Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.480
Peer-review started: December 8, 2020
First decision: January 11, 2021
Revised: January 25, 2021
Accepted: March 8, 2021
Article in press: March 8, 2021
Published online: April 15, 2021
Processing time: 121 Days and 18.1 Hours
Type 2 diabetes (T2D) is characterized by insufficient insulin secretion caused by defective pancreatic β-cell function or insulin resistance, resulting in an increase in blood glucose. However, the mechanism involved in this lack of insulin secretion is unclear. The level of vascular endothelial growth factor B (VEGF-B) is significantly increased in T2D patients. The inactivation of VEGF-B could restore insulin sensitivity in db/db mice by reducing fatty acid accumulation. It is speculated that VEGF-B is related to pancreatic β-cell dysfunction and is an important factor affecting β-cell secretion of insulin. As an in vitro model of normal pancreatic β-cells, the MIN6 cell line can be used to analyze the mechanism of insulin secretion and related biological effects.
To study the role of VEGF-B in the insulin secretion signaling pathway in MIN6 cells and explore the effect of VEGF-B on blood glucose regulation.
The MIN6 mouse pancreatic islet β-cell line was used as the model system. By administering exogenous VEGF-B protein or knocking down VEGF-B expression in MIN6 cells, we examined the effects of VEGF-B on insulin secretion, Ca2+ and cyclic adenosine monophosphate (cAMP) levels, and the insulin secretion signaling pathway.
Exogenous VEGF-B inhibited the secretion of insulin and simultaneously reduced the levels of Ca2+ and cAMP in MIN6 cells. Exogenous VEGF-B also reduced the expression of phospholipase C gamma 1 (PLCγ1), phosphatidylinositol 3-kinase (PI3K), serine/threonine kinase (AKT), and other proteins in the insulin secretion pathway. Upon knockdown of VEGF-B, MIN6 cells exhibited increased insulin secretion and Ca2+ and cAMP levels and upregulated expression of PLCγ1, PI3K, AKT, and other proteins.
VEGF-B can regulate insulin secretion by modulating the levels of Ca2+ and cAMP. VEGF-B involvement in insulin secretion is related to the expression of PLCγ1, PI3K, AKT, and other signaling proteins. These results provide theoretical support and an experimental basis for the study of VEGF-B in the pathogenesis of T2D.
Core Tip: Type 2 diabetes has elicited worldwide public health concerns, and mechanism regulating insulin secretion is unclear. We found that vascular endothelial growth factor B (VEGF-B) prevents MIN6 cells from secreting insulin through the PI3K-AKT (phosphatidylinositol 3-kinase-serine/threonine kinase) pathway. We have provided mechanistic insights into the effect of VEGF-B on insulin secretion and suggest VEGF-B as a new target that affects the occurrence and development of type 2 diabetes.
- Citation: Jia JD, Jiang WG, Luo X, Li RR, Zhao YC, Tian G, Li YN. Vascular endothelial growth factor B inhibits insulin secretion in MIN6 cells and reduces Ca2+ and cyclic adenosine monophosphate levels through PI3K/AKT pathway. World J Diabetes 2021; 12(4): 480-498
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/480.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.480
Diabetes and diabetic complications are serious threats to human health. Due to the high fatality rate, disability rate, and medical costs, diabetes has become a common health problem faced by countries worldwide. The International Diabetes Federation predicts that by 2040, there will be 642 million adults with diabetes worldwide, of which more than 90% will comprise patients with type II diabetes, which is caused by insulin secretion defects or decreased insulin sensitivity[1]. However, the mechanism of insulin secretion is still not completely clear. Further exploration of the mechanism of insulin secretion has been an important topic in various disciplines and has attracted the attention of scholars worldwide.
In 2012, Hagberg et al[2] proposed for the first time that abnormal lipid deposits in peripheral tissues can impair insulin sensitivity, which in turn affects glucose uptake, and is one of the factors that triggers type 2 diabetes (T2D).
Researchers discovered that reducing vascular endothelial growth factor B (VEGF-B) levels in db/db mice could restore insulin sensitivity by reducing lipid accu
Although the specific mechanism of VEGF-B involvement in insulin secretion and insulin resistance is not yet clear, studies have confirmed that VEGF-B affects insulin secretion by regulating fatty acid (FA) metabolism, and can improve obesity-related metabolic complications and restore insulin sensitivity[2,5]. VEGF-B belongs to the VEGF family. Compared with VEGF-A, VEGF-B does not regulate the growth of blood vessels but can regulate the uptake of endothelial FAs and promote the survival of cardiomyocytes, retinal neurons, and other cells, showing a proliferative effect on cells[3]. VEGF-B is mainly expressed in tissues with high metabolic activity, such as the myocardium, skeletal muscle, and brown fat. VEGF-B/VEGFR (VEGF receptor) signaling plays a certain role in inhibiting Ang II-induced cardiac hypertrophy[6,7], promoting lipid absorption in skeletal muscle, inhibiting FA re-esterification, and promoting triglyceride participation in the process of oxidation and decomposition[8]. At present, VEGF-B has been predicted to be a promising candidate to treat T2D, but there have been few studies on VEGF-B and insulin secretion. Some of the questions to be addressed include whether VEGF-B participates in glucose-induced insulin secretion or in regulating the signaling pathway of insulin secretion. Studies on these topics will provide the basis for understanding the role of VEGF-B in insulin secretion and diabetes.
This study therefore explored the role of VEGF-B in the insulin secretion signaling pathway and blood glucose regulation by interfering with the expression of VEGF-B in MIN6 cells. We observed changes in insulin secretion, Ca2+ and cyclic adenosine monophosphate (cAMP) levels, and the expression of proteins related to the insulin secretion signaling pathway. Studies have shown that exogenous VEGF-B protein inhibits the secretion of insulin in MIN6 cells, and insulin secretion is increased after knocking down VEGF-B. Moreover, the effect of VEGF-B on Ca2+ and cAMP is consistent with the changes in insulin secretion. We observed that VEGF-B affected the expression of VEGF receptor 1 (VEGFR1), neuropilin 1 (NRP1), phospholipase C gamma 1 (PLCγ1), phosphatidylinositol 3-kinase (PI3K), serine/threonine kinase (AKT), and other proteins. Thus, we determined that VEGF-B could participate in the insulin secretion by binding to VEGFR1 and NRP1 and consequently affecting the expression of proteins related to the insulin secretion signaling pathway.
MIN6 mouse insulinoma cells (Wuhan Feien Biotechnology, China) were cultured in RPMI 1640 (HyClone, United States) supplemented with 10% fetal bovine serum (FBS; Gibco, Brazil), 1% penicillin, and 1% streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. VEGF-B protein (PeproTech, United States) treatment groups were divided into five concentrations: 0, 12.5, 25, 50, and 100 ng/mL. There were three small interfering RNA (siRNA) knockdown groups — Si1, Si2, and Si3 — according to the different Si-M-VEGF-B primer target sequences (Supplementary Table 1); a control group was also established.
