Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.741
Peer-review started: January 3, 2023
First decision: January 17, 2023
Revised: February 24, 2023
Accepted: April 11, 2023
Article in press: April 11, 2023
Published online: June 15, 2023
Processing time: 163 Days and 3.2 Hours
Diabetic neuropathy (DN) is a devastating disorder with an increasing prevalence globally. This epidemic can pose a critical burden on individuals and com-munities, subsequently affecting the productivity and economic output of a country. With more people living a sedentary lifestyle, the incidence of DN is escalating worldwide. Many researchers have relentlessly worked on ways to combat this devastating disease. Their efforts have given rise to a number of commercially available therapies that can alleviate the symptoms of DN. Unfortunately, most of these therapies are only partially effective. Worse still, some are associated with unfavorable side effects. This narrative review aims to highlight current issues and challenges in the management of DN, especially from the perspective of molecular mechanisms that lead to its progression, with the hope of providing future direction in the management of DN. To improve the approaches to diabetic management, the suggested resolutions in the literature are also discussed in this review. This review will provide an in-depth understanding of the causative mechanisms of DN, apart from the insights to improve the quality and strategic approaches to DN management.
Core Tip: This review elaborates on the current aspects regarding diabetic neuropathy (DN), especially issues pertaining to the treatments and current challenges in the management of DN with some suggested recommendations on strategies to slow down DN progression. In order to increase the understanding of DN, current lines of therapy, the understanding of its pathophysiology, and future direction of its management are also included. Perhaps, this review may provide insights to understand the important information regarding DN and give ideas for improvement of treatments and management of DN.
- Citation: Ismail CAN. Issues and challenges in diabetic neuropathy management: A narrative review. World J Diabetes 2023; 14(6): 741-757
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/741.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.741
Diabetes mellitus (DM), a global public health issue, affects up to half a million people worldwide. According to the World Health Organization (WHO), there was a marked increase in the number of individuals suffering from DM from 108 million in 1980 to as high as 422 million in 2014[1]. In the United States, the Center for Disease Control and Prevention reported that 37.3 million (11.3%) people of the whole United States population are suffering from DM[2]. Diabetic neuropathy (DN) is a common complication of DM that encompasses various patterns of neuropathy as categorised by the location of nerve damage. A recent cross-sectional study among 473 type 2 DM patients from the United Kingdom between 2015 and 2020 demonstrated that the prevalence of diabetic peripheral neuropathy (DPN) was 26.6%, whereby more than half were male patients (52.3%). In terms of DPN severity, 17.3%, 8.2%, and 1.1% of the patients suffered from mild, moderate, and severe DPN, respectively[3]. These statistics showed a huge increase in DN among the DM patient population. Such a worrying trend warrants urgent attention to slowing the DN progression among affected individuals.
Generally, DN can be asymptomatic and only manifests when any disability arises. This disorder affects sensory nerves and it may progress from mild numbness to dysaesthesia, pain, and allodynia eventually. Furthermore, it commonly begins in the feet and lower limbs before spreading proximally[4]. Apart from that, DN may also interrupt motor functions, leading to weakness, atrophy, gait abnormality, and loss of coordination. As a result of the difficulties in performing daily routines, many patients experience a poor quality of life (QOL). DN is also classified as a “length-dependent” neuropathy as it starts at the distal nerve endings of the longest nerve in the lower limbs and extends proximally[5]. In addition, DN can vary in its clinical manifestations. It is categorised either as “painful DN” that manifests as positive symptoms and gain of function (e.g., pain, allodynia, and hyperalgesia) or “painless or insensate DN” that appears as negative symptoms and loss of functions (e.g., numbness and dysaesthesia). Painless DN is a result of the predominant loss of small and large nerve fibres[6] starting at the distal nerve of the limbs before it progresses to the proximal ends in a “glove and stocking” distribution[7]. Despite massive research aimed at identifying the key culprits of DN, its underlying mechanisms remain complicated and unclear[8,9]. Several reviews of DN highlighted the shift in the management towards molecular-oriented approaches. However, the molecular mechanism leading to the progression of DN and its complications remains poorly understood. Consequently, the prevalence of DN continues to escalate and there is a very minimal enhancement in the management of DN. In this review, we aim to highlight current issues and challenges in the management of DN, especially from the perspective of molecular mechanisms that lead to its progression, with the hope of providing the future direction in the management of DN.
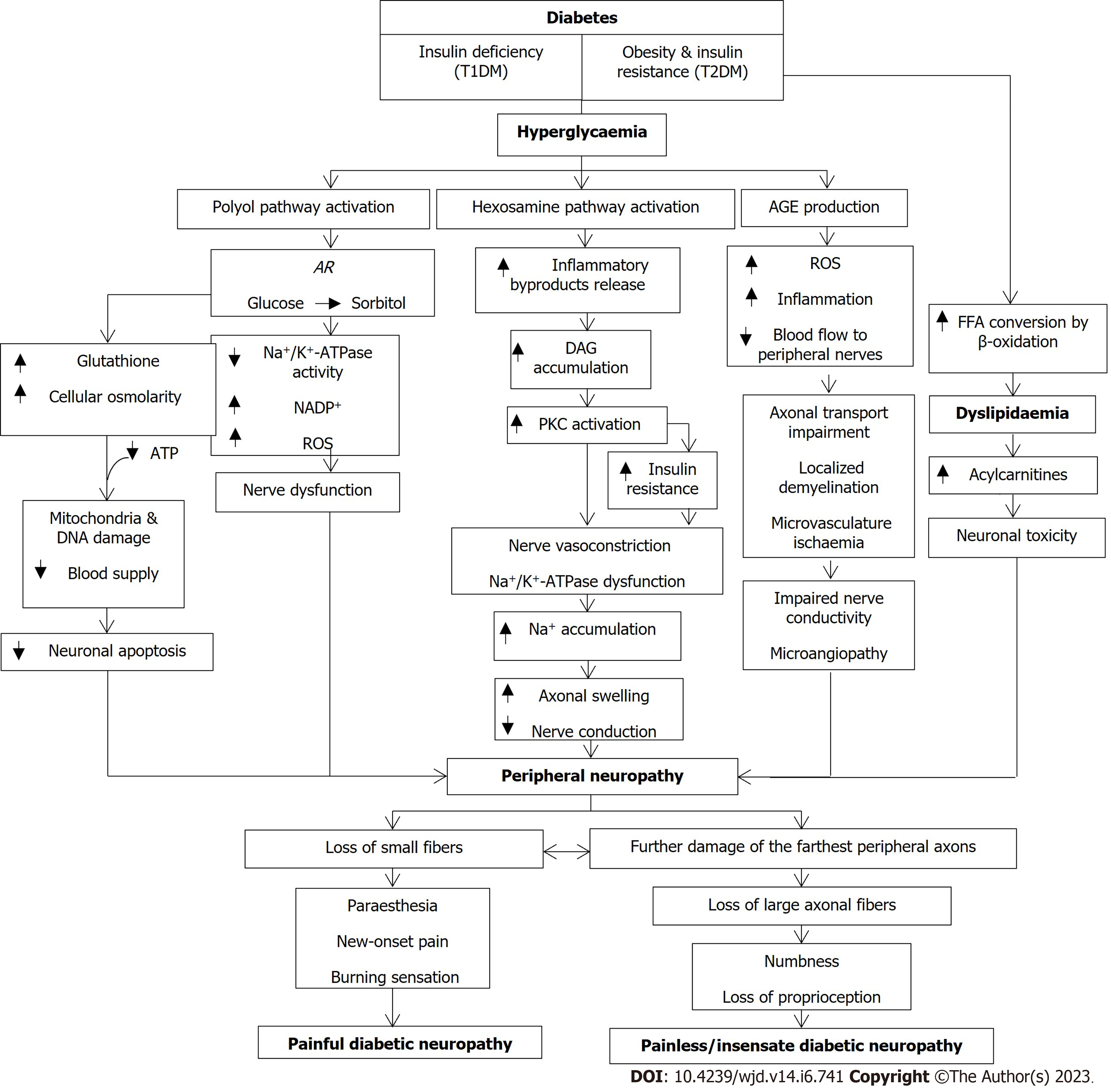
The underlying metabolic abnormalities in DM patients can synergistically drive the development of DN. These abnormalities start with the development of obesity and insulin resistance in type 2 DM (T2DM) or insulin deficiency in T1DM, all of which can result in glucose dysregulation and subsequently, hyperglycaemia and dyslipidaemia[4,10]. In a healthy individual, insulin induces the release of neurotrophic and neuroprotective factors that ensure neuronal survival, as well as C-peptide that restores the structure and function of defective axons. In T1DM patients, as the insulin level falls, the sodium-potassium ATPase (Na+/K+-ATPase) and nitric oxide will be disrupted, leading to neuronal dysfunction, oxidative stress, axonal swelling, and apoptosis[5]. Similarly, insulin resistance in T2DM patients may also reduce the anti-oxidant Akt, consequently producing mitochondrial dysfunction, oxidative stress overproduction, and neuronal apoptosis[11]. In addition, the concomitant dyslipidaemia in T2DM patients occurs when free fatty acids are excessively converted by β-oxidation. The acetyl-CoA transformation during the conversion leads to a great increase in acylcarnitines that are toxic to neurons and Schwann cells[12].
