, the clinical diagnosis of this disease is mainly based on fundus photography and fluorescein angiography, but the application process is complicated and may cause adverse reactions in diabetic patients. In addition, there is a lack of convenient and intuitive biochemical markers providing guidance for the diagnosis of DR[4]. Therefore, it is important to identify relevant biochemical markers to predict DR. Urinary β2 microglobulin (β2MG) has been found to be closely associated with microvascular complications such as diabetic nephropathy. It is known that DR is a microvascular complication, so it is assumed that the pathogenesis of the two diseases is similar and β2MG may be a useful marker for predicting DR[5]. Retinol-binding protein (RBP), a lipid-derived cytokine, has been shown to be closely associated with the development of diabetes mellitus and diabetic vasculopathy[6]. Urinary microalbumin (U-mALB), urinary creatinine (U-CR) and the mALB/U-CR ratio are predictors of diabetic vasculopathy and are risk factors for endothelial cell function and microvascular function[7]. In this study, we aimed to examine the correlation between glycated hemoglobin A1c (HbA1c), β2MG, RBP, mALB, U-CR, mALB/U-CR and DR lesions in patients with DR. The innovation of this study is determination of the predictive value of the combined detection of HbA1c, mALB, mALB/U-CR, U-CR, β2MG, and RBP in DR using real clinical data. The clinical significance is to provide a scientific basis and guidance for the clinical use of the combined detection of HbA1c, mALB, mALB/U-CR, U-CR, β2MG, and RBP to evaluate the risk of DR.
A total of 180 type 2 diabetic patients attending the Second People’s Hospital of Hefei from January 2022 to August 2022 were enrolled retrospectively, including 68 patients with diabetes without retinopathy (NDR group), 54 patients with non-proliferative diabetic retinopathy (NPDR group), and 58 with proliferative diabetic retinopathy (PDR group).
(1) The study subjects met the diagnostic criteria for type 2 diabetes mellitus[8]; (2) The diagnosis of DR was based on the International Clinical Classification Criteria for Diabetic Retinopathy[9]. NPDR: microaneurysm alone was observed or 4 quadrants with intraretinal hemorrhage and microangioma; or moderate retinal mesangiopathy occurring in more than 2 or more quadrants; PDR: If the retina had new abnormal blood vessels, this was considered PDR. The diagnosis was confirmed by satisfying one or more of the following: neovascularization, vitreous hematopoiesis or anterior retinal hemorrhage; and (3) None of the study subjects had a history of trauma or ocular surgery.
(1) Those with combined non-fundus pathology, e.g., cataract, glaucoma; (2) Those with poorly graded fundus visual field images due to blurring of large blood vessels adjacent to the optic disc, and whose diagnosis was more difficult to further confirm on fundus examination; (3) Those with organ disease, such as coronary artery disease, heart failure, diabetic nephropathy, etc.; (4) Combined with diabetic complications, such as diabetic gangrene, stroke, or atherosclerosis; and(5) Difficult to cooperate in the completion of the study.
General information of the patients was collected, including age, gender, duration of disease, systolic and diastolic blood pressure. Blood was collected in the morning after a 12-h fast to measure HbA1c, fasting blood glucose (FPG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG) using a glycated hemoglobin analyzer and supporting reagents.
Urinary mALB and U-CR concentrations were measured using a special protein analyzer and the urinary mALB/U-CR ratio was calculated three times. β2MG was measured by the immunoturbidimetric method and RBP was measured using an automatic biochemical analyzer.
General information: age, gender, duration of disease, systolic and diastolic blood pressure. Clinical indicators: FPG, HDL-C, LDL-C, TC, TG, and HbA1c. Combined indicators: mALB, mALB/U-CR, U-CR, β2MG, and RBP levels.
GraphPad Prism 9 was used to analyze the study data and for image export. The measurement data were expressed as mean ± SD, and compared by one-way ANOVA for multiple groups of data or for two groups of data. The count data were expressed by n (%), and compared using the χ2 test. Correlation analysis and risk factor identification were performed using Pearson’s correlation method and multiple linear regression, respectively. A receiver operator characteristic (ROC) curve was plotted to predict the value of PDR. P < 0.05 was considered statistically significant.
