Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1514
Peer-review started: June 20, 2023
First decision: July 7, 2023
Revised: July 19, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: October 15, 2023
Processing time: 111 Days and 3.9 Hours
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease featured by insulin resistance (IR) and decreased insulin secretion. Currently, vitamin D deficiency is found in most patients with T2DM, but the relationship between vitamin D and IR in T2DM patients requires further investigation.
To explore the risk factors of IR and the effects of vitamin D supplementation on glucose and lipid metabolism in patients with T2DM.
Clinical data of 162 T2DM patients treated in First Affiliated Hospital of Harbin Medical University between January 2019 and February 2022 were retrospectively analyzed. Based on the diagnostic criteria of IR, the patients were divided into a resistance group (n = 100) and a non-resistance group (n = 62). Subsequently, patients in the resistance group were subdivided to a conventional group (n = 44) or a joint group (n = 56) according to the treatment regimens. Logistic regression was carried out to analyze the risk factors of IR in T2DM patients. The changes in glucose and lipid metabolism indexes in T2DM patients with vitamin D deficiency were evaluated after the treatment.
Notable differences were observed in age and body mass index (BMI) between the resistance group and the non-resistance group (both P < 0.05). The resistance group exhibited a lower 25-hydroxyvitamin D3 (25(OH)D3) level, as well as notably higher levels of 2-h postprandial blood glucose (2hPG), fasting blood glucose (FBG), and glycosylated hemoglobin (HbA1c) than the non-resistance group (all P < 0.0001). Additionally, the resistance group demonstrated a higher triglyceride (TG) level but a lower high-density lipoprotein-cholesterol (HDL-C) level than the non-resistance group (all P < 0.0001). The BMI, TG, HDL-C, 25(OH)D3, 2hPG, and HbA1c were found to be risk factors of IR. Moreover, the post-treatment changes in levels of 25(OH)D3, 2hPG, FBG and HbA1c, as well as TG, total cholesterol, and HDL-C in the joint group were more significant than those in the conventional group (all P < 0.05).
Patients with IR exhibit significant abnormalities in glucose and lipid metabolism parameters compared to the non-insulin resistant group. Logistic regression analysis revealed that 25(OH)D3 is an independent risk factor influencing IR. Supplementation of vitamin D has been shown to improve glucose and lipid metabolism in patients with IR and T2DM.
Core Tip: A retrospective analysis was conducted on 162 type 2 diabetes mellitus (T2DM) patients to analyze the risk factor for insulin resistance (IR) and to investigate the effects of vitamin D supplementation on glucose and lipid metabolism in patients with T2DM and IR. It was found that 25-hydroxyvitamin D3 and body mass index were risk factors for IR in T2DM patients, and vitamin D supplementation improved the glucose and lipid metabolism in patients with IR. The treatment regimen with vitamin D supplementation led to more significant decreases in 2-h postprandial blood glucose, fasting blood glucose, glycosylated hemoglobin, triglyceride, and total cholesterol levels and more increase in high-density lipoprotein-cholesterol than the conventional regimen. It is suggested that vitamin D supplementation may be an effective intervention for T2DM patients with vitamin D deficiency and IR.
- Citation: Sun LJ, Lu JX, Li XY, Zheng TS, Zhan XR. Effects of vitamin D supplementation on glucose and lipid metabolism in patients with type 2 diabetes mellitus and risk factors for insulin resistance. World J Diabetes 2023; 14(10): 1514-1523
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1514.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1514
Diabetes mellitus (DM) is a chronic disease affecting hundreds of millions of patients worldwide[1]. According to the data from the World Health Organization, there are approximate 425 million DM patients globally, and it is estimated that this number will increase to 700 million by 2045[2]. Over the past few years, with the improvement of living standards and changes in lifestyle, the number of DM patients in China has increased dramatically[3]. According to an estimation, there are approximate 114 million DM patients in China, accounting for 1/4 of the global number of patients[4]. As a public health problem, DM places a heavy burden on the economic and medical systems[5]. Without timely and effective treatment, patients with DM are prone to various complications, such as cardiovascular diseases, nephropathy, retinopathy, and neuropathy, which seriously compromise the quality of life and lifespan of the patients[6,7].
Insulin, a hormone produced by the pancreas, plays a crucial role in facilitating the absorption of blood glucose by cells for energy production. Insulin resistance (IR) refers to a condition in which the body becomes less responsive to insulin[8], leading to an increase in blood glucose level. In response to hyperglycemia, the pancreas tries to compensates by secreting higher amounts of insulin[9]. However, over time, the pancreas may not be able to produce sufficient insulin to meet the demand, resulting in DM[10]. Patients with type 2 diabetes mellitus (T2DM) often develop IR first, followed by a gradual decline in insulin secretion, which eventually triggers the inability to effectively regulate blood glucose levels[11].
Vitamin D is a fat-soluble hormone that plays a crucial role in the metabolism of calcium and phosphorus, and it is also implicated in many physiological processes, including immune regulation, inflammation and insulin synthesis and secretion[12]. In recent years, vitamin D supplementation has attracted much attention in promoting blood glucose control, suppressing inflammation, enhancing insulin secretion and improving muscle function in T2DM patients[13]. Reportedly, about 50% of T2DM patients have vitamin D deficiency, and approximately 1/3 to 2/3 of them are accompanied with decreased bone density, which increases the risk of falls, fractures and death in elderly patients[14].
This study aimed to analyze the risk factors for IR in T2DM patients and the effects of vitamin D supplementation on glucose and lipid metabolism in the patients.
Clinical data of 332 T2DM patients treated in First Affiliated Hospital of Harbin Medical University between January 2019 and February 2022 were retrospectively analyzed. This study was performed with approval from the Medical Ethics Committee of First Affiliated Hospital of Harbin Medical University.
Patients were eligible if they met the diagnostic criteria in Guidelines for Prevention and Treatment of Type 2 Diabetes Mellitus in China (2020)[15] and held complete clinical data.
Patients were excluded if they had diabetes other than T2DM, recently suffered from acute infection or acute complications of DM, or had a history of mental illness.
The homeostasis model assessment of IR (HOMA-IR) was adopted for the evaluation of the IR degree. According to the Consensus of Chinese Diabetes Experts, IR is indicated by HOMA-IR ≥ 2.69.
According to the criteria of vitamin D deficiency in the Consensus of Clinical Application of Vitamin D and Its Analogs[16], vitamin D deficiency is indicated by serum 25-hydroxyvitamin D (25(OH)D3) less than 50 nmol/L.
A total of 332 patients were screened based on the inclusion and exclusion criteria, and 162 patients who met the criteria were finally included. Based on the criteria of IR, the patients were divided into a resistance group (n = 100) and non-resistance group (n = 62). Subsequently, patients in the resistance group were subdivided into a conventional group (conventional treatment for DM, n = 44) or a joint group (conventional treatment for DM plus vitamin D supplementation, n = 56) according to the treatment regimens. The patient screening flow chart is shown in Figure 1.
Patients in both groups received routine treatment and nursing interventions, and healthcare records were established for each patient during hospitalization. All patients were given metformin [Merck & Co. Inc, State Food and Drug Administration (SFDA) approval number: H20023370] and insulin pump (biosynthetic human insulin, Novo Nordisk, SFDA approval number: S20153001). Metformin was administered with a small initial dosage (0.50 g, twice daily, or 0.85 g, once daily, taken with meals), and the dosage was gradually increased based on the patient’s conditions. Insulin is administered subcutaneously using an insulin pump at a dose of 0.15 IU/kg of body weight. The patients were required to take the medication as prescribed, and provided with relevant healthcare and exercise instruction manuals for disease knowledge education. After discharge, the patients were followed up every month and provided with personalized diet and exercise advice according to the changes in blood glucose level. Each patient received continuous intervention for 3 months, during which they were reminded to regularly monitor their blood glucose levels, maintain a reasonable diet, and engage in regular exercise.
Patients in the joint group received additional vitamin D supplementation by giving oral calcium carbonate D3 tablets (Wyeth Company, SFDA approval number: H10950029), once a day, one tablets each time, for three consecutive months.
The laboratory indicators and baseline data of patients were collected from the hospital electronic medical records. The laboratory indicators included 25(OH)D3, 2-h postprandial blood glucose (2hPG), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and HOMA-IR, Using a Hitachi 7600 fully automatic biochemical analyzer for testing. The baseline data included sex, age, course of disease, body mass index (BMI), smoking history and alcoholism history.
Primary outcome measure was the risk factors for IR, which was analyzed by Logistic regression analysis in the included patients.
Secondary outcome measures were changes in glucose metabolism indexes (25(OH)D3, 2hPG, FBG, and HbA1c) and lipid metabolism indexes (TG, TC, HDL-C, and LDL-C) in T2DM patients with IR after treatment.
SPSS 26.0 software was used for statistical analysis. The measurement data conforming to a normal distribution were expressed by mean ± SD, and the inter-group comparisons were conducted using t test. Counting data were described by cases (%), and their inter-group comparisons were conducted using chi-square test. Logistic regression analysis was used for analyzing the risk factors for IR in T2DM patients. GraphPad Prism 9.0 software was adopted for data visualization. P < 0.05 was considered a significant difference.
The baseline data were compared between the resistance group and the non-resistance group. The results showed that there were no statistically significant differences in gender, disease duration, smoking history, and alcohol consumption between the Resistance group and the Non-resistance group (all P > 0.05, Table 1). However, the proportion of patients aged > 60 years and with BMI > 25 kg/m2 was higher in the Resistance group compared to the Non-resistance group. Additionally, the Resistance group had lower levels of 25 (OH) D3 compared to the Non-resistance group (all P < 0.05, Table 1).
| Factors | Resistance group (n = 100) | Non-resistance group (n = 62) | P value | |
| Age | 0.041a | |||
| > 60 yr old | 60 | 27 | ||
| ≤ 60 yr old | 40 | 35 | ||
| Sex | 0.240 | |||
| Male | 61 | 32 | ||
| Female | 39 | 30 | ||
| BMI | < 0.0001d | |||
| > 25 kg/m2 | 44 | 10 | ||
| ≤ 25 kg/m2 | 56 | 52 | ||
| Course of disease | 0.458 | |||
| > 5 yr | 38 | 20 | ||
| ≤ 5 yr | 62 | 42 | ||
| Smoking history | 0.240 | |||
| Yes | 61 | 32 | ||
| No | 39 | 30 | ||
| Alcoholism history | 0.988 | |||
| Yes | 8 | 5 | ||
| No | 92 | 57 | ||
| 25(OH)D3 | 35.92 ± 7.12 | 44.78 ± 4.52 | < 0.001c |
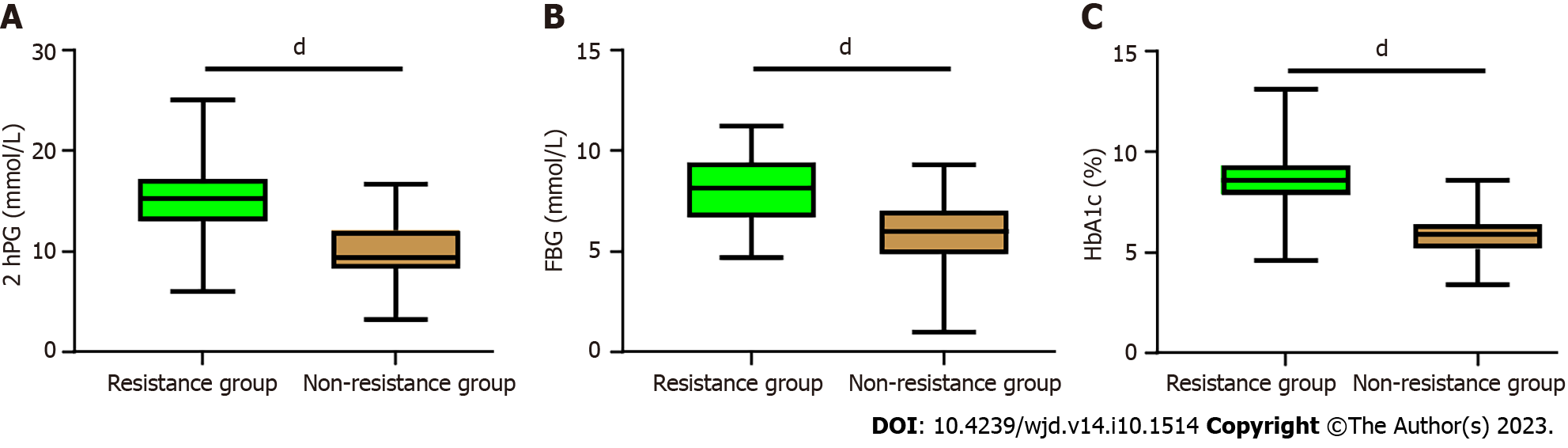
Comparison of glucose metabolism indexes between the Resistance group and Non-resistance group. The results showed that Patients in the Resistance group had higher levels of 2hPG, FBG, and HbA1c compared to the non-resistance group (all P < 0.0001, Figure 2).
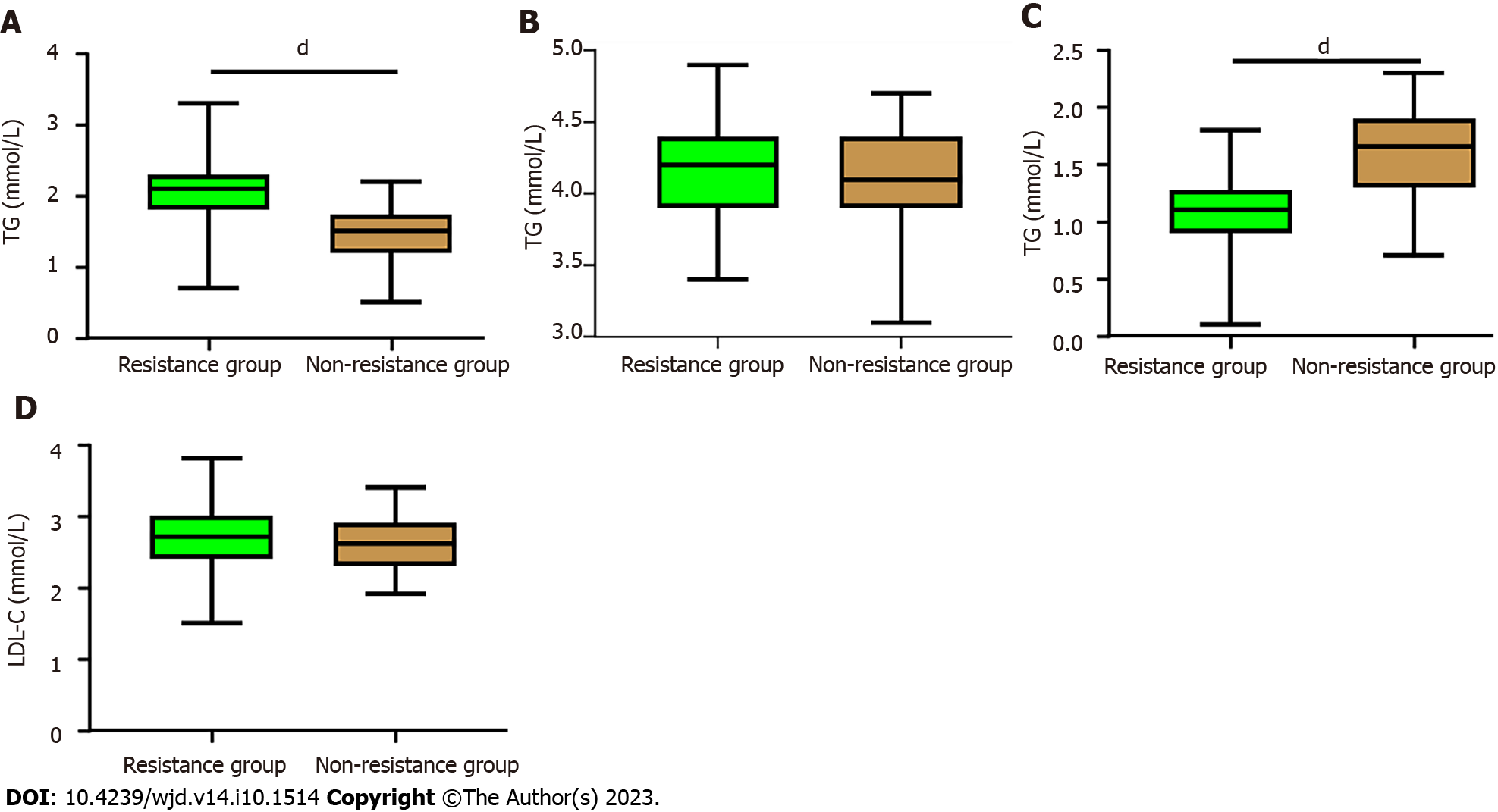
When comparing the lipid metabolism indexes between the Resistance group and the Non-resistance group, no significant differences were found in the levels of LDL-C and TC (all P > 0.05). However, the Resistance group exhibited significantly higher levels of TG compared to the Non-resistance group, while the Resistance group had lower levels of HDL-C than the Non-resistance group (all P < 0.0001, Figure 3).
A logistic regression analysis was performed using the collected and assigned significant variables (Table 2). The results revealed that BMI (OR:16.802, 95%CI: 2.557-110.43), HDL-C (OR:0.069, 95%CI: 0.009-0.540), 25(OH)D3 (OR:26.109, 95%CI: 4.285-159.098), 2hPG(OR:31.804, 95%CI: 5.567-181.709) and HbA1c (OR:90.379, 95%CI: 13.622-599.650) (all P < 0.05, Table 3).
| Factors | Assignment |
| Age | > 60 = 1, ≤ 60 = 0 |
| BMI | > 25 kg/m2 = 1, ≤ 25 kg/m2 = 0 |
| 25(OH)D3 | Data belonging to continuous variables were analyzed with their raw data |
| 2hPG | Data belonging to continuous variables were analyzed with their raw data |
| FBG | Data belonging to continuous variables were analyzed with their raw data |
| HbA1c | Data belonging to continuous variables were analyzed with their raw data |
| TG | Data belonging to continuous variables were analyzed with their raw data |
| HDL-C | Data belonging to continuous variables were analyzed with their raw data |
| Insulin resistance | Yes = 1, No = 0 |
| Factors | β | Standard error | χ2 | P value | OR value | 95%CI | |
| Lower limit | Upper limit | ||||||
| Age | 0.257 | 0.770 | 0.111 | 0.739 | 1.293 | 0.286 | 5.851 |
| BMI | 2.822 | 0.961 | 8.626 | 0.003b | 16.802 | 2.557 | 110.430 |
| TG | 1.148 | 0.853 | 1.808 | 0.179 | 3.151 | 0.591 | 16.783 |
| HDL-C | -2.68 | 1.053 | 6.476 | 0.011a | 0.069 | 0.009 | 0.540 |
| 25(OH)D3 | 3.262 | 0.922 | 12.517 | < 0.001c | 26.109 | 4.285 | 159.098 |
| 2hPG | 3.460 | 0.889 | 15.137 | < 0.001c | 31.804 | 5.567 | 181.709 |
| FBG | 0.751 | 0.756 | 0.985 | 0.321 | 2.119 | 0.481 | 9.329 |
| HbA1c | 4.504 | 0.965 | 21.762 | < 0.001c | 90.379 | 13.622 | 599.650 |
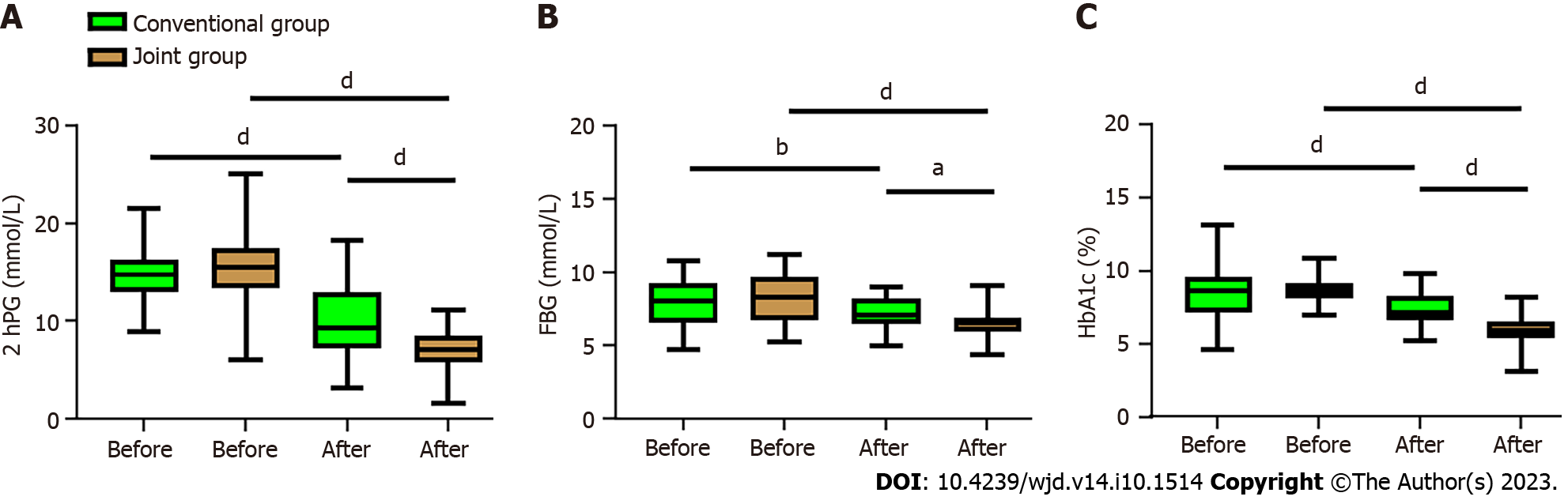
In order to determine the effects of vitamin D supplementation on glucose metabolism in patients with T2DM and IR, we analyzed the changes of glucose metabolism indicators before and after the treatment. It was found that the levels of 2hPG, FBG, and HbA1c in both the conventional group and the joint group decreased notably after treatment (all P < 0.01, Figure 4), but the joint group demonstrated more significant decreases in all the three indices than the conventional group (all P < 0.05, Figure 4).
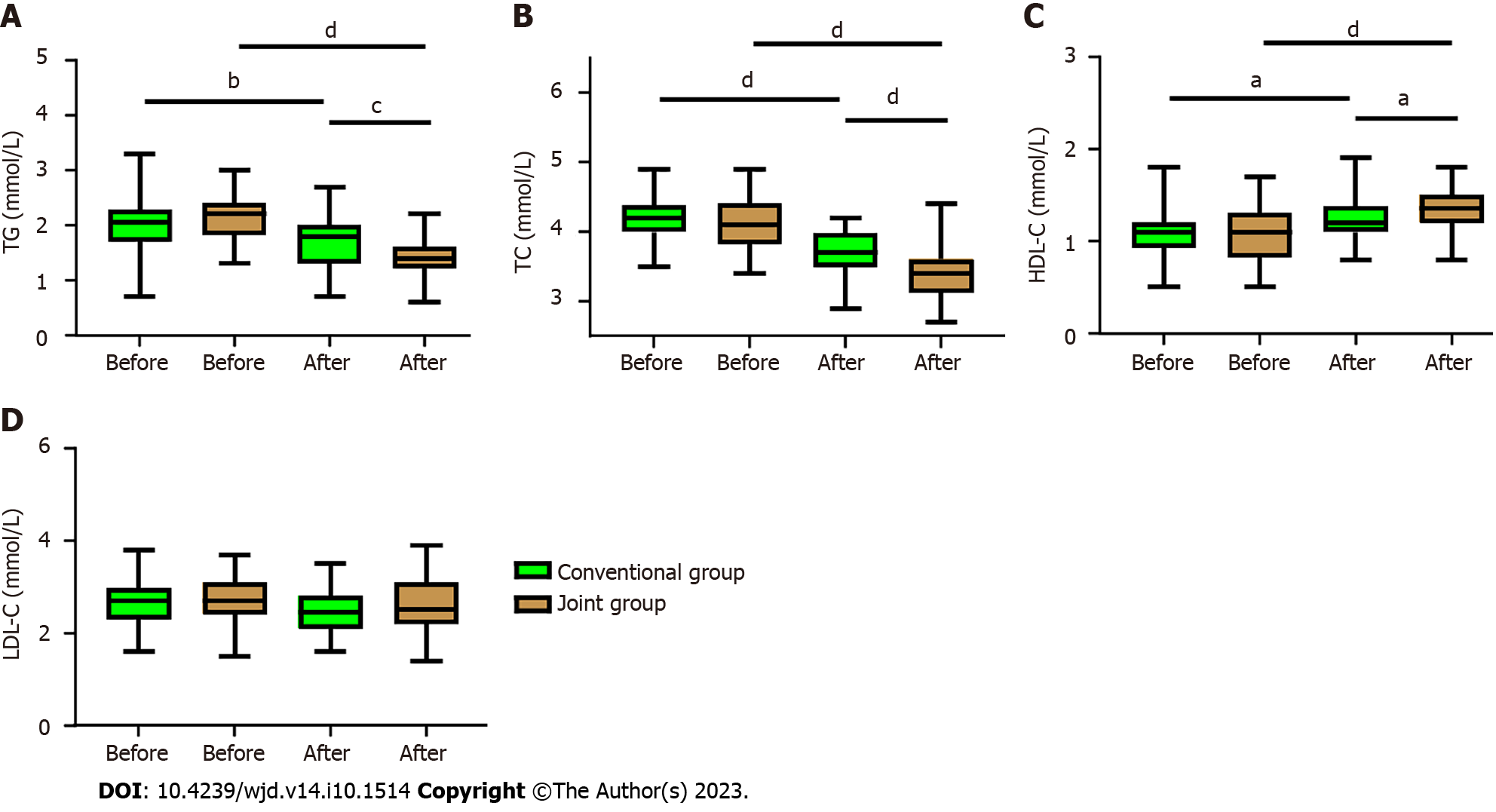
In order to determine the effect of vitamin D supplementation on lipid metabolism in T2DM patients with IR, we analyzed the changes in lipid metabolism before and after treatment. It was found that both groups exhibited decreased TG and TC, as well as increased HDL-C after treatment (all P < 0.05, Figure 5). Furthermore, the joint group presented notably lower TG and TC levels but a considerably higher HDL-C level than the conventional group (all P < 0.05, Figure 5). However, no significant different was observed in the LDL-C level between the two groups and between before and after treatment.
IR plays a crucial role in the development of T2DM[17-19]. Primarily, IR is manifested with a decrease of insulin sensitivity in the body, which leads to the secretion of a large amount of insulin by the pancreas to maintain the stability of blood glucose, thus triggering hyperinsulinemia[20]. IR affects the efficiency of glucose intake in DM patients and further triggers metabolic disorders of glucose, fat and protein, which underlie the common pathogenesis of hypertension, dyslipidemia, coronary heart diseases and obesity[21-23]. Therefore, effectively improving IR is crucial for the treatment of T2DM and contributes to the prevention and treatment of related diseases.
This study evaluated the risk factors for IR in T2DM patients and found that BMI, TG, HDL-C, 25(OH)D3, 2hPG, and HbA1c were the independent risk factors for IR. Previous studies also reported TG, HDL-C, 2hPG and HbA1c as risk factors for IR in T2DM patients[24,25]. We also investigated the effects of vitamin D supplementation on glucose and lipid metabolism in the patients with T2DM and IR. Vitamin D is a fat-soluble vitamin that is transformed into the active form 1,25(OH)2D3 through the action of the liver and kidney[26]. According to prior research[27], vitamin D is essential to stimulate insulin secretion and maintain normal glucose tolerance under physiological conditions. Vitamin D deficiency can lead to a decrease in β cell insulin secretion, exacerbating IR and increasing the risk of DM[28]. By binding to the receptor, vitamin D regulates the expression of immune- and apoptosis- related genes in pancreatic tissues, protecting islet β cells and weakening apoptosis[29]. In addition, vitamin D can affect insulin secretion and release by regulating intracellular calcium levels, and further improves insulin sensitivity by regulating insulin receptor expression and glucose transport sensitivity[30]. In this study, BMI was found to be an independent risk factor for IR in T2DM patients[31]. A previous study[32] also reported that the increase of BMI was associated with the increased risk of IR, especially for those of overweight and obesity. A high BMI is often indicative of excessive body fat, especially visceral fat. Adipocytes can secrete pro-inflammatory factors and hormones that may interfere with insulin signal transduction, thus leading to IR.
Recent studies[33,34] have revealed that approximately 50% of T2DM patients have vitamin D deficiency, and the level of vitamin D impacts insulin secretion, sensitivity and resistance. In this study, after vitamin D supplementation, the joint group showed more notable decreases in 2hPG, FBG, HbA1c and a more notable increase in 25(OH)D3 than the conventional group. In addition, the joint group presented lower TG and TC levels but a higher HDL-C level than the conventional group. These results indicate that vitamin D supplementation can improve the glucose and lipid metabolism in T2DM patients with IR. We believe this is mainly because vitamin D can improve IR and insulin sensitivity, posing a positive effect on glucose metabolism. By increasing the expression of insulin receptor and promoting glucose transport, vitamin D is helpful to reduce blood glucose level and alleviate the glucose metabolic disorder in T2DM patients. In addition, vitamin D also has a regulatory effect on lipid metabolism. It can lower the level of inflammatory factors released by adipocytes, thus improving IR. Moreover, vitamin D can regulate the adipohormone secreted by adipocytes, promote the oxidation of fatty acids, and help to lower the blood lipid level[35,36].
However, the study still has some limitations. Firstly, the long-term prognosis of patients was followed up in this study, so whether vitamin D supplementation has a long-term efficacy still needs to be further studied. Secondly, this is a single-center study with limited participants, which may lead to bias in the results. We hope to carry out further clinical experiments in future to verify and improve the research conclusions.
Patients with IR exhibit significant abnormalities in glucose and lipid metabolism parameters compared to the non-insulin resistant group. Logistic regression analysis revealed that 25(OH)D3 is an independent risk factor influencing IR. Supplementation of vitamin D has been shown to improve glucose and lipid metabolism in patients with IR and T2DM.
This study was founded on the understanding of the crucial role of insulin resistance (IR) in the development of type 2 diabetes mellitus (T2DM). A lack of insulin sensitivity in the body leads to increased insulin secretion by the pancreas, triggering hyperinsulinemia, and affecting the efficiency of glucose intake, ultimately leading to metabolic disorders.
Given that these metabolic disorders underlie several other conditions such as hypertension, dyslipidemia, coronary heart diseases, and obesity, finding effective ways to improve IR is a critical part of treating T2DM and preventing related diseases. The motivation was to evaluate risk factors for IR and study the effects of vitamin D supplementation on glucose and lipid metabolism in patients with T2DM and IR.
To identify independent risk factors for IR in T2DM patients and investigate the effects of vitamin D supplementation on their glucose and lipid metabolism.
The study carried out a comprehensive evaluation of risk factors for IR in T2DM patients, including parameters like BMI, TG, HDL-C, 25(OH)D3, 2hPG, and HbA1c. Furthermore, it explored the impact of vitamin D supplementation on glucose and lipid metabolism in T2DM patients with IR.
The study found that BMI, TG, HDL-C, 25(OH)D3, 2hPG, and HbA1c were independent risk factors for IR. After vitamin D supplementation, the test group showed notable decreases in 2hPG, FBG, HbA1c and a notable increase in 25-hydroxyvitamin D (25(OH)D3), as well as lower TG and TC levels but higher HDL-C level than the control group.
Patients with IR exhibit significant abnormalities in glucose and lipid metabolism parameters compared to the non-insulin-resistant group. The study concluded that 25(OH)D3 is an independent risk factor influencing IR and supplementation of vitamin D has been shown to improve glucose and lipid metabolism in patients with IR and T2DM.
While promising, the study has some limitations including the need for long-term patient prognosis and the possibility of bias due to it being a single-center study with limited participants. Further clinical experiments are needed to verify and improve the research conclusions, especially to assess the long-term efficacy of vitamin D supplementation.
| 1. | Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 2. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 1027] [Article Influence: 146.7] [Reference Citation Analysis (1)] |
| 3. | Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, Tabesh M, Koye DN, Shaw JE. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 2019;366:l5003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 4. | Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, Zhang X, Li C, Huang Z, Sun X, Wang L, Zhou M, Wu J, Wang Y. Prevalence and Treatment of Diabetes in China, 2013-2018. JAMA. 2021;326:2498-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (2)] |
| 5. | Refardt J, Winzeler B, Christ-Crain M. Diabetes Insipidus: An Update. Endocrinol Metab Clin North Am. 2020;49:517-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Kothari V, Cardona Z, Eisenberg Y. Adipsic diabetes insipidus. Handb Clin Neurol. 2021;181:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Tomkins M, Lawless S, Martin-Grace J, Sherlock M, Thompson CJ. Diagnosis and Management of Central Diabetes Insipidus in Adults. J Clin Endocrinol Metab. 2022;107:2701-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Ding PF, Zhang HS, Wang J, Gao YY, Mao JN, Hang CH, Li W. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front Endocrinol (Lausanne). 2022;13:1092431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Lei WS, Kindler JM. Insulin resistance and skeletal health. Curr Opin Endocrinol Diabetes Obes. 2022;29:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lubawy M, Formanowicz D. Insulin Resistance and Urolithiasis as a Challenge for a Dietitian. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Cui Y, Tang TY, Lu CQ, Ju S. Insulin Resistance and Cognitive Impairment: Evidence From Neuroimaging. J Magn Reson Imaging. 2022;56:1621-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Chen YQ, Zhang Q. Association between vitamin D and insulin resistance in adults with latent tuberculosis infection: Results from the National Health and Nutrition Examination Survey (NHANES) 2011-2012. J Infect Public Health. 2022;15:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 13. | Di Filippo L, De Lorenzo R, Giustina A, Rovere-Querini P, Conte C. Vitamin D in Osteosarcopenic Obesity. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Rodrigues CZ, Cardoso MA, Maruyama JM, Neves PAR, Qi L, Lourenço BH. Vitamin D insufficiency, excessive weight gain, and insulin resistance during pregnancy. Nutr Metab Cardiovasc Dis. 2022;32:2121-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi. 2022;61:12-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 16. | Chinese Nephrologist Association; The Working Group of Chinese Practice Program of Vitamin D. [The application of vitamin D and its analogues in patients with chronic kidney disease: the Chinese practice program (2019)]. Zhonghua Nei Ke Za Zhi. 2020;59:104-116. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 18. | Mannino GC, Andreozzi F, Sesti G. Pharmacogenetics of type 2 diabetes mellitus, the route toward tailored medicine. Diabetes Metab Res Rev. 2019;35:e3109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Hou YY, Ojo O, Wang LL, Wang Q, Jiang Q, Shao XY, Wang XH. A Randomized Controlled Trial to Compare the Effect of Peanuts and Almonds on the Cardio-Metabolic and Inflammatory Parameters in Patients with Type 2 Diabetes Mellitus. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Pearson ER. Type 2 diabetes: a multifaceted disease. Diabetologia. 2019;62:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Michailidis M, Moraitou D, Tata DA, Kalinderi K, Papamitsou T, Papaliagkas V. Alzheimer's Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer's Disease and Type 2 Diabetes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 22. | Gordon PS, Farkas GJ, Gater DR Jr. Neurogenic Obesity-Induced Insulin Resistance and Type 2 Diabetes Mellitus in Chronic Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2021;27:36-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Sharma S, Taliyan R. Histone deacetylase inhibitors: Future therapeutics for insulin resistance and type 2 diabetes. Pharmacol Res. 2016;113:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235-239. [PubMed] |
| 25. | Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 26. | Zhu Y, Li L, Li P. Vitamin D in gestational diabetes: A broadened frontier. Clin Chim Acta. 2022;537:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Bikle DD. Vitamin D Regulation of Immune Function. Curr Osteoporos Rep. 2022;20:186-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 28. | Bacchetta J, Edouard T, Laverny G, Bernardor J, Bertholet-Thomas A, Castanet M, Garnier C, Gennero I, Harambat J, Lapillonne A, Molin A, Naud C, Salles JP, Laborie S, Tounian P, Linglart A. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch Pediatr. 2022;29:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Hussain S, Yates C, Campbell MJ. Vitamin D and Systems Biology. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Liu D, Meng X, Tian Q, Cao W, Fan X, Wu L, Song M, Meng Q, Wang W, Wang Y. Vitamin D and Multiple Health Outcomes: An Umbrella Review of Observational Studies, Randomized Controlled Trials, and Mendelian Randomization Studies. Adv Nutr. 2022;13:1044-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Alami F, Alizadeh M, Shateri K. The effect of a fruit-rich diet on liver biomarkers, insulin resistance, and lipid profile in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Scand J Gastroenterol. 2022;57:1238-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 32. | Deng K, Shuai M, Zhang Z, Jiang Z, Fu Y, Shen L, Zheng JS, Chen YM. Temporal relationship among adiposity, gut microbiota, and insulin resistance in a longitudinal human cohort. BMC Med. 2022;20:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Pieńkowska A, Janicka J, Duda M, Dzwonnik K, Lip K, Mędza A, Szlagatys-Sidorkiewicz A, Brzeziński M. Controversial Impact of Vitamin D Supplementation on Reducing Insulin Resistance and Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Fong C, Alesi S, Mousa A, Moran LJ, Deed G, Grant S, Tapia K, Ee C. Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Sharafi SM, Yazdi M, Goodarzi-Khoigani M, Kelishadi R. Effect of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D and Homeostatic Model of Insulin Resistance Levels in Healthy Pregnancy: A Systematic Review and Meta-Analysis. Iran J Med Sci. 2023;48:4-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Ebadi SA, Sharifi L, Rashidi E, Ebadi SS, Khalili S, Sadeghi S, Afzali N, Shiri SM. Supplementation with vitamin D and insulin homeostasis in healthy overweight and obese adults: A randomized clinical trial. Obes Res Clin Pract. 2021;15:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhadada SK, India; Negera WG, Germany S-Editor: Chen YL L-Editor: A P-Editor: Yu HG