Meanwhile, hyperglycaemia can also activate some pathways that produce excessive polyol, glycation, protein kinase C (PKC), poly (ADP-ribose) polymerase (PARP), and hexosamine, all of which can simultaneously cause overproduction of oxidative stress in the nerves and microvessels[4,5]. In the polyol pathway, the enzyme aldose reductase (AR) converts glucose to sorbitol. This conversion affects a number of downstream reactions that depletes N+/K+-ATPase activity, thus reducing nicotinamide adenine dinucleotide phosphate (NADP+) and enhancing the production of reactive oxygen species (ROS), eventually impairing nerve functions[5,6,13,14] and leading to DN. Besides, excessive glucose molecules will enter the hexosamine pathway to produce inflammatory by-products and induce PKC activation secondary to the accumulation of diacylglycerol. Following this activation, insulin resistance is augmented in a way that interrupts the biology of growth factors and causes vasoconstriction of the nerves[10] on top of Na+/K+-ATPase dysfunction. As a result, the accumulation of Na+ leads to axonal swelling and reduced nerve conductivity[5]. Furthermore, the elevated sorbitol and decreased NADH levels trigger ROS increment, glutathione reduction, and cellular osmolarity. Coupled with a decrease in ATP production, these effects can damage mitochondria and DNA as well as reduce the blood supply, eventually speeding up neuronal apoptosis[5]. Additionally, excessive glucose molecules also contribute to the formation of advanced glycation end products (AGEs). When they bind to the receptors (RAGEs), excessive ROS production leads to downstream inflammation that limits blood flow to the peripheral nerves[15]. Although the glycation pathway may take place in the cells of several organs, its effects in DN are more prominent on both myelinated and unmyelinated axons, endothelial cells, pericytes, and Schwann cells[16]. Furthermore, the interference of AGEs on neurofilaments and microtubules of the nerves impedes the axonal transport whilst AGEs formation on the myelin sheath results in localised demyelination[5,16]. Besides, the attack of AGEs on the microvessels increases vascular permeability, hinders vasodilation, stimulates cytokine production, and amplifies oxidative stress levels, all of which lead to a blood flow restriction to the nerves[16]. As more blood capillaries are damaged, the closely connected microvasculature undergoes ischaemia because of the abnormal modification of basement membrane density, pericyte and endothelial cell functions, and arteriovenous shunt formation[5]. All these changes diminish the neuroprotective role of angiogenic factors such as vascular endothelial growth factor. Therefore, the severity of microangiopathy is shown to be associated with impaired nerve conductivity.
The overall pathomechanisms eventually affect the nerves, especially in the peripheral nervous system. Peripheral axons are more fragile compared to motor neurons since they are placed outside the blood-brain barrier. The location also predisposes peripheral axons to injury secondary to DM[10]. Among the different types of peripheral nerves, small unmyelinated C-fibres termed “small fibres” are the most common sensory axons. However, large fibres comprised of small and thinly myelinated Aδ-fibres as well as fully myelinated Aα- and Aβ-fibres are also prone to DN. Patients with DN may experience degeneration and loss of small fibres that result in new-onset pain and prickling or burning sensations (i.e., dysesthesias) in the feet, followed by the initial demyelination or remyelination of the large fibres[12]. Most of the time, the axons that are farthest from the cell body (i.e., located in the feet) are the most severely affected since the number of functional mitochondria produced in their neuronal cell bodies tracking down the axons would be depleted, causing energy deprivation. Amongst the nerve fibres, small fibres are the earliest to be affected due to their structures (i.e., lack of myelination and encapsulation of Schwann cells). Schwann cell encapsulates large fibres to protect axons from external damage and toxic substances. This is an important step in slowing down diabetic-induced progressive energy loss. Therefore, this explains why patients with painful DN often experience pain and dysesthesia as their first symptoms[10]. As diabetes progresses, the myelin sheaths of the nerve fibres undergo degeneration with the detachment of Schwann cells[9]. Subsequently, this leads to even fewer neurotrophic factors being released and eventually, neuronal apoptosis[9]. Consequently, the loss of large axonal fibres causes the patient to experience numbness and loss of proprioception distally in the feet that gradually progress proximally with time. The symptoms usually occur in a symmetrical, distal-to-proximal pattern in all populations of nerves, beginning at the tip of the toes and progressing proximally, giving rise to the “stocking-and-glove” clinical presentation[5,10,17]. Such symptom presentation is regarded as insensate or painless DN whereby the loss of sympathetic regulation of the arteriovenous shunt of the vessels and sweat glands in the foot predisposes the patients to bacterial infections that can later culminate in cellulitis and ulcers[18]. Simplified pathomechanisms of the development of DN are summarised in Figure 1.
Moreover, DN patients are at a high risk of developing diabetic polyradiculopathy, a syndrome that appears together with severe disabling pain in one or more than one distribution of nerve roots and is possibly linked with motor weakness[19]. Besides that, patients with uncontrolled DM and peripheral neuropathy are prone to Charcot neuroarthropathy (CN), also known as Charcot foot, a dreadful condition that can easily originate from microtrauma and neurovascular modifications (i.e., arteriovenous shunting causing the escalation of blood flow and bone resorption)[17,20]. In due time, CN can result in deformities such as collapsed joints and pedal disfigurement[4,17].
In view of the wide range of disabling symptoms of DN, various management strategies have been recommended by healthcare professionals to alleviate the symptoms so that the QOL of the affected individuals can be improved.
To date, the management of DN emphasises delaying the progression of neuropathy, reducing the symptoms, and alleviating the complications arising from insensate or painless DN[18,19]. The success of DN management depends on the individual’s pathogenic processes[18]. Currently, various clinical guidelines on strategies to prevent and manage DN are available worldwide based on the available published literature. The guidelines encompass a wide range of strategies to prevent the development of DN symptoms, hamper the DN progression, and cure the DN symptoms. Thus, the management of DN can be categorised as preventive or symptomatic approaches[19]. Nevertheless, there are very limited treatment options available for DN that are aimed at underlying nerve impairment. To date, most of the management strategies emphasise the best way to slow down the progression of DN. Screening for any signs or symptoms of DN is crucial in clinical practice to detect the earliest signs of neuropathy so that prompt intervention can be started[6]. Table 1 outlines the current management to prevent DN progression as elaborated in the literature.
| Strategies | Description/indication | Intervention/strategies | Ref. |
| Glucose level monitoring | Prevents distal symmetric polyneuropathy and cardiovascular autonomic neuropathy developments in patients with T1DM, and delays the progression of distal symmetric polyneuropathy in T2DM patients | Treatments (insulin, anti-diabetic medications, electrical stimulation, and percutaneous nerve stimulation; non-treatments (lifestyle modifications such as glucose-dietary control, exercises, and physiotherapy); pancreas transplant; bariatric surgery | [6,4,132] |
| Lifestyle modifications | Reduce risk of DN and cardiometabolic causes | Glucose-dietary control; counselling; supervised training programs including physiotherapy/rehabilitation | [4] |
| Diabetic foot care | Delays or lowers the risk of amputations | Five key elements for prevention of DFUs: (1) Recognition of the at-risk foot; (2) consistent check and examination of the at-risk foot; (3) education of patients, their family, and healthcare providers; (4) routine of wearing suitable footwear; and (5) management of pre-ulceration signs | [47] |
| Pharmacologic therapeutics | Manage diabetes and neuropathy and treat symptomatic pain | Three suggested phases can be useful: Step 1: Treatment with first-line therapy of TCAs (e.g., amitriptyline), SNRIs (e.g., duloxetine), pregabalin, and gabapentin; step 2: Treatment with second-line therapy including tramadol (weak opioids and SNRIs); step 3: Treatment with last line therapy including strong opioids, cannabinoids, and anticonvulsants | [89,90,133] |
| Alternatives (anti-oxidant supplementations): α-lipoic acid; acetyl-L-carnitine vitamin B12 |
First and foremost, DN prevention strategies should begin with blood glucose monitoring and lifestyle modifications[4,6]. Reduction of sweet food can hinder the progression of distal symmetrical polyneuropathy and cardiovascular autonomic neuropathy in patients with T1DM and T2DM[6,21]. However, based on the Diabetes Control and Clinical Trials, this strategy appears to be more effective in T1DM patients whereby their clinical neuropathy is reduced by 60% within 6.5 years following the intensive therapy[22]. In 1998, The United Kingdom Prospective Diabetes Study reported that T2DM patients with neuropathy showed improvement in vibration perception after improvement in blood glucose levels with intensive treatment[23]. However, there was no significant impact of tight glycaemic control on neuropathy among T2DM patients from 1998 to 2015[24]. Although it is suggested that tight glycaemic control could prevent or delay the progression of DN among DM patients, Rodríguez-Gutiérrez et al[24] believed that this strategy alone is inadequate for T2DM patients since they are more likely to suffer from other risk factors such as cardiometabolic factors that are unaddressed[25]. The finding that glycaemic monitoring alone is incapable to slow down the progression of DN in T2DM patients appears to be a new consensus. These patients often suffer from metabolic syndrome that includes obesity, hyperglycaemia, and dyslipidaemia, all of which are critical risk factors for neuropathy[10] as shown in several clinical trials conducted in various countries[24,26-31]. In the United States, The American Diabetes Association (ADA) has implemented different glycaemic target guidelines for children, teenagers, adults, pregnant ladies, and senior citizens in an effort to promote customised care based on individualised glycaemic targets[4,32-35].