Cell counting kit-8 (CCK-8) was used to detect the effects of exogenous VEGF-B and VEGF-B knockdown on the proliferation of MIN6 cells. MIN6 cells were seeded in a 96-well plate at a cell density of 2 × 105/mL. After culture in a cell incubator at 37 °C for 6 h, MIN6 cells were treated with exogenous VEGF-B protein or subjected to knockdown of VEGF-B protein. The original medium was discarded and the cells were washed with PBS buffer. Next, the medium was mixed with CCK-8 solution (Bimake, United States) at a ratio of 9:1 and 100 μL was added to each well. The plates were incubated for another 2 h before they were placed in a microplate reader (Bio Tek, United States) to detect the optical density at 450 nm.
After MIN6 cells were seeded in a 12-well plate and incubated for 12 h, they were transfected for 48 h with medium containing 500 nmol/L siRNA and jetPRIM reagent (Polyplus, France); the sequences of siRNAs used are listed in Supplementary Table 2. Then, the cells were used for different assays or lysed for RNA or protein isolation.
MIN6 cells were processed and total RNA was extracted. After reverse transcription with RNA-easy Isolation Reagent (Vazyme, China), 1000 ng of cDNA was obtained with the TB Green premix Ex Taq II kit (Takara, Japan) in a QuantStudio 3 PCR machine (Thermo Fisher, United States) for real-time polymerase chain reaction (PCR) amplification (Supplementary Tables 3 and 4).
Enzyme-linked immunoassay kit (ELISA) kits (mlbio, China) were used to detect insulin secretion and intracellular Ca2+ and cAMP contents in MIN6 cells. The supernatant was collected after cells were subjected to the indicated treatments. For insulin secretion, cells were centrifuged at 2000-3000 r/min for 20 min, and an Ins ELISA kit was used. For determining the intracellular Ca2+ and cAMP content, cells underwent repeated freeze/thaw cycles before they were centrifuged at 2000-3000 r/min for 20 min; the supernatant was collected and processed with Ca2+ and cAMP kits, respectively. The absorbance (OD value) was measured with a microplate reader at a wavelength of 450 nm, standard curves were established for each kit, and the insulin, Ca2+, and cAMP levels of each group of samples were calculated from the respective standard curves. Statistical analysis was then performed on the resulting data.
The protein of MIN6 cells was extracted and the protein concentration was measured with a BCA kit (Takara, Japan). Specific antibodies against VEGFR1, NRP1, PI3K-p85, PI3K-p110γ, PLCγ1, phosphorylated (p)-PLCγ1, AKT, and p-AKT were used for the corresponding protein detection, and β-actin was used as the internal reference for normalization. Antibody information and concentration are listed in Supplementary Table 5. The antigens were visualized using an ECL plus detection system (Tanon-5200).
The effect of exogenous VEGF-B on MIN6 cell apoptosis was detected. After centrifugation at 2000 r/min, the cell density was adjusted to 5 × 105/mL. After treatment with the Apoptosis Detection Kit (KeyGEN BioTECH, China), apoptosis was detected on a flow cytometer (BD Canto II).
The data were statistically analyzed using SPSS 22 and are reported as the mean ± SE. One-way analysis of variance was used to analyze the differences between multiple groups, while the least-significant difference method was used to analyze normally distributed data. P < 0.05 indicated the statistical difference and P < 0.001 was considered statistically significant.
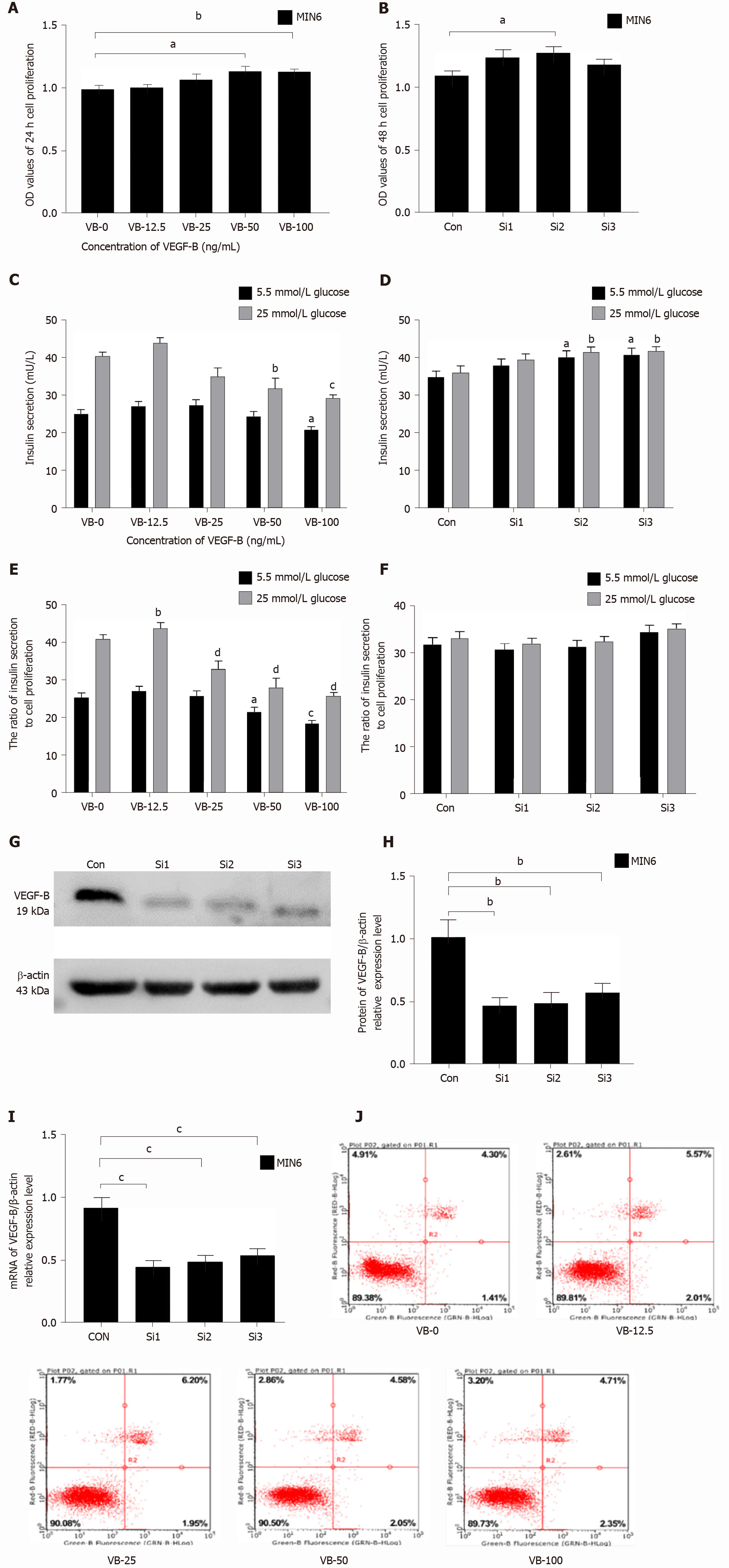
Among MIN6 cells stimulated with five concentrations of VEGF-B protein for 24 h, the number of MIN6 cells in the VB (VEGF-B)-50 and VB-100 group was significantly increased compared with that in the VB-0 group (P < 0.05 and P < 0.01, respectively) (Figure 1A). After VEGF-B expression in MIN6 cells was knocked down by siRNA, cell proliferation showed an increasing trend (Figure 1B).