The differences in age, gender, systolic and diastolic blood pressure between the three groups were not significant (P > 0.05), but the differences in disease duration were significant (P < 0.05, Table 1).
| Group | Age (yr) | Sex (M/F) | Duration of illness (yr) | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) |
| NDR (n = 68) | 57.71 ± 7.18 | 37/31 | 4.21 ± 0.81 | 117.47 ± 19.38 | 76.05 ± 9.48 |
| NPDR (n = 54) | 58.00 ± 8.93 | 29/25 | 6.22 ± 1.26 | 118.32 ± 16.02 | 75.34 ± 11.91 |
| PDR (n = 58) | 56.59 ± 7.12 | 31/37 | 8.12 ± 1.47 | 111.33 ± 18.09 | 75.69 ± 7.96 |
| F/χ2 value | 0.534 | 0.013 | 169.133 | 2.606 | 0.178 |
| P value | 0.587 | 0.994 | < 0.001 | 0.078 | 0.836 |
No significant differences in FPG, HDL-C, LDL-C, TC and TG were observed among the groups (P > 0.05); HbA1c in the PDR group was higher than that in the NPDR and NDR groups (P < 0.05, Table 2).
| Group | HbA1c (%) | FPG (mmol/L) | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | HDL-C (mmol/L) |
| NDR (n = 68) | 8.01 ± 1.86 | 8.60 ± 1.96 | 4.86 ± 0.98 | 1.68 ± 0.21 | 2.61 ± 0.42 | 1.15 ± 0.22 |
| NPDR (n = 54) | 9.14 ± 2.12 | 8.55 ± 1.94 | 4.42 ± 0.75 | 1.69 ± 0.27 | 2.62 ± 0.41 | 1.24 ± 0.20 |
| PDR (n = 58) | 10.28 ± 2.66 | 8.92 ± 2.16 | 4.55 ± 0.84 | 1.77 ± 0.29 | 2.74 ± 0.54 | 1.22 ± 0.27 |