Apart from glycaemic monitoring, lifestyle modification is also recommended to reduce cardiometabolic risk factors among T2DM patients to lower the risk of DN and delay its progression. Lifestyle modifications can be in the form of regular exercise and a balanced diet[10]. In animal studies, sustained exercise has been found to: (1) Decrease hyperglycaemia and overproduction of oxidative and nitrosative stress; (2) enhance mitochondrial bioenergetics in the nerve cell body and distal axon; (3) improve microvascular vasoreactivity and reduce nerve ischaemia; (4) elevate axonal transport; (5) counteract the inflammatory effects of dyslipidaemia, lipotoxicity, and obesity; and (6) improve nerve regeneration following metabolic injury[10,36-38]. However, clinical studies involving human subjects reported various outcomes. In 2006, a clinical trial investigating the effect of long-term exercise training on DPN patients reported a significant improvement in peroneal and sural motor nerve functions in the patients[39]. Over the four years of the study period, the development of motor and sensory neuropathy slowed down, thus suggesting that exercise may change the natural course of DN. However, recent studies reported contradicting findings on the effect of exercise on DN. In a randomised controlled trial (RCT) by Stubbs et al[40], 12-wk physical exercise training regardless of type (i.e., sedentary controls, aerobic, isokinetic strength, or a combination of aerobic-isokinetic strength training) did not improve or exacerbate the sensory or motor nerve electrodiagnostic findings (i.e., sural, median, and ulnar sensory nerve responses) in older T2DM patients with length-dependent distal symmetric polyneuropathy. However, a short-term structured program of aerobic exercise was found to selectively improve the sensory nerve functions in a subset of patients. This finding was supported by a recent meta-analysis that included 13 RCTs from 2014 to 2022 with 592 patients that underwent peripheral nerve conduction tests. Exercise, when combined with endurance and sensorimotor training programme, was found to improve balance, glycaemic control, and peripheral nerve conduction, especially in DN patients[41]. Unfortunately, the implementation of such supervised exercise training among the general population in the healthcare system outside of the research setting can be challenging due to patient compliance and shortage of funding, infrastructure, and staff to supervise the patients[4].
In the literature, suggestions have been put forth to include diet observation as part of the prevention strategies in delaying the progression of DN. However, there is a lack of evidence on the effect of diet as the sole prevention strategy for DN since most of the studies incorporated diet as one of the multifactorial lifestyle strategies. For instance, the Diabetes Prevention Program demonstrated that the combination of exercise and diet counselling can reverse the symptoms of metabolic syndrome and lower the incidence of T2DM[42,43]. On a similar note, the ADA also recommends restriction of high-calorie and processed food intake to reduce the risk factors for DN. In turn, the patients should consume food rich in polyunsaturated fats and antioxidants to prevent the development of DN[10]. It is known that lipid metabolites and chronic cellular hyperglycaemia may induce pro-inflammatory cellular injury responses and generate oxidative stress that further diminishes the roles of mitochondria in distal axons[21]. Several dietary supplements are recommended to fight against oxidative damage, including the anti-oxidant α-lipoic acid (ALA). Besides, supplements containing nicotine riboside, a key generator of nicotinamide adenine dinucleotide (NAD+), are also recommended as they can activate certain molecular pathways that shield against dyslipidaemia and obesity[44], resulting in the prevention of oxidative damage in the neurons and delaying the onset of DN[45]. Therefore, dietary management can be effective in alleviating DN. However, it is best to be combined with exercise-based intervention to ensure a long-term positive impact on glucose and lipid metabolism, as well as axonal regeneration in BM patients[21].
In addition, patients with DN are predisposed to a higher risk of lower extremity amputations. A recent systematic review that evaluated the 5-year mortality rate of patients with non-traumatic below-the-knee amputation and above-the-knee amputation was 40%-82% and 40%-90%, respectively[46], emphasising the importance of annual foot examination and routine foot care in the prevention of lower limb amputations[17]. Education on proper diabetic foot care should be provided to DM patients, including the identification of the at-risk foot, daily examination and inspection, and the use of suitable footgear, as well as accurate and early treatment of pre-ulcerative lesions[47]. The education should also be extended to family members and healthcare providers. Despite the available guidelines on foot care, there is a lack of comprehensive evidence on the best ways to hamper diabetic foot complications. A systematic review of 19 studies demonstrated a reduction in amputation severity, duration of hospital stay, and death rates with proper diabetic foot care. However, the studies were of low quality[48]. In addition, another systematic review of 12 RCTs revealed inadequate high-quality evidence on whether the application of educational strategies alone may minimise the incidence of diabetic foot ulcerations (DFUs) and amputations. The authors agreed that educational interventions should be combined with other interventions in the prevention of DFUs[49].
Although some non-pharmacological approaches have been introduced to manage the signs and symptoms of DN, anti-diabetic drugs remain the mainstay of DN treatment. Furthermore, there is a paucity of management strategies for individuals with painless or insensate DN as the current therapy focuses on the painful type of DN. Several antidepressants [tricyclic anti-depressants (TCAs), i.e., duloxetine, venlafaxine, and amitriptyline], analgesics (morphine, oxycodone, and tramadol), and anti-convulsants (gabapentin, pregabalin, topiramate, and valproic acid) are prescribed for patients with painful DN. Table 2 summarises the available treatments for DN. Since there is a huge variability in pain between the patients, various types of medications are given to lower painful DN.
| Management strategy | Therapeutic approach | Description | Contraindications/issues |
| Pharmacological | Anti-convulsants: Gabapentin; pregabalin | First line medication for painful DN[4,51]; gold standard for pain management[50,51] | Reports on misuse and increased death rate in patients[54] |
| SSRI and SNRIs: Duloxetine; venlafaxine | First- and second-line therapy for painful DN[56,57] | Low evidence on venlafaxine effectiveness for painful DN treatment[58] | |
| TCAs: Amitriptyline; desapramine | First and second-line therapy for painful DN | Associated with constipation, dry mouth, sleep disturbance, sexual dysfunction, somnolence, headaches, arrhythmias, constipation, sleep disturbances, and postural hypotension[4,63] | |
| Opioids: Tramadol; trapentadol | Opted as acute salvage treatment or as a part of drug combination for painful DN treatment | Strong opioids are frequently associated with therapeutic abuse and misuse[68]; use of tramadol is more preferred due to reduced risk of abuse or misuse[68] | |
| Sympathetic blocking agents (α-adrenergic antagonists): Clonidine; regitine; phenoxybenzamine | One of the opted therapies for complex regional pain syndrome treatment[72] | Limited evidence in RCT testing the drug’s efficacy in painful DN patients; efficiency of clonidine depends on relative functionality of nociceptors in painful DN patients, however no statistical significance is achieved although the trends of efficacy is shown[70] | |
| Non-pharmacological | Sympathetic nerves blockade: Lumbar sympathetic nerves blockade; combined strategies of lumbar sympathetic pulsed radiofrequency and continuous epidural infusion; combined treatment of continuous sympathetic block and neurolysis with alcohol | Recommended for severe painful DN patients who failed to any pharmacological treatments | Patients demonstrated improved life expectancy, greater DN symptom improvement, satisfactory safety, rapid recovery, and rapid relief of pain[73-76]; associated with several limitations of additional diagnostic tools, small size population, short period of follow-up, and issue regarding combined treatment duration[75,76] |
| Capsaicin | Recommended for patients with intolerable oral therapeutic consumption[4] | Low to moderate level of evidence for topical capsaicin efficacy[82,83]; associated with small nerve fibers injury and disturbed nociceptive signaling[84] | |
| Neuromodulation devices: FREMS; SCS, NMES; TENS | Studies on their efficacy in painful DN is still on-going | Not yet approved for clinical guidelines for painful DN treatment due to very low evidence of efficacy[4,85,86] | |
| Nutraceuticals: ALA; ALC; vitamin B12 | ALA improves numbness and paraesthesia with reduced side effects[89]; vitamin B12 is recommended to T2DM patients with metformin prescription[90] | There is a lack of standardization in quality and manufacturing of nutraceuticals[91,92]; low safety level due to less evidence of high-quality studies[87,93] |
Generally, DN will first afflict small nerve fibres such as unmyelinated C-fibres before large fibres (myelinated A fibres), thus explaining the complaints of burning and discomfort among patients with painful DN[18,19]. Pregabalin and gabapentin are the gold standard drugs for pain management[50,51] and are therefore the first- and second-line medications to treat painful DN[4,51]. The exact mechanism of how these anticonvulsants alleviate DN symptoms is unclear. It is postulated that they bind to the α2δ subunit of calcium channels on presynaptic nerve terminals[52] to induce analgesia. However, these drugs are associated with adverse effects such as tachyphylaxis, somnolence, drowsiness, headache, dizziness, nausea, and diarrhoea[4,51,53]. Furthermore, pregabalin has been linked to misuse and a higher prevalence of deaths, thus there have been calls for its reclassification as a Class C controlled substance in the United Kingdom[54,55].