Under the same VEGF-B protein treatment conditions, the insulin secretion of each group was measured. The results showed that as the concentration of VEGF-B protein increased, insulin secretion showed a decreasing trend. Among the groups, the VB-100 group showed a significantly lower level of insulin secretion in response to 5.5 mmol/L glucose than the control group (P < 0.05); in the medium containing 25 mmol/L glucose, the insulin secretion in the VB-50 and VB-100 groups was significantly reduced compared with the VB-0 group (P < 0.01 and P < 0.001, respectively) (Figure 1C). Upon siRNA-mediated knockdown of VEGF-B in MIN6 cells and subsequent culture in 5.5 mmol/L or 25 mmol/L glucose, insulin secretion in the Si2 and Si3 groups was significantly higher than that of the control (Con) group (P < 0.05 for both) (Figure 1D).
Insulin secretion per unit cell proliferation level in each group was analyzed. In a medium containing 5.5 mmol/L glucose and after 24 h of treatment with different concentrations of VEGF-B, insulin secretion showed a decreasing trend, and insulin secretion in the VB-100 group was significantly reduced compared to that in the VB-0 group (P < 0.05). In the presence of 25 mmol/L glucose, VB-25 group had higher insulin secretion compared with the VB-0 group, whereas insulin secretion in the other groups was significantly reduced (P < 0.001) (Figure 1E).
After knocking down VEGF-B, there was no statistical difference in insulin secretion or cell proliferation ratio between the Con group and knockdown groups (Figure 1F).
Verification of siRNA knockdown of VEGF-B showed that the levels of mRNA and protein expression of VEGF-B in transfected MIN6 cells were significantly lower than those in the Con cells (P < 0.01 and P < 0.001, respectively) (Figure 1G-I).
The flow cytometry results showed that after 24 h of exogenous VEGF-B stimulation in MIN6 cells, the ratio of viable, early apoptotic, late apoptotic, and necrotic cells in the VEGF-B-treated groups (at different concentrations) was not statistically significant compared with the VB-0 group (Figure 1J).
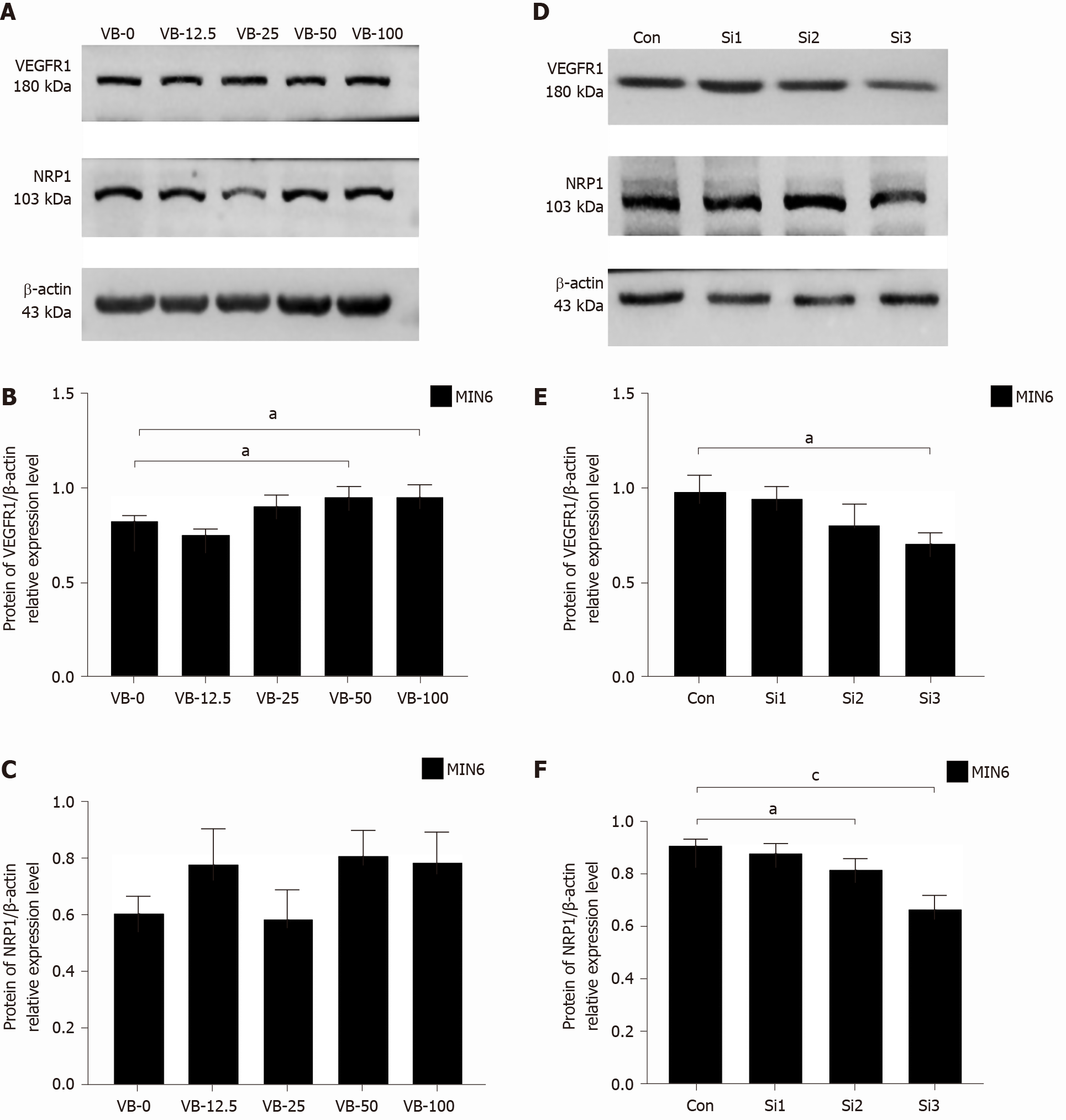
Western blot analysis revealed that in MIN6 cells stimulated with exogenous VEGF-B for 24 h, when the VEGF-B protein concentration exceeded 25 ng/mL, the expression level of VEGFR1 protein was higher than that of the VB-0 group. The VB-50 and VB-100 groups showed statistical differences (P < 0.05). Except for the VB-25 treatment, all the VEGF-B-treated groups exhibited slightly higher protein expression levels of NRP1 than the VB-0 group (Figure 2A-C).
After undergoing siRNA-mediated knockdown of VEGF-B, MIN6 cells were cultured in 5.5 mmol/L and 25 mmol/L glucose for 24 h. Compared with those of the Con group, the expression levels of VEGFR1 and NRP1 were reduced in the VEGF-B-knockdown groups (P < 0.05 and P < 0.001, respectively; Figure 2D-F).
The expression levels of PI3K-p85 in MIN6 cells stimulated with gradient concen
MIN6 cells were stimulated with VEGF-B for 24 h, and the expression levels of AKT and p-AKT in the VB-12.5 group were higher than those in the VB-0 group, while decreased in the other three groups compared to those in the VB-0 group. The expression levels of p-AKT in the VB-50 and VB-100 groups were obviously lower than that of the VB-0 group (P < 0.05). The p-AKT/AKT ratio in MIN6 cells treated with VEGF-B protein was lower than that of VB-0, and the VB-50 group had the lowest ratio (P < 0.05) (Figure 3D-F).