| F value | 15.385 | 0.572 | 0.319 | 2.216 | 1.476 | 1.073 |
| P value | < 0.001 | 0.565 | 0.726 | 0.112 | 0.231 | 0.344 |
The levels of mALB, β2MG, RBP, mALB/U-CR, and U-CR in the PDR group were higher than those in the NPDR and NDR groups (P < 0.05, Table 3).
| Group | mALB (mg/L) | mALB/U-CR (mg/mmoL) | U-CR (μmol/L) | β2MG (mg/L) | RBP (μg/L) |
| NDR (n = 68) | 15.04 ± 1.94 | 2.19 ± 0.86 | 6.86 ± 1.67 | 2.28 ± 0.66 | 12.29 ± 2.82 |
| NPDR (n = 54) | 65.69 ± 7.30 | 3.29 ± 1.26 | 19.97 ± 5.81 | 3.13 ± 0.84 | 21.58 ± 4.83 |
| PDR (n = 58) | 170.29 ± 11.63 | 5.09 ± 1.02 | 33.35 ± 11.45 | 4.53 ± 0.97 | 36.78 ± 7.84 |
| F value | 147.103 | 121.668 | 206.027 | 117.619 | 69.460 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
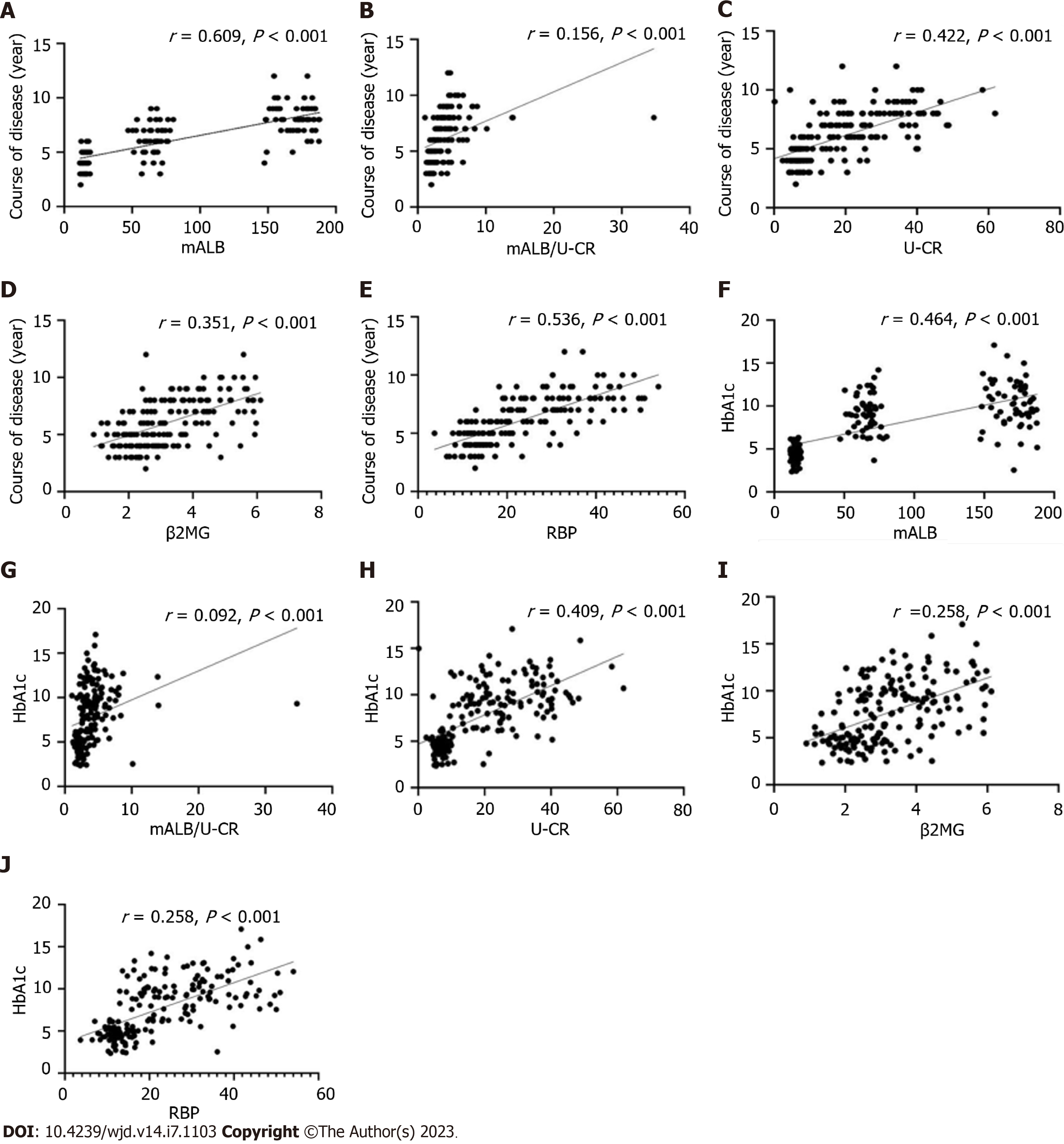
By Pearson's correlation analysis, mALB, mALB/U-CR, U-CR, β2MG, and RBP were positively correlated with disease duration and HbA1c, (P < 0.05, Figure 1).
With PDR as the dependent variable (yes = 1, no = 0) and the above meaningful results as independent variables all included as original values, multiple linear regression analysis was performed and the results revealed that disease duration, HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR were all risk factors for the development of PDR (Table 4).