Apart from that, antagonists of serotonin and norepinephrine reuptake (SNRIs) are also used to reduce DN pain. Similar to pregabalin and gabapentin, duloxetine is recommended as the mainstay of treatment for painful DN. It attenuates the descending pain mechanisms and moderately hinders dopamine reuptake. Apart from producing similar side effects as anticonvulsants, this drug also unfavourably affects sexual functions and sleep[6]. Another selective serotonin reuptake inhibitor, venlafaxine, is also recommended by the European Federation of Neurological Societies Task Force and the American Academy of Neurology as a therapy for painful DN[56,57]. However, based on a previous Cochrane systematic review of six RCTs and 460 participants comparing the placebo effect with a venlafaxine dosage of 150-225 mg, the level of evidence for its effectiveness is low[58].
Apart from that, tricyclic antidepressants such as amitriptyline are also recommended for painful DN[4], especially acute pain[59]. Several RCTs have reported its effectiveness in alleviating painful DN[60]. In a RCT, Kaur et al[61] compared the efficiency of duloxetine and amitriptyline. They found a similar efficacy of these drugs in treating patients with painful DN. The mechanism of TCAs in targeting painful DN is not understood, but amitriptyline is found to attenuate the reuptake of serotonin and noradrenaline at the nerve terminals and ion channels (sodium and potassium ion channels), as well as N-methyl-D-aspartate receptors (NMDARs) in the central nervous system[62]. However, amitriptyline is associated with side effects such as constipation, dry mouth, sleep disturbance, sexual dysfunction, somnolence, headaches, arrhythmias, sleep disturbances, and postural hypotension[63]. Apart from amitriptyline, other TCAs such as desipramine and nortriptyline have also been investigated as potential treatments for painful DN. Several RCTs reported a reduction in painful DN symptoms following desipramine treatment[64-66], making it likely to be as effective as amitriptyline[64] with lesser side effects[65].
On top of that, some clinical guidelines recommended opioids be included as one of the treatments with or without other drugs for DN patients with severe pain intensity[67]. However, opioid is frequently associated with therapeutic abuse and misuse. Tramadol, one of the opioids, is fairly acceptable in the treatment of moderate to severe pain as it has a lower risk of abuse or misuse. It reduces pain by binding to opioid receptors (i.e., ҡ-, δ-, and µ-receptors) centrally besides mitigating the serotonin and norepinephrine reuptake, thus augmenting the inhibitory effects of pain transmission in the spinal cord dorsal horn[68]. Apart from that, tapentadol is also suggested for the treatment of painful DN in the US. It shares a similar mechanism of action with tramadol, except for a higher affinity for µ-receptors. However, the level of evidence to show the efficacy of these opioids was low based on the above-mentioned Cochrane systematic review that included six RCTs and 438 participants[69].
Pertaining to the potential to target the C-fibres in peripheral sympathetic nerves, the use of sympathetic blocking medications (α-adrenergic antagonists) such as clonidine, regitine, or phenoxybenzamine is recommended in some studies to improve the pain secondary to the spontaneous firing of the affected nerve fibres[18,19]. An RCT conducted by Campbell et al[70] demonstrated that the level of foot pain subsided after the topical application of clonidine gel among patients with painful DN. However, the effectiveness of this medication relies on the relative functionality level of nociceptors (i.e., functional and possibly sensitised nociceptors in the affected skin). This trial failed to achieve significant results despite showing some evidence of the drug’s efficacy. Another earlier RCT on transdermal clonidine application in diabetic polyneuropathy patients also failed to achieve promising results as drug withdrawal effects and pain recurrence were reported among the trial participants[71]. Even though this sympathetic blocking agent can be used to treat other complex regional pain syndromes, there is still very scarce analysis with regard to painful RN in the Cochrane database. This was concurred by Mackey et al[72] who reported that not only this class of medication did not show any efficacy in treating neuropathic pain, its use was challenging due to the side effects profile.
In some cases of patients with persistent severe painful DN despite multiple pharmacological approaches, shifting the pain relief mechanism to the sympathetic nervous system can possibly assist the management of the severe pain. In a clinical trial, permanent lumbar epidural blockade was found to produce satisfactory outcomes when several other pharmacotherapeutics failed to treat patients with painful DN[73]. In another case reported by Cheng et al[74], a painful DN patient who was unresponsive to several medications showed significant pain relief following the blockade of nine lumbar sympathetic nerves over a 26-mo duration. His QOL was further improved over the two years. Further advancement of this approach, i.e., lumbar sympathetic pulsed radiofrequency combined with continuous epidural infusion, appeared to successfully manage painful symptoms of DN in the patients[75]. Meanwhile, the combined treatment of continuous lumbar sympathetic block and neurolysis with alcohol also produced a greater improvement of DN symptoms and rapid recovery in the patients, not to mention its satisfactory safety profile[76]. However, there are certain limitations to this approach, such as the requirement for additional tools to assess and diagnose the severity and duration of DN. Furthermore, the small size population, short period of follow-up, and duration of the combined treatment strategies in the previous studies[75,76] restrict the generalisability of the results, thus further research is warranted.
Additionally, unmyelinated C-fibres release neurotransmitter substance P during the transmission of pain signals from the periphery to higher centres. This pathway could be blocked by the topical application of capsaicin[77], especially for patients with localised pain who are unable to tolerate oral medications[4]. Previous reports have demonstrated its effectiveness in improving nerve functions and lowering pain sensations in painful DN patients at a dosage of 0.075% four times a day[78,79]. Meanwhile, DN can also affect myelinated A-fibres that produce deep-seated, dull, and distressing pain that is usually unresponsive to sympathetic blocking agents and capsaicin[18,19]. This natural product blocks pain transmission by modifying the membrane potential of vanilloid receptor subtype 1 and certain ion channels, as well as the neurotrophic signalling at the nerve fibres[19,80]. Besides, it can also initiate acute production of vasoactive peptides from perivascular sensory terminals following topical application[81]. The use of topical capsaicin to treat painful DN is approved by the Food and Drug Administration and the level of evidence for its efficacy ranges from moderate to low[82,83]. On the downside, several reports have emerged regarding the potential side effects of topical capsaicin in damaging small nerve fibre and interrupting nociceptive signalling[84].
Besides the above-mentioned pharmacological strategies, there are other alternative approaches to alleviate the symptoms of painful DN. Neuromodulation strategies using specific devices such as frequency-modulated electromagnetic neural stimulation (FREMS), spinal cord stimulation (SCS), neuromuscular electrical stimulation (NMES), and transcutaneous electrical nerve stimulation (TENS) represent new hopes for DN patients[4]. However, these strategies are still under investigation and not included in any clinical guidelines to treat DN as the level of evidence is very low[4,85,86]. Similarly, alternative complementary approaches such as acupuncture and static magnetic field therapy have also been used to manage painful DN[19]. Nevertheless, data on these management strategies are also limited.
Furthermore, a series of clinical trials have demonstrated the efficacy of the antioxidant nutritional supplement, i.e., ALA, acetyl-L-carnitine, and vitamin B12 in alleviating the pain linked to DN[57,87,88]. An oral supplement of ALA at 600 mg per day may reduce DN pain within 2 wk, besides improving numbness and paraesthesia symptoms with minimal adverse effects[89]. Similarly, ALA lowers pain intensity by decreasing oxidative stress that afflicts nerves and microvessels after metabolic modifications[4]. Meanwhile, the regular supplementation of vitamin B12 is recommended especially for T2DM patients who are on metformin to offset the side effect of vitamin B12 deficiency[90]. Despite promising outcomes, worldwide availability, affordable cost, and being regarded as a “safer option”, there are concerns regarding these nutraceuticals in terms of lack of regulations including standardisation in manufacturing and quality control[91,92]. Furthermore, the safety profile of these nutraceuticals remains unclear due to the lack of high-quality clinical trials[87,93].