MIN6 cells with siRNA knockdown of VEGF-B showed higher expression levels of PI3K-p85 and PI3K-p110γ than the Con cells at 48 h after transfection (P < 0.01 and P < 0.05, respectively) (Figure 3G-I).
After siRNA knockdown of VEGF-B in MIN6 cells for 48 h, there were higher expression levels of AKT and p-AKT than those in the Con cells (P < 0.05, P < 0.01, and P < 0.001, respectively). The p-AKT/AKT ratio was significantly lower in the VEGF-B-knockdown cells than in Con cells (P < 0.01) (Figure 3J-L).
When the glucose concentration was 5.5 mmol/L, MIN6 cells stimulated with 50 and 100 ng/mL exogenous VEGF-B showed significantly lower cAMP levels than the VB-0 cells (P < 0.05 for both). When the glucose concentration was 25 mmol/L, the cAMP levels of all the VEGF-B-treated cell groups decreased compared with those of the VB-0 group, and the decrease in cAMP level in the VB-100 group was significantly different (P < 0.01) (Figure 4A).
At a glucose concentration of 5.5 mmol/L, MIN6 cells stimulated with exogenous VEGF-B showed decreased Ca2+ levels compared with those of the control cells; the decreases in the VB-50 and VB-100 groups were significantly reduced (P < 0.01) (Figure 4C).
After siRNA knockdown of VEGF-B in MIN6 cells, the cells were cultured in 5.5 mmol/L or 25 mmol/L glucose for 24 h. Compared with those of the Con groups, intracellular Ca2+ and cAMP levels were increased (P < 0.05, P < 0.01, and P < 0.001) (Figure 4B and D).
Compared with those of the VB-0 group, the PLCγ1 and p-PLCγ1 protein expression levels and the p-PLCγ1/PLCγ1 ratio in the VEGF-B-stimulated groups were all reduced; the reductions in the VB-50 and VB-100 groups were statistically different compared with those of the VB-0 treatment (P < 0.05) (Figure 4E-G).
MIN6 cells with siRNA-mediated knockdown of VEGF-B showed increasing protein expression of PLCγ1 and p-PLCγ1 compared to the control cells at 48 h after transfection (P < 0.05). However, the p-PLCγ1/PLCγ1 ratios were not statistically different between the VEGF-B-knockdown and Con groups (Figure 4H-J).
Seven VEGF family members have been identified in mammals: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor (PIGF)[9,10]. The binding of members of the VEGF family with three tyrosine kinase receptors, namely, VEGFR1, VEGFR2, and VEGFR3, results in downstream biological activity. In addition, neuropilins, such as NRP1 and NRP2, can cooperate with VEGFR[11-13]. VEGF-B, a recently discovered factor in the VEGF family, is a glycoprotein composed of homodimers that are covalently linked by disulfide bonds[14]. VEGF-B is expressed in tissues with high metabolic activity, such as heart, skeletal muscle, and brown fat, but unlike other VEGF family members, VEGF-B does not significantly promote angiogenesis under physiological conditions. Once apoptosis and other degenerative pathological changes occur, VEGF-B protects cells from apoptosis and exerts a prosurvival effect[8,15]. By contrast, if there is a high concentration of growth-promoting factors, VEGF-B can inhibit growth and prevent excessive growth. VEGF-B acts to maintain the normal state of the body; for example, VEGF-B protects the nervous system while playing a protective role in blood vessels, and it does not cause cardiovascular, cerebrovascular, or nerve damage, showing superior safety as a drug target[16-18]. In addition, VEGF-B can also act as an antioxidant to protect tissues and cells from damage induced by oxidative stress[19,20]. In pathological conditions such as diabetes, obesity, and cardiovascular disease, VEGF-B regulates lipid accumulation, but its mechanism of action is still unclear in these processes[21]. In 2010, Hagberg et al[22] first proposed in “Nature” that VEGF-B affects the abnormal metabolism of FAs in endodermal cells. Subsequently, the team discovered that abnormal lipid deposition in peripheral tissues can impair insulin sensitivity and affect glucose uptake[2]. In 2016, the Mehlem et al[3] further confirmed that inactivation of VEGF-B could improve insulin resistance, and high levels of VEGF-B could cause insulin resistance and blood glucose deterioration. In the same year, Robciuc et al[5] performed a study on obese and insulin-resistant mice and showed that VEGF-B gene transfer and endothelial VEGFR1 conduction blockade led to weight loss and reduced metabolic-related complications. These findings reveal the therapeutic role of the VEGF-B/VEGFR1 pathway in obesity and T2D. VEGF-B may be a considerable factor in the influence of insulin secretion and is expected to become an important factor for maintaining blood glucose balance and affecting insulin secretion[21,23].
In 2020, Chen et al[24] published a study on a mouse model with fat-specific VEGF-B inhibition to better elucidate the role of VEGF-B in fat development and energy metabolism. After inhibiting VEGF-B, the mice had larger bodies, more white adipose tissue appeared in the mice, and the morphology and function of brown fat changed to those of white fat. It was proposed that VEGF-B expressed in fat is a function of fat development and a main regulator of fat function[24]. In a study of a mouse model of pancreatic β-cell-specific VEGF-B deficiency, Ning et al[4] found that the specific deletion of VEGF-B can increase insulin secretion but does not have much effect on the glucose tolerance or insulin sensitivity of mice. Moreover, the study also showed that VEGF-B-deficient mice fed an HFD exhibited no alterations in glucose homeostasis, and pancreatic islet lipid uptake was not altered[4]. In addition to the study of model mice, in 2017, Wu et al[25] found that the plasma VEGF-B level of T2D patients was significantly higher than that of subjects with normal glucose tolerance, and VEGF-B was related to the first stage of glucose-stimulated β-cell insulin secretion, indicating that VEGF-B may be related to abnormal glucose metabolism.
Dijkstra et al[26] hold a different view from Hagberg's team. Researchers believe that VEGF-B has little effect on changes in lipid metabolism in a mouse model of HFD-induced diabetes. In 2014, Sun et al[27] also showed that VEGF-B (-/-) model mice did not show much change in body weight or glucose metabolism upon HFD consump
Based on studies of the phenotypes of animal models of diabetes and humans with diabetes, the mechanism by which VEGF-B affects glucose and lipid metabolism is still controversial. Research on VEGF-B involvement in insulin sensitivity has attracted much attention in recent years. The MIN6 cell line was derived from mouse insulinoma and is very similar in function to pancreatic islets in mice. This cell line can be used as a model to simulate β-cell functions and study insulin secretion in vitro. We added VEGF-B to subcultured MIN6 cells and observed changes in cell proliferation and apoptosis. After stimulation of MIN6 cells with 50 ng/mL and 100 ng/mL VEGF-B for 24 h, cell proliferation was higher than that of cells without VEGF-B treatment, and there was no significant change in apoptotic cells. Although studies have found that VEGF-B affects the proliferation of cultured MIN6 cells, the effect of VEGF-B on insulin secretion in MIN6 cells needs to be further examined.