| Independent variable | B value | SE | β value | t value | P value |
| Course of disease | 1.203 | 0.293 | 0.220 | 4.106 | < 0.001 |
| HbA1c | 0.942 | 0.192 | 0.755 | 4.906 | < 0.001 |
| mALB | 0.874 | 0.128 | 0.256 | 6.828 | < 0.001 |
| mALB/U-CR | 0.743 | 0.284 | 0.525 | 6.959 | < 0.001 |
| U-CR | 0.842 | 0.121 | 0.254 | 6.959 | < 0.001 |
| β2MG | 1.048 | 0.123 | 0.157 | 8.520 | < 0.001 |
| RBP | 1.262 | 0.184 | 0.215 | 3.271 | < 0.001 |
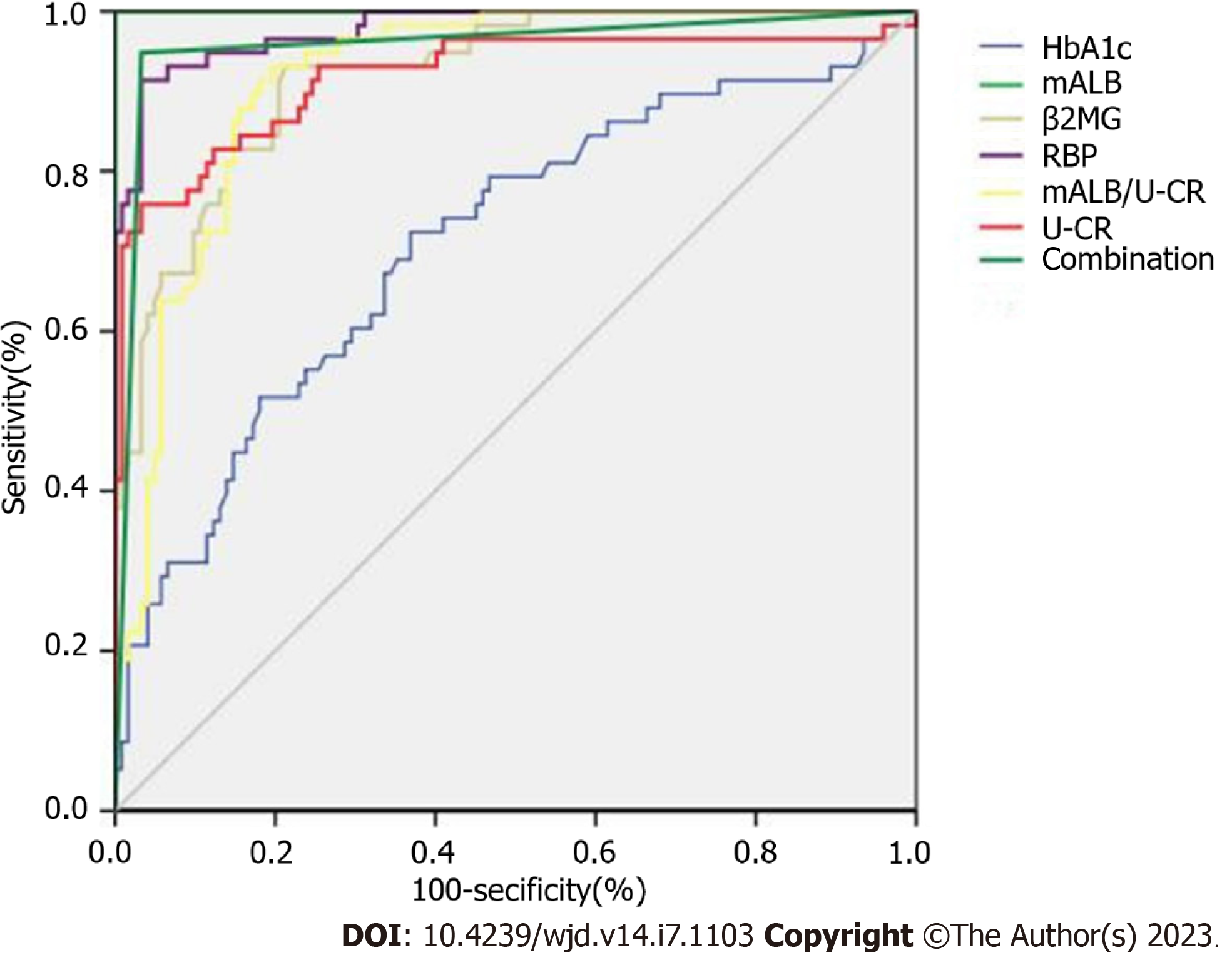
As shown in Table 5 and Figure 2, the ROC curve indicated that the combined diagnostic area under the curve of the indicators was 0.904, with a sensitivity of 92.53% and specificity of 90.65%, which was higher than the prediction of HbA1c, mALB, mALB/U-CR, U-CR, β2MG and RBP alone (P < 0.05).