Since the prevalence of DN is rapidly rising, multiple strategies in terms of treatments, new therapeutic approaches, patient access to healthcare facilities, and provision of knowledge regarding DN have been introduced to slow down the disease progression. Unfortunately, several ongoing issues must be resolved in the management of DN. This section elaborates on the issues and challenges in improving the management of DN from the aspect of treatment, patient adherence, access to facilities, and knowledge.
In the literature, a number of observational and interventional studies revealed that half of the patients with DM develop the signs and symptoms of DN during their lifetime[6,87,94-96]. The prevalence of DN is high (approximately 20%-30% in newly diagnosed and early-stage T2DM)[30]. Additionally, it is challenging to treat DN patients with symptomatic (painful) variants since the pain can be debilitating and excruciating. They often complain about pain sensation over the lower extremities that is apparent at rest and intensifies during night time[19]. Unfortunately, the exact pathogenesis of this illness is unknown. Many clinical trials failed despite promising outcomes in pre-clinical studies. Therefore, novel disease-modifying medications are scarcely developed because of the doubts surrounding pharmacological targets.
On a further note, since the role of aldose reductase in the pathogenesis of DN was discovered by Dvornik et al[97], it has been extensively investigated due to its promising effects in reversing DN. Combating DN by antagonising this enzyme seems to be a promising step[14]. The application of aldose reductase inhibitors (ARIs) has been shown to hamper the overactivity of the polyol pathway. However, a previously published systematic review did not pinpoint a single RCT showing any superiority in ARIs compared to placebo in DN patients[98]. Although it has been three decades since the first discovery of ARIs, these drugs are still not established as the mainstay of DN treatment due to a high incidence of side effects[99]. Similar issues were also raised for other potential therapeutics involving the antagonism of PKC activation resulting from excessive diacylglycerol accumulation. A systematic review of RCTs on the application of the PKC inhibitor ruboxistaurin (RBX) has reported its therapeutic effects on DN. However, the evidence from those studies was insufficient to establish its efficiency in treating DN[100]. Moreover, RBX has been shown to be more effective in relieving symptoms among patients with less severe DN[100,101].
Last but not least, other potential new drugs targeting RAGEs activation have also been extensively explored in animal models[102,103], some of which have produced encouraging therapeutic effects in patients[104]. However, the high toxic contents of these drugs become a major problem in human trials[10,15]. Due to these uncertainties and suboptimal therapeutic efficiency in improving nerve functions in T2DM-induced DN[15], the industry refuses to invest further in such drugs[5]. Thus, it limits the available medication option for patients. They have to rely on the combination of anti-diabetic medications with other management strategies to delay the progression of DN.
Although diabetic management guidelines have been established worldwide, not all patients can adhere to the recommended strategies due to many factors. Patients’ non-adherence to T2DM treatment regimens continues to be a major issue in most countries[105,106]. It is closely related to poor knowledge regarding diabetes aetiology and disease progression, unstable socioeconomic status, poor family support, patient-staff engagement barriers, complex therapeutic regimens, and lack of medical insurance coverage[105-108]. Some patients even voluntarily stopped the treatment plan and shifted to traditional herbs following their concerns about the side effects of the medications.
Moreover, unsatisfactory healthcare also contributes to the non-adherence to self-care diabetic management[106]. Even with free medications provided by the government, patient adherence can be compromised if there is ineffective communication between the patients and healthcare providers[106]. It is undeniable that myths and cultural beliefs would influence the faith of a patient in doctors’ prescriptions and recommendations, especially if the patient lacks an understanding of disease progression[105,109]. Therefore, it is vital to provide appropriate health education and counselling to increase the patient’s adherence rate. As proven by Awodele and Osuolale[110], patients’ clinical outcomes improved significantly (i.e., 86.8% adherence rate) following health education and counselling.
Besides that, a complex treatment regimen can also contribute to non-adherence. Patients with multiple comorbidities generally have more medications from different pharmacological classes, giving rise to polypharmacy. A cross-sectional study among diabetic patients with no comorbidities demonstrated a higher adherence to diabetic medications[111] as compared to patients with com-orbidities who required multiple medications[112,113]. This is further complicated by the poor awareness of the importance of diabetic medications, especially in rural areas of low-income countries[105,114,115]. However, this issue can be addressed by involving the community and healthcare providers to improve the awareness of the patients. Evidently, encouragement from family and friends has been linked with an improvement in patients’ knowledge and adherence to dietary recommendations[106]. Moreover, elderly patients with multiple comorbidities displayed better medication adherence when provided with more information on the benefits[116,117].
Although comprehensive diabetic management has been established and practised globally, not all are fully attainable, especially in low-income or developing countries with high rates of poverty. Financial restraint often leads to the non-adherence of patients. In Nigeria, 51% of diabetic patients, most of who were women and unemployed, could not afford DM medications. Another 69% had to purchase their medications in smaller dosages due to high costs[110]. To minimise these obstacles, support from high-income countries is crucial. National programmes in medical schools, health centres, and hospitals can be put in place under international collaborative partnerships[118]. Evidently, a 12-mo Kerala Diabetes Prevention Programme made up of a peer support education group led to significantly improved lifestyle changes and lower cardiovascular factors among the participants. However, there was an insignificant outcome for diabetic symptom improvement[119].
It is undeniable that the coronavirus disease 2019 (COVID-19) pandemic has cast a huge impact on the healthcare and management of many diseases, including DM. During the pandemic, a prolonged lockdown was implemented. In many low-income countries, there was a lack of proper guidelines for DM patients to attend follow-ups in hospitals. Furthermore, with the low coverage of sick pay or social security, people from low-income countries were less likely to practise preventive measures such as social distancing, the use of protective gear, and visiting emergency health services. Furthermore, since diabetic management requires a visit to healthcare centres for drug prescription, many patients faced restricted access to medications. Insulin was especially restricted during the COVID-19 outbreak. At some point, many outpatient clinics and endocrinologists at private hospitals were temporarily shut down while the focus of emergency services shifted to the treatment of COVID-19 patients. These difficulties affected the care of diabetic patients, especially those who required hospital admission[120]. In short, the interruption of routine diabetic care created stress among patients, not to mention worsening obesity due to physical inactivity, both of which worsened their hyperglycaemic conditions and diabetes-related complications[121].
As the crisis of COVID-19 unfolds over the past two years, new strategies were developed to enhance diabetes care, including the use of telehealth, remote patient monitoring, online glucose monitoring via wearable technologies supported by the internet and smartphones, and free educational videos and e-books on self-management of diabetes via mobile applications[122-124]. However, these guidelines are established in developed countries, making them less suitable for patients in low-income countries with issues like poverty, poor education level, and suboptimal healthcare planning. Several suggestions were put forth to potentially improve the care of DM patients, such as replacing active follow-up with passive care, establishing community centres for patient visit and training purposes outside the hospitals (e.g., in mosques, churches, and community centres), and setting up more outpatient clinics and primary healthcare centres for the treatment of non-communicable diseases. At these centres, innovative steps were proposed and implemented, including self-monitoring of blood glucose levels without additional charges, guidelines for physicians on clinical management cases during disease outbreaks, needs assessment survey by trained investigators, and contacting patients via landlines for consultation with physicians and endocrinologists, as well as spreading educational and intervention information via text messages for patients with smartphones[120].
It is crucial to implement strategies to prevent and slow the progression of DN, especially since severe DN can be challenging to treat. There are many suggestions to achieve this. In T1DM patients, not only are the insulin-producing pancreatic β-cells destroyed, but the blood capillaries are also hugely affected. Blood capillaries are critical in insulin production; thus, it is vital to manage capillary destruction. A new drug from bone marrow stem cells has been developed to replenish the cells of blood capillaries and increase the production of β-cells. This intervention is based on the concept of introducing the formed β-cells in the form of “immunoprotective capsules” to avoid destruction by auto-immune cells[125]. This research is still ongoing. Issues related to the capability of multipotent stem cells in the formation of β-cells that can potentially proliferate into cancerous cells need to be fully addressed before the application of this drug[126]. Besides that, other proposed methods include dietary changes in DM patients, such as the consumption of amino acid arginine to facilitate the metabolism of glucose as has been proven in animal studies[125]. Arginine stimulates the production of glucagon-like peptide-1 from endocrine cells in the gut following nutrient ingestion that can promote insulin secretion, reduce food intake, increase β-cell production, and minimise β-cell apoptosis[127].
Lastly, there is growing research in the area of metabolomics technology that may aid in the diagnosis and biomarker discovery of DM. Since metabolites reflect the whole body’s functions, it is hypothesised that they can provide a comprehensive picture of what happens in the body. The combination of metabolomics detection technology with computational biology and orthogonal experiments allows the screening of diabetic metabolites and evaluation of the related metabolic pathways[128]. Evidently, through metabolomics research, it is discovered that T1DM children who developed auto-antibodies before the age of 2 had twice the depletion rate of methionine level compared to the children who developed autoantibodies in later childhood or children who were auto-antibody-negative. The same research also speculated that the methionine pathway could be involved in the generation of antibodies during early infancy[129]. Following that, a metabolomics study using transgenic and knock-out mouse models that resembled early stages of human T1DM also revealed metabolomics disturbances before the onset of T1DM. In their study, Overgaard et al[130] found a reduced level of lysophosphatidylcholine and methionine as compared to an elevated level of ceramides before the onset of T1DM. Meanwhile, in a study on insulin autoantibody seroconversion among diabetic children, Li et al[131] discovered that the rapid growth of children’s height is linked to an increased risk of islet autoimmunity and progression of T1DM. These published studies represent the growing metabolomics research that has made great progress in the identification of the main factors and metabolites that helps to identify the pathophysiological process, aetiology, early prevention, and assessment of the treatment effects of diabetes.
Primary resources of diabetic care from published studies serve as the general guidelines for better diabetic prevention strategies and patient care worldwide. Along with lifestyle and dietary modifications, additional strategies need to be added to the guidelines for the betterment of diabetes care. For instance, glucose monitoring is one of the current strategies that has been proven effective in controlling blood and dietary glucose in the previous literature for T1DM patients. However, this strategy is more beneficial in reducing diabetic complications and progression for T1DM patients because the pathogenesis of DN differs between T1DM and T2DM. For example, hyperglycaemia is not the key factor to all the complications suffered by T2DM patients. In view of this, the general management of DN among T1DM and T2DM should be tailored accordingly. It is also important to note that over-aggressive glucose control can lead to hypoglycaemia-induced neuropathy in T2DM patients. It is especially devastating for neurons in the brain that use more glucose than other cells to fulfill their functions.
There are certain misconceptions regarding the dietary monitoring of glucose intake among DM patients. Most diabetic patients eliminated sugars in their beverages but fail to reduce the consumption of carbohydrate-rich meals and sugar-rich fruits, especially in countries where carbohydrate-rich food and exotic fruits are the staple diets. The consumption of these foods may complicate the diabetic condition and accelerate the progression of DN. Therefore, it is critical to disseminate accurate knowledge about dietary glucose through social media to avoid any misconceptions among diabetic patients.
Besides that, poor treatment adherence is also a major challenge in the prevention and management of DN as discussed in the previous section. In countries with traditional lifestyles such as Asian countries, many patients opted for herbal medicine rather than modern medications, possibly due to concern about side effects and a lack of trust towards modern medicine. Although some of the traditional herbs demonstrate a potent anti-diabetic effect, the herb preparation by local manufacturers may contain additional harmful substances such as steroids that can lead to other complications. Furthermore, the crude extracts of certain herbs can be unsafe as some of the unknown metabolites can worsen the diabetic condition. Therefore, governmental agencies should conduct strict screening of the content of traditional anti-diabetic herbs before it is commercialised to reduce the risk of complications. More importantly, patient education and continuous research on these new anti-diabetic agents should be emphasised by the government as a step to improve diabetic management.
The increasing prevalence of DN and its complications among DM patients is alarming and can be costly to individuals and countries alike. Recently, psychosocial impact and morbidity from DN have also received widespread concern. Current clinical guidelines focus on preventing the progression of DN and managing the DN symptoms in patients. However, most of these guidelines fail to address the underlying factors contributing to DN, thus compromising the effectiveness of current management. Therefore, it is crucial to identify the mechanisms and risk factors of DN so that issues hindering the success of the current management of DN can be resolved. This review outlines various challenges in the management of DN on top of the pathomechanisms of DN. With a better understanding of DN pathogenesis, DN management can be enhanced. It is hoped that the additional recommendations pertaining to the raised issues can be addressed for the betterment of the quality of care and patients’ health.
| 1. | World Health Organization. Cardiovascular diseases (CVDs). 2022. [cited 11 June 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). |
| 2. | Centers for Disease Control and Prevention. What is Diabetes? 2022. [cited 11 June 2022]. Available from: https://www.cdc.gov/diabetes/basics/diabetes.html. |
| 3. | Dinh Le T, Phi Thi Nguyen N, Thanh Thi Tran H, Luong Cong T, Ho Thi Nguyen L, Do Nhu B, Tien Nguyen S, Van Ngo M, Trung Dinh H, Thi Nguyen H, Trung Nguyen K, Le DC. Diabetic Peripheral Neuropathy Associated with Cardiovascular Risk Factors and Glucagon-Like Peptide-1 Concentrations Among Newly Diagnosed Patients with Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2022;15:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Smith S, Normahani P, Lane T, Hohenschurz-Schmidt D, Oliver N, Davies AH. Prevention and Management Strategies for Diabetic Neuropathy. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 5. | Smith S, Normahani P, Lane T, Hohenschurz-Schmidt D, Oliver N, Davies AH. Pathogenesis of Distal Symmetrical Polyneuropathy in Diabetes. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1426] [Cited by in RCA: 1578] [Article Influence: 175.3] [Reference Citation Analysis (2)] |
| 7. | Wang M, Zhang Z, Mi J, Wang G, Tian L, Zhao Y, Li X, Wang X. Interventional Clinical Trials on Diabetic Peripheral Neuropathy: A Retrospective Analysis. J Pain Res. 2021;14:2651-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93:1296-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 673] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 9. | Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Pop-Busui R, Ang L, Boulton AJ, Feldman EL, Marcus RL, Mizokami-Stout K, Singleton JR, Ziegler D. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy. ADA Clin Compendia. 2022;2022:1-32. [DOI] [Full Text] |
| 11. | Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 989] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 13. | Mizukami H, Osonoi S. Pathogenesis and Molecular Treatment Strategies of Diabetic Neuropathy Collateral Glucose-Utilizing Pathways in Diabetic Polyneuropathy. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Zhu CJ. Aldose Reductase Inhibitors as Potential Therapeutic Drugs of Diabetic Complications. In: Oguntibeju OO. Diabetes mellitus Insights and Perspectives. Croatia: Intech Open, 2013: 17-46. [DOI] [Full Text] |
| 15. | Kobayashi M, Zochodne DW. Diabetic polyneuropathy: Bridging the translational gap. J Peripher Nerv Syst. 2020;25:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Song K, Chambers AR. Diabetic Foot Care. 2022 Jul 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 18. | Fonseca VA, Fonseca VA. Clinical Diabetes: Translating Research into Practice. Ann Intern Med. 2007;146:; 152. [DOI] [Full Text] |
| 19. | Jain E, Goldstein L, Jain E. Pain Management of Diabetic Neuropathy. Pract Pain Manag. 2012;12. [DOI] [Full Text] |
| 20. | Trieb K. The Charcot foot: pathophysiology, diagnosis and classification. Bone Joint J. 2016;98-B:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Zilliox LA, Russell JW. Physical activity and dietary interventions in diabetic neuropathy: a systematic review. Clin Auton Res. 2019;29:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16443] [Article Influence: 498.3] [Reference Citation Analysis (4)] |
| 23. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12805] [Article Influence: 457.3] [Reference Citation Analysis (0)] |
| 24. | Rodríguez-Gutiérrez R, Montori VM. Glycemic Control for Patients With Type 2 Diabetes Mellitus: Our Evolving Faith in the Face of Evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Dziemidok P, Szcześniak G, Kostrzewa-Zabłocka E, Paprzycki P, Korzon-Burakowska A. Current glycaemic control has no impact on the advancement of diabetic neuropathy. Ann Agric Environ Med. 2012;19:742-745. [PubMed] |
| 27. | Callaghan BC, Xia R, Banerjee M, de Rekeneire N, Harris TB, Newman AB, Satterfield S, Schwartz AV, Vinik AI, Feldman EL, Strotmeyer ES; Health ABC Study. Metabolic Syndrome Components Are Associated With Symptomatic Polyneuropathy Independent of Glycemic Status. Diabetes Care. 2016;39:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Jaiswal M, Fufaa GD, Martin CL, Pop-Busui R, Nelson RG, Feldman EL. Burden of Diabetic Peripheral Neuropathy in Pima Indians With Type 2 Diabetes. Diabetes Care. 2016;39:e63-e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, Villegas-Umana E, Pop-Busui R, Feldman EL. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016;73:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, Jensen TM, Finnerup NB, Jensen TS, Lauritzen T, Feldman EL, Callaghan BC, Charles M. Risk Factors for Incident Diabetic Polyneuropathy in a Cohort With Screen-Detected Type 2 Diabetes Followed for 13 Years: ADDITION-Denmark. Diabetes Care. 2018;41:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 31. | Callaghan BC, Gao L, Li Y, Zhou X, Reynolds E, Banerjee M, Pop-Busui R, Feldman EL, Ji L. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5:397-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S73-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 590] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 33. | American Diabetes Association. 12. Older adults: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (20)] |
| 34. | Järvisalo J, Saris NE. Action of propranolol on mitochondrial functions--effects on energized ion fluxes in the presence of valinomycin. Biochem Pharmacol. 1975;24:1701-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S200-S210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 36. | Semeraro MD, Almer G, Kaiser M, Zelzer S, Meinitzer A, Scharnagl H, Sedej S, Gruber HJ, Herrmann M. The effects of long-term moderate exercise and Western-type diet on oxidative/nitrosative stress, serum lipids and cytokines in female Sprague Dawley rats. Eur J Nutr. 2022;61:255-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Singleton JR, Foster-Palmer S, Marcus RL. Exercise as Treatment for Neuropathy in the Setting of Diabetes and Prediabetic Metabolic Syndrome: A Review of Animal Models and Human Trials. Curr Diabetes Rev. 2022;18:e230921196752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Sherizadeh H. What is axoplasmic transport? Considering the role of exercise training: A mini review. J Exerc Organ Cross Talk. 2022;2:123-131. [DOI] [Full Text] |
| 39. | Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 262] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Stubbs EB Jr, Fisher MA, Miller CM, Jelinek C, Butler J, McBurney C, Collins EG. Randomized Controlled Trial of Physical Exercise in Diabetic Veterans With Length-Dependent Distal Symmetric Polyneuropathy. Front Neurosci. 2019;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Streckmann F, Balke M, Cavaletti G, Toscanelli A, Bloch W, Décard BF, Lehmann HC, Faude O. Exercise and Neuropathy: Systematic Review with Meta-Analysis. Sports Med. 2022;52:1043-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 42. | Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S; Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 622] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 43. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12679] [Article Influence: 528.3] [Reference Citation Analysis (1)] |
| 44. | Chandarasekaran K, Chen C, Sagi A, Russel J. A nicotinamide adenine nucleotide (NAD+) precursor is a potential therapy for diabetic neuropathy. J Neuromusc Dis. 2016;3:S86. [DOI] [Full Text] |
| 45. | Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 991] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 46. | Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality After Nontraumatic Major Amputation Among Patients With Diabetes and Peripheral Vascular Disease: A Systematic Review. J Foot Ankle Surg. 2016;55:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 47. | Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K; International Working Group on the Diabetic Foot. Prevention and management of foot problems in diabetes: a Summary Guidance for Daily Practice 2015, based on the IWGDF Guidance Documents. Diabetes Metab Res Rev. 2016;32 Suppl 1:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 48. | Buggy A, Moore Z. The impact of the multidisciplinary team in the management of individuals with diabetic foot ulcers: a systematic review. J Wound Care. 2017;26:324-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2014;2014:CD001488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Siddiqui A, Suresh S. Pregabalin for acute pain management: a shift in paradigm. Br J Anaesth. 2009;102:144; author reply 144. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Azmi S, ElHadd KT, Nelson A, Chapman A, Bowling FL, Perumbalath A, Lim J, Marshall A, Malik RA, Alam U. Pregabalin in the Management of Painful Diabetic Neuropathy: A Narrative Review. Diabetes Ther. 2019;10:35-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Göthert M. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 53. | Azmi S, Ferdousi M, Petropoulos IN, Ponirakis G, Fadavi H, Tavakoli M, Alam U, Jones W, Marshall A, Jeziorska M, Boulton AJ, Efron N, Malik RA. Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care. 2015;38:e3-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Manders B. Statistical bulletin: deaths related to drug poisoning in England and Wales: 2016 registrations. Office for National Statistics. August 4, 2017. [cited 20 October 2022]. Available from: https://www.gov.uk/government/statistics/deaths-related-to-drug-poisoning-in-england-and-wales-2017-registrations. |
| 55. | Iacobucci G. UK government to reclassify pregabalin and gabapentin after rise in deaths. BMJ. 2017;358:j4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113-1e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1214] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 57. | Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2011;3:345-352, 352.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 58. | Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;2015:CD011091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, Dubner R. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 422] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011;34:818-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Su M, Liang L, Yu S. Amitriptyline Therapy in Chronic Pain. Int Arch Clin Pharmacol. 2015;1:1-15. [DOI] [Full Text] |
| 63. | Sankar V, Oommen AE, Thomas A, Nair J, James JS. Efficacy, safety and cost effectiveness of amitriptyline and pregabalin in patients with diabetic peripheral neuropathy. Indian J Pharm Sci. 2017;79:646-650. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Max MB, Kishore-Kumar R, Schafer SC, Meister B, Gracely RH, Smoller B, Dubner R. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 192] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 661] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 66. | Sindrup SH, Gram LF, Skjold T, Grodum E, Brøsen K, Beck-Nielsen H. Clomipramine vs desipramine vs placebo in the treatment of diabetic neuropathy symptoms. A double-blind cross-over study. Br J Clin Pharmacol. 1990;30:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJ; Toronto Expert Panel on Diabetic Neuropathy. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 68. | Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 813] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 69. | Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, Petersen KL, Rowbotham MC, Campbell JN. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Zeigler D, Lynch SA, Muir J, Benjamin J, Max MB. Transdermal clonidine versus placebo in painful diabetic neuropathy. Pain. 1992;48:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Mackey S, Feinberg S. Pharmacologic therapies for complex regional pain syndrome. Curr Pain Headache Rep. 2007;11:38-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Cha Y, Lee SD, Kim CS. Lumbar Sympathetic Block for the Pain Management of Diabetic Neuropathy: A case report. Korean J Anaesthesiol. 1996;30:498-501. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 74. | Cheng J, Daftari A, Zhou L. Sympathetic blocks provided sustained pain relief in a patient with refractory painful diabetic neuropathy. Case Rep Anesthesiol. 2012;2012:285328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Li SJ, Feng D. Lumbar sympathetic pulsed radiofrequency combined with continuous epidural infusion for treatment of painful diabetic neuropathy: A report of two cases and a literature review. J Int Med Res. 2020;300060518786903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 76. | Sun H, He M, Pang J, Guo X, Huo Y, Ma J. Continuous Lumbar Sympathetic Blockade Enhances the Effect of Lumbar Sympatholysis on Refractory Diabetic Neuropathy: A Randomized Controlled Trial. Diabetes Ther. 2020;11:2647-2655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 116] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Capsaicin Study Group. Diabetes Care. 1992;15:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Arch Intern Med. 1991;151:2225-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Dludla PV, Nkambule BB, Cirilli I, Marcheggiani F, Mabhida SE, Ziqubu K, Ntamo Y, Jack B, Nyambuya TM, Hanser S, Mazibuko-Mbeje SE. Capsaicin, its clinical significance in patients with painful diabetic neuropathy. Biomed Pharmacother. 2022;153:113439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 81. | Zochodne DW. Microangiopathy, diabetes, and the peripheral nervous system. In: Veves A, Malik R (eds) Contemporary diabetes: diabetic neuropathy: Clinical Management. 2nd ed. Totowa: Humana Press, 2007: 207-229. [DOI] [Full Text] |
| 82. | Freynhagen R, Argoff C, Eerdekens M, Engelen S, Perrot S. Progressive Response to Repeat Application of Capsaicin 179 mg (8% w/w) Cutaneous Patch in Peripheral Neuropathic Pain: Comprehensive New Analysis and Clinical Implications. Pain Med. 2021;22:2324-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | van Nooten F, Treur M, Pantiri K, Stoker M, Charokopou M. Capsaicin 8% Patch Versus Oral Neuropathic Pain Medications for the Treatment of Painful Diabetic Peripheral Neuropathy: A Systematic Literature Review and Network Meta-analysis. Clin Ther. 2017;39:787-803.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 84. | Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 85. | Crasto W, Altaf QA, Selvaraj DR, Jack B, Patel V, Nawaz S, Murthy N, Sukumar N, Saravanan P, Tahrani AA. Frequency Rhythmic Electrical Modulation System (FREMS) to alleviate painful diabetic peripheral neuropathy: A pilot, randomised controlled trial (The FREMSTOP study). Diabet Med. 2022;39:e14710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Stein C, Eibel B, Sbruzzi G, Lago PD, Plentz RD. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: systematic review and meta-analysis. Braz J Phys Ther. 2013;17:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Rolim LC, da Silva EM, Flumignan RL, Abreu MM, Dib SA. Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst Rev. 2019;6:CD011265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Yang W, Cai X, Wu H, Ji L. Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J Diabetes. 2019;11:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Rutkove SB. A 52-year-old woman with disabling peripheral neuropathy: review of diabetic polyneuropathy. JAMA. 2009;302:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S34-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 91. | Yilmaz Z, Piracha F, Anderson L, Mazzola N. Supplements for Diabetes Mellitus: A Review of the Literature. J Pharm Pract. 2017;30:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Le Y, Wang B, Xue M. Nutraceuticals use and type 2 diabetes mellitus. Curr Opin Pharmacol. 2022;62:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 93. | Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Ziegler D, Strom A, Lobmann R, Reiners K, Rett K, Schnell O. High prevalence of diagnosed and undiagnosed polyneuropathy in subjects with and without diabetes participating in a nationwide educational initiative (PROTECT study). J Diabetes Complications. 2015;29:998-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 384] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 96. | Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, Green J, Palumbo P, Perkins BA, Whitehouse F, Jones TL; BARI 2D Study Group. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013;36:3208-3215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 97. | Dvornik E, Simard-Duquesne N, Krami M, Sestanj K, Gabbay KH, Kinoshita JH, Varma SD, Merola LO. Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science. 1973;182:1146-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Chalk C, Benstead TJ, Moore F. Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev. 2007;2007:CD004572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Veves A. Aldose Reductase Inhibitors for the Treatment of Diabetic Neuropathy. In: Veves A, Malik RA (eds) Diabetic neuropathy. Clinical Diabetes. Totowa: Humana Press, 2007: 309-320. [DOI] [Full Text] |
| 100. | Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes Metab J. 2013;37:375-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ 3rd; MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27:1164-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Saleh I, Maritska Z, Parisa N, Hidayat R. Inhibition of Receptor for Advanced Glycation End Products as New Promising Strategy Treatment in Diabetic Retinopathy. Open Access Maced J Med Sci. 2019;7:3921-3924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, Brussee V, Liu W, Durand J, Nguyen MD, Schmidt AM, Zochodne DW. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 104. | El-Mesallamy HO, Hamdy NM, Ezzat OA, Reda AM. Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Investig Med. 2011;59:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 105. | Whittemore R, Vilar-Compte M, De La Cerda S, Marron D, Conover R, Delvy R, Lozano-Marrufo A, Pérez-Escamilla R. Challenges to diabetes self-management for adults with type 2 diabetes in low-resource settings in Mexico City: a qualitative descriptive study. Int J Equity Health. 2019;18:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 106. | Barasa Masaba B, Mmusi-Phetoe RM. Determinants of Non-Adherence to Treatment Among Patients with Type 2 Diabetes in Kenya: A Systematic Review. J Multidiscip Healthc. 2020;13:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Mirghani HO. An evaluation of adherence to anti-diabetic medications among type 2 diabetic patients in a Sudanese outpatient clinic. Pan Afr Med J. 2019;34:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Aminde LN, Tindong M, Ngwasiri CA, Aminde JA, Njim T, Fondong AA, Takah NF. Adherence to antidiabetic medication and factors associated with non-adherence among patients with type-2 diabetes mellitus in two regional hospitals in Cameroon. BMC Endocr Disord. 2019;19:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 109. | Rezaei M, Valiee S, Tahan M, Ebtekar F, Ghanei Gheshlagh R. Barriers of medication adherence in patients with type-2 diabetes: a pilot qualitative study. Diabetes Metab Syndr Obes. 2019;12:589-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Awodele O, Osuolale JA. Medication adherence in type 2 diabetes patients: study of patients in Alimosho General Hospital, Igando, Lagos, Nigeria. Afr Health Sci. 2015;15:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 111. | Alqarni AM, Alrahbeni T, Qarni AA, Qarni HMA. Adherence to diabetes medication among diabetic patients in the Bisha governorate of Saudi Arabia - a cross-sectional survey. Patient Prefer Adherence. 2019;13:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Ahmad NS, Ramli A, Islahudin F, Paraidathathu T. Medication adherence in patients with type 2 diabetes mellitus treated at primary health clinics in Malaysia. Patient Prefer Adherence. 2013;7:525-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | Hatah E, Rahim N, Makmor-Bakry M, Mohamed Shah N, Mohamad N, Ahmad M, Haron NH, Sze Hwe C, Tan Meng Wah A, Hassan F, Shaik Rahmat S, Robert SA, Abdullah N. Development and validation of Malaysia Medication Adherence Assessment Tool (MyMAAT) for diabetic patients. PLoS One. 2020;15:e0241909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 114. | Divya S, Nadig P. Factors contributing to non-adherence to medication among type 2 diabetes mellitus in patients attending tertiary care hospital in South India. Asian J Pharm Clin Res. 2015;8:274-276. [DOI] [Full Text] |
| 115. | Benrazavy L, Khalooei A. Medication adherence and its predictors in type 2 diabetic patients referring to urban primary health care centers in Kerman City, Southeastern Iran. Shiraz Med J. 2019;20:e84746. [DOI] [Full Text] |
| 116. | Rwegerera GM. Adherence to anti-diabetic drugs among patients with Type 2 diabetes mellitus at Muhimbili National Hospital, Dar es Salaam, Tanzania- A cross-sectional study. Pan Afr Med J. 2014;17:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Huber CA, Reich O. Medication adherence in patients with diabetes mellitus: does physician drug dispensing enhance quality of care? Evidence from a large health claims database in Switzerland. Patient Prefer Adherence. 2016;10:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Karachaliou F, Simatos G, Simatou A. The Challenges in the Development of Diabetes Prevention and Care Models in Low-Income Settings. Front Endocrinol (Lausanne). 2020;11:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 119. | Thankappan KR, Sathish T, Tapp RJ, Shaw JE, Lotfaliany M, Wolfe R, Absetz P, Mathews E, Aziz Z, Williams ED, Fisher EB, Zimmet PZ, Mahal A, Balachandran S, D'Esposito F, Sajeev P, Thomas E, Oldenburg B. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15:e1002575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 120. | Nouhjah S, Jahanfar S. Challenges of diabetes care management in developing countries with a high incidence of COVID-19: A brief report. Diabetes Metab Syndr. 2020;14:731-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Ghosal S, Sinha B, Majumder M, Misra A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: A simulation model using multivariate regression analysis. Diabetes Metab Syndr. 2020;14:319-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 122. | Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 123. | Gentile S, Strollo F, Ceriello A. COVID-19 infection in Italian people with diabetes: Lessons learned for our future (an experience to be used). Diabetes Res Clin Pract. 2020;162:108137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 124. | Hartmann-Boyce J, Morris E, Goyder C, Kinton J, Perring J, Nunan D, Mahtani K, Buse JB, Del Prato S, Ji L, Roussel R, Khunti K. Diabetes and COVID-19: Risks, Management, and Learnings From Other National Disasters. Diabetes Care. 2020;43:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 125. | George CM. Future trends in diabetes management. Nephrol Nurs J. 2009;36:477-483. [PubMed] |
| 126. | Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 127. | Hui H, Zhao X, Perfetti R. Structure and function studies of glucagon-like peptide-1 (GLP-1): the designing of a novel pharmacological agent for the treatment of diabetes. Diabetes Metab Res Rev. 2005;21:313-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Wu F, Liang P. Application of Metabolomics in Various Types of Diabetes. Diabetes Metab Syndr Obes. 2022;15:2051-2059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Pflueger M, Seppänen-Laakso T, Suortti T, Hyötyläinen T, Achenbach P, Bonifacio E, Orešič M, Ziegler AG. Age- and islet autoimmunity-associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes. 2011;60:2740-2747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Overgaard AJ, Weir JM, De Souza DP, Tull D, Haase C, Meikle PJ, Pociot F. Lipidomic and metabolomic characterization of a genetically modified mouse model of the early stages of human type 1 diabetes pathogenesis. Metabolomics. 2016;12:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Li Z, Veijola R, Koski E, Anand V, Martin F, Waugh K, Hyöty H, Winkler C, Killian MB, Lundgren M, Ng K, Maziarz M, Toppari J. Childhood Height Growth Rate Association With the Risk of Islet Autoimmunity and Development of Type 1 Diabetes. J Clin Endocrinol Metab. 2022;107:1520-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 132. | Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 609] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 133. | Ansari P, Hannan JMA, Azam S, Jakaria. Challenges in diabetic micro-complication management: Focus on diabetic neuropathy. Int J Transl Med. 2021;1:175-186. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Society for the Study of Pain, No. 65470; Malaysian Society of Neurosciences, No. MSN/1211/O; and Malaysian Society of Pharmacology and Physiology, No. MSPP/M/0012/2022.
Specialty type: Research and experimental medicine
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liao Z, Singapore; Patel MV, India; Zheng L, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YX