Insulin secretion and its regulation are important mechanisms for maintaining glucose homeostasis in the body. Insufficient insulin secretion can lead to non-insulin-dependent diabetes. Insulin is encapsulated in vesicles with dense cores, and pancreatic β-cells regulate insulin secretion by regulating the exocytosis and secretion of these vesicles. Intracellular Ca2+ is an important factor affecting insulin secretion; pancreatic islet β-cells change the intracellular Ca2+ concentration mainly through ATP-sensitive calcium channels on the plasma membrane and intracellular calcium store activities, thereby regulating insulin secretion[28,29]. Under physiological conditions, an increase in the Ca2+ concentration can directly trigger the secretion of insulin by pancreatic β-cells. When the glucose concentration is higher than the stimulation threshold, ATP production in β-cells increases through metabolic processes[30], thereby closing the K-ATP channel on the cell membrane, depolarizing the membrane, and inducing action potentials to open L-type Ca2+ channels; this results in extracellular Ca2+ influx and an increase in intracellular Ca2+, which further activates the release of intracellular Ca2+ pools and finally activates insulin vesicles to release a large amount of insulin via exocytosis to accelerate the absorption and utilization of glucose by all human tissues (except brain)[31-35].
In addition to causing changes in Ca2+, glucose also increases the cAMP levels in pancreatic β-cells. Glucose in pancreatic β-cells can cause rapid changes in intracellular Ca2+ and cAMP concentrations. These effects are independent of glucose metabolism and are mediated by glucose-sensitive receptors[36]. Increased cAMP in the cytoplasm is induced by a high concentration of glucose and is secondary to the increase in Ca2+; that is, the increase in Ca2+ increases the production of cAMP[37,38]. On the other hand, cAMP can also increase the sensitivity of mouse pancreatic β-cell exocytosis to Ca2+[39]. In our study, we found that after 24 h of glucose induction, MIN6 cells treated with 50 ng/mL and 100 ng/mL VEGF-B exhibited decreased insulin secretion. By contrast, after siRNA-mediated knockdown of VEGF-B for 48 h, MIN6 cells showed increased insulin secretion. This was consistent with the study of Ning et al[4], who found that in a VEGF-B-deficient mouse model, the specific deletion of VEGF-B in β-cells could upregulate insulin gene expression and increase insulin secretion. Our research also showed that after 24 h of glucose induction, stimulation with 50 ng/mL and 100 ng/mL exogenous VEGF-B can cause decreases in the intracellular Ca2+ and cAMP concentrations in MIN6 cells. After siRNA-mediated knockdown of VEGF-B for 48 h, the Ca2+ and cAMP concentrations in MIN6 cells were higher than those in Con cells. The results of the study showed that after exogenous VEGF-B stimulation or siRNA-mediated knockdown of VEGF-B in MIN6 cells, the changes in insulin secretion and intracellular Ca2+ and cAMP levels were consistent. We found that VEGF-B is involved in insulin secretion in MIN6 cells and that VEGF-B may affect insulin secretion by changing the intracellular Ca2+ concentration and cAMP level. In the same year, Robciuc et al[5] studied obese and insulin-resistant mice and found that VEGF-B gene transfer and VEGFR1 gene deletion led to weight loss and reduced metabolic complications. These findings reveal the therapeutic role of the VEGF-B/VEGFR1 pathway in obesity and T2D.
As a glycoprotein, VEGF-B mainly acts by binding to the membrane receptors VEGFR1 and NRP1. The full-length VEGFR1 gene is 7680 bp, which encodes 1338 amino acids. The seven Ig-like domains in the extracellular region are responsible for binding the VEGF ligand and promoting angiogenesis[40]. VEGFR1 is mainly expressed in endothelial cells, blastoderm trophoblasts, renal interstitial cells, and other cells[41]. The functions and properties of VEGFR1 can be determined according to the developmental stages of different cells in different animals. VEGFR1 can bind to not only VEGF-A but also PLGF and VEGF-B, but neither PIGF nor VEGF-B can bind VEGFR2. NRP1 is a non-tyrosine kinase single-pass transmembrane glycoprotein approximately 130-140 KD in size that was originally discovered as a receptor for nerve guidance factors (semaphorin 3A, Sema3A) involved in regulating nerve cell guidance and axon growth. NRP1 is composed of three parts: An intracellular domain, a transmembrane domain, and an extracellular domain (a1/a2, b1/b2, and c domain). The a1/a2 domain, also known as the CUB domain, mainly binds to Sema3A; the b1/b2 domain is mainly related to the binding of VEGF family members and mediating angiogenesis; and the c domain mainly mediates the dimerization of NRP1 and is closely related to its signal transduction[42]. In 2016, Robciuc et al[5] reported on cellular metabolism and stated that increasing VEGF-B levels do not affect the weight of mice but can improve glucose metabolism, enhance insulin sensitivity, reduce inflammation, and significantly improve the metabolic health of obese mice. Moreover, deletion of the VEGFR1 gene in endothelial cells enhances the effect of VEGF-B, activates thermogenesis in subcutaneous fat tissue, increases the basal metabolic rate, and prevents obesity and related metabolic complications caused by diet.
Robciuc et al[5] believed that VEGF-B competes with VEGF-A to bind VEGFR1 and promotes the binding of VEGF-A and VEGFR2 to increase angiogenesis and perfusion of adipose tissues to improve insulin secretion and signal transduction through vascular remodeling. The binding of VEGF-B and VEGFR1 activates the VEGF/ VEGFR2 pathway and increases capillary density in adipose tissue, tissue blood circulation, and signal transduction.
VEGFR1 was the first VEGF receptor discovered. It has a higher affinity for the VEGF family of ligands than VEGFR2 but has weak tyrosine kinase activity, and the expression level of VEGFR1 on endothelial cells is lower than that of VEGFR2. Many biological functions of VEGF are mainly mediated by VEGFR2. VEGFR1 also has a synergistic effect with VEGFR2, and the two receptors can jointly regulate multiple signal transduction pathways[43]. For many years, VEGFR1 has been considered to have only modified auxiliary functions. However, in recent years, scientific exploration has found that the expression of VEGFR1 is increased in many pathological conditions, and this receptor can mediate some biological functions, such as angiogenesis in ischemic diseases, remodeling after vascular injury, atherosclerosis, and certain inflammatory diseases[44,45]. Studies have shown that VEGFR1 can also bind to proteins such as PI3K, PLCγ, Grb2, SHP2, and Nck, but the downstream signal transduction pathways involved are unclear[17,46]. In our study, we found that after 24 h of treatment with 50 ng/mL or 100 ng/mL VEGF-B, the protein expression level of VEGFR1 in MIN6 cells was significantly increased, and the protein expression level of NRP1 also increased. We further observed the effect of VEGF-B on PLCγ1, PI3K, AKT, and other molecules downstream of the VEGF-B/VEGFR1 signaling pathway in this study. Our study found that exogenous VEGF-B protein reduced the expression of the PI3K kinase isoforms p85 and p110γ in MIN6 cells, and after knocking down VEGF-B, the expression of p85 and p110γ increased significantly. The study also showed that exogenous VEGF-B simultaneously reduced the expression of AKT and P-AKT in MIN6 cells. Upon knockdown of VEGF-B, the expression of these proteins in MIN6 cells also increased significantly.
A large number of studies have shown that the PI3K-AKT signaling pathway is related to cell proliferation, for instance, lactoglobulin-mediated promotion of C2C12 cell proliferation[47]. By inhibiting the activation of PI3K/AKT, the resultant downregulation of LRRC8A (leucine-rich repeat-containing 8A) activity can inhibit the angiotensin II-induced proliferation of cerebral vascular smooth muscle cells[48]. FER1L4 (Fer-1-like family member 4) inhibits the proliferation and metastasis of lung cancer cells by regulating the PI3K/AKT pathway[49]. Prucalopride inhibits the proliferation, invasion, and migration of lung cancer cells by inhibiting the PI3K/AKT pathway[50].
CCK-8 analysis showed that in MIN6 cells with VEGF-B knockdown, exogenous VEGF-B administration induced MIN6 cell proliferation. We hypothesize that the MIN6 cell proliferation observed in this study was not necessarily related to the PI3K/AKT pathway. The results of Western blot indicated that after VEGF-B intervention, the protein expression of PI3K, P-PI3K, AKT, and P-AKT was consistent with the trend of insulin secretion. When exogenous VEGF-B was used to stimulate MIN6 cells, the secretion of insulin was reduced, and the expression of PI3K/AKT pathway related proteins was also reduced. After siRNA-mediated knockdown of VEGF-B, the amount of insulin secretion and the protein expression of PI3K, P-PI3K, AKT, and P-AKT increased.
PI3K is a kinase that specifically catalyzes phosphatidylinositol. In glucose homeostasis, insulin can regulate β-cell function and insulin secretion through the PI3K/AKT pathway[51,52]. MacDonald et al[53] showed that the p110γ isoform of PI3K kinase can positively regulate glucose-stimulated insulin secretion. Inhibition of the PI3K catalytic subunit p110γ can significantly reduce insulin secretion and exocytosis induced by depolarization and directly eliminate exocytosis caused by Ca2+ influx, thereby inhibiting insulin secretion[54]. Our research results are consistent with these findings.
Phosphatidylinositol is very important in the regulation of vesicle transport during the release of insulin secretory vesicles caused by a large influx of Ca2+[55]. In pancreatic cells, glucose can activate PLC in the cytoplasm, and the influx of calcium also activates PLC, causing the production of inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 in turn induces the endoplasmic reticulum to release intracellular Ca2+, and DAG induces the transport of protein kinase C to the plasma membrane and promotes insulin secretion[56,57]. Fiume et al[58] further proved that insulin secretion in MIN6 cells was regulated by PLC. The inhibition of PLC leads to impaired insulin secretion, and among the isoforms of PLC, PLCγ1 has the greatest impact on insulin secretion[59]. Therefore, we explored the expression changes of PLCγ1 and P-PLCγ1 as it relates to the mechanism by which VEGF-B affects insulin expression and found that after stimulation with exogenous VEGF-B, the protein expression of PLCγ1 and p-PLCγ1 in MIN6 cells decreased. After knocking down VEGF-B in these cells, the protein expression of PLCγ1 and p-PLCγ1 increased. We hypothesize that the inhibition of insulin secretion by VEGF-B is also related to the PLC signaling pathway.
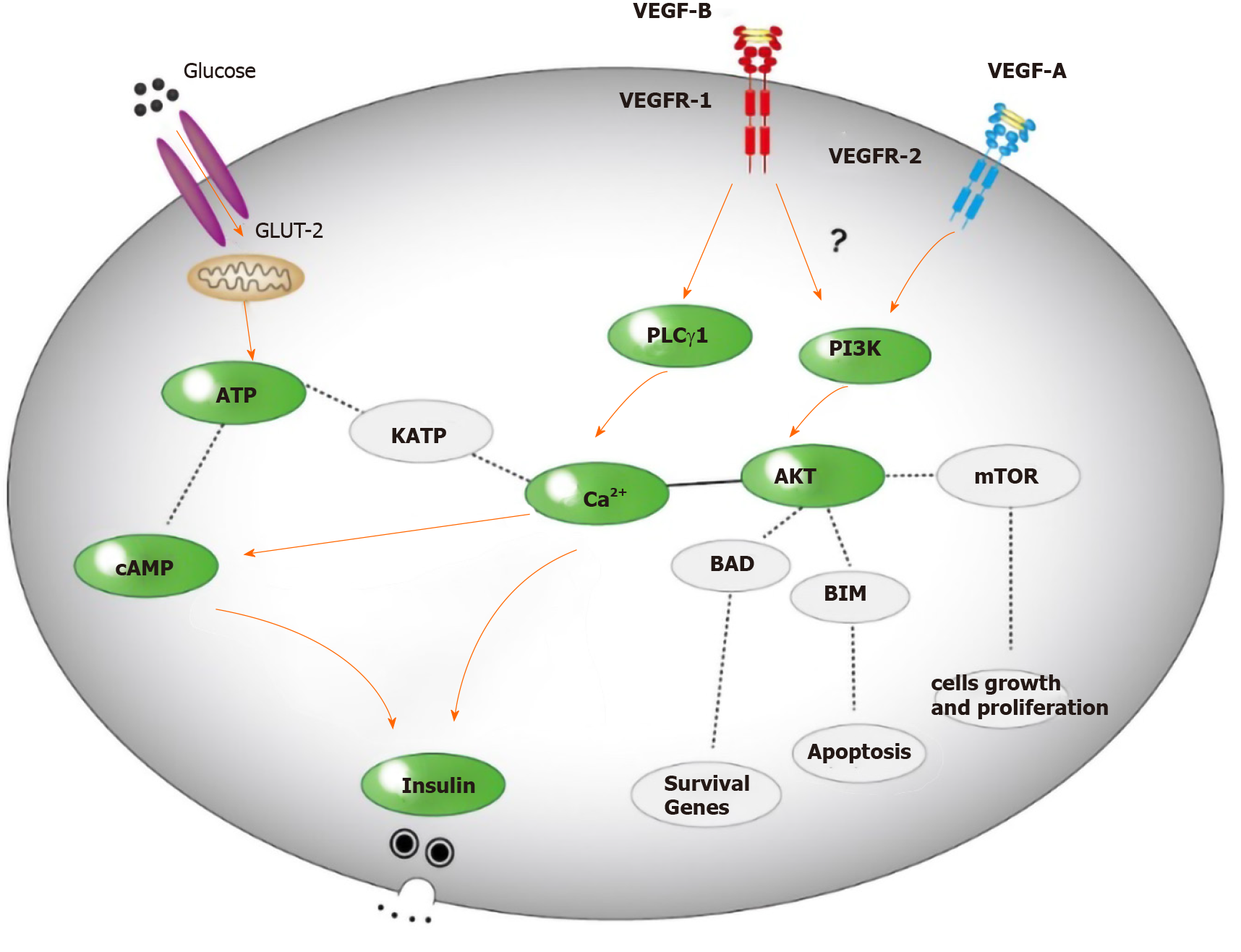
Our research indicated that the effects of VEGF-B on insulin secretion and Ca2+ and cAMP levels in MIN6 cells were mediated by the PI3K/AKT pathway. First, stimulation with exogenous VEGF-B decreased insulin secretion from MIN6 cells, and the levels of Ca2+ and cAMP related to insulin secretion in these cells also decreased. Moreover, the changes in the expression of key proteins in insulin secretion signaling pathways, such as PLCγ1 and PI3K/AKT, were consistent with the changes in insulin levels. In addition, the increased insulin secretion of MIN6 cells with downregulated VEGF-B expression was confirmed by the direct transfection of VEGF-B siRNA. The changes in the levels of Ca2+ and cAMP and the expression of PLCγ1, PI3K/AKT and other proteins were also consistent with the changes in insulin levels. These results confirm our hypothesis that VEGF-B affects insulin secretion by MIN6 cells through the PI3K/AKT signaling pathway (Figure 5), which may provide an explanation for the involvement of VEGF-B in the pathogenesis of T2D. However, whether VEGF-B directly functions by binding VEGFR1 or competing with VEGF-A to promote the binding of VEGF-A and VEGFR2 to activate downstream signaling pathways remains to be further studied[5]. In addition, these findings encourage us to continue to explore the effect of VEGF-B on insulin secretion by regulating the expression level of VEGF-B in mice.
The results of this study show that VEGF-B can regulate insulin secretion by modulating the levels of Ca2+ and cAMP. VEGF-B involvement in insulin secretion is related to the expression of PLCγ1, PI3K, AKT, and other signaling proteins. These results provide theoretical support and an experimental basis for the study of VEGF-B in the pathogenesis of T2D.
Type 2 diabetes (T2D), which is characterized by defective pancreatic β-cell function or insulin resistance leading to insufficient insulin secretion and increased blood sugar, has elicited worldwide public health concerns. Despite the knowledge available on T2D, the mechanism of insulin secretion is still unclear. In this study, we studied the mechanism by which vascular endothelial growth factor B (VEGF-B) affects the insulin secretion signaling pathway in MIN6 cells and explored the role of VEGF-B in blood glucose regulation.
We explored the role of VEGF-B in the insulin secretion signaling pathway and provided mechanistic insights into the occurrence and development of insulin secretion and T2D.
Our aim was to explore the mechanism of insulin secretion, study the effect of VEGF-B on insulin secretion, and provide a new strategy to prevent the progression of T2D.
This study was performed with in vitro cultures of MIN6 cells. By studying the effect of VEGF-B on insulin secretion in MIN6 cells and detecting the levels of Ca2+ and cyclic adenosine monophosphate (cAMP) in the insulin secretion signaling pathway and the expression of key proteins in the PI3K-AKT (phosphatidylinositol 3-kinase-serine/threonine kinase) signaling pathway, the effect of VEGF-B on the insulin secretion mechanism was discussed. Statistical analyses were performed using SPSS statistical software (version 22.0).
In this study, we found that exogenous VEGF-B treatment inhibited the secretion of insulin and simultaneously reduced the levels of Ca2+ and cAMP in MIN6 cells. In MIN6 cells with VEGF-B knockdown, insulin secretion and Ca2+ and cAMP levels increased. The effect of VEGF-B on insulin occurred through the PI3K-AKT pathway.
VEGF-B can inhibit insulin secretion through the PI3K-AKT pathway and may become a new target for the study of T2D. Our study provides new mechanistic insight into insulin secretion.
This study could provide new insights into the mechanism of insulin secretion and form the foundation for new ideas for the prevention of T2D.
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4594] [Article Influence: 574.3] [Reference Citation Analysis (8)] |
| 2. | Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, Scotney P, Nyqvist D, Samén E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjöholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Mehlem A, Palombo I, Wang X, Hagberg CE, Eriksson U, Falkevall A. PGC-1α Coordinates Mitochondrial Respiratory Capacity and Muscular Fatty Acid Uptake via Regulation of VEGF-B. Diabetes. 2016;65:861-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Ning FC, Jensen N, Mi J, Lindström W, Balan M, Muhl L, Eriksson U, Nilsson I, Nyqvist D. VEGF-B ablation in pancreatic β-cells upregulates insulin expression without affecting glucose homeostasis or islet lipid uptake. Sci Rep. 2020;10:923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Robciuc MR, Kivelä R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppänen VM, Käkelä R, Eklund L, Wasserman DH, Groen AK, Alitalo K. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab. 2016;23:712-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Dmytriyeva O, de Diego Ajenjo A, Lundø K, Hertz H, Rasmussen KK, Christiansen AT, Klingelhofer J, Nielsen AL, Hoeber J, Kozlova E, Woldbye DPD, Pankratova S. Neurotrophic Effects of Vascular Endothelial Growth Factor B and Novel Mimetic Peptides on Neurons from the Central Nervous System. ACS Chem Neurosci. 2020;11:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shen Z, Zhang Z, Wang X, Yang K. VEGFB-VEGFR1 ameliorates Ang II-induced cardiomyocyte hypertrophy through Ca2+ -mediated PKG I pathway. J Cell Biochem. 2018;119:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Li LJ, Ma J, Li SB, Chen X, Zhang J. Vascular endothelial growth factor B inhibits lipid accumulation in C2C12 myotubes incubated with fatty acids. Growth Factors. 2019;37:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Li X, Kumar A, Zhang F, Lee C, Tang Z. Complicated life, complicated VEGF-B. Trends Mol Med. 2012;18:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 7055] [Article Influence: 306.7] [Reference Citation Analysis (0)] |
| 11. | Claesson-Welsh L. VEGF receptor signal transduction - A brief update. Vascul Pharmacol. 2016;86:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 513] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 13. | Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000;26:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Jensen LD, Nakamura M, Bräutigam L, Li X, Liu Y, Samani NJ, Cao Y. VEGF-B-Neuropilin-1 signaling is spatiotemporally indispensable for vascular and neuronal development in zebrafish. Proc Natl Acad Sci USA. 2015;112:E5944-E5953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 16. | Li X. VEGF-B: a thing of beauty. Cell Res. 2010;20:741-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Li X, Aase K, Li H, von Euler G, Eriksson U. Isoform-specific expression of VEGF-B in normal tissues and tumors. Growth Factors. 2001;19:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Li X, Claesson-Welsh L, Shibuya M. VEGF receptor signal transduction. Methods Enzymol. 2008;443:261-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Huang D, Zhao C, Ju R, Kumar A, Tian G, Huang L, Zheng L, Li X, Liu L, Wang S, Ren X, Ye Z, Chen W, Xing L, Chen Q, Gao Z, Mi J, Tang Z, Wang B, Zhang S, Lee C. VEGF-B inhibits hyperglycemia- and Macugen-induced retinal apoptosis. Sci Rep. 2016;6:26059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Arjunan P, Lin X, Tang Z, Du Y, Kumar A, Liu L, Yin X, Huang L, Chen W, Chen Q, Ye Z, Wang S, Kuang H, Zhou L, Xu K, Chen X, Zeng H, Lu W, Cao Y, Liu Y, Zhao C, Li X. VEGF-B is a potent antioxidant. Proc Natl Acad Sci USA. 2018;115:10351-10356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol Res. 2019;143:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I, van Meeteren LA, Samen E, Lu L, Vanwildemeersch M, Klar J, Genove G, Pietras K, Stone-Elander S, Claesson-Welsh L, Ylä-Herttuala S, Lindahl P, Eriksson U. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 23. | Albrecht I, Kopfstein L, Strittmatter K, Schomber T, Falkevall A, Hagberg CE, Lorentz P, Jeltsch M, Alitalo K, Eriksson U, Christofori G, Pietras K. Suppressive effects of vascular endothelial growth factor-B on tumor growth in a mouse model of pancreatic neuroendocrine tumorigenesis. PLoS One. 2010;5:e14109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Chen Y, Zhao M, Wang C, Wen H, Zhang Y, Lu M, Adlat S, Zheng T, Zhang M, Li D, Lu X, Guo M, Chen H, Zhang L, Feng X, Zheng Y. Adipose vascular endothelial growth factor B is a major regulator of energy metabolism. J Endocrinol. 2020;244:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wu J, Wei H, Qu H, Feng Z, Long J, Ge Q, Deng H. Plasma vascular endothelial growth factor B levels are increased in patients with newly diagnosed type 2 diabetes mellitus and associated with the first phase of glucose-stimulated insulin secretion function of β-cell. J Endocrinol Invest. 2017;40:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Dijkstra MH, Pirinen E, Huusko J, Kivelä R, Schenkwein D, Alitalo K, Ylä-Herttuala S. Lack of cardiac and high-fat diet induced metabolic phenotypes in two independent strains of Vegf-b knockout mice. Sci Rep. 2014;4:6238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Sun CY, Lee CC, Hsieh MF, Chen CH, Chou KM. Clinical association of circulating VEGF-B levels with hyperlipidemia and target organ damage in type 2 diabetic patients. J Biol Regul Homeost Agents. 2014;28:225-236. [PubMed] |
| 28. | Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes. 2016;65:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 29. | Seino S, Sugawara K, Yokoi N, Takahashi H. β-Cell signalling and insulin secretagogues: A path for improved diabetes therapy. Diabetes Obes Metab. 2017;19 Suppl 1:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Velasco M, Díaz-García CM, Larqué C, Hiriart M. Modulation of Ionic Channels and Insulin Secretion by Drugs and Hormones in Pancreatic Beta Cells. Mol Pharmacol. 2016;90:341-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca2+ homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017;24:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | Schulla V, Renström E, Feil R, Feil S, Franklin I, Gjinovci A, Jing XJ, Laux D, Lundquist I, Magnuson MA, Obermüller S, Olofsson CS, Salehi A, Wendt A, Klugbauer N, Wollheim CB, Rorsman P, Hofmann F. Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844-3854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Nitert MD, Nagorny CL, Wendt A, Eliasson L, Mulder H. CaV1.2 rather than CaV1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J Mol Endocrinol. 2008;41:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Ashcroft FM, Rorsman P. K(ATP) channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol. 2013;9:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 35. | Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. The relationship between serum calcium level and early-phase insulin secretion in normoglycemic and pre-diabetic individuals. Exp Clin Endocrinol Diabetes. 2015;123:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Nakagawa Y, Nagasawa M, Medina J, Kojima I. Glucose Evokes Rapid Ca2+ and Cyclic AMP Signals by Activating the Cell-Surface Glucose-Sensing Receptor in Pancreatic β-Cells. PLoS One. 2015;10:e0144053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol. 2010;654:281-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Landa LR Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem. 2005;280:31294-31302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Skelin M, Rupnik M. cAMP increases the sensitivity of exocytosis to Ca²+ primarily through protein kinase A in mouse pancreatic beta cells. Cell Calcium. 2011;49:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Owen LA, Uehara H, Cahoon J, Huang W, Simonis J, Ambati BK. Morpholino-mediated increase in soluble Flt-1 expression results in decreased ocular and tumor neovascularization. PLoS One. 2012;7:e33576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 485] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 42. | Erskine L, François U, Denti L, Joyce A, Tillo M, Bruce F, Vargesson N, Ruhrberg C. VEGF-A and neuropilin 1 (NRP1) shape axon projections in the developing CNS via dual roles in neurons and blood vessels. Development. 2017;144:2504-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 449] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 44. | Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J Cell Physiol. 2001;188:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (3)] |
| 45. | Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 785] [Article Influence: 32.7] [Reference Citation Analysis (8)] |
| 46. | Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 Ligands. Sci Signal. 2009;2:re1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Li H, Xu W, Ma Y, Zhou S, Xiao R. Milk fat globule membrane protein promotes C2C12 cell proliferation through the PI3K/Akt signaling pathway. Int J Biol Macromol. 2018;114:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Lu J, Xu F, Zhang J. Inhibition of angiotensin II-induced cerebrovascular smooth muscle cell proliferation by LRRC8A downregulation through suppressing PI3K/AKT activation. Hum Cell. 2019;32:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Gao X, Wang N, Wu S, Cui H, An X, Yang Y. Long noncoding RNA FER1L4 inhibits cell proliferation and metastasis through regulation of the PI3K/AKT signaling pathway in lung cancer cells. Mol Med Rep. 2019;20:182-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Chen M, Zhu LL, Su JL, Li GL, Wang J, Zhang YN. Prucalopride inhibits lung cancer cell proliferation, invasion, and migration through blocking of the PI3K/AKT/mTor signaling pathway. Hum Exp Toxicol. 2020;39:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Abeyrathna P, Su Y. The critical role of Akt in cardiovascular function. Vascul Pharmacol. 2015;74:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 52. | Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483-1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 1167] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 53. | MacDonald PE, Joseph JW, Yau D, Diao J, Asghar Z, Dai F, Oudit GY, Patel MM, Backx PH, Wheeler MB. Impaired glucose-stimulated insulin secretion, enhanced intraperitoneal insulin tolerance, and increased beta-cell mass in mice lacking the p110gamma isoform of phosphoinositide 3-kinase. Endocrinology. 2004;145:4078-4083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Rückle T, Woo M, Manning Fox JE, MacDonald PE. Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human beta-cells. Diabetes. 2009;58:2084-2092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2210] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 56. | Ganesan S, Calle R, Zawalich K, Smallwood JI, Zawalich WS, Rasmussen H. Glucose-induced translocation of protein kinase C in rat pancreatic islets. Proc Natl Acad Sci USA. 1990;87:9893-9897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Ganesan S, Calle R, Zawalich K, Greenawalt K, Zawalich W, Shulman GI, Rasmussen H. Immunocytochemical localization of alpha-protein kinase C in rat pancreatic beta-cells during glucose-induced insulin secretion. J Cell Biol. 1992;119:313-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Fiume R, Ramazzotti G, Faenza I, Piazzi M, Bavelloni A, Billi AM, Cocco L. Nuclear PLCs affect insulin secretion by targeting PPARγ in pancreatic β cells. FASEB J. 2012;26:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Yamazaki H, Zawalich KC, Zawalich WS. Physiologic implications of phosphoinositides and phospholipase C in the regulation of insulin secretion. J Nutr Sci Vitaminol (Tokyo). 2010;56:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pasini L S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