| Item | Cut-off | Standard error | AUC | 95%CI | Sensitivity (%) | Specificity (%) |
| mALB | 56.84 mg/L | 0.040 | 0.641 | 0.530-0.688 | 68. 82 | 71.24 |
| mALB/U-CR | 2.45 mg/mmoL | 0.046 | 0.726 | 0.728-0.876 | 70.38 | 73.85 |
| U-CR | 25.96 μmol/L | 0.004 | 0.757 | 0.508-0.722 | 72.49 | 75.58 |
| β2MG | 3.18 mg/L | 0.027 | 0.748 | 0.637-0.882 | 76.84 | 79.84 |
| RBP | 26.58 μg/L | 0.036 | 0.807 | 0.637-0.882 | 82.48 | 79.38 |
| HbA1c | 9.05% | 0.043 | 0.710 | 0.638-0.775 | 72.41 | 63.11 |
| Combination | - | 0.017 | 0.958 | 0.917-0.982 | 94.83 | 96.72 |
DR is a diabetes-induced retinal vascular complication and causes irreversible visual impairment and vision loss[10]. Currently, irreversible visual impairment due to DR accounts for approximately 1.9% worldwide, while visual loss accounts for approximately 2.6% worldwide. However, there are significant reported differences in the prevalence of DR in China and abroad[11]. Some scholars have reported that the prevalence of DR in diabetes is about 34.6% globally, and is 16.4% and 25.9% in the UK and Australia, respectively. The incidence of PDR is approximately 7.0%. In China, the results of the six provinces of the Guangdong Provincial Flow Survey showed that the prevalence of DR in 13473 diabetic patients ranged from 33.28% to 34.88%[12,13]. The above studies suggest that DR is a common and highly prevalent chronic microangiopathy, which endangers public health safety. Therefore, early diagnosis of DR in diabetic patients is essential in clinical settings.
In recent years, studies have found that persistent poor glycemic control was a risk factor for the development and progression of DR, disrupting polyol metabolic pathways, contributing to the release of protein kinase C in large amounts and stimulating the onset of oxidative stress, inflammatory cell infiltration and other metabolic imbalances[14]. The above cascade of reactions further affects endothelial cells and microcirculatory function, leading to abnormal retinal microvascular biology and hemodynamics, and the development of DR. It has been found that persistent poor glycemic control is associated with alterations in mALB and U-CR, which are stimulated by oxidative stress and inflammation, and persistent high expression of mALB and U-CR[15]. The mALB/U-CR ratio is a novel index that is more accurate and reliable than traditional 24 h urine protein quantification, and is a valid marker for qualitative or quantitative prediction of proteinuric changes in the clinic[16]. DR severity has been reported to be positively correlated with decreased renal function and is independent of renal pathology[17]. An 8-year follow-up study reported that patients with DR with upregulated expression of mALB/U-CR had a progressively reduced glomerular filtration rate[18]. In the current results, mALB, mALB/U-CR, U-CR, β2MG, and RBP levels were found to be consistently increased as DR progressed from NDR, NPDR, to the PDR stage. It is hypothesized that mALB, mALB/U-CR, U-CR, β2MG, and RBP upregulated expression in DR patients is closely associated with progressive loss of renal function in diabetic patients.
Urinary β2MG was also expressed at high levels with the progressive of DR, which is a recognized early predictor of diabetic nephropathy in the clinic with high sensitivity and specificity[19]. This is consistent with previous studies by Cheng et al[20] and others, although altered β2MG levels have been associated with systemic lupus erythematous nephritis and globular nephropathy. However, the present study combined urinary mALB, mALB/U-CR, U-CR, and RBP to positively verify the association between DR occurrence and altered renal function. RBP is a low molecular mass vitamin A transporter protein, synthesized by the liver, expressed in large amounts in urine, blood, and cerebrospinal fluid, and reaches the blood via retinol in the liver[21]. It has been found that free RBP can normally be filtered by the glomerulus in healthy populations[22]. Lu et al[23] reported that urinary RBP correlated significantly with changes in renal function as the disease progressed in patients with diabetic nephropathy, elevating the rate of thylakoid cell proliferation, basement membrane synthesis and impaired glomerular filtration in patients with diabetic nephropathy, with subsequent upregulation of urinary RBP. Our study showed that mALB was involved in the regulation of renal function.
In addition, the results showed that mALB, mALB/U-CR, U-CR, β2MG and RBP were related to disease duration and HbA1c (P < 0.05); and disease duration, HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR were risk factors for the development of PDR. This indicates that the progression of diabetic microangiopathy is related to duration of the disease and the degree of abnormal glucose metabolism. It was found that persistent elevation of HbA1c accelerates damage to structural proteins in the glomerular basement membrane, causing disruption of polyol pathways, oxidative stress onset, and inflammatory infiltration involved in microvascular injury[24]. With the onset and progression of DR, disease duration and HbA1c levels increased abnormally, suggesting that persistent disease duration and abnormal HbA1c expression are involved in the development of diabetic microangiopathy, consistent with the findings of Casadei et al[25] and others. mALB, mALB/U-CR, U-CR, β2MG, RBP, disease duration and HbA1c were positively correlated in DR patients suggesting a synergistic role in promoting disease progression. The physiological characteristics of the glomerular and retinal vasculature, both of which are microcirculatory systems, suggest that persistent disease progression and elevated HbA1c levels induce disruption of the body's metabolic homeostasis and activation of oxidative stress, leading to damage to the vascular endothelium and the release of large amounts of inflammatory cytokines, inducing damage to the blood-retinal barrier and the glomerular filtration membrane barrier. In a state of persistently high glucose levels, oxides in vascular endothelial cells cannot be excreted, activating multiple signaling pathways and accelerating the impairment of vascular endothelial function, which may manifest as diabetic nephropathy if the abnormality is only in the kidney, or as DR if it occurs in the retina. Therefore, further studies found that the combination of HbA1c, mALB, mALB/U-CR, U-CR, β2MG and RBP levels is predictive of the occurrence of PDR and can be used as a biochemical marker of DR. However, this study is a single center small sample study, and the results require further verification by follow-up multicenter and large sample studies.
HbA1c, mALB, mALB/U-CR, U-CR, β2MG and RBP levels were up-regulated in DR patients, and their levels were closely related to disease duration, HbA1c and severity, all of which are risk factors for the development of PDR and can be used as markers to screen for DR progression. In the future, multi-center or propensity matching methods will be adopted to exclude the interference of multiple factors and provide new directions for clinical targeted therapy.
Diabetic retinopathy (DR) is a common complication of diabetes, which can eventually lead to blindness and seriously affect the quality of life of diabetic patients. Therefore, identification of the risk factors of DR is significant for early intervention.
This study explored the risk factors for DR and their predictive effect on retinopathy.
This study aimed to investigate the correlation between glycated hemoglobin A1c (HbA1c), urinary microalbumin (U-mALB), urinary creatinine (U-CR), mALB/U-CR ratio, β2 microglobulin (β2MG), retinol binding protein (RBP) and DR.
Based on real population data, a retrospective study was carried out.
Duration of disease, HbA1c, mALB, β2MG, RBP, mALB/U-CR and U-CR were found to be risk factors for the development of DR. The area under the curve of the combined indices (HbA1c + mALB + mALB/U-CR + U-CR + β2MG + RBP) was 0.958.
The combination of HbA1c, mALB, mALB/U-CR, U-CR, β2MG and RBP has predictive value for proliferative DR.
Large multicenter studies are needed to further verify these results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vinceti M, Italy; Yoon S, South Korea S-Editor: Wang JL L-Editor: A P-Editor: Chen YX
| 1. | Fung TH, Patel B, Wilmot EG, Amoaku WM. Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond). 2022;22:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Wang F, Mao Y, Wang H, Liu Y, Huang P. Semaglutide and Diabetic Retinopathy Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig. 2022;42:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Sugimoto M, Sampa K, Tsukitome H, Kato K, Matsubara H, Asami S, Sekimoto K, Kitano S, Yoshida S, Takamura Y, Hirano T, Murata T, Shimizu M, Kinoshita T, Kusuhara S, Sawada O, Ohji M, Yoshikawa R, Kimura K, Ishikawa H, Gomi F, Terasaki H, Kondo M, Ikeda T; On Behalf Of The Writing Committee Of Japan-Clinical Retina STudy Group J-Crest. Trends in the Prevalence and Progression of Diabetic Retinopathy Associated with Hyperglycemic Disorders during Pregnancy in Japan. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, Lin H, Loewenstein A, Mohan S, Pearce IA, Sakamoto T, Schlottmann PG, Silverman D, Sun JK, Wells JA, Willis JR, Tadayoni R; YOSEMITE and RHINE Investigators. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399:741-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 5. | Tzvi-Behr S, Ivgi H, Frishberg Y, Ben Shalom E. First-week urine beta-2 microglobulin levels in term healthy neonates. Pediatr Nephrol. 2021;36:1511-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Boonloh K, Lee ES, Kim HM, Kwon MH, Kim YM, Pannangpetch P, Kongyingyoes B, Kukongviriyapan U, Thawornchinsombut S, Lee EY, Kukongviriyapan V, Chung CH. Rice bran protein hydrolysates attenuate diabetic nephropathy in diabetic animal model. Eur J Nutr. 2018;57:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Zhang P, Meng J, Duan M, Li D, Wang R. Efficacy of Yishen Huashi Granules Combined with Linagliptin Tablets on Blood Glucose and Renal Function in Patients with Type 2 Diabetic Nephropathy. Comput Intell Neurosci. 2022;2022:4272520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1980] [Cited by in RCA: 2387] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 10. | Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12:1322-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 11. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 1243] [Article Influence: 248.6] [Reference Citation Analysis (1)] |
| 12. | Dag U, Çaglayan M, Alakus MF, Öncül H. The relationship between reduced choroidal thickness due to high plasma asymmetrical dimethylarginine level and increased severity of diabetic retinopathy. Arq Bras Oftalmol. 2023;86:27-32. [PubMed] |
| 13. | Wang Q, Zeng N, Tang H, Yang X, Yao Q, Zhang L, Zhang H, Zhang Y, Nie X, Liao X, Jiang F. Diabetic retinopathy risk prediction in patients with type 2 diabetes mellitus using a nomogram model. Front Endocrinol (Lausanne). 2022;13:993423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes Metab. 2019;21:454-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (1)] |
| 15. | Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA(1c) Change and Diabetic Retinopathy During GLP-1 Receptor Agonist Cardiovascular Outcome Trials: A Meta-analysis and Meta-regression. Diabetes Care. 2021;44:290-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Tang H, Zhao Y, Tan C, Liu Y. Significance of Serum Markers and Urinary Microalbumin in the Diagnosis of Early Renal Damage in Patients with Gout. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Saigo S, Kino T, Uchida K, Sugawara T, Chen L, Sugiyama M, Azushima K, Wakui H, Tamura K, Ishigami T. Blood Pressure Elevation of Tubular Specific (P)RR Transgenic Mice and Lethal Tubular Degeneration due to Possible Intracellular Interactions between (P)RR and Alternative Renin Products. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Jiang H, Zhang Y, Xu D, Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J Clin Lab Anal. 2021;35:e23650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Yang Z, Lou X, Zhang J, Nie R, Liu J, Tu P, Duan P. Association Between Early Markers of Renal Injury and Type 2 Diabetic Peripheral Neuropathy. Diabetes Metab Syndr Obes. 2021;14:4391-4397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Cheng Z, Qian S, Qingtao M, Zhongyuan X, Yeda X. Effects of ATRA on diabetic rats with renal ischemia-reperfusion injury. Acta Cir Bras. 2020;35:e202000106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Schiborn C, Weber D, Grune T, Biemann R, Jäger S, Neu N, Müller von Blumencron M, Fritsche A, Weikert C, Schulze MB, Wittenbecher C. Retinol and Retinol Binding Protein 4 Levels and Cardiometabolic Disease Risk. Circ Res. 2022;131:637-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 22. | Huang H, Xu C. Retinol-binding protein-4 and nonalcoholic fatty liver disease. Chin Med J (Engl). 2022;135:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Lu J, Wang D, Ma B, Gai X, Kang X, Wang J, Xiong K. Blood retinol and retinol-binding protein concentrations are associated with diabetes: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2022;61:3315-3326. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Kaya B, Paydas S, Kuzu T, Basak Tanburoglu D, Balal M, Eren Erdogan K, Gonlusen G. Primary glomerulonephritis in diabetic patients. Int J Clin Pract. 2021;75:e13713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Casadei G, Filippini M, Brognara L. Glycated Hemoglobin (HbA1c) as a Biomarker for Diabetic Foot Peripheral Neuropathy. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |