Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.466
Peer-review started: November 30, 2020
First decision: January 25, 2021
Revised: February 3, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: April 15, 2021
Processing time: 129 Days and 21.8 Hours
Atherosclerosis is a major cause of mortality worldwide and is driven by multiple risk factors, including diabetes, which results in an increased atherosclerotic burden, but the precise mechanisms for the occurrence and development of diabetic atherosclerosis have not been fully elucidated.
To summarize the potential role of retinol binding protein 4 (RBP4) in the pathogenesis of diabetic atherosclerosis, particularly in relation to the RBP4-Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway.
Male Wistar rats were randomly divided into three groups, including a control group (NC group), diabetic rat group (DM group), and diabetic atherosclerotic rat group (DA group). The contents of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), fasting insulin (FINS), fasting plasma glucose, and hemoglobin A1c (HbA1c) were measured. Moreover, the adipose and serum levels of RBP4, along with the expression levels of JAK2, phosphorylated JAK2 (p-JAK2), STAT3, phosphorylated STAT3 (p-STAT3), B-cell lymphoma-2 (Bcl-2), and Cyclin D1 in aortic tissues were also measured. Besides, homeostasis model assessment of insulin resistance (HOMA-IR) and atherogenic indexes (AI) were calculated.
Compared with the NC and DM groups, the levels LDL-c, TG, TC, FINS, HOMA-IR, RBP4, and AI were upregulated, whereas that of HDL-c was downregulated in the DA group (P < 0.05); the mRNA levels of JAK2, STAT3, Cyclin D1, and Bcl-2 in the DA group were significantly increased compared with the NC group and the DM group; P-JAK2, p-JAK2/JAK2 ratio, p-STAT3, p-STAT3/STAT3 ratio, Cyclin D1, and Bcl-2 at protein levels were significantly upregulated in the DA group compared with the NC group and DM group. In addition, as shown by Pearson analysis, serum RBP4 had a positive correlation with TG, TC, LDL-c, FINS, HbA1C, p-JAK2, p-STAT3, Bcl-2, Cyclin D1, AI, and HOMA-IR but a negative correlation with HDL-c. In addition, multivariable logistic regression analysis showed that serum RBP4, p-JAK2, p-STAT3, and LDL-c were predictors of the presence of diabetic atherosclerosis.
RBP4 could be involved in the initiation or progression of diabetic atherosclerosis by regulating the JAK2/STAT3 signaling pathway.
Core Tip: Atherosclerosis is a major cause of mortality worldwide and is driven by multiple risk factors including diabetes, which entails increased atherosclerotic burden, but the precise mechanisms for the occurrence and development of diabetic atherosclerosis are yet to be fully made clear. Retinol binding protein 4 is clinically associated with obesity, insulin resistance, type 2 diabetes, and cardiovascular diseases. This study aimed to explore the expression regulation and mechanism of retinol binding protein 4 that is involved in diabetic macrovascular disease in order to find therapeutic targets for diabetic macrovascular disease.
- Citation: Zhou W, Ye SD, Wang W. Elevated retinol binding protein 4 levels are associated with atherosclerosis in diabetic rats via JAK2/STAT3 signaling pathway. World J Diabetes 2021; 12(4): 466-479
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/466.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.466
Atherosclerosis is a chronic disorder that involves inflammatory cell recruitment, endothelial dysfunction, local cytokine generation, and lipid accumulation in the vascular wall intima[1]. Retinol binding protein 4 (RBP4) is a novel adipokine released from hepatocytes and adipocytes and is considered as an emerging cardiometabolic risk factor that is correlated with obesity, insulin resistance (IR), impaired glucose tolerance, and type 2 diabetes (T2DM). This molecule was first reported by Yang et al[2], who, using a mouse model, found that the increased RBP4 expression in circulation resulted in IR by suppressing the activity of phosphatidylinositol 3-kinase (PI3K) in the skeletal muscle while upregulating the hepatic level of phosphoenol
Recent studies have shown that the increased proliferation and migration of vascular smooth muscle cells (VSMCs) are a key event in the progression of cardiovascular diseases[6]. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, which can regulate various pathophysiological processes, has been implicated in mediating cell migration and proliferation in VSMCs. Binding of cytokines to their receptors induces their autoactivation through transphosphorylation. Once activated, JAK2 is then rapidly activated in VSMCs, and STAT3 is phosphorylated and translocated to the nucleus in a JAK2-dependent manner[7].
Therefore, the aim of the present study was to determine the expression and function of RBP4 in diabetic rats with atherosclerosis, and to confirm whether the role of RBP4 in the development of atherosclerosis is mediated via the JAK/STAT signaling pathway.
The study was approved by the Ethics Committee of Anhui Provincial Hospital Medical Institution Animal Care and Research Advisory Committee (Hefei, China) and was carried out in compliance with the Animal Research: Reporting in vivo Experiments Guidelines (ARRIVE Guidelines). Altogether 70 2-mo-old male Wistar rats weighing 190-210 g were purchased from the Experimental Animal Center of Anhui Medical University and raised in clean plastic cages at a temperature of 20 ± 1 °C, humidity of 47% ± 9%, and photoperiod of 14 h (light)/10 h (dark) during the entire experiment. The animals were divided into a normal control (NC, n = 20) or observation (n = 50) group. The NC group was given a normal diet, whereas the remaining animals were given a high-glucose and high-fat diet composed of 78.3% carbohydrates, 10% lard, 6% sugar, 5% cholesterol, 0.2% propylthiouracil, and 0.5% sodium cholate. The rats were acclimatized to the experimental conditions, and then, 30 mg/kg streptozotocin (STZ; Sigma-Aldrich, United States) was used to induce diabetes in rats randomly selected from the observation group. To confirm T2DM, we used a glucometer (Roche, Diagnostics GmbH, Germany) to carry out glucose tolerance tests on the rats fasted for 12 h. As a result, the 50 rats were shown to have T2DM (fasting plasma glucose, FPG > 7.8 mmol/L). The observation group rats continued on the high-glucose-high-fat diet and were randomly divided into diabetic rats (DM group, n = 25) and diabetic rats with atherosclerosis (DA group, n = 25). The rats in the DA group were then intraperitoneally injected with recombinant RBP4 (ab109146, abcam, United Kingdom) at 3 µg/g every 12 h for 3 wk.
At the end of week 19, each rat was anaesthetized intraperitoneally with 2% sodium pentobarbital sodium at a dose of 30 mg/kg. Then, blood was sampled to detect the related serum component contents. Except for samples for hematoxylin and eosin (HE) staining and immunohistochemistry, samples for Western blot assays of JAK2, phosphorylated JAK2 (p-JAK2), STAT3, phosphorylated STAT3 (p-STAT3), Cyclin D1, and B-cell lymphoma-2 (Bcl-2) were obtained when the thoracic aorta was separated and used to extract mRNA and protein. In addition, visceral adipose tissue was extracted for the measurement of the RBP4 mRNA level and the quantitative protein expression of RBP4. All methods were performed in accordance with the relevant guidelines and regulations.
The levels of low-density lipoprotein cholesterol (LDL-c), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), and triglycerides (TG) were detected with an automatic biochemical analyzer (Hitachi 7600-020, Japan). Additionally, the levels of fasting insulin (FINS) were tested with an insulin radioimmunoassay kit (Atom Hi-Tech, China). The level of serum RBP4 was evaluated by enzyme-linked immuno
BCA assays were conducted to determine the protein levels in cell lysates. After separation through 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the separated proteins were transferred onto a PVDF (polyvinylidene fluoride) membrane. At 4 °C, the membrane was simultaneously exposed overnight to solution containing primary antibodies [1:300, anti-JAK2 (Bioss, China); 1:5000, anti-p-JAK2 (abcam, United States); 1:1000, anti-STAT3 (Bioss, China); 1:10000, anti-p-STAT3 (abcam, United States); 1:300, anti-Cyclin D1 (Bioss, China); 1:1000, anti- Bcl-2 (Bioss, China); 1:1000, anti-RBP4 (Bioss, China)]. After the membrane was washed with Tris-buffered saline (TBS)-0.1% Tween 20 solution four times every 5 min, it was incubated with the corresponding secondary antibodies conjugated to horseradish peroxidase (1:10000, Zs-BIO, China). Western blots were treated with an ECL (electro-chemiluminescence) detection kit (Thermo, United States) to induce the chemilumine
TRIzol (Invitrogen, United States) was used to extract total RNA. Thereafter, the RevertAidTM First Strand cDNA Synthesis Kit (Thermo, United States) was used to prepare cDNA in accordance with specific protocols. Moreover, the Novostart SYBR qPCR SuperMix Plus (Novoprotein, China) was adopted for real-time quantitative polymerase chain reaction (PCR) by using the fluorescence quantitative LightCycler 96 Real-Time PCR System (Thermo, United States). The primer sequences are listed in Table 1. The relative gene expression was calculated by using the 2-ΔΔCt method.
| Gene | Amplicon size (bp) | Forward primer (5'→3') | Reverse primer (5'→3') |
| β-actin | 150 | CCCATCTATGAGGGTTACGC | TTTAATGTCACGCACGATTTC |
| Cyclin D1 | 138 | TCAAGTGTGACCCGGACTG | GACCAGCTTCTTCCTCCACTT |
| STAT3 | 115 | GCAATACCATTGACCTGCCG | AACGTGAGCGACTCAAACTG |
| RBP4 | 131 | GCGAGGAAACGATGACCACT | TGGGGTCACGAGAAAACACA |
| JAK2 | 179 | ACAAGCAGGACGGGAAGGTC | AATTGGGCCGTGACAGTTGC |
| Bcl2 | 102 | GAGTACCTGAACCGGCATCT | GAAATCAAACAGAGGTCGCA |
The serum RBP4 expression was detected by ELISA in accordance with specific instructions. After sample collection and standard preparation, the prepared products were added to specific wells. Thereafter, the substrate solution, together with detection reagents A and B, was added, for incubation at 37 °C for 30 min. Then, the stop solution was added to terminate the reaction, and the absorbance (OD) value was detected at 450 nm using a microplate reader.
The fixed tissue was washed, cleared, dehydrated, paraffin embedded, and cut into sections. Each tissue section was subjected to deparaffinization and treated with 3% H2O2 for 10 min to inactivate endogenous peroxidases, followed by 30 min of heating at 120 °C in 10 mmol/L citrate buffer to retrieve the antigen. Subsequently, primary antibodies dissolved in the phosphatebuffered saline (PBS) were added to incubate the sections at 4 °C overnight. The sections were then washed with PBS thrice, followed by 20 min of incubation with secondary antibodies and visualization with diamino
Variables are presented as the mean ± SD or median. Two groups were compared by t-tests, and multiple groups were analyzed by one-way analysis of variance (ANOVA). Pearson’s correlation coefficient was used to assess the relationship between RBP4 and other markers. Multivariable logistic regression analysis was used to calculate the odds ratios and 95% confidence intervals for diabetic atherosclerosis; P < 0.05 was considered statistically significant. All statistical analyses were carried out with SPSS 23.0 statistical software (IBM, Armonk, NY, United States).
As depicted in Table 2, the levels of FPG, HbA1C, TG, LDL-c, FINS, RBP4, AI, and HOMA-IR increased, while the level of HDL-c decreased in the DM and DA groups compared to the NC group (P < 0.05). When compared with the DM group, the levels of LDL-c, TG, TC, FINS, HOMA-IR, RBP4, and AI were significantly increased; conversely, the level of HDL-c was increased.
| Index | Group NC (n = 20) | Group DM (n = 25) | Group DA (n = 25) | F | P value |
| Weight (kg) | 491.84 ± 80.82 | 504.61 ± 63.16 | 526.07 ± 85.66 | 1.155 | 0.321 |
| TG (mmol/L) | 0.65 ± 0.10 | 1.36 ± 0.17a | 1.82 ± 0.28a,b | 191.339 | < 0.001 |
| LDL-c (mmol/L) | 0.35 ± 0.12 | 0.49 ± 0.12a | 0.57 ± 0.14a,b | 17.562 | < 0.001 |
| HDL-c (mmol/L) | 1.07 ± 0.19 | 0.98 ± 0.20 | 0.71 ± 0.11a,b | 27.856 | < 0.001 |
| TC (mmol/L) | 1.98 ± 0.39 | 2.23 ± 0.42 | 2.95 ± 0.50 a,b | 30.234 | < 0.001 |
| FPG (mmol/L) | 5.42 ± 0.82 | 13.37 ± 2.16a | 14.04 ± 2.40a | 125.463 | < 0.001 |
| FINS (mU/L) | 9.84 ± 1.99 | 14.42 ± 2.12a | 19.24 ± 3.17a,b | 77.990 | < 0.001 |
| HbA1C (%) | 5.10 ± 0.81 | 9.95 ± 2.02a | 10.86 ± 1.65a | 78.800 | < 0.001 |
| RBP4 (ng/mL) | 15.37 ± 2.07 | 21.23 ± 2.70a | 32.28 ± 4.68a,b | 144.583 | < 0.001 |
| AI | 0.58 ± 0.23 | 1.71 ± 0.71a | 3.31 ± 0.76a,b | 105.551 | < 0.001 |
| HOMA-IR | 2.38 ± 0.62 | 8.49 ± 1.36a | 12.24 ± 2.82a,b | 149.994 | < 0.001 |
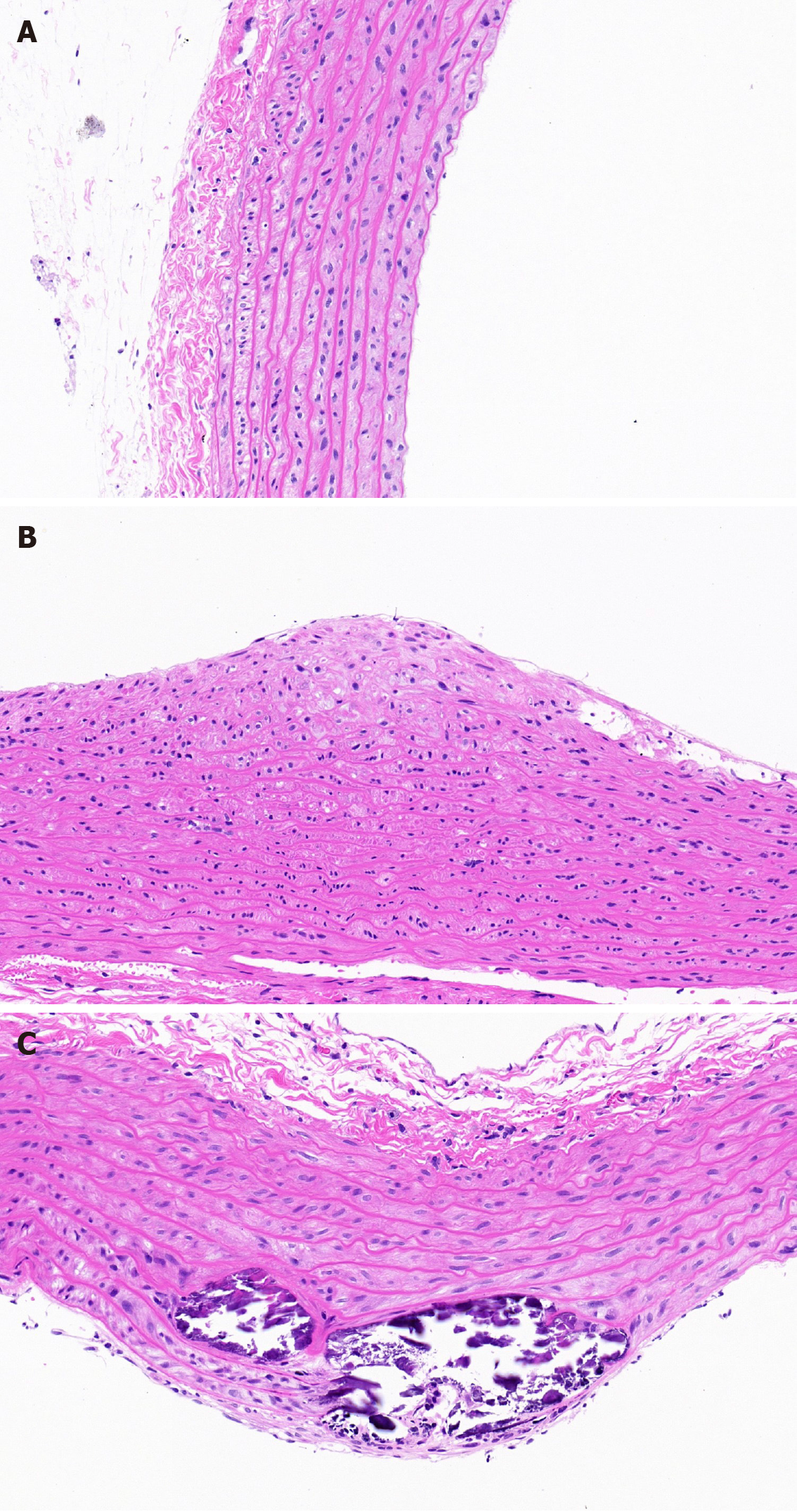
As illustrated in Figure 1A-C, the vessels had no obvious intimal thickening or lumen stenosis in the NC group. However, the intima became thicker and the structure and arrangement of VSMCs were disordered in the DM group. The lumen became narrower and a large number of VSMCs migrated and proliferated in the DA group.
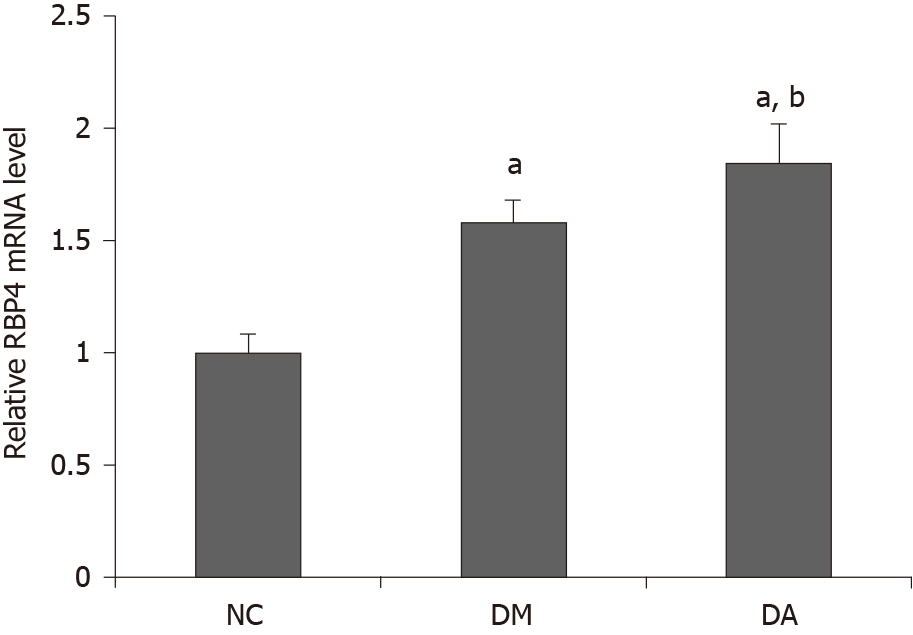
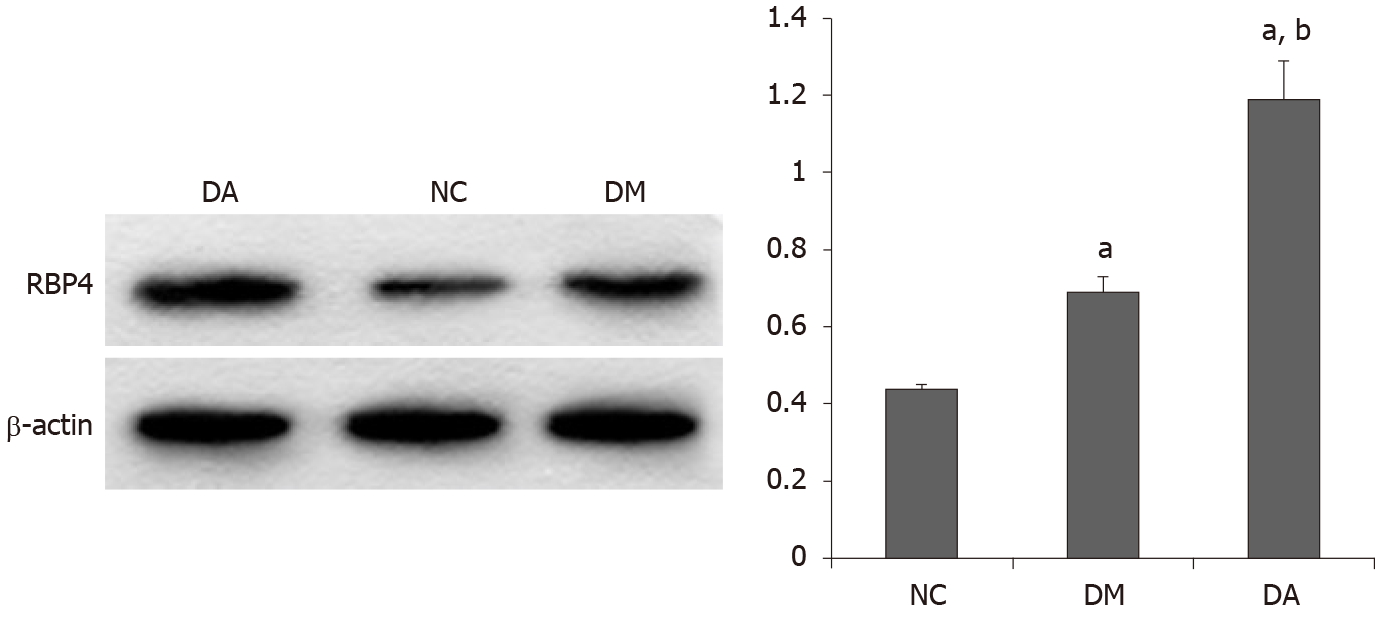
As shown in Figure 2, the mRNA expression of RBP4 in adipose tissue was higher in the DA group (1.85 ± 0.17) than in the NC group (1.0 ± 0.08) and the DM group (1.58 ± 0.10). As illustrated in Figure 3, the protein expression of RBP4 was low in the NC group but dramatically increased in the DM and DA groups, and the increase was more significant in the DA group.
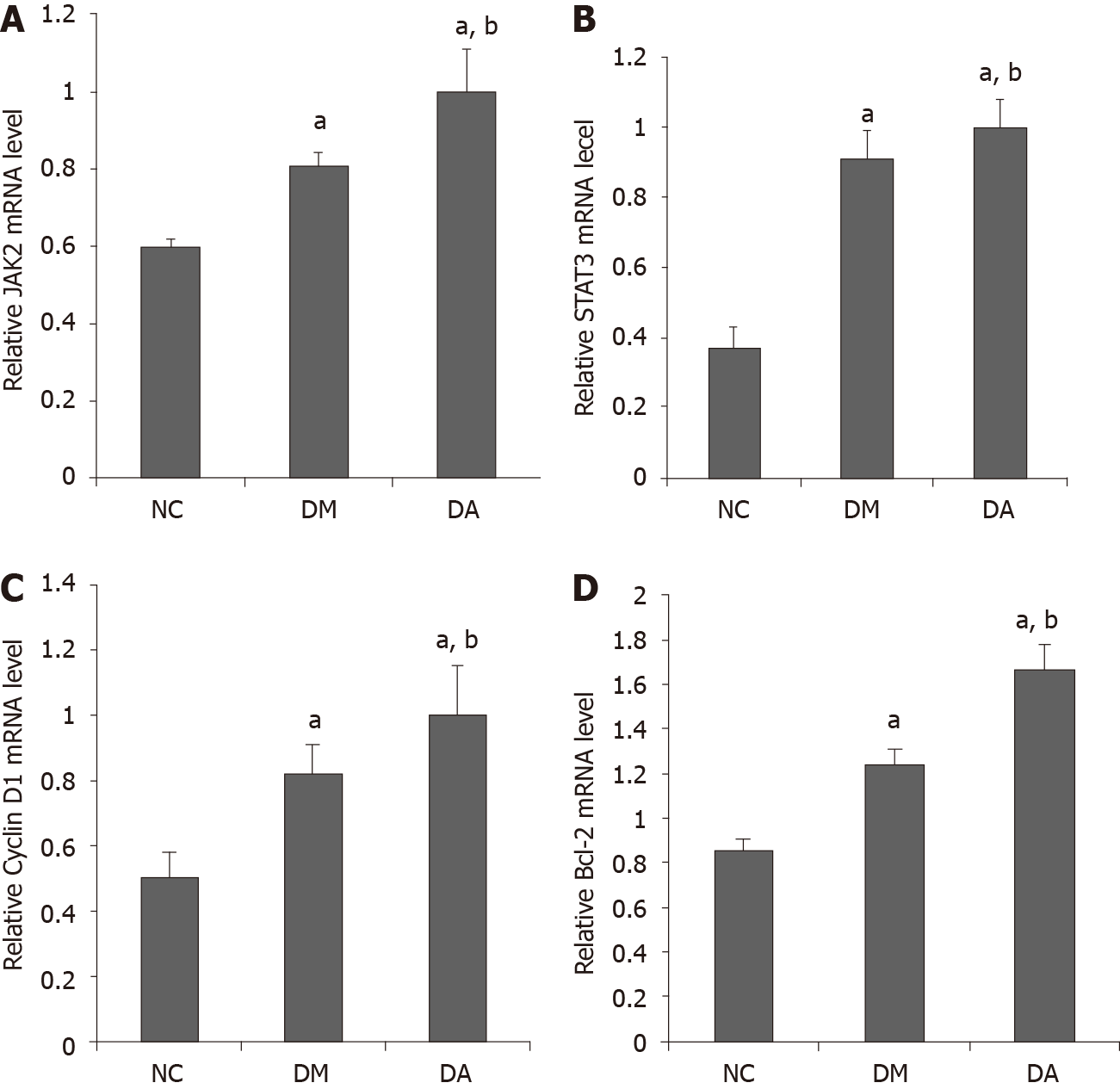
mRNA expression: The data revealed that the mRNA expression of JAK2 in aortic tissues was 0.60 ± 0.02, 0.81 ± 0.03, and 0.99 ± 0.11 in the NC, DM, and DA groups, respectively. The mRNA expression of STAT3 in aortic tissues was higher in the DA group (1.0 ± 0.08) than in the NC group (0.37 ± 0.06) and the DM group (0.92 ± 0.08). In addition, the mRNA expression of Cyclin D1 (1.0 ± 0.15) and Bcl-2 (1.67 ± 0.11) in the DA group was significantly increased compared with the NC group (Cyclin D1: 0.5 ± 0.08; Bcl-2: 0.85 ± 0.03) and the DM group (Cyclin D1: 0.82 ± 0.09; Bcl-2: 1.25 ± 0.05) (Figure 4).
Protein expression by Western blot: P-JAK2, the p-JAK2/JAK2 ratio, p-STAT3, and the p-STAT3/STAT3 ratio were significantly increased in the DA group compared with the NC group and the DM group, while there was no significant difference in JAK2 and STAT3 expression among the groups (Figure 5A and B). The protein expression levels of Cyclin D1 and Bcl-2 in the DA group were significantly increased compared with the NC group and the DM group (Figure 5C).
Protein expression by immunohistochemistry: The positive protein expression of Cyclin D1 distributed in the nucleus (Figure 6A-C) and that of Bcl-2 distributed in the cytoplasm (Figure 6D-E), which were stained brown-yellow, were highly expressed in the aortic tissues of the rats in the DA group but were dramatically decreased in the DM and NC groups. The corresponding statistics are recorded in Table 3.
As shown in Table 4, the results of correlation analysis revealed that serum RBP4 were positively correlated with TG, TC, LDL-c, FINS, HbA1C, p-JAK2, p-STAT3, Bcl-2, Cyclin D1, AI, and HOMA-IR but negatively correlated with HDL-c. Multiple logistic regression analysis was also used to determine the independent associations with the presence of diabetic atherosclerosis. The occurrence of atherosclerosis was set as a dependent variable, and the factors in the DM and DA groups were selected as independent variables. The results revealed that serum RBP4, LDL-c, p-JAK2, and p-STAT3 were risk factors for atherosclerosis (Table 5).
| Indicator | r | P value |
| Weight (kg) | 0.121 | 0.401 |
| TG (mmol/L) | 0.622 | < 0.001 |
| LDL-c (mmol/L) | 0.395 | 0.005 |
| HDL-c (mmol/L) | -0.566 | < 0.001 |
| TC (mmol/L) | 0.586 | < 0.001 |
| AI | 0.716 | < 0.001 |
| FPG (mmol/L) | 0.111 | 0.444 |
| FINS (mU/L) | 0.556 | < 0.001 |
| HOMA-IR | 0.541 | < 0.001 |
| HbA1C (%) | 0.284 | 0.045 |
| JAK2 | 0.239 | 0.094 |
| p-JAK2 | 0.433 | 0.002 |
| STAT3 | -0.040 | 0.781 |
| p-STAT3 | 0.539 | < 0.001 |
| Cyclin D1 | 0.476 | < 0.001 |
| Bcl-2 | 0.325 | 0.021 |
| Variable | β value | SE | χ2 | OR | 95%CI | P value |
| RBP4 | 0.951 | 0.338 | 7.933 | 2.589 | 1.335-5.019 | 0.005 |
| LDL-c | 5.211 | 2.421 | 4.631 | 18.253 | 1.592-39.597 | 0.031 |
| p-JAK2 | 2.040 | 0.866 | 5.554 | 7.693 | 1.410-41.979 | 0.018 |
| p-STAT3 | 2.734 | 1.187 | 5.305 | 15.402 | 1.503-57.794 | 0.021 |
| Constant | -21.007 | 7.916 | 7.041 | 0.000 | 0.008 |
Atherosclerosis is a major cause of mortality worldwide and is driven by multiple risk factors, including diabetes, which results in an increased atherosclerotic burden, but the precise mechanisms for the occurrence and development of diabetic atherosclerosis have not been fully elucidated. Dyslipidemia and inflammation have important synergistic effects in deteriorating vascular walls when diabetic atherosclerosis develops[8]. In addition, VSMCs play a critical role in various processes, including abnormal cell migration and proliferation, and synthesis of the extracellular matrix, which contribute to the progression of diabetic atherosclerosis[9]. JAK2/STAT3 is an important signal transduction pathway that mediates VSMC proliferation[10,11]. STAT3 can be activated when the signal transduction protein JAK2 is stimulated by cytokines to phosphorylate the STAT3 tyrosine 705 residue. Consequently, activated STAT3 forms dimers, translocates to the nucleus, and binds to downstream targets such as Bcl-2, Cyclin D1, c-Myc, and survivin to promote the proliferation and differentiation of VSMCs[12,13]. As previously reported, the JAK/STAT signal transduction pathway participates in the regulation of the inflammatory response of the arterial adventitia in diabetic patients. Banes et al[14] observed an increase in JAK2 and STAT3 tyrosine phosphorylation levels in STZ-induced rat thoracic aortae. These researchers also found that the treatment of diabetic rats with ketanserin prevented the increase in p-STAT3 and p-JAK2 levels in the aorta [14]. Recent studies have indicated that mice with endothelial STAT3 knockdown display decreased atherosclerotic lesions, lessened formation of fatty streaks, and intensified staining for activated STAT3 within inflammatory areas in atherosclerotic lesions relative to regions in their wild-type counterparts[15]. In the present study, both the mRNA and phosphorylated protein expression of JAK2 and STAT3 was significantly upregulated in the DA group compared with the NC group and the DM group, which was in accordance with previous studies.
RBP4 is clinically associated with obesity, IR, T2DM, and cardiovascular diseases. Furthermore, patients with diabetes complicated with macroangiopathy have enhanced serum RBP4 levels compared to diabetic patients with mild or no macroangiopathy[16-18], suggesting that RBP4 is involved in the pathogenesis of diabetes with macrovascular complications. As the causative factor and marker of vascular injury, RBP4 is related to IR[19]. Mohapatra et al[20] reported that concentrations of RBP4 in T2DM complicated with cardiovascular diseases were significantly increased and associated with glycolipid imbalances. RBP4 can also promote atherogenesis by inducing macrophage-derived foam cell formation[21]. As reported by Zabetian-Targhi et al[22], holo-RBP4 and apo-RBP4 result in the production of specific risk markers for inflammation and cardiovascular diseases, such as interleukin-6 and tumor necrosis factor-alpha in macrophages as well as vascular cell adhesion molecule-1 and intercellular cell adhesion molecule-1 in endothelial cells. Furthermore, RBP4 may directly induce cardiovascular diseases through inflammatory pathways. In our previous research, we found that RBP4 may contribute to the development of diabetic atherosclerosis through some mechanisms, such as IR, inflammatory reactions, and glycolipid metabolic disorder[23]. There are few studies on the potential role of RBP4 in the development of cardiovascular diseases, except for inflammation, IR, and glycolipid metabolism disorder. Gao et al[24] showed that RBP4 can promote the proliferation of VSMCs through the PI3K/AKT signaling pathway, which further leads to the occurrence of atherosclerosis. Moreover, Li et al[25] found that insulin induced the proliferation of VSMCs through the JAK2/STAT3 pathway and that RBP4 significantly promoted the hyperinsulinism-induced proliferation of VSMCs. These two studies are both consistent with the hypothesis that RBP4 may activate proliferation and migration of VSMCs through the JAK/STAT pathway. We therefore aimed to verify whether the JAK2/STAT3 signal is responsible for RBP4-induced proliferation and migration in VSMCs. In the present study, signs of atherosclerosis were present in the rats after 3 wk of intraperitoneal recombinant RBP4 injection. Statistical analysis also showed that RBP4 was positively correlated with p-JAK2, p-STAT3, Bcl-2, Cyclin D2, and AI and that RBP4 was one of the predictors of the presence of diabetic atherosclerosis, which implies that RBP4 can increase the degrees of phosphorylation of JAK2 and STAT3 and participate in the formation of atherosclerosis through the JAK2/STAT3 pathway. Previous studies have shown that elevated RBP4 may lead to IR and dyslipidemia through the JAK/STAT signaling pathway. It has been demonstrated that treatment of cultured adipocytes with RBP4 triggers the phosphorylation of retinoic acid gene 6 (STRA6), activation of JAK2 and STAT, and upregulation of suppressor of cytokine signaling 3 (SOCS3), leading to the suppression of insulin responses. Similarly, RBP4 injection in mice led to the activation of STRA6, resulting in the phosphorylation of JAK2 and STAT and the subsequent upregulation of SOCS3 expression in muscle and adipose tissue[26]. One study showed that the RBP4/retinol complex stimulates the expression of SOCS3 through JAK/STAT signaling, which has been implicated in IR. These observations further revealed that activation of a JAK/STAT cascade by RBP-retinol leads to the upregulation of the expression of STAT target genes, which can inhibit insulin signaling or control lipid homeostasis[27]. Zargha et al[28] found that RBP4 can affect lipid metabolism through the JAK/STAT3 pathway mediated by STRA6. Leptin can affect vascular endothelial cell function and VSMC proliferation through the JAK/STAT pathway, which indicates that JAK2/STAT3 is an adipocyte-derived signaling pathway[29]. There are few studies investigating whether RBP4 can promote VSMC proliferation by using JAK/STAT signaling. We investigated the association between diabetic vascular complications and the RBP4-JAK2/STAT3 signaling pathway in STZ-induced diabetic rats and found that the expression of JAK2, STAT3, Bcl-2, and Cyclin D1 was increased after intraperitoneal injection of recombinant RBP4. Therefore, we speculate that RBP4 may be the upstream signal of the VSMC proliferation pathway by triggering the phosphorylation of JAK2 and STAT3, which leads to the upregulation of the expression of Bcl-2 and Cyclin D1 and accelerates the occurrence of diabetic atherosclerosis. It is important to explore the regulation and mechanism of RBP4 expression in diabetic macrovascular disease to find therapeutic targets for diabetic macrovascular disease.
In summary, increased secretion of RBP4 stimulates the expression of JAK2, STAT3, Bcl-2, and Cyclin D1 in diabetic rats and promotes the development of atherosclerosis. RBP4 may contribute to the development of diabetes complicated with cardiovascular disease, particularly through the RBP4-JAK2/STAT3 signaling pathway.
With the increasing incidence of diabetes, the incidence of diabetic macroangiopathy continues to rise, which entails and increases atherosclerotic burden. Retinol binding protein 4 (RBP4) is clinically associated with obesity, insulin resistance, type 2 diabetes, and cardiovascular diseases. However, the precise role of RBP4 in the initiation and progression of atherosclerosis remains elusive.
We tried to provide new insight into the mechanism of diabetic atherosclerosis.
This study aimed to explore the expression regulation and mechanism of RBP4 in the diabetic rats with atherosclerosis, and to examine whether the role of RBP4 in the progression of atherosclerosis is mediated via the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway.
Male Wistar rats were randomly divided into a control group (NC group), diabetic rats (DM group), and diabetic atherosclerosis rats (DA group). At the end of week 19, serum RBP4, fasting insulin (FINS), fasting plasma glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), and hemoglobin A1c were measured. Except for hematoxylin and eosin staining and immunohistochemistry, the thoracic aorta was separated and extracted for mRNA and Western blot assay of JAK2, phosphorylated-JAK2 (p-JAK2), STAT3, phosphorylated-STAT3 (p-STAT3), Cyclin D1, and B-cell lymphoma-2 (Bcl-2). In addition, visceral adipose tissue was extracted for the measurement of RBP4 mRNA and the quantitative protein expression of RBP4. Homeostasis model assessment of insulin resistance (HOMA-IR) and atherogenic index (AI) were calculated.
Compared with the NC group and DM group, the levels of LDL-c, TG, TC, FINS, HOMA-IR, RBP4, and AI increased, while the level of HDL-c decreased in the DA group. The mRNA expression of JAK2, STAT3, Cyclin D1, and Bcl-2 in the DA group was significantly increased compared with the NC group and DM group. P-JAK2, p-JAK2/JAK2 ratio, p-STAT3, p-STAT3/STAT3 ratio, Cyclin D1, and Bcl-2 in the DA group were significantly increased at protein levels compared with the NC group and DM group. Pearson analysis showed that serum RBP4 was positively correlated with TG, TC, LDL-c, FINS, hemoglobin A1c, p-JAK2, p-STAT3, Bcl-2, CyclinD1, AI, and HOMA-IR but negatively correlated with HDL-c. In addition, multivariable logistic regression analysis showed that serum RBP4, p-JAK2, p-STAT3, and LDL-c were predictors of the presence of diabetic atherosclerosis.
The current study demonstrated that RBP4 could be involved in the initiation or progression of diabetic atherosclerosis by regulating the JAK2/STAT3 signaling pathway.
These results provide important insights into the mechanism of diabetic atherosclerosis and may help find therapeutic targets for diabetic macrovascular disease.
| 1. | Chen WJ, Yin K, Zhao GJ, Fu YC, Tang CK. The magic and mystery of microRNA-27 in atherosclerosis. Atherosclerosis. 2012;222:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1555] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 3. | Xiao ML, Lin JS, Li YH, Liu M, Deng YY, Wang CY, Chen YM. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr. 2020;23:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Takebayashi K, Sohma R, Aso Y, Inukai T. Effects of retinol binding protein-4 on vascular endothelial cells. Biochem Biophys Res Commun. 2011;408:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Park SE, Kim DH, Lee JH, Park JS, Kang ES, Ahn CW, Lee HC, Cha BS. Retinol-binding protein-4 is associated with endothelial dysfunction in adults with newly diagnosed type 2 diabetes mellitus. Atherosclerosis. 2009;204:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Silvério de Barros R, Dias GS, Paula do Rosario A, Paladino FV, Lopes GH, Campos AH. Gremlin-1 potentiates the dedifferentiation of VSMC in early stages of atherosclerosis. Differentiation. 2019;109:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Lu QB, Wang HP, Tang ZH, Cheng H, Du Q, Wang YB, Feng WB, Li KX, Cai WW, Qiu LY, Sun HJ. Nesfatin-1 functions as a switch for phenotype transformation and proliferation of VSMCs in hypertensive vascular remodeling. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2154-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Spartalis M, Spartalis E, Tzatzaki E, Tsilimigras DI, Moris D, Kontogiannis C, Kaminiotis VV, Paschou SA, Chatzidou S, Siasos G, Voudris V, Iliopoulos DC. The Beneficial Therapy with Colchicine for Atherosclerosis via Anti-inflammation and Decrease in Hypertriglyceridemia. Cardiovasc Hematol Agents Med Chem. 2018;16:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 451] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 10. | Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4322] [Cited by in RCA: 4644] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 11. | Lichtor T, Libermann TA. Coexpression of interleukin-1 beta and interleukin-6 in human brain tumors. Neurosurgery. 1994;34:669-672; discussion 672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 954] [Article Influence: 39.8] [Reference Citation Analysis (7)] |
| 13. | Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Banes AK, Shaw SM, Tawfik A, Patel BP, Ogbi S, Fulton D, Marrero MB. Activation of the JAK/STAT pathway in vascular smooth muscle by serotonin. Am J Physiol Cell Physiol. 2005;288:C805-C812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, Wei L, Pfeffer LM, Berliner JA. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282:31460-31468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Codoñer-Franch P, Carrasco-Luna J, Allepuz P, Codoñer-Alejos A, Guillem V. Association of RBP4 genetic variants with childhood obesity and cardiovascular risk factors. Pediatr Diabetes. 2016;17:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Majerczyk M, Olszanecka-Glinianowicz M, Puzianowska-Kuźnicka M, Chudek J. Retinol-binding protein 4 (RBP4) as the causative factor and marker of vascular injury related to insulin resistance. Postepy Hig Med Dosw (Online). 2016;70:1267-1275. [PubMed] |
| 18. | Ali EY, Hegazy GA, Hashem EM. Evaluation of irisin, retinol-binding protein 4, and leptin serum levels as biomarkers of macrovascular complications involvement in Saudi type 2 diabetes mellitus. A case-control study. Saudi Med J. 2020;41:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Domingos MAM, Queiroz M, Lotufo PA, Benseñor IJ, Titan SMO. Serum RBP4 and CKD: Association with insulin resistance and lipids. J Diabetes Complications. 2017;31:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Mohapatra J, Sharma M, Acharya A, Pandya G, Chatterjee A, Balaraman R, Jain MR. Retinol-binding protein 4 : a possible role in cardiovascular complications. Br J Pharmacol. 2011;164:1939-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Zhong Y, Chen H, Wang D, Wang M, Ou JS, Xia M. Retinol-Binding Protein-Dependent Cholesterol Uptake Regulates Macrophage Foam Cell Formation and Promotes Atherosclerosis. Circulation. 2017;135:1339-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Zabetian-Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv Nutr. 2015;6:748-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Zhou W, Ye SD, Chen C, Wang W. Involvement of RBP4 in Diabetic Atherosclerosis and the Role of Vitamin D Intervention. J Diabetes Res. 2018;2018:7329861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Gao D, Hao G, Meng Z, Ning N, Yang G, Liu Z, Dong X, Niu X. Rosiglitzone suppresses angiotensin II-induced production of KLF5 and cell proliferation in rat vascular smooth muscle cells. PLoS One. 2015;10:e0123724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Li F, Xia K, Sheikh SA, Cheng J, Li C, Yang T. Involvement of RBP4 in hyperinsulinism-induced vascular smooth muscle cell proliferation. Endocrine. 2015;48:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, Usheva A, Davis RJ, Smith U, Kahn BB. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 27. | Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim Biophys Acta. 2012;1821:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond). 2008;114:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Trovati M, Doronzo G, Barale C, Vaccheris C, Russo I, Cavalot F. Leptin and vascular smooth muscle cells. Curr Pharm Des. 2014;20:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ohashi N S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH