Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.578
Peer-review started: December 26, 2020
First decision: January 18, 2021
Revised: February 8, 2021
Accepted: April 5, 2021
Article in press: April 5, 2021
Published online: May 15, 2021
Processing time: 131 Days and 3.9 Hours
In this review, we discuss the chronic care model (CCM) in relation to the diabetes pay-for-performance (P4P) program in Taiwan. We first introduce the 6 components of the CCM and provide a detailed description of each of the activities in the P4P program implemented in Taiwan, mapping them onto the 6 components of the CCM. For each CCM component, the following three topics are described: the definition of the CCM component, the general activities implemented related to this component, and practical and empirical practices based on hospital or local government cases. We then conclude by describing the possible successful features of this P4P program and its challenges and future directions. We conclude that the successful characteristics of this P4P program in Taiwan include its focus on extrinsic and intrinsic incentives (i.e., shared care network), physician-led P4P and the implementation of activities based on the CCM components. However, due to the low rate of P4P program coverage, approximately 50% of patients with diabetes cannot enjoy the benefits of CCM-related activities or receive necessary examinations. In addition, most of these CCM-related activities are not allotted an adequate amount of incentives, and these activities are mainly implemented in hospitals, which compared with primary care providers, are unable to execute these activities flexibly. All of these issues, as well as insufficient implementation of the e-CCM model, could hinder the advanced improvement of diabetes care in Taiwan.
Core Tip: Most studies have shown that pay-for-performance (P4P) can reduce diabetes-related complications. The successful characteristics of this P4P program in Taiwan include its focus on extrinsic and intrinsic incentives (i.e., shared care network), physician-led P4P and the implementation of activities based on the chronic care model components. However, the P4P coverage rate should be steadily improved, and Taiwanese government should invest more in primary care to help these facilities participate in the P4P program and have the capacity to implement chronic care model -related activities.
- Citation: Chen TT, Oldenburg B, Hsueh YS. Chronic care model in the diabetes pay-for-performance program in Taiwan: Benefits, challenges and future directions. World J Diabetes 2021; 12(5): 578-589
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/578.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.578
There are two kinds of pay-for-performance (P4P) designs[1]. The first type of design is called indicator-based P4P, which is designed to provide extra incentives and establish a fee for service or capitation to meet objectives; examples are the P4P systems adopted in the United Kingdom[2], the United States [not including patient-centered medical homes (PCMH)][3], France[4], South Korea[5], etc. The second type is called participatory P4P, which is especially for diseases such as diabetes and has been employed in the PCMH model in the United States[6], Australia[7], Ontario of Canada[8], Tuscany in Italy[9], and Taiwan; this design is focused on patient engagement and simply rewards participation in care-improvement activities without necessarily linking bonuses to the attainment of objectives based on specific measures. Both of these types of P4P designs have their own distinct advantages. For example, the former design can steer the provider toward the predefined goals, and the latter design places fewer limitations on professional autonomy and fosters cooperation between providers and other medical staff (e.g., health educators)[1,10]. In general, the effect of P4P designs on outcomes is still arguable, and most studies have been conducted in the United States and the United Kingdom[11]. Participatory P4P research outside United States and United Kingdom is still in its infancy, and further research is needed[1].
In participatory P4P, some of these countries have achieved better outcomes for diabetes; for instance, in 2010, the Tuscany region implemented this kind of P4P design to improve general practitioner (GP) management of chronic diseases[9]. In Taiwan, more than 10 studies have shown that this kind of P4P design can reduce negative outcomes. For example, most studies have shown that P4P can reduce diabetes-related complications[12-14], emergency department visits or hospital admis
In Taiwan, most studies related to the diabetes P4P system have tended to focus on the effectiveness of the integrative model (i.e., have mainly focused on outcomes)[28]. However, a detailed understanding of the P4P system in Taiwan is more important because it may provide insights into how to build a P4P system featuring incentives for care compliance and care activities based on the CCM. In addition, if the beneficial activities associated with all CCM components are clearly mapped from a nationwide perspective, the subsequent implementation of these activities could be more informed[29]. Moreover, recent scoping research has indicated that empirical studies on the successful application of the CCM have mainly focused on two dimensions, namely, patient self-management and provider service delivery[30], and have addressed other components less. Hence, in this review, we aim to discuss the CCM in the diabetes P4P program in Taiwan. We first introduce the 6 components of the CCM and provide a detailed description of each of the activities in the P4P program implemented in Taiwan, mapping them onto the 6 components of the CCM. For each CCM component, the following three topics are described: the definition of this specific CCM component, the general activities implemented related to this component, and practical and empirical practices based on hospital or local government cases. We then conclude by describing the possible successful features of this P4P program and its challenges and future directions.
Many of the initial disease management strategies for improving quality of care were cooperative and were referenced in Wagner’s CCM proposed in mid-1990[31]. Many activities implemented by disease management programs for improving chronic care have been based on the different levels and components of the CCM, which often includes four levels for classifying different activities: (1) the system; (2) the physician/facility; (3) the patient; and (4) the community[32]. Furthermore, the CCM has 6 interrelated components: (1) a health care system (at the system level); (2) a coordinated care/delivery system design (at the hospital/physician level); (3) a decision support system (DSS) (at the hospital/physician level) to support physician guideline adherence; (4) patient self-management (at the patient level); (5) community resources (at the community level); and (6) a clinical information system (CIS) (at the hospital/physician level)[32,33].
We searched PubMed using the following keywords: “share care/shared care/case management/care system/comprehensive care and Taiwan and diabetes”. Articles found in the different searches were used as the materials for this review. For the mapping of activities to the 6 components of CCM, at least 9 articles can be referenced, and we described empirical practices based on these articles (see below)[28,34-41].
CCM component 1 — the health system in Taiwan (system level): The health system focuses on creating a culture, organization and mechanisms that foster productive interactions with consumers and promote safe, high-quality care, which includes incentives based on quality of care, visible support for improvements provided by senior leadership, and the development of agreements that facilitate care coordination within and across organizations[31,33,42,43]. We discuss incentives based on quality of care of CCM component 1 below in detail (the leadership issue will be mentioned in the Discussion section).
Regarding incentives based on the quality of care, the P4P program has undergone a two-stage evolution, with the initial establishment of disease management activities the shared care network (SCN) followed by the integration of reward systems for examinations and these activities. The pilot SCN proposed in Taiwan, called the Lan-Yang Diabetes Shared Care System (LYDSCS), which was first experimentally implemented in I-Lan County in 1996, was collaboratively executed by governmental authorities (the central Bureau of Health Promotion and local government) and hospitals[36]. After the successful implementation by I-Lan County, the National Health Insurance Administration (NHIA) integrated external incentives with this SCN as the first generation of the diabetes P4P program in 2001, which not only enforces the execution of suggested activities from the SCN but also highlights the adherence to guidelines for physicians to conduct the necessary examinations[35,36,41]. Based on the regulations for the incentive structure described in the 4th to 9th proposals for the diabetes P4P programs from 2006 to 2012, a team can receive a one-time sign-on payment of US $13 per patient enrolled in the P4P program at the hospital. In addition to the regular fee-for-service charge from the annual global budget, a team earns US $108 (not including the enrollment fees) for each patient who completes the cycle of care in a year and whom the team sees at least four times per year. The incentives (US $108) include three follow-up fees (total US $21), a one-time yearly evaluation fee (US $27), and physician fees that are paid four times, once for every patient visit except for visits to stand-alone clinics (total US $60)[14].
The P4P proposal suggests once a patient was enrolled as a participant or had undergone a yearly assessment, the patient must be given 11 essential lab tests A1c, fasting plasma glucose, fasting lipid profile (total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein), serum creatinine, serum glutamic-pyruvic transaminase, urinalysis, microalbumin, and a dilated eye examination[14]. Regarding the three follow-up visits, only two necessary examinations need be done, the A1c and glucose. In August 2006, there was a physician-level outcome incentive for the two poor outcomes used, which were “Percentage of A1C ≥ 9.5 percent” and “Percentage of low-density lipoprotein ≥ 130 mg/dL”[44].
Regarding the development of agreements that facilitate care coordination within and across organizations, some local counties, such as Changhua, have expanded the role of public health nurses and dietitians in health centers to cooperate with private primary care physicians[22]. In addition, some small facilities cannot hire a diabetes team due to cost considerations; however, by sharing labor from large facilities with small facilities, adequate economies of scale can be attained[36].
CCM component 2 — patient self-management support/patient education in Taiwan (patient level): Patient self-management support includes the following activities: information provision, patient education (general, disease, and self-management), behavioral/motivational support, patient-centered care (i.e., goal setting), and the provision of self-management tools[31,33,45].
The P4P proposal suggests the following evaluations, which fit with the principles above: (1) short- and long-term goals; (2) adherence to self-management training; (3) self-management of blood sugar results; (4) knowledge of diabetes; and (5) self-management skills. The P4P proposal also suggests the following educational steps that should be recorded into patients’ charts: (1) describe the disease progression and treatment options; (2) make lifestyle changes and diagnose personal problems; (3) use a coping strategy to solve problems in daily life; and (4) implement patient self-management from these principles[14].
Special hospital cases to help patients perform self-management are as follows. (1) In the Case 1 hospital, education is conducted in a small group of 8-10 patients around a table using an interactive approach that is intended to resolve the problems associated with self-care, reinforce knowledge of diabetes, and encourage participants to share experiences. The content of discussion includes motivation to exercise and make dietary changes, the suggestion of at least 150 min/wk of exercise, and the selection of a type of exercise depending on the patient’s lifestyle and preferences. Each patient is encouraged to monitor his or her own blood glucose[28]; And (2) In the Case 2 hospital, patients use a self-management tool called an insulin rotary disc, which is made by this hospital. Patients adjust their insulin dosage according to the suggestion displayed by the tool when the dial is aligned with the average of the past fasting glucose values[34].
CCM component 3 — a coordinated care/delivery system design/practice redesign in Taiwan (hospital/provider level): The coordinated care/delivery system design includes the following activities: team-based care provision (professionals play roles on a team), individualized care (case management), follow-up, adjustment based on health literacy and cultural background, and nurse/physician-led care[31,33,45].
The P4P proposal strictly requires that the care of diabetes patients be performed by a team consisting of trained professions[14]. The members of a team should include at least one physician of any specialty (endocrinology, family medicine or internal medicine), health educators (registered nurses), and dietitians, and they must register with the division of health of the local government. Communication between team members is facilitated through clinical training, periodic conference meetings, panel discussions, and the circulation of newsletters, and physicians within the P4P system use shared referral protocols and referral sheets[36,40]. In addition, the proposal also suggests that the delivery system be tailored to make psychosocial adjustments according to the patient’s condition[14]. Through training in the relevant knowledge and skills above, in 2013, more than 4000 health professionals, including physicians, nurses, dieticians, and pharmacists, were qualified by the Taiwan Association of Diabetes Educators (TADE), which indicates that they completed the training to gain the relevant knowledge and skills, as well as the Taiwan Bureau of Health Promotion, Department of Health (this unit has now been promoted to the Ministry of Health and Welfare)[35].
Special hospital cases for redesigning delivery systems are as follows. (1) Regarding individualized care in the Case 1 hospital, taking nutrition therapy as an example, the dietitians in this hospital provide individualized nutrition plans that are prescribed based on recommendations and that are adjusted based on a patient’s preferences; ideal body weight; and demographic, religious and socioeconomic factors[28]. Furthermore, the nurse case manager and dietitian in this hospital are sent to visit patients who express difficulty in identifying “high-sodium foods” in their diet[22]; And (2) In the Case 2 hospital, regarding adjustments based on health literacy, educational materials are translated into different languages; materials are also adapted to different literacy levels[34]. In addition, the hospital also focuses on patients’ emotional well-being by supporting them as they adjust their psychological conditions and social relationships in daily life; the hospital receives patient feedback or elicits patients’ feelings about insulin injection or glucose monitoring[34]. For remote patients, the same hospital even formed the Healthcare Diabetes e-Institute to enhance self-management through telecare for patients living in an underserved rural community[38].
CCM component 4 — the clinical information system in Taiwan (hospital/provider level): The CIS focuses on organizing data to facilitate efficient and effective care, including summarizing data to help track and plan care and facilitate performance monitoring and quality improvement efforts[31].
In Taiwan, systems that are used to monitor patient records or hospital performance, such as the CIS, are built at three levels. (1) At the national level, hospitals must report patient clinical outcomes via the virtual private network (VPN). The NHIA provides a website for every hospital to track and query their patients[41]; (2) At the local government level, some counties, such as Changhua County, have built the diabetes care management information system (DCMIS) to promote the use of clinical information in primary care. The DCMIS includes functions such as registration, reminders, descriptive statistics, and quality report production[39]; And (3) At the hospital level, hospital-made systems are usually richer than nationwide-level VPN systems. Hospitals can set up a diabetes registry that automatically captures their hospital information system records and monitors data for patient follow-up visits, such as patient demographics, telephone interview records, clinical chemistry values (outcomes), and health education records[34].
CCM component 5 — the DSS/expert system in Taiwan (hospital/provider level): The DSS focuses on promoting clinical care that is consistent with scientific evidence, which includes evidence-based guidelines for daily clinical practice and proven provider education methods[31].
In Taiwan, systems designed to provide performance feedback and/or reminders, such as the DSS, are built at two levels. (1) At the nationwide level, a public report card system is used in Taiwan[46] through which hospitals can receive feedback on diabetes quality, conduct benchmarking, and improve their performance[34,35,39]; And (2) At the hospital level, hospitals may make their own DSS, which can require the implementation of alert functions and reminders for guideline adherence[34,35]. All of these monitoring measures align with evidence-based guidelines.
CCM component 6 — community resources in Taiwan (community level): At the community level, the focus is on the mobilization of community resources to meet the needs of patients, which includes encouraging patients to participate in effective community programs and forming partnerships with community organizations to support and develop interventions that fill gaps in needed services[31].
Community resources focus on patient self-support groups, which have undergone 2 stages and have developed since the early era of LYDSCS. In the early period, the era of the LYDSCS brought about the planning of patient self-support groups[36]. With support from the Bureau of Health Promotion, TADE represents over 450 diabetic patient groups in different regions of Taiwan and aims to improve diabetes self-care and high risk awareness[22,36,41]. In the latter period, most patient peer groups were generated by the hospital itself. Hospitals invited patients’ caregivers or peer groups to supervise patients, participate in the discussion groups, and provide information about compliance[28,37]. Based on these activities, patients’ families or peer groups gradually became members of the medical team, and health professionals now consider them to be partners[40].
There are several policy implications for countries outside Taiwan, including the dual role of incentives as P4P features and physician-led quality improvement. However, these policy implications cannot be generalized throughout the world and are only referenced by countries with similar participatory P4P systems. In addition, there are also some disadvantages of diabetes P4P programs that need to be improved in the future (see the final paragraph).
Although reward systems are for both examinations and CCM-oriented activities in Taiwan, these kinds of incentives are mainly used for examinations because the low amount of incentives cannot sufficiently cover the cost of CCM-oriented activities. Extrinsic incentives (money) that target examinations alone without complex CCM-oriented activities are not sufficient to drive the improvement of patient outcomes [10,47] unless a large amount money is paid[11]. Usually, an extrinsic incentive is not sufficiently large enough to drive the execution of CCM-oriented activities, and compliance regarding the execution of these activities should also rely on the power of intrinsic incentives. Physicians have intrinsic motivations, such as professional autonomy/professionalism, inherent desires, and providing good-quality care, for performing these CCM-oriented activities[48]. However, purely relying on CCM-oriented activities provided by disease management programs may not work because there is always a quality gap when the provider has to adhere to guidelines to conduct the examinations[49]. In summary, the diabetes P4P program in Taiwan has dual characteristics that integrate extrinsic (guideline adherence) and intrinsic (CCM-oriented activities) activities at the same time, which is probably what makes the program successful[22].
We mapped the activities in the P4P program implemented in Taiwan onto the 6 components of the CCM. These 6 components are important because recent meta-analyses and systematic reviews have shown that only the integrated execution of activities corresponding to the 6 components could achieve better patient outcomes[32,50]. Another recent systematic review also demonstrated that integrating all of these components in an intervention could have greater benefits for improved patient outcomes than conducting an intervention featuring a single or some CCM components[33]. Hence, the issue of how to drive more CCM components is an important topic; the secret is perhaps found in the conclusions of Baptista et al[33]’s systematic review research, which highlighted the value of starting with this component (i.e., leadership) and continuing to subsequently implement all the other components. Based on this research, the CCM leadership component is the key to driving the other 5 components. In addition to the incentives that are based on quality of care, part of this health system component emphasizes visible support for improvements provided by senior leadership[31,33,42]; this provider-driven leadership is important for provider engagement, and it enhances professional collaborations between providers and other medical staff (e.g., health educators)[51]. Physicians, as health professional team leaders, strategically lead teams to provide coordinated care and make quality improvements[9,33].
Another example that highlights the importance of physician leadership is Wagner[52]’s revised CCM model, which was published in 1998. Interventions in health care organizations, including the delivery system design, decision support and information system categories, can help to ensure that teams are prepared and proactive, which can allow them to provide effective self-management support and access to community services to help patients be informed and activated. However, empirical research has shown that the revised CCM model still cannot address effective leadership and the robust measurement of clinical quality or effective clinical teams, which indicates the importance of starting from physician-driven leadership to lead professional teams in executing all CCM-related components[52]. Based on this research, the revised CCM model has been incorporated into the PCMH model, which emphasizes effective leadership and team building, which are essential for CCM implementation[45]; however, more evidence is needed on the effects of PCMHs in reducing patient adverse outcomes[53].
The P4P program, especially for the SCN in Taiwan, might provide physicians with the intrinsic motivation to offer provider-driven leadership to implement relevant activities because the system meets physicians’ needs to allow them to provide good-quality care and promotes providers’ vision and enthusiasm to become leaders[14], which is a vital part of the successful execution of different CCM-related activities[36]. Physician-led teams also have benefits for patients because they increase patient trust and consequently promote patients’ willingness to take on self-management through regular contact sessions and supervision[37].
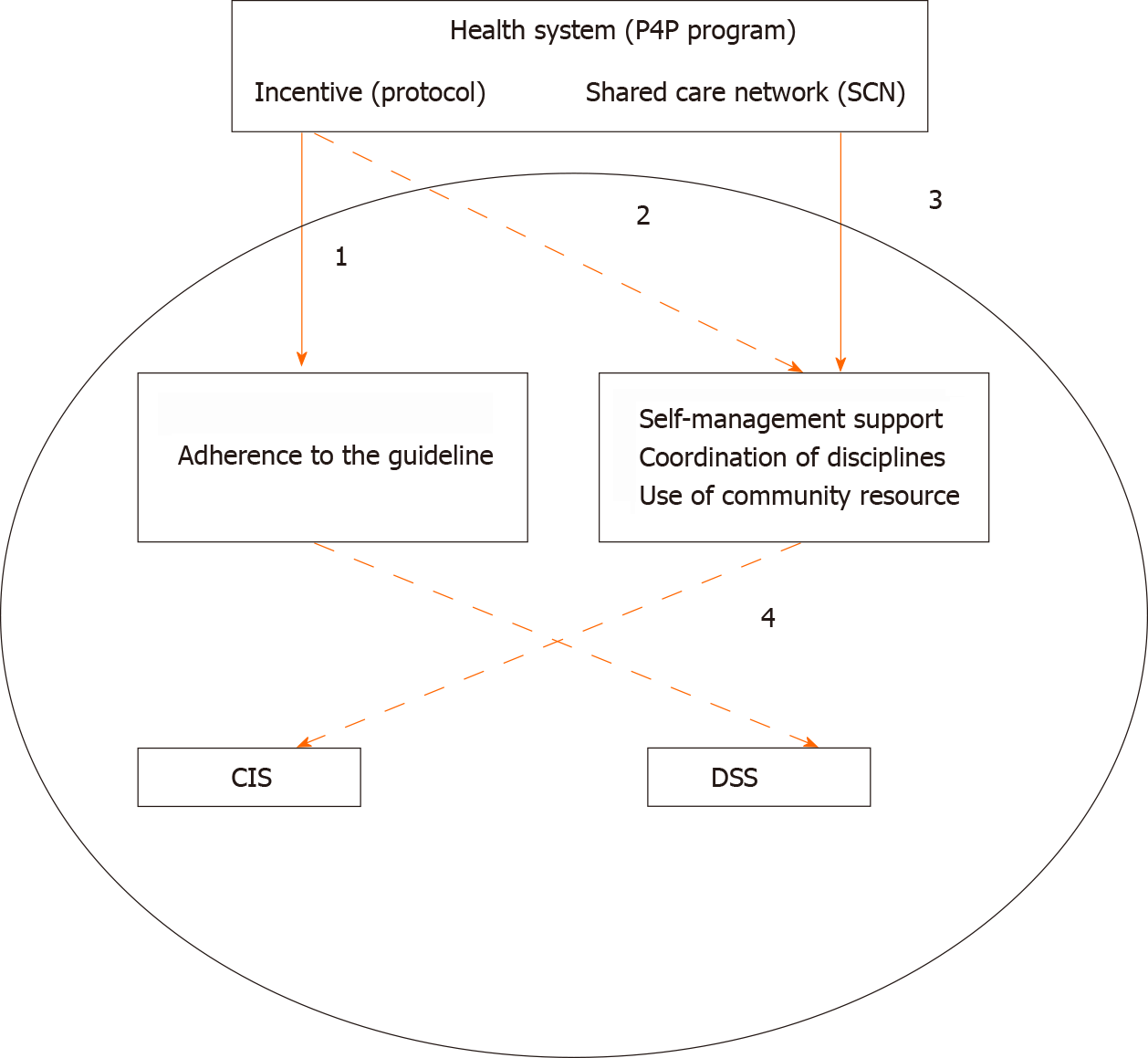
The practical framework and examples of driving other CCM components from system components can be seen in Figure 1, which starts with two health system components: incentive and SCN. In Figure 1, the top solid line represents the main target or support, and the top dashed line represents the principle provision (suggestion). The incentives mainly target guideline adherence (path 1). Other activities, including self-management support, coordination of different disciplines, and use of community resources, are only suggested in the P4P protocol (path 2). Although these activities are not the main incentive target, which means they are not paid per activity base, they are still strengthened and supported by SCN (path 3). For example, regarding coordinated care, the P4P protocol regulates team-based implementation, and the SCN provides support for team building via nationwide recognition. Another example is the component of self-management, where SCN provides training for coordinated care teams to boost specific caring skills, and the P4P protocol establishes the principles of patient self-management. The lower levels are the hospital-based CIS and DSS components, which are not strictly paid the incentive or supported by SCN; however, there is perhaps a spillover or induced effects on these two activities (path 4). For example, hospitals can automatically set up a DSS to promptly encourage physicians to adhere to the guidelines[34,35]. Future studies should investigate the efficiency of the framework.
Taiwan’s diabetes P4P framework still has room for improvement. First, approximately 30% of patients with diabetes in Taiwan were covered by the P4P program in 2010[41] and up to 43% were covered in 2015[54]. Hence, although the coverage rate of the P4P program in Taiwan approached approximately 50%, the other 50% of patients who were not covered by the program did not receive these CCM-related activities and perhaps had worse outcomes. This is probably why Taiwan is not high in the overall ranking of diabetes care across the globe[55]. However, the P4P model in Taiwan has been proven to be effective because it has the distinctive characteristics of including all CCM components. In the future, the P4P coverage rate should be steadily improved.
Second, more patients with diabetes choose to visit hospitals rather than clinics. Taiwanese citizens can visit physicians at stand-alone clinics or hospital outpatient departments for free based on their preference. Sixty percent of patients with diabetes receive care in hospitals[56]. Hospital physicians may need to treat many patients, from at least 60 to 100 or more in one day[57]. In 2018, only 8% (900/11000) of stand-alone clinics participated in the P4P program[57], and only 10% of all patients were enrolled in the P4P program in primary care (a 25% enrollment rate in primary care). Because of the limited capacity and time strain in hospital care[57], it is unlikely feasible for large hospitals (i.e., tertiary hospitals) to conduct a perfect delivery system redesign, such as one that includes individualized care (case management) and tailored care based on patients’ needs, which are very time-consuming. It is also unlikely for hospitals to be able to more flexibly and efficiently use community services than stand-alone clinics or practice teams since stand-alone clinics are located elsewhere in urban or rural communities. However, most of the primary care in Taiwan lacks the resources to implement activities suggested by the SCN. Since primary care may play an important role in caring for patients with diabetes[58,59], in the future, Taiwanese governments should invest more in primary care to help these facilities participate in the P4P program and have the capacity to implement CCM-related activities[60].
Third, the estimation of the incentive amount is mainly based on the number of patient visits and is not truly estimated based on the actual cost of CCM-based activities. On average, only a small amount of extra money, i.e., US $100 per patient per year, can be received by the facility if the incentive amount is based on the number of patient visits[41]. Although we do not have data on the portion of P4P earnings related to the total income by physicians in Taiwan, we know that the annual amount of the financial incentive averaged only 2%-3% of the total diabetes care expenditures (approximately US $0.4 billion]) in 2003[61], and the investment in P4P rewards was small compared to that in other countries, such as the United Kingdom, which has an indicator-based P4P system and invests large extra rewards in quality competition; for example, every GP in the United Kingdom could earn an average bonus of ₤77 thousand[62,63], and this approach was successful[1]. In contrast, in theory, the value creation of other P4P systems such as participatory P4P relies heavily on beneficial activities implemented for patients; more investment should be made in the core part of P4P program activities but not in measures for quality competition. If the investment is right for participatory P4P design, this design could achieve much better success. In the future, incentives should be invested directly in value-created CCM-oriented activities in Taiwan.
Fourth, the use of IT (Internet technology) to support CCM-related activities is still lacking. Systematic and meta-analyses have shown that the use of IT, such as telemedicine and mobile phones, can increase the effectiveness of diabetes patients’ self-management[64]. Gee et al[65] proposed a future e-CCM model that consists of the use of the internet to seek health information, social networking, telehealth mobile health (mHealth), and patient portals (PRs) or patient health records (PHRs) to enhance the use of the original CCM model. The features of PRs include the tracking of patients’ clinical results, proactive uptake of preventive care and screening, and suggestions for treatment strategies[66]. For example, regarding patients’ self-management support, the use of PHRs, telemedicine, and mHealth could enhance patients’ self-management and thus strengthen patient activation or self-perceived confidence in care[67,68] and improve patient outcomes[69]. In the future, the Taiwanese government or facilities should develop a comprehensive IT infrastructure for the e-CCM model.
We conclude that the successful characteristics of this P4P program in Taiwan include its focus on extrinsic and intrinsic incentives (i.e., SCN), physician-led P4P and the implementation of activities based on the CCM components. However, due to the low rate of P4P program coverage, approximately 50% of patients with diabetes cannot enjoy the benefits of CCM-related activities or receive necessary examinations. In addition, most of these CCM-related activities are not allotted an adequate amount of incentives, and these activities are mainly implemented in hospitals, which compared with primary care providers, are unable to execute these activities flexibly. All of these issues, as well as insufficient implementation of the e-CCM model, could hinder the advanced improvement of diabetes care in Taiwan.
| 1. | Iezzi E, Lippi Bruni M, Ugolini C. The role of GP's compensation schemes in diabetes care: evidence from panel data. J Health Econ. 2014;34:104-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Ryan AM, Doran T. The effect of improving processes of care on patient outcomes: evidence from the United Kingdom's quality and outcomes framework. Med Care. 2012;50:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tompkins CP, Higgins AR, Ritter GA. Measuring outcomes and efficiency in medicare value-based purchasing. Health Aff (Millwood). 2009;28:w251-w261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Girault A, Bellanger M, Lalloué B, Loirat P, Moisdon JC, Minvielle E. Implementing hospital pay-for-performance: Lessons learned from the French pilot program. Health Policy. 2017;121:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Yang JH, Kim SM, Han SJ, Knaak M, Yang GH, Lee KD, Yoo YH, Ha G, Kim EJ, Yoo MS. The impact of Value Incentive Program (VIP) on the quality of hospital care for acute stroke in Korea. Int J Qual Health Care. 2016;28:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Friedberg MW, Schneider EC, Rosenthal MB, Volpp KG, Werner RM. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Scott A, Schurer S, Jensen PH, Sivey P. The effects of an incentive program on quality of care in diabetes management. Health Econ. 2009;18:1091-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Kiran T, Victor JC, Kopp A, Shah BR, Glazier RH. The relationship between financial incentives and quality of diabetes care in Ontario, Canada. Diabetes Care. 2012;35:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Profili F, Bellini I, Zuppiroli A, Seghieri G, Barbone F, Francesconi P. Changes in diabetes care introduced by a Chronic Care Model-based programme in Tuscany: a 4-year cohort study. Eur J Public Health. 2017;27:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chen TT, Hsueh YA, Ko CH, Shih LN, Yang SS. The effect of a hepatitis pay-for-performance program on outcomes of patients undergoing antiviral therapy. Eur J Public Health. 2017;27:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Mendelson A, Kondo K, Damberg C, Low A, Motúapuaka M, Freeman M, O'Neil M, Relevo R, Kansagara D. The Effects of Pay-for-Performance Programs on Health, Health Care Use, and Processes of Care: A Systematic Review. Ann Intern Med. 2017;166:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Lin TY, Chen CY, Huang YT, Ting MK, Huang JC, Hsu KH. The effectiveness of a pay for performance program on diabetes care in Taiwan: A nationwide population-based longitudinal study. Health Policy. 2016;120:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Hsieh HM, Lin TH, Lee IC, Huang CJ, Shin SJ, Chiu HC. The association between participation in a pay-for-performance program and macrovascular complications in patients with type 2 diabetes in Taiwan: A nationwide population-based cohort study. Prev Med. 2016;85:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kornelius E, Chiou JY, Yang YS, Lu YL, Peng CH, Huang CN. The Diabetes Shared Care Program and Risks of Cardiovascular Events in Type 2 Diabetes. Am J Med 2015; 128: 977-85. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Huang YC, Lee MC, Chou YJ, Huang N. Disease-specific Pay-for-Performance Programs: Do the P4P Effects Differ Between Diabetic Patients With and Without Multiple Chronic Conditions? Med Care. 2016;54:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Chen CC, Cheng SH. Does pay-for-performance benefit patients with multiple chronic conditions? Health Policy Plan. 2016;31:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Hsieh HM, Chiu HC, Lin YT, Shin SJ. A diabetes pay-for-performance program and the competing causes of death among cancer survivors with type 2 diabetes in Taiwan. Int J Qual Health Care. 2017;29:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lo HY, Yang SL, Lin HH, Bai KJ, Lee JJ, Lee TI, Chiang CY. Does enhanced diabetes management reduce the risk and improve the outcome of tuberculosis? Int J Tuberc Lung Dis. 2016;20:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Pan CC, Kung PT, Chiu LT, Liao YP, Tsai WC. Patients with diabetes in pay-for-performance programs have better physician continuity of care and survival. Am J Manag Care. 2017;23:e57-e66. [PubMed] |
| 20. | Chen YC, Lee CT, Lin BJ, Chang YY, Shi HY. Impact of pay-for-performance on mortality in diabetes patients in Taiwan: A population-based study. Medicine (Baltimore). 2016;95:e4197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Liao PJ, Lin TY, Wang TC, Ting MK, Wu IW, Huang HT, Wang FC, Chang HC, Hsu KH. Long-Term and Interactive Effects of Pay-For-Performance Interventions among Diabetic Nephropathy Patients at the Early Chronic Kidney Disease Stage. Medicine (Baltimore). 2016;95:e3282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Hsu CC, Tai TY. Long-term glycemic control by a diabetes case-management program and the challenges of diabetes care in Taiwan. Diabetes Res Clin Pract. 2014;106 Suppl 2:S328-S332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Hsieh HM, Tsai SL, Shin SJ, Mau LW, Chiu HC. Cost-effectiveness of diabetes pay-for-performance incentive designs. Med Care. 2015;53:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Tan EC, Pwu RF, Chen DR, Yang MC. Is a diabetes pay-for-performance program cost-effective under the National Health Insurance in Taiwan? Qual Life Res. 2014;23:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Hsieh HM, Gu SM, Shin SJ, Kao HY, Lin YC, Chiu HC. Cost-Effectiveness of a Diabetes Pay-For-Performance Program in Diabetes Patients with Multiple Chronic Conditions. PLoS One. 2015;10:e0133163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Mesana L, Cheung L, Syed IA, Gauthier A, Pruce D. Value-Based Health Care in Asia: Outcomes from Taiwan's Pay-for-Performance Programs. Value Health. 2018;21:S54-S54. |
| 27. | Economist. Value-based healthcare in Taiwan Towards a leadership role in Asia. [cited November 11, 2020]. In: Economist [Internet]. Available from: https://eiuperspectives.economist.com/healthcare/value-based-healthcare-taiwan-towards-leadership-role-asia/white-paper/value-based-healthcare-taiwan-towards-leadership-role-asia. |
| 28. | Tien KJ, Hung HC, Hsiao JY, Hsu SC, Hsin SC, Shin SJ, Hsieh MC. Effectiveness of comprehensive diabetes care program in Taiwanese with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Busetto L, Luijkx KG, Elissen AMJ, Vrijhoef HJM. Context, mechanisms and outcomes of integrated care for diabetes mellitus type 2: a systematic review. BMC Health Serv Res. 2016;16:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Struckmann V, Leijten FRM, van Ginneken E, Kraus M, Reiss M, Spranger A, Boland MRS, Czypionka T, Busse R, Rutten-van Mölken M; SELFIE consortium. Relevant models and elements of integrated care for multi-morbidity: Results of a scoping review. Health Policy. 2018;122:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Improving Chronic Illness Care. The Chronic Care Model. [cited November 11, 2020]. In: Improving Chronic Illness Care [Internet]. Available from: http://www.improvingchroniccare.org/index.php?p=The_Chronic_Care_Model&s=2. |
| 32. | Bongaerts BW, Müssig K, Wens J, Lang C, Schwarz P, Roden M, Rathmann W. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and meta-analysis. BMJ Open. 2017;7:e013076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Baptista DR, Wiens A, Pontarolo R, Regis L, Reis WC, Correr CJ. The chronic care model for type 2 diabetes: a systematic review. Diabetol Metab Syndr. 2016;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Kuo JF, Tu ST, Hsu SR, Mao IC, Li YC, Lin GY, Tian JY, Syu YY, Chen WH, Hsu CC, Syu BL, Wu TY, Cho YW. Hospital-based integrated diabetes care management: an overview. Diabetes Res Clin Pract. 2014;106 Suppl 2:S323-S327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Wang CY, Yu NC, Sheu WH, Tsai ST, Tai TY. Team care of type 2 diabetes mellitus in Taiwan. Diabetes Res Clin Pract. 2014;106 Suppl 2:S309-S313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Chiou ST, Lin HD, Yu NC, Hseuh HK, Lin LH, Lin LT, Chen TJ, Lai MS. An initial assessment of the feasibility and effectiveness of implementing diabetes shared care system in Taiwan--some experiences from I-Lan County. Diabetes Res Clin Pract. 2001;54 Suppl 1:S67-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Hao LJ, Tien KJ, Chao H, Hong CJ, Chou FS, Wu TJ, Chao JK, Shi MD, Chai KL, Ko KC, Cheng JS, Ma MC. Metabolic outcome for diabetes shared care program outpatients in a veterans hospital of southern Taiwan. J Chin Med Assoc. 2011;74:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Liou JK, Soon MS, Chen CH, Huang TF, Chen YP, Yeh YP, Chang CJ, Kuo SJ, Hsieh MC. Shared care combined with telecare improves glycemic control of diabetic patients in a rural underserved community. Telemed J E Health. 2014;20:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Yeh YP, Chang CJ, Hsieh ML, Wu HT. Overcoming disparities in diabetes care: eight years' experience changing the diabetes care system in Changhua, Taiwan. Diabetes Res Clin Pract. 2014;106 Suppl 2:S314-S322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Chang HC, Chang YC, Lee SM, Chen MF, Huang MC, Peng CL, Yan CY. The effectiveness of hospital-based diabetes case management: an example from a northern Taiwan regional hospital. J Nurs Res. 2007;15:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Jiang YD, Shiu RS, Chuang LM, Lin BJ. Is the development of a diabetes care system important for quality care? J Diabetes Investig. 2011;2:79-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Barr VJ, Robinson S, Marin-Link B, Underhill L, Dotts A, Ravensdale D, Salivaras S. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 43. | Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1479] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 44. | Chen TT, Chung KP, Lin IC, Lai MS. The unintended consequence of diabetes mellitus pay-for-performance (P4P) program in Taiwan: are patients with more comorbidities or more severe conditions likely to be excluded from the P4P program? Health Serv Res. 2011;46:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Wagner EH. Organizing Care for Patients With Chronic Illness Revisited. Milbank Q. 2019;97:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Chen TT, Hsueh YA, Liaw CK, Shih LN, Huang LY. Does public report card matter? Eur J Public Health. 2020;30:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Chen TT, Yang JJ, Hsueh YA, Wang V. The effects of a schizophrenia pay-for-performance program on patient outcomes in Taiwan. Health Serv Res. 2019;54:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Conrad DA. The Theory of Value-Based Payment Incentives and Their Application to Health Care. Health Serv Res. 2015;50 Suppl 2:2057-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 49. | McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3308] [Cited by in RCA: 3153] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 50. | Flanagan S, Damery S, Combes G. The effectiveness of integrated care interventions in improving patient quality of life (QoL) for patients with chronic conditions. An overview of the systematic review evidence. Health Qual Life Outcomes. 2017;15:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Skillman M, Cross-Barnet C, Singer RF, Ruiz S, Rotondo C, Ahn R, Snyder LP, Colligan EM, Giuriceo K, Moiduddin A. Physician Engagement Strategies in Care Coordination: Findings from the Centers for Medicare & Medicaid Services' Health Care Innovation Awards Program. Health Serv Res. 2017;52:291-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2-4. [PubMed] |
| 53. | Sinaiko AD, Landrum MB, Meyers DJ, Alidina S, Maeng DD, Friedberg MW, Kern LM, Edwards AM, Flieger SP, Houck PR, Peele P, Reid RJ, McGraves-Lloyd K, Finison K, Rosenthal MB. Synthesis Of Research On Patient-Centered Medical Homes Brings Systematic Differences Into Relief. Health Aff (Millwood). 2017;36:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | American Public Health Association 2017 Annual Meeting and Expo. Board 2 -Performance of Diabetes Shared Care in Taiwan. [cited November 11, 2020]. In: American Public Health Association [Internet]. https://apha.confex.com/apha/2017/meetingapp.cgi/Paper/381674. |
| 55. | GBD 2015 Healthcare Access and Quality Collaborators; GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet. 2017;390:231-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 56. | Hou YH, Lin SP, Chiu YL, Hsu YJ, Cheng LL. Investigating the Quality of Outpatient Care for Diabetic Patients in Different Health Care Organizations in Taiwan. Chengching Yixue Zazhi. 2011;7:32-41. [DOI] [Full Text] |
| 57. | Hsh HC, Lee YJ. The hierarchy of medical care for diabetes care. Taiwan Yixue Zazhi. 2019;62:44-49. |
| 58. | Russell AW, Baxter KA, Askew DA, Tsai J, Ware RS, Jackson CL. Model of care for the management of complex Type 2 diabetes managed in the community by primary care physicians with specialist support: an open controlled trial. Diabet Med. 2013;30:1112-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Schäfer WLA, Boerma WGW, Schellevis FG, Groenewegen PP. GP Practices as a One-Stop Shop: How Do Patients Perceive the Quality of Care? Health Serv Res. 2018;53:2047-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Jan CF, Lee MC, Chiu CM, Huang CK, Hwang SJ, Chang CJ, Chiu TY. Awareness of, attitude toward, and willingness to participate in pay for performance programs among family physicians: a cross-sectional study. BMC Fam Pract. 2020;21:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Chen TT, Lai MS, Chung KP. Participating physician preferences regarding a pay-for-performance incentive design: a discrete choice experiment. Int J Qual Health Care. 2016;28:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Epstein AM. Paying for performance in the United States and abroad. N Engl J Med. 2006;355:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Roland M. Linking physicians' pay to the quality of care--a major experiment in the United kingdom. N Engl J Med. 2004;351:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 472] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 64. | Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do Mobile Phone Applications Improve Glycemic Control (HbA1c) in the Self-management of Diabetes? Diabetes Care. 2016;39:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 65. | Gee PM, Greenwood DA, Paterniti DA, Ward D, Miller LM. The eHealth Enhanced Chronic Care Model: a theory derivation approach. J Med Internet Res. 2015;17:e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 66. | Greenberg AJ, Falisi AL, Finney Rutten LJ, Chou WS, Patel V, Moser RP, Hesse BW. Access to Electronic Personal Health Records Among Patients With Multiple Chronic Conditions: A Secondary Data Analysis. J Med Internet Res. 2017;19:e188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Davis S, Roudsari A, Raworth R, Courtney KL, MacKay L. Shared decision-making using personal health record technology: a scoping review at the crossroads. J Am Med Inform Assoc. 2017;24:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Archer N, Fevrier-Thomas U, Lokker C, McKibbon KA, Straus SE. Personal health records: a scoping review. J Am Med Inform Assoc. 2011;18:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 69. | Lee AA, Piette JD, Heisler M, Janevic MR, Rosland AM. Diabetes self-management and glycemic control: The role of autonomy support from informal health supporters. Health Psychol. 2019;38:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji G S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