Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1832
Peer-review started: May 3, 2021
First decision: June 16, 2021
Revised: June 26, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: November 15, 2021
Processing time: 196 Days and 0.4 Hours
Diabetes is one of the four major non-communicable diseases, and appointed by the world health organization as the seventh leading cause of death worldwide. The scientists have turned over every rock in the corners of medical sciences in order to come up with better understanding and hence more effective treatments of diabetes. The continuous research on the subject has elucidated the role of immune disorders and inflammation as definitive factors in the trajectory of diabetes, assuring that blood glucose adjustments would result in a relief in the systemic stress leading to minimizing inflammation. On a parallel basis, microbial infections usually take advantage of immunity disorders and propagate creating a pro-inflammatory environment, all of which can be reversed by antimicrobial treatment. Standing at the crossroads between diabetes, immunity and infection, we aim in this review at projecting the interplay between immunity and diabetes, shedding the light on the overlapping playgrounds for the activity of some antimicrobial and anti-diabetic agents. Furthermore, we focused on the anti-diabetic drugs that can confer antimicrobial or anti-virulence activities.
Core Tip: Understanding the mutual interplay between diabetes and microbial infection is necessary to control both and to avoid a lot of serious complications that may happen in such clinical conditions. Repurposing of approved drugs and investigation of their new application represents a promising approach for maximizing treatment outcomes. In this review, we shed light on the overlapping areas of efficacy between anti-diabetics and antimicrobials.
- Citation: Hegazy WAH, Rajab AAH, Abu Lila AS, Abbas HA. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World J Diabetes 2021; 12(11): 1832-1855
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1832.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1832
Diabetes is a chronic metabolic disorder associated with high blood glucose levels. Diabetes is a lifelong condition which requires proper monitoring and change in the patient’s diet and routine habits. The world health organization reported an increase in the incidence of diabetes over the last decades worldwide that affected all social, economic and ethnic backgrounds. The universal prevalence of diabetes has nearly doubled since 1980 to 2014, rising from 4.7% to 8.5% in the adult population, and expected to rise to 10.4% in 2040[1]. This alarming uprise in the statistics of diabetes is owed to the global shift towards urban habits generally characterized by unbalanced diet, stress and reduced physical activity. Before the coronavirus disease 2019 (COVID-19) pandemic 650 million adults (13% of the world's adult population) were obese and it was estimated that 19.7% of the world's population will be obese by the year of 2030[2]. The COVID-19 pandemic has been associated with increased risk of obesity and associated health hazards mainly diabetes. The global application of quarantine requirements forced billions of people into a new life style of isolation where people are forced to spend more time indoors with minimum physical activities and limited contact with others. The quarantine related frustration pushed people to consume larger amounts of high sugar foods which is reflected as higher incidence of obesity[3,4]. Moreover, many studies have outlined the role of obesity and diabetes as important risk factors in COVID-19 infections[5,6].
Diabetes is commonly divided into two major categories depending on the age of onset and the pathophysiological cascade of events giving rise to diabetes; type I diabetes (T1DM), also known as juvenile diabetes, is characterized by the inability of pancreas to secrete insulin due to damage of β-cells mostly caused by an autoimmune disorder. The onset of T1DM appears usually in childhood and requires lifelong insulin injections. On the other hand, type II diabetes (T2DM) is characterized by insulin resistance that can be accompanied by reduced insulin secretion from the pancreas. T2DM is more common than T1DM, its onset appears in adulthood and its treatment involves diet control, medications for control of blood glucose level and eventually insulin injection is required in late stages[7].
The delay in diagnosis and treatment of diabetes can lead to irreversible damage to many of the body organs; some of the complications of diabetes include neuropathy, retinopathy, nephropathy, cardiovascular diseases, peripheral insufficiency, and diabetic foot ulcers. Failure to control diabetes can eventually lead to life threatening complications like kidney failure, lower limbs gangrene, heart attacks and stroke[8]. Some of the most distinguished complications of diabetes include: Immunodeficiency, high risk of infection and longer recovery period, all of which represent lifelong companions of diabetic patients. The most frequent infections in diabetic patients are respiratory tract infections, urinary tract infections, skin and soft tissues infections, diabetic foot ulcers, otitis and periodontal infections[9]. Many mechanisms were proposed for the reasons behind the high risk of contracting infection in diabetic individuals like the high blood glucose level, the lower-than-normal pH in body fluids[9,10], in addition to poor vascularity of peripheral tissues[11], all of which support pathogenic infestation. However, the most profound factor is the impaired immune functions. The relation between microbes and diabetes is bidirectional. In other words, the high blood glucose levels complicate infections and also some microbial infections can contribute to the etiology of diabetes[12-16]. The undeniable role of inflammation in both diabetes and infection have been extensively portrayed, which lead to the conclusion that the use of anti-inflammatory agents can represent a rationale treatment approach for better control of diabetes or even delaying its complications. Generally, anti-inflammatory agents are essential members in any anti-diabetic regimen; non-steroidal anti-inflammatory drugs and salicylates are commonly prescribed anti-inflammatory agents for better control of the diabetes associated inflammation[17].
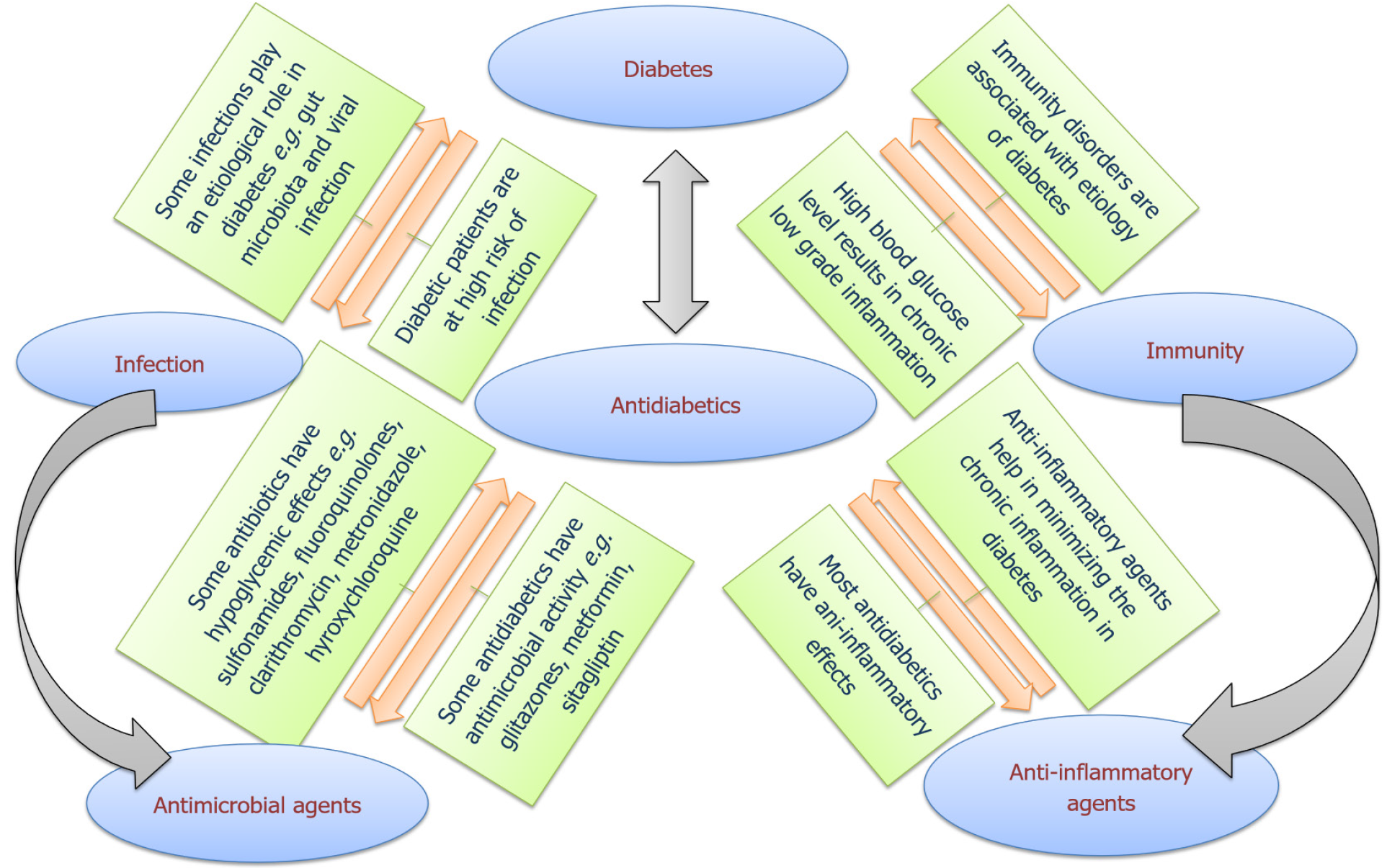
As diabetics are immunocompromised chronic patients and are more susceptible to microbial infections, exploring antimicrobial activities of approved anti-diabetic agents may be highly appreciated by clinicians. Preferential selection of anti-diabetic agents that have additional antimicrobial activities for diabetic patients can offer multiple advantages, including antimicrobial protection enhancement and decreasing the treatment costs[18-21]. In this work we intend to discuss the multiple facets of the relation between infection and diabetes. We shed the light on the interplay between immunity, diabetes and microbial infections, discussing the influence of diabetes on worsening of microbial infections. Additionally, the antimicrobial agents that can harbor anti-diabetic activities were discussed, with special interest in the anti-diabetic drugs which have antimicrobial activities, enhance immune responses or mitigate microbial virulence (Figure 1).
The interplay between immune-dysfunction and diabetes has a deep complicated background that exceeds our full understanding. The question whether immune-dysfunction is the cause or the effect of diabetes was always questioned. It is widely accepted that an immune disorder is responsible for T1DM via T cell-mediated selective destruction of pancreatic β-cells[15]. The activation of such destructive autoimmune behavior is based on a genetic factor in addition to a triggering environmental event[22]. Meta-analysis of genomic data has identified the genetic loci related to high risk of T1DM, the most prevailing are haplotypes in human leukocyte antigen class II, other common risk loci include mutations in INS-gene leading to preproinsulin misfolding and polymorphisms in Protein tyrosine phosphatase, non-receptor type (PTPN-22), interleukin-2 (IL-2), renalase (RNLS) and CTLA-4 genes[9,15,23]. The presence of a single or multiple risk loci would lead to an unfortunate sequence of immunological reactions starting by loss of tolerance to pancreatic islets β-cell antigens, the production of anti-diabetic islet antibodies from plasma B-cells and the active involvement of autoreactive CD4- and CD8-T cells, eventually leading to steady rate damage in pancreatic β-cells[24]. This steady autoimmune destructive pattern remains hidden from the individual up until a critical damage limit is reached in the pancreatic islets after which hyperglycemia prevails and external insulin dependence becomes crucial[25]. During the prolonged silent preclinical period, early detection and reversal of the disease is possible by screening for the genetic risk loci, circulating anti-islet autoantibodies, and auto-reactive CD4 and CD8-T cells[15,25,26]. The presence of high-risk genetic loci only presents a predisposing factor to the disease; as a matter of fact, an individual carrying a high-risk gene could enjoy a delay in the onset of symptoms if he was lucky enough to escape the triggering factors associated with T1DM[22,27]. The triggering event can be an alteration in gut microbiota, obesity, a dietary factor like gluten or early introduction of cow’s milk in infancy, toxins or a viral infection especially by dsRNA virus[28-31]. It is widely conceived that such events could trigger abnormal immunogenicity of β-cells and the loss of tolerance to pancreatic islets β-cell antigens which marks the onset of the autoimmune response[22,24,27].
On the other hand, T2DM etiology involves weaker dependence on genetic factors and more correlation to life style factors[32]. The genetic risk factors predisposing to T2DM were outlined by genome-wide association studies as polymorphisms in TCFL2, ABCC8, CAPN10, PPAR, CDNKN2A/B, CDKAL1, and IGF2BP2 genes[33]. How- ever, the genetic susceptibility factor plays little role in the development of T2DM and again immunity related disorders play the leading role in the pathogenesis of the disease[9]. T2DM is generally characterized by a chronic low grade of inflammation arising from the immune response to hyperglycemia, aging, obesity and stress[7,34]. A growing mass of evidence suggests the involvement of both innate and adaptive immune-responses in the inflammatory trajectory of T2DM. The diabetes related dysfunctions in adaptive immunity include decreased γδ-T cell function, increased inflammatory T-helper phenotypes, decreased regulatory T-cells, and impaired B-cells function[7,35-37]. On the other hand, T2DM innate response defects come with altered neutrophil function, increased pro-inflammatory M1 macrophages, abnormal natural killer cell phenotypes, and increased inflammatory dendritic cells[7,35,36,38]. It should be noted that systemic inflammation is less projected in T1DM due to stimulated production of IL-10 from dendritic cells which is reflected as low incidence of insulin resistance[7,36]. Moreover, an autoimmune response characterized by circulating autoantibodies has been increasingly recognized in many T2DM patients[35,36,38]. However, the autoimmune pathway differs in T1DM compared to T2DM, since autoimmunity in T2DM patients is more related to obesity-activated chronic inflammatory responses and β-cells fatigue[36,38].
The gut microbiota represents an ecosystem of trillions of inhabitants that co-exist in our gastrointestinal track in perfect balance with other body systems, actively engaging in a mutually beneficial relationship[39,40]. The formation of the gut microbiota starts in infancy and continues to develop and diversify throughout our lifetime[41]. The complex and dynamic population of the gut microbiota includes bacteria, fungi, protists, archaea, and viruses with bacteria comprising the vast majority in the gut population[42]. The composition of the gut microbiota is subjected to continuous alterations and development depending on age, diet, geographical distribution, infection history, antimicrobial treatments, medication regimen, stress and physical activity among many other parameters[43,44], leading to huge composition variability between individuals in a pattern that can resemble fingerprint uniqueness[45]. Indeed, the gut microbiota plays an undeniable role in many metabolic and immune related disorders e.g., metabolic syndrome, diabetes, inflammatory bowel diseases and obesity[46-48]. However, the exact contribution of gut microbiota to the pathophysiology of diabetes is widely variable due to the individual variations on the matter. That being said, a handful of gut microbiota members have shown repeated signals in multiple researches, where the results suggested some bacterial genus to impact protective effects against T2DM e.g. Bifidobacterium, Lactobacillus, Bacteroides, Roseburia, Faecalibacterium, Clostridium cluster IV and subcluster XIVa and Akkermansia[49], such members were suggested as probiotics treatment with high association to improved glucose homeostasis and protection against T2DM, bearing in mind the importance of species-dependent variation in the result outcomes[49,50].
The suggested mechanisms for the protective effect of these bacterial groups against T2DM involves multiple pathways; observations have recorded an increase in the anti-inflammatory cytokines IL-10 and IL-22, enhanced T regulatory cell function, increased transforming growth factor-beta (TGF-β), suppressed intestinal inflammation, decreased gut permeability and increased insulin sensitivity[49]. On the other hand, other bacterial genera were repeatedly associated with impaired glucose homeostasis and increased risk of obesity and diabetes e.g., Ruminococcus, Fusobacterium, Blautia and Firmicutes[49,51,52]. The involved mechanisms are not clearly elucidated; however, some studies suggested that the introduction of a dietary factor like gluten, unbalanced high fat diet or the reduction in gut pH can significantly increase the pro-diabetic bacterial population at expense of the balance and diversity in the gut microbiota[14], with many observations relating these effects to increased pro-inflammatory cytokines and induction of antigen-specific T cells-initiated destruction of the pancreatic β-cells in T1DM[14,16], increased bowel permeability[53], endotoxemia[54,55], and altered metabolism of bile acids[56,57]. Moreover, the shift in balance in the gut microbiota can lead to overgrowth of bacteria that has an increased capacity to harvest energy from the diet, such members of microbiota can boost energy uptake from diet by hydrolysing the undigested plant polysaccharides (cellulose, xylan and pectin) thus contributing to higher risk of obesity and subsequently higher risk of T2DM[58,59].
Many of the risk factors related to diabetes have been studied and identified like the genetic risk loci, obesity and stress among others. Nevertheless, the role of some microbial related events has been repeatedly outlined as potential triggers in both T1DM and T2DM. In the following segment we discuss examples of the identified microbial suspects in the etiology of diabetes.
One of the leading triggers of T1DM is believed to be an enterovirus infection by Coxsackievirus B (CVB), rotavirus, mumps or cytomegalovirus[60-62]. This idea was first conceived when an observation in the Finnish population, where the highest incidence of T1DM is reported, lead to linking the first signs of autoantibodies in genetically susceptible children to the seasonal pattern of enterovirus infections, especially by CVB-1[13,60]. Additionally, enteric infections by CVB-4 were repeatedly associated with pancreatic islets inflammation and infiltration mediated by β-cell specific autoantigens and subsequent β-cell apoptosis[62]. Another study has outlined the positive correlation between enterovirus (A) overpopulation in the gut and an autoimmune response in the pancreatic islets of genetically susceptible individuals[63]. Some enterovirus can directly infect the β-cells via targeting specific pancreatic receptors such as the poliovirus receptor and integrin αvβ3, hence initiating an inflammatory autoimmune response[64,65]. This effect was clearer in some individuals who suffer a chronic viral induced β-cell inflammation that can be detected by tracing enteroviral major capsid protein VP1, enteroviral RNA and the over-expression of the major histocompatibility complex-1[12,66,67].
The association between hepatitis C virus (HCV) and T2DM was repeatedly studied; it is known that some extra-hepatic manifestations of HCV are related to impaired glucose homeostasis, decreased glucose uptake and increased insulin receptor damage[68,69]. Molecular investigations into the underlying mechanisms have revealed that HCV core protein enhances the production of reactive oxygen species (ROS) in the mitochondria and endoplasmic reticulum of hepatocytes. The accumulating oxidative stress results in propagating hepatic cirrhosis and fibrosis with impairment in liver mediated glucose homeostasis[70]. HCV core protein also activates serine phosphorylation with subsequent deterioration of insulin receptor substrate (IRS)-1 and consecutive blocking of insulin signal propagation at the insulin receptors[68,71]. Additionally, the function of (IRS)-1 is further impaired due to degradation mediated by the inflammatory mediator tumour necrosis factor (TNF)-α[69,71]. Moreover, HCV induces gluconeogenesis, reduced glucose uptake and accumulation of lipid droplets via up-regulation of the enzymes glucose 6 phosphatase (G6P) and phosphoenolpyruvate carboxykinase 2 (PCK2), and down regulation of glucose transporters (GLUT)-2 and (GLUT)-4[68]. The preceding information leads to the general conclusion that treatment of HCV infection could impose improvement in glucose homeostasis and insulin resistance, that was indeed observed in patients receiving anti-HCV antiviral regimens as shall be discussed shortly.
In an epidemiological study, an inverse relationship has been established between the decreasing prevalence of helminth infections and the increasing prevalence of metabolic diseases as diabetes[9]. But the controversy about the influence of Helicobacter pylori (H. pylori) bacteria on diabetes is more interesting. H. pylori, Gram-negative bacteria, is the most common causative agent of peptic ulcer and chronic gastritis[72]. H. pylori infection persists in the gastric epithelium generating local and systemic inflammation induced by multiple mediators[73-76] in addition to molecular antigenicity which provokes autoimmune responses[77,78]. All of these abnormalities predispose to a storm of inflammatory manifestations that has been linked to multiple extra-gastrointestinal disorders such as diabetes, cardiovascular disease, metabolic syndrome, atherosclerosis, neurodegenerative disorders, idiopathic iron deficiency anemia and vitamin B12 deficiency[79]. The relation between H. pylori infection and diabetes was proposed and discussed multiple times with conflicting significances being presented. Multiple meta-analyses have established positive correlation between chronic H. pylori infections and T2DM with less significant correlation to T1DM[80,81]. The correlation was more obvious in studies performed on data from Asian, European and African cases, with contradictory results obtained from United States patients[80,81]. There are two main proposed mechanisms for the diabetogenic effect of H. pylori: The diffuse inflammation stress induced by the infection and gastric hormones imbalance[82,83]. It can be expected that the pro-inflammatory environment caused by H. pylori would impact insulin receptors, leading to impaired insulin sensitivity. This hypothesis was confirmed in multiple studies that highlighted the positive correlation between H. pylori infections and insulin resistance[84,85]. Furthermore, one study reported that the presence of H. pylori antibodies was linked to 2.5 higher levels of insulin resistance[86]. H. pylori infection was also associated with higher incidence of chronic complications in T2DM patients, and associated with higher mean glycated hemoglobin (HbA1c), an indicator of chronic hyperglycemia in Prediabetic individuals[75]. On the other hand, it was reported that eradication of H. pylori by antibiotic treatment courses was not associated with improved insulin sensitivity[87]. In addition to the inflammatory pathways connecting H. pylori to T2DM, a parallel hormonal mechanism was proposed; H. pylori infection was reportedly associated with imbalance in secretion of the gastric hormones, with increased secretion of gastrin and decreased secretion of ghrelin, leptin and somatostatin[83,88,89]. The H. pylori induced imbalance in these hormones was associated with impaired insulin release from pancreatic islets, increased appetite and fat deposition[90-92]. More studies are required to clarify the exact pathways for H. pylori triggered insulin resistance and the interactions between gastric hormone imbalance and insulin release from the pancreatic islets, by which H. pylori interferes with insulin release from the pancreatic islets.
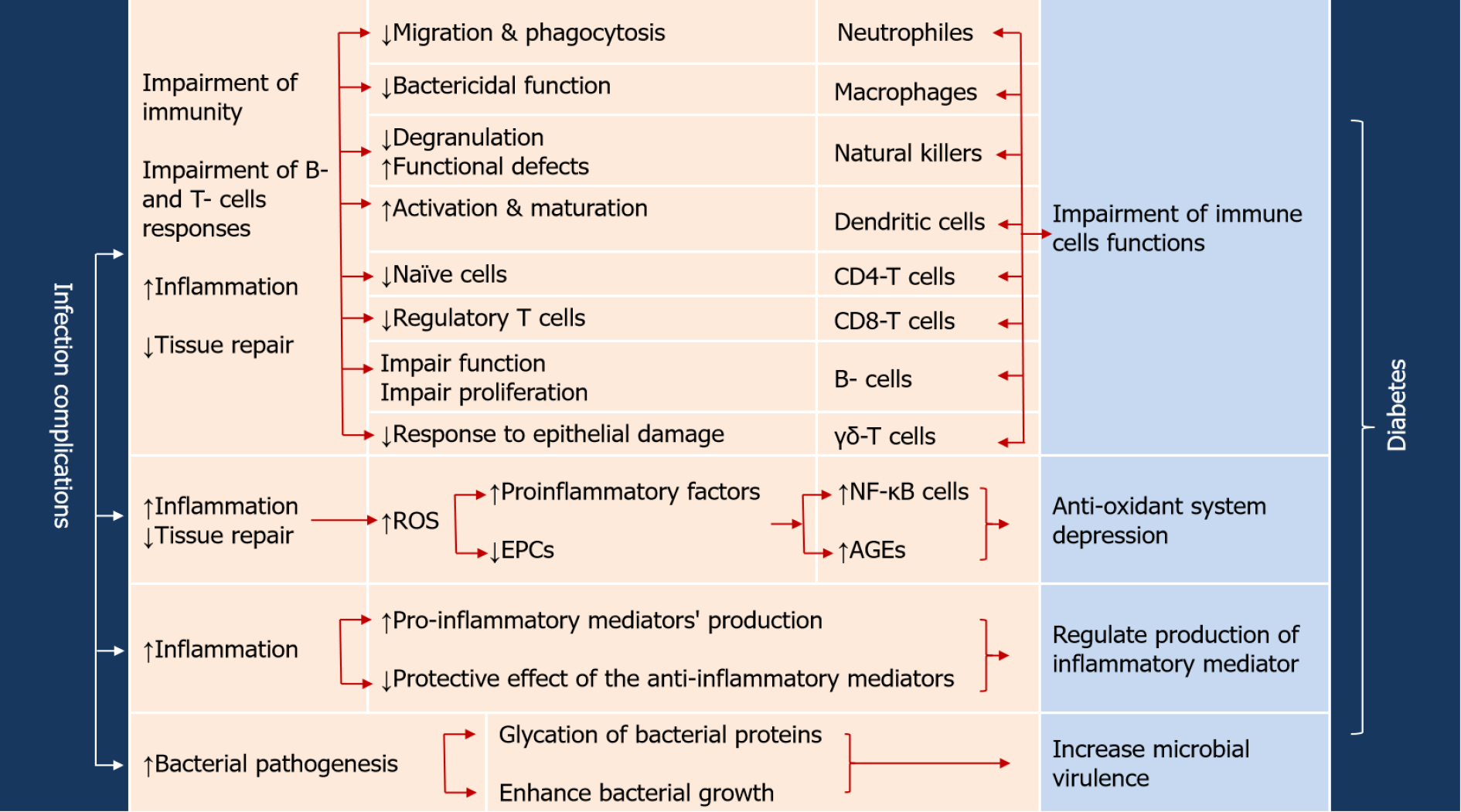
The evidence of bidirectional link between diabetes and viral, bacterial, fungal, and parasitic infectious agents has been proven and extensively documented[9,10,93,94]. This bidirectional link between diabetes and infection is governed by the inflammatory mediators that link inflammation process and diabetes vulnerability to infection[9,10,23]. In other words, diabetes augments the outcome of microbial infections and vice versa (Figure 2). As a consequence of diabetes, immune alterations would lead to (1) Increased activity of ROS; (2) Increased production of the pro-inflammatory mediators TNF-α, INF-γ, IL-1β, IL-6, IL-8, IL-12 and IL-17; (3) Reduced protective effect of the anti-inflammatory mediators interferon-1, IL-2, IL-10 and IL-22; (4) Reduced expression of cathelicidins in macrophage leading to impaired bactericidal activity and chemotaxis; and (5) Reduced glutathione and non-enzymatic glycation of complement factor thus inhibiting its activation[7,9,35,36,38]. Such ramifications are responsible for impaired function of the first line antimicrobial defense, higher susceptibility to pathogens and delayed healing[7,35]. Long term hyperglycemia will cause advanced glycation end (AGE) products of proteins such as AGE-albumin which hinders trans-endothelial migration in macrophages[35].
Just as diabetes weakens both humoral and cellular immune responses, hyperglycemia can enhance the microbial virulence. Generally, diabetic patients with higher HbA1c (> 6.5%) are at higher risk of hospital-acquired and community-acquired infections and sepsis[10,95,96]. Elevated HbA1c represent a risk factor for bacteremia and sepsis in diabetic patients who suffer from urinary tract infections[96]. The increased susceptibility to E. coli infections is owed to glycation of E. coli fimbrial FimH adhesin which promotes the bacterial adhesion to urinary tract epithelial cells[97]. In periodontitis, diabetes enhances expression of IL-17 and increases pathogenicity of the oral microbiome[98]. In respiratory infections, the elevation of blood glucose in diabetic mice promotes the Staphylococcus aureus growth in the airways increasing the possibility of infection[99]. Moreover, the influences of diabetes on patient's immunity are considered in defensing mechanisms against mycotic, parasitic and viral infections. For instance, Candida spp. constitute the most frequent isolates from urogenital tract of hyperglycemic patients[100,101] and the severity of infection is in correlation with glucose level[102]. The adhesion of Candida spp is enhanced in presence of high glucose which increases the expression of intercellular adhesion molecule-1[103]. Diabetes changes the morphology of Leishmania major lesions[104] and particularly causes severe cutaneous Leishmania infantum lesions[105]. Chickenpox complications such as postherpetic neuralgia are more severe and persistent in diabetics[106], and T1DM vascular complications confers an additional virulence to herpes zoster[107]. The severity of liver damage is more observed in HCV patients with uncontrolled glucose levels[108].
In context with prominent effects of diabetes on both innate and adaptive immunity, diabetic patients are more susceptible than nondiabetics to all types of infections such as nosocomial infections[95], sepsis[10] tuberculosis[109-111], Legionella infections[112], gum infections[113], fungal infections[114], dengue fever[115], influenza virus[116], herpes zoster[106,117,118], and other infections reviewed in[9]. Moreover, the risk of post-sepsis infections increases due to alterations in innate and adaptive immune responses resulting in chronic inflammation and persistence of causative microbe[10,96]. As a consequence of impaired immunity, diabetic patients are prone to more aggressive, recurrent and life uncommon life-threatening infections[9,10,95,119,120]. Conclusively, diabetes augments the bacterial virulence either by impairing the patient's immune responses or even by enhancing the invasion and spreading of bacterial[9,10,120,121].
Diabetes complications favor the microbial pathogenesis due to decreased blood supply to the affected areas and reduced neural sensation[122]. Furthermore, the development of resistance to antimicrobial agents is more common in diabetic patients as compared to nondiabetics[123]. The prevalence methicillin-resistant S. aureus[124-126], vancomycin-resistant Enterococci, carbapenem-resistant Enterobacteria, extended-spectrum β-lactamases-producing Enterobacteria, and non-fermenting Gram-negative bacilli are elevated in diabetic patients[9,120,127,128]. This is owed to the impaired immunity of diabetic patients which principally leads to increasing their susceptibility to infectious agents and failure in complete eradication of persistent infections, this results in more exposure to antimicrobial agents and subsequently higher risk of antimicrobial resistance. Examples for the antimicrobial resistance development in treatment of surgical infections and diabetic foot are tremendous and very serious as reviewed[9,10,120,129]. Although H. pylori is well known for its susceptibility to usual therapy regimen, it resists eradication in diabetics which require specific modified antimicrobial regimen[130].
Antimicrobial agents can induce multiple pharmacological effects beyond their lethality to invading pathogens; these effects can be reflected as metabolic changes which sometimes affect glucose homeostasis. Recently, it was shown that the exposure to antibiotics in childhood has been linked to increased risk of metabolic disorders later in life and associated with changes in development of pancreas[63]. Additionally, some antibiotics may alter the antidiabetic plasma levels; diabetic patients with tuberculosis were advised to take rifampicin and metformin with sufficient time interval[131]. Clarithromycin is another antimicrobial agent associated with severe hypoglycemia in diabetic patients receiving hypoglycemic medications, the risk increases with renal impairment and in elderly patients[132]. The suggested mechanism for clarithromycin induced hypoglycemia is the inhibition of the cytochrome-P450 enzyme which is responsible for metabolic inactivation of sulfonylurea or meglitinide hypoglycemics, this leads to increased plasma concentration of these medications and subsequent hypoglycemia[133]. Similar hypoglycemic effects were reported for metronidazole which also inhibits CYP2C9 inhibitor which interferes with the metabolism of hypoglycemic agents[134]. It can be concluded from the above that clarithromycin and metronidazole don’t have a direct hypoglycemic effect, rather they increase the systemic concentration of sulfonylurea or meglitinide drugs as a result of the delay in their metabolism[134]. More considerably, some antibiotics impose disrupting effects on gut microbiota with alterations in the expression of their key metabolic pathways which influences both their response to antibiotics and the glucose metabolism[135-137]. In addition to the above mentioned indirect hypoglycemic effects of some antimicrobial agents, others have showed direct hypoglycemic effects. In the next paragraphs we will give a glance at some of these drugs.
Sulfonamides are of the oldest known antimicrobial agents. During their long use, clinical observations revealed other clinical effects of sulfonamides including anti-carbonic anhydrase, anti-obesity, diuretic, hypoglycemic, antithyroid, antitumor, anti-neuropathic and anti-inflammatory activities[138]. The hypoglycemic activity of some sulfonamides received the most attention from the mid-20th century scientists, it was concluded that sulfonylureas have the best hypoglycemic activity through stimulating insulin secretion from pancreatic β-cells and decrease in hepatic clearance of insulin[139]. Further investigations into the hypoglycemic effects of sulfonylureas lead to the development of first and second generations of hypoglycemic sulfonylureas which constitute an important group of anti-diabetic agents that are still widely used today for the treatment of T2DM[140]. It was repeatedly advised to be cautious while combining sulfonamides with other hypoglycemic agents due to the synergistic effects that can lead to life threatening hypoglycemia, which is more common in case of elderly and renal dysfunction patients[141-143].
Fluoroquinolones represent a group of broad-spectrum antimicrobial agents that are widely used for treatment of respiratory tract and urinary tract infections. Despite the fact that this group has outstanding antimicrobial efficiency against a wide range of infections, they suffer from serious risk factors like the significant risk of aortic aneurysm, neuropathy, tendinopathy, and interference with glucose homeostasis[127,144,145]. Fluoroquinolones can induce life threatening hypoglycemia in diabetic patients, and dysglycemia in nondiabetic individuals. The suggested mechanism of hypoglycemia is via blocking the ATP-sensitive K+ channels in the pancreatic β-cell in the pancreas which boosts insulin secretion, however the mechanism behind the hyperglycemic effect is unclear[146]. The FDA repeatedly reported the high risk of dysglycemia associated with different members of fluoroquinolones. The multiple reports of severe hypo- and hyperglycemic clinical observations were the reasons behind the withdrawal of oral and systemic gatifloxacin preparations from the markets in 2006[147].
Hydroxychloroquine is an antimalarial drug that shows additional anti-inflammatory, immunomodulatory, anti-rheumatic and hypolipidemic activities. Hydroxychloroquine is also known to exert a significant hypoglycemic effect[148]. The exact mechanism of the hypoglycemic effect is not known; however, it is suspected to increase insulin receptors sensitivity, decrease hepatic clearance of insulin and reduce systemic inflammation[148-150]. Benzimidazoles are group of medications mostly used as anti-helminthic. It was reported that they have hypoglycemic activity mediated by augmenting insulin secretion and activity[151,152].
Telaprevir is a protease inhibitor effective against HCV genotype 1. Some studies reported the development of hypoglycemia in diabetic patients receiving the antiviral telaprevir treatment course. One case study reported a female diabetic patient with obesity and HCV-related cirrhosis. She was given triple antiviral treatment by interferon-α, ribavirin and telaprevir. During the course of treatment multiple episodes of severe hypoglycaemia were recorded, however this effect disappeared after course completion which drove the general conclusion that telaprevir could impose a hypoglycemic effect[153]. Similar conclusions were obtained from another case study of a male diabetic patients receiving anti-HCV triple treatment with interferon, ribavirin and boceprevir. The study reported reversal of diabetes and termination of the anti-diabetic treatment after the successful viral treatment. The study suggests a relation between HCV and diabetes and possible reversibility of glucose abnormalities with successful eradication of HCV[154]. Two years later, similar outcomes were reproducible with another diabetic male also receiving triple HCV treatment in addition to anti-diabetic regimen including insulin and metformin. After the successful antiviral treatment, the patient was able to gradually withdraw insulin from his anti-diabetic treatment regimen and continued only the oral hypoglycemic linagliptin. This study weighs on the possibility of reduced glucose imbalance and even reversal of diabetes as an unexpected outcome after the antiviral treatment, the study attributes the improved glucose homeostasis due to retained normal functions of the liver after the termination of the viral infection[155]. Similar conclusions were reached in another retrospective study that included 65 diabetic patients subjected to the anti HCV triple-treatment including sofosbuvir, the results indicated improved blood glucose levels in all enrolled cases after the antiviral treatment as a result of retained normal liver activity[156].
Just as there are antimicrobials that can induce dysglycemia, some anti-diabetic can alter the antibacterial metabolism[157]. Searching into new fields for application of currently approved medicinal drugs, scientists have been more interested in drug repurposing as an elegant strategy for applying maximum use of already approved medicinal agents[158,159]. The benefits may be augmented by repurposing routinely used anti-diabetics as antimicrobial agents, this decreases the dose and number of administrated drugs that results in saving time and cost, decreasing the drug-drug interactions and enhancing the patients’ compliance with the applied treatment regimens.
Likewise, most of the commonly used anti-diabetic agents offer additional anti-inflammatory activity as a favorable side effect during the treatment; the anti-inflammatory properties of glitazones, metformin, sulfonylureas and Dipeptidyl peptidase (DPP)-4 inhibitors were authenticated and appraised. The hypoglycemic effect of these agents is usually associated with decreased oxidative stress, decreased pro-inflammatory and increased anti-inflammatory mediators[160].In this context, it is highly valuable to clearly identify anti-diabetic agents that have additional antimicrobial activity, which is considered an interesting and promising area of active research[18,19]. During this search, we are interested in projecting the antimicrobial activities of some anti-diabetics (Table 1).
| Antidiabetic drug | Antimicrobial activity | Proposed mechanism | Ref. |
| Metformin | Antibacterial | Activation of the AMPK-mediated phagocytosis and production of mROS | [170] |
| Disruption of the outer membrane permeability | [170] | ||
| Down regulation of the Q.S encoding genes and mitigate the bacterial virulence | [19,186] | ||
| Anti-TB | Increasing the production of β-defensin-2, -3 and -4 which diminish bacterial growth and multiplication | [178] | |
| Inhibition of mitochondrial complex-I which is analogous to mycobacterial NDH-I complex | [176] | ||
| Activation of T regulatory and CD8 memory T cells responses activity | [177] | ||
| Sitagliptin | Antibacterial | Downregulation of the Q.S encoding genes, occupy the Q.S receptors and diminish bacterial virulence | [18,19,186] |
| Anti-COVID-19 | Reduction of the inflammation intensity | [190,191] | |
| Targeting viral proteins | [192] | ||
| Binding to viral spikes | [193] | ||
| Antibiofilm | Targeting enzyme XPDAP, analogous to mammalian enzyme DPP IV | [187] | |
| Vildagliptin | Antibiofilm | Targeting enzyme XPDAP, analogous to DPP IV | [187] |
| Anti-amoebic | - | [188] | |
| Saxagliptin | Antibiofilm | Targeting enzyme XPDAP, analogous to DPP IV | [187] |
| Pioglitazone | Antibacterial | Increasing phagocytosis and production of reactive oxygen species in phagocytes | [197,198] |
| Tolbutamide | Antibacterial and antifungal | - | [205] |
| 2nd generation sulfonylureas | Antifungal | Inhibition of the NLRP3 inflammasome | [206] |
| Antibacterial | Prevention of inflammasome effector IL-1β | [207] | |
| Glimepiride | Anti-amoebic | - | [188] |
| Repaglinide | Anti-amoebic | - | [188] |
| Glucagon-like Peptide-1 | In HIV treatment | Reduction of HIV-associated metabolic adverse effects | [212] |
| α-glucosidase inhibitors | Antibacterial | Targeting bacterial glucosidase | [220] |
| Antiviral and anti-COVID | Alter glycosylation in viral life cycle | [221] |
Insulin is a peptide hormone produced by β-cells of the pancreatic islets. It regulates glucose metabolism in all body cells[161]. Yano et al[162], showed the antibacterial activity of insulin on surgical site S. aureus infection via restoring neutrophil phagocytosis and bactericidal activity[162]. In 1946, Bollenback and Fox[163] showed the antibacterial activity of protamine zinc insulin against Lactobacillus arabinousus, S. aureus and E. coli. they owed the antibacterial activity to the additive protamine sulphate not to insulin itself[163]. Similar conclusion was derived from another study performed on commercial U.S.P. insulin, the bactericidal activity against Staphylococcus epidermidis, S. aureus and E. coli was secondary to the preservatives placed in the insulin and not to the insulin itself[164]. In general, most studies suggest that insulin doesn’t have a direct antimicrobial effect, rather an indirect antimicrobial effect can be expected due to the adjustment of hyperglycemia and relief in inflammation and oxidative stress. It is noteworthy to highlight the intrinsic anti-inflammatory nature of insulin as opposed to the inflammatory downfalls of hyperglycemia, additionally insulin promotes protein and lipid biosynthesis thus improving wound healing. Moreover, insulin induces the expression of the anti-inflammatory cytokines IL-4/IL-13, IL-10 and down regulates the pro-inflammatory cytokines IL-6 and IL-10[17,165]. However, research groups are invited to further investigate the insulin effects on microbial growth and virulence.
Metformin is a hypoglycemic drug used as first line treatment in T2DM. The hypoglycemic activity is owed to the suppression of hepatic glucose production, the reduced intestinal absorption of glucose and the increase in peripheral glucose uptake, however, the exact molecular mechanism of metformin is still the focus of active research[166]. Metformin also showed multiple beneficial effects that extend beyond diabetes control, with increasing studies referring to anti-inflammatory, cardio- and nephro-protective, anti-proliferative, antifibrotic and antioxidant effects. Moreover, metformin was suggested as an anti-aging compound with promises of increased lifespan and delayed onset of aging-associated diseases[167-169]. The repurposing of metformin extended to explore its antimicrobial activity, which also presented promising antimicrobial effects. A late study has shown the ability of metformin to restore tetracycline susceptibility in multidrug resistant strains of S. aureus, Enterococcus faecalis, E. coli, and Salmonella enteritidis both in vivo and in vitro. The study proposed the disruption of outer membrane permeability in resistant bacteria as a mechanism for reversing the bacterial intrinsic resistance to tetracyclines. Furthermore, the study reported that metformin imposed anti-inflammatory and improved innate immunity responses due to activation of the adenosine monophosphate-activated protein kinase (AMPK)-mediated phagocytosis and production of mitochondrial ROS (mROS)[170]. Metformin is associated with reduced serum levels of C reactive protein (CRP) and monocyte release of TNF-α, IL-1β, IL-6, MCP-1, and IL-8 in pre-diabetic patients[171]. Another study reported elevated bactericidal and anti-inflammatory outcomes upon combining metformin with photodynamic therapy for the treatment of chronic resistant periodontitis[172].
The antibacterial activity of metformin also attracted the attention of scientists as an adjuvant in tuberculosis (TB) treatment regimens[173]. Meta-analysis studies revealed reduced mortality rates and improved treatment outcomes in diabetic patients subjected to anti-TB regimen combined with metformin as anti-diabetic drug, also metformin administration was linked to reduced risk of TB disease among diabetics[174,175]. One suggested explanation was based on the fact that metformin is an inhibitor of mitochondrial complex-I which is analogous to mycobacterial NDH-I complex, hence giving the way for another mechanism of bactericidal activity of metformin[176]. Another study suggested that metformin had an immunomodulating effect by activating T regulatory and CD8 memory T cells responses activity leading to decreased pro-inflammatory responses which is reflected as reduction in lungs’ lesions[177]. In another study, metformin was observed to reduce TB bacilli load in lung epithelial cells in relation to increased production of β-defensin-2, -3 and -4 which restrain bacterial growth and multiplication[178]. Contrary to expected, metformin has an enrichment rather than inhibition effect on gut microbiota, shifting the balance towards more short chain fatty acids-producing bacteria which are reported to confer protection against inflammation, maintain intestinal barrier integrity and augment insulin production from b-cells due to stimulation of glucagon-like peptide 1 (GLP-1) secretion[179,180].
In recent studies, the anti-virulence effects of metformin have been extensively studied. Significantly, metformin mitigated the virulence of Pseudomonas aeruginosa in-vitro[19,181]. It reduced the production extracellular virulence enzymes such as protease, elastase and hemolysin and inhibited bacterial motility and biofilm formation. The anti-virulence activity of metformin was owed to its ability to downregulate the quorum sensing (Q.S) encoding genes[19]. Q.S is a process uses chemical language by which bacterial populations can communicate. This intercellular communication is performed through signaling molecules produced by bacterial cell called autoinducers that are detected by receptors on another cell. The Q.S signaling system controls various virulence factors and physiological functions in both Gram-positive and Gram-negative bacteria. Q.S targeting has been proposed as an effective strategy to cripple the bacterial virulence[18,182,183]. Despite the in vitro metformin success in mitigation of Q.S, it failed to confer the protection to mice from P. aeruginosa. The in vivo failure of metformin was owed to its chemical nature which changed by the change of pH during bacterial growth, these changes hinder the complete blocking of Q.S receptors by metformin molecule[19]. Taking in consideration that metformin was not tested in combination with other antibiotics and was used in sub-MIC concentrations (10 mg/mL) which can be increased to enhance its efficacy, we encourage research group for further investigation of anti-virulence effects of metformin.
Gliptins are oral hypoglycemic medications used for management of T2DM, they act by selective inhibition of DPP-IV leading to increased plasma GLP-1 and gastrointestinal insulinotropic peptide (GIP) levels, hence increased β-cell activity and suppression of glucagon secretion[184]. The alteration effects of gliptins on the composition of gut microbiota developed functional shifts in the microbiome, that improves the glucose homeostasis[185]. Interestingly, DPP-4 inhibitors are associated with reduced inflammatory effects in adipose tissue and pancreatic islets through reduced expression of inflammatory cytokines and adjusted macrophage activity[171]. Among the thirteen members of gliptins, sitagliptin has an attractive chemical structure that may antagonize the Q.S receptors, plus it is the most prescribed gliptin. Hegazy et al[186], investigated the sitagliptin effects on the virulence behavior of Serratia marcescens. Interestingly, sitagliptin showed a significant capability of quenching the bacterial virulence both in vitro and in vivo via significant downregulation of the virulence encoding genes[18,186]. These findings encouraged us to further investigate another gliptin member: Vildagliptin in comparison to sitagliptin on virulent Pseudomonas aeruginosa[18,181]. Despite the marked downregulation effect of both vildagliptin and sitagliptins on Q.S encoding genes, vildagliptin failed to attain significant inhibition of bacterial virulence in vitro and in vivo as compared to the effects of sitagliptin. Docking studies provided us with the satisfying explanation that sitagliptin structure offers better fitting onto Q.S receptors as compared to the weak association of vildagliptin on the same receptors. We hypothesized that the anti-virulence or anti-Q.S activity of sitagliptin is not only due to its down regulation of responsible genes, but also due to its ability to block the Q.S receptors[19].
The potent competitive inhibition of bacterial virulence determinants of other bacterial species by sitagliptin and other gliptins were demonstrated (unpublished data). In a separate work, saxagliptin, vildagliptin and sitgliptin decreased ex vivo the biofilm formation by dental caries causing odontopathogen Streptococcus mutans. As bacterial enzyme X-prolyl dipeptidyl peptidase (XPDAP) is analogous to mammalian enzyme DPP-IV, it was hypothesized that anti-protease activity of gliptins may affect XPDAP and bacterial growth[187]. In a separate prospect, vildagliptin reduced the numbers of viable Acanthamoeba castellanii that causes fatal granulomatous amoebic encephalitis. The amoebicidal activity of vildagliptin was significantly enhanced when formulated as vildagliptin-conjugated silver nanoparticles[188]. Surprisingly, some studies linked between reduction in COVID-19 mortality rates and treatment with sitagliptin[189]. Gliptins, especially sitagliptin, reduced the inflammation intensity and may control cytokine storms mostly via nuclear factor kappa B signaling pathway[190,191]. Nevertheless, cheminformatics suggested sitagliptin as anti-severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)[192] as a result of the expected potential molecular binding between sitagliptin and viral spikes[193]. Nar et al[194], showed the ineffectiveness of gliptins against SARS-CoV-2 protease[194]. An enzymatic assay was performed to measure the sitagliptin, linagliptin, alogliptin and saxagliptin inhibitory effects on catalytic activity of SARS-CoV-2 main protease Mpro, significantly tested gliptins were inactive. They owed this inactivity due to lack of apparent structural similarity between Mpro and DPP-IV[194]. Regardless of the controversy about the efficacy of gliptins as anti-COVID-19, more investigations are required to explore whether gliptins harbor anti-viral activity or not. That being said, we consider gliptins in general and sitagliptin in particular to be promising targets for drug repurposing as bacterial anti-virulence agents.
Thiazolidinediones (TZDs), also known as glitazones, are a group of oral hypoglycemic agents used in T2DM. Glitazones work by restoring insulin sensitivity through the selective activation of the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) which controls the transcription of genes regulating glucose and lipids metabolism[195]. Glitazones also exert anti-inflammatory effects through suppression of IL-6 and reduction in circulating CRP levels[196]. One study reported direct impact of TZDs via increasing phagocytosis by liver recruited macrophages, increased production of ROS in phagocytes and decreased serum pro-inflammatory cytokines (TNF-α, IL-12, IFN-γ)[197]. It was shown that pioglitazone has antibacterial activity against Streptococcus pneumoniae, E. coli and Klebsiella pneumoniae. Moreover, pretreatment of bacteria with a suboptimal concentration of pioglitazone enhanced the antibacterial activity of some antibiotics[198]. In another study, pioglitazone was used as an adjuvant to amphotericin B to ameliorate cryptococcosis in a murine model, that may indicate its promising application as an adjuvant for controlling fungal infection[199]. Interestingly, thiazolidinedione nucleus is present in several antimicrobial compounds, e.g., antibacterial, anti-mycobacterium, anti-malarial and antiviral, which was reviewed[200]. However, the mechanism of the antimicrobial activity is not fully understood since prokaryotes lack the PPAR-γ receptor which is the target site for glitazones, the antibacterial activity of glitazones may be owed to thiazolidinedione nucleus[197,200]. Although there are no deep studies on the antimicrobial or anti-virulence activities of glitazones, we predict that their antimicrobial activities are owed to thiazolidinedione nucleus.
Sulfonylureas antidiabetic drugs stimulate insulin secretion from the pancreatic β-cells, they bind to ATP-sensitive K-channels in the β-cell membrane, depolarizes the cells and open voltage-gated Ca2+ channels that results in insulin release. Sulfonylureas anti-diabetics, especially the second generation, are widely used in the management of T2DM[201]. Multiple antidiabetic sulfonylurea derivatives showed significant bactericidal[202,203] and fungicidal activities[203,204]. The first generation of sulfonylurea antidiabetic tolbutamide analogues were screened for their antibacterial and antifungal activities, they showed activity against S. aureus, E. coli, Pseudomonas aeruginosa, Bacillus subtilis and C. albicans[205]. Interestingly, Lowes et al[206], and others suggested repurposing the secondgeneration sulfonylurea anti-diabetics (glyburide, glisoxepide, gliquidone, and glimepiride) to treat fungal and bacterial infections[206-208]. They demonstrated the sulfonylurea anti-diabetics capability to inhibit activation of the NLRP3 inflammasome in various disease models such as vaginal candidiasis[206] and Burkholderia pseudomallei infection (melioidosis)[207]. Sulfonylureas were reported to decrease M1 macrophage activity and reduce IL-1β synthesis, pioglitazone is a direct PPAR-γ inhibitor that reduces adipose tissue inflammation[171]. It was supposed that sulfonylurea anti-diabetics prevent the release of major inflammasome effector IL-1β. Considerably, sulfonylurea anti-diabetics lack antimicrobial activity against C. albicans or Burkholderia pseudomallei and their anti-inflammatory activity was not specific to microbial infections, that means the possibility of repurposing these drugs against infectious and other immunopathological diseases[206,207]. Moreover, glimepiride was repurposed as amoebicidal agent, it decreased the numbers and encysts of Acanthamoeba castellanii[188].
Meglitinides mechanism of action closely resembles that of the sulfonylureas, they stimulate the insulin release from the pancreatic β-cells. Meglitinides are used orally and comprise nateglinide and repaglinide[209]. Unfortunately, there is a shortage in reports discussing the meglitinides' effects on both immune system and microbes. One study, the anti-amoebic activity of repaglinide was assessed against Acanthamoeba castellanii. It showed significant amoebicidal activity comparable to vildagliptin and glimepiride[188].
Gut enteroendocrine cells release GLP-1 to control the meal related hyperglycemia through inhibition of glucagon and enhancement of insulin secretions. GLP-1 receptor agonists or incretin mimetics such as exenatide, liraglutide and albiglutide are used for the treatment of T2DM[210]. Liraglutide can lead to wight loss by changing the overall composition of gut microbiota as well as the relative abundance of weight-relevant phylotypes[211]. Generally, the hypoglycemic effects of GLP-1 agonists improve the immune status in diabetic patients to counteract the microbial infections. The associated metabolic activities of these drugs may be helpful in the treatment of human immunodeficiency virus (HIV)-associated metabolic adverse effects[212], and PEGylated exendin-4 has the potential to treat sepsis[213].
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, represented by canagliflozin, empagliflozin and dapagliflozin, are hypoglycemic agents that work by increasing renal clearance of glucose through decreasing the renal tubular glucose reabsorption, hence reducing blood glucose level[214]. The interaction between SGLT-2 inhibitors and antibiotics is bidirectional, while the pharmacokinetic profile of SGLT-2 inhibitors may be influenced by co-administration of some antibiotics as rifampicin[215], they confer protection from gentamicin induced nephrotoxicity[216]. There are no reports documenting direct anti-microbial activities of SGLT-2 inhibitors. On the other hand, SGLT-2 inhibitors associated glucosuria increases the risk of urogenital infections especially in postmenopausal women with T2DM[217].
α-glucosidase inhibitors (AGI) reversibly bind to oligosaccharide binding sites of glucosidase enzymes, resulting in delaying the polysaccharide degradation to glucose, slowing down the food digestion and decrease blood glucose levels after meals[218]. Amylin analogs such as pramlintide affect glucose levels via several mechanisms, including slowed gastric emptying, regulation of postprandial glucagon, and reduction of food intake[219]. It has been hypothesized that the interaction capabilities of AGI to glucosidase enzymes may be beneficial in targeting bacterial glucosidase[220] and altering glycosylation in viral life cycle, showing anti-viral activity against HIV, HBV and COVID-19[221]. However, we shortage in reporting antibacterial or antiviral activities of used antidiabetic AGI like acarbose, several studies indicated the antibacterial, anti-biofilm[222] and antifungal[223] activities of other similar AGI.
The relationship between diabetes, immunity and infection is complicated and bidirectional in most cases. This fact is clearly presented in the tangled interactions between diabetes and immunity disorders where each can potentially contribute to the other in a kind of “the egg or the chicken” dilemma. On a parallel basis, antimicrobial and anti-diabetic agents showed a grey area of overlapping activities that should be subjected to further investigations. It would be of great value to submit such information into practical applications by refining the currently used anti-diabetic regimens to include the anti-diabetic agents which offer antimicrobial protection as an accessory benefit. The systematic application of this approach would minimize the wide margins of morbidity and mortality usually associated with diabetes, in addition to reduced treatment costs and overall better treatment outcomes. In this review, we tried to aggregate the available information which would support this approach in addition to highlighting the proved antimicrobial activities of multiple anti-diabetic agents. By putting this information in the hands of clinicians and researchers, more attention can be paid during selection of the treatment options in order to offer diabetic patients the best outcomes and help in better containment of the emerging statistics of the diabetes pandemic.
| 1. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2599] [Article Influence: 288.8] [Reference Citation Analysis (5)] |
| 2. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2121] [Article Influence: 117.8] [Reference Citation Analysis (2)] |
| 3. | Mattioli AV, Pinti M, Farinetti A, Nasi M. Obesity risk during collective quarantine for the COVID-19 epidemic. Obes Med. 2020;20:100263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Mediouni M, Madiouni R, Kaczor-Urbanowicz KE. COVID-19: How the quarantine could lead to the depreobesity. Obes Med. 2020;19:100255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Vas P, Hopkins D, Feher M, Rubino F, B Whyte M. Diabetes, obesity and COVID-19: A complex interplay. Diabetes Obes Metab. 2020;22:1892-1896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Santos A, Magro DO, Evangelista-Poderoso R, Saad MJA. Diabetes, obesity, and insulin resistance in COVID-19: molecular interrelationship and therapeutic implications. Diabetol Metab Syndr. 2021;13:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | de Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, Spagnuolo MI, Colamatteo A, Fusco C, Micillo T, Bruzzaniti S, Ceriello A, Puca AA, Matarese G. Type 2 Diabetes: How Much of an Autoimmune Disease? Front Endocrinol (Lausanne). 2019;10:451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1086] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 9. | Toniolo A, Cassani G, Puggioni A, Rossi A, Colombo A, Onodera T, Ferrannini E. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol. 2019;30:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 10. | Trevelin SC, Carlos D, Beretta M, da Silva JS, Cunha FQ. Diabetes Mellitus and Sepsis: A Challenging Association. Shock. 2017;47:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Dryden M, Baguneid M, Eckmann C, Corman S, Stephens J, Solem C, Li J, Charbonneau C, Baillon-Plot N, Haider S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21 Suppl 2:S27-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Siewko K, Maciulewski R, Zielinska-Maciulewska A, Poplawska-Kita A, Szumowski P, Wawrusiewicz-Kurylonek N, Lipinska D, Milewski R, Gorska M, Kretowski A, Szelachowska M. Interleukin-6 and Interleukin-15 as Possible Biomarkers of the Risk of Autoimmune Diabetes Development. Biomed Res Int. 2019;2019:4734063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sioofy-Khojine AB, Lehtonen J, Nurminen N, Laitinen OH, Oikarinen S, Huhtala H, Pakkanen O, Ruokoranta T, Hankaniemi MM, Toppari J, Vähä-Mäkilä M, Ilonen J, Veijola R, Knip M, Hyöty H. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia. 2018;61:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 699] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 16. | Zheng P, Li Z, Zhou Z. Gut microbiome in type 1 diabetes: A comprehensive review. Diabetes Metab Res Rev. 2018;34:e3043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Kaur P, Choudhury D. Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics. Biomol Concepts. 2019;10:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Abbas HA, Hegazy WAH. Repurposing anti-diabetic drug "Sitagliptin" as a novel virulence attenuating agent in Serratia marcescens. PLoS One. 2020;15:e0231625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Hegazy WAH, Khayat MT, Ibrahim TS, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 20. | Khayyat AN, Hegazy WAH, Shaldam MA, Mosbah R, Almalki AJ, Ibrahim TS, Khayat MT, Khafagy ES, Soliman WE, Abbas HA. Xylitol Inhibits Growth and Blocks Virulence in Serratia marcescens. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 21. | Hegazy WAH, Abbas HA. Evaluation of the role of SsaV ‘Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr J Biotechnol. 2017;718. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 474] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 23. | Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 24. | Roep BO, Peakman M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb Perspect Med. 2012;2:a007781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes. 2015;6:380-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 26. | Aldawsari MF, Alalaiwe A, Khafagy ES, Al Saqr A, Alshahrani SM, Alsulays BB, Alshehri S, Abu Lila AS, Danish Rizvi SM, Hegazy WAH. Efficacy of SPG-ODN 1826 Nanovehicles in Inducing M1 Phenotype through TLR-9 Activation in Murine Alveolar J774A.1 Cells: Plausible Nano-Immunotherapy for Lung Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Saberzadeh-Ardestani B, Karamzadeh R, Basiri M, Hajizadeh-Saffar E, Farhadi A, Shapiro AMJ, Tahamtani Y, Baharvand H. Type 1 Diabetes Mellitus: Cellular and Molecular Pathophysiology at A Glance. Cell J. 2018;20:294-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 28. | Shimobayashi M, Albert V, Woelnerhanssen B, Frei IC, Weissenberger D, Meyer-Gerspach AC, Clement N, Moes S, Colombi M, Meier JA, Swierczynska MM, Jenö P, Beglinger C, Peterli R, Hall MN. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128:1538-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 359] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 29. | Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflam. 2011;2011:529061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 31. | Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol. 2019;10:1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 768] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 32. | Kolb H, Martin S. Environmental/Lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (1)] |
| 33. | Al-Sinani S, Woodhouse N, Al-Mamari A, Al-Shafie O, Al-Shafaee M, Al-Yahyaee S, Hassan M, Jaju D, Al-Hashmi K, Al-Abri M, Al-Rassadi K, Rizvi S, Loic Y, Froguel P, Bayoumi R. Association of gene variants with susceptibility to type 2 diabetes among Omanis. World J Diabetes. 2015;6:358-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Pereira SS, Alvarez-Leite JI. Low-Grade Inflammation, Obesity, and Diabetes. Curr Obes Rep. 2014;3:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 596] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 36. | Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol. 2018;104:525-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 37. | Al Saqr A, Khafagy ES, Alalaiwe A, Aldawsari MF, Alshahrani SM, Anwer MK, Khan S, Lila ASA, Arab HH, Hegazy WAH. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Palmer JP, Hampe CS, Chiu H, Goel A, Brooks-Worrell BM. Is latent autoimmune diabetes in adults distinct from type 1 diabetes or just type 1 diabetes at an older age? Diabetes. 2005;54 Suppl 2:S62-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2239] [Article Influence: 248.8] [Reference Citation Analysis (8)] |
| 40. | Aldawsari MF, Khafagy ES, Saqr AA, Alalaiwe A, Abbas HA, Shaldam MA, Hegazy WAH, Goda RM. Tackling Virulence of Pseudomonas aeruginosa by the Natural Furanone Sotolon. Antibiotics (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1250] [Article Influence: 138.9] [Reference Citation Analysis (2)] |
| 42. | Matijašić M, Meštrović T, Paljetak HČ, Perić M, Barešić A, Verbanac D. Gut Microbiota beyond Bacteria-Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 43. | Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (4)] |
| 44. | Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front Microbiol. 2018;9:2013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 45. | Hampton-Marcell JT, Lopez JV, Gilbert JA. The human microbiome: an emerging tool in forensics. Microb Biotechnol. 2017;10:228-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 46. | Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079-16094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 342] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (6)] |
| 47. | Rapozo DC, Bernardazzi C, de Souza HS. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J Gastroenterol. 2017;23:2124-2140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (3)] |
| 48. | Zuo T, Ng SC. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front Microbiol. 2018;9:2247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 429] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 49. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1386] [Cited by in RCA: 1187] [Article Influence: 197.8] [Reference Citation Analysis (1)] |
| 50. | Ganesan K, Chung SK, Vanamala J, Xu B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 51. | Ma S, You Y, Huang L, Long S, Zhang J, Guo C, Zhang N, Wu X, Xiao Y, Tan H. Alterations in Gut Microbiota of Gestational Diabetes Patients During the First Trimester of Pregnancy. Front Cell Infect Microbiol. 2020;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 52. | Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuño MI. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 574] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 53. | Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 54. | Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 616] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 55. | Fuke N, Nagata N, Suganuma H, Ota T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (1)] |
| 56. | Gérard C, Vidal H. Impact of Gut Microbiota on Host Glycemic Control. Front Endocrinol (Lausanne). 2019;10:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Liu H, Hu C, Zhang X, Jia W. Role of gut microbiota, bile acids and their cross-talk in the effects of bariatric surgery on obesity and type 2 diabetes. J Diabetes Investig. 2018;9:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 58. | Dahiya DK, Renuka, Puniya M, Shandilya UK, Dhewa T, Kumar N, Kumar S, Puniya AK, Shukla P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front Microbiol. 2017;8:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 59. | Harsch IA, Konturek PC. The Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabetes Mellitus: New Insights into "Old" Diseases. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 60. | Bergamin CS, Dib SA. Enterovirus and type 1 diabetes: What is the matter? World J Diabetes. 2015;6:828-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57:2863-2871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 63. | Kim KW, Horton JL, Pang CNI, Jain K, Leung P, Isaacs SR, Bull RA, Luciani F, Wilkins MR, Catteau J, Lipkin WI, Rawlinson WD, Briese T, Craig ME. Higher abundance of enterovirus A species in the gut of children with islet autoimmunity. Sci Rep. 2019;9:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus--why the β cells? Nat Rev Endocrinol. 2016;12:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 65. | Petzold A, Solimena M, Knoch KP. Mechanisms of Beta Cell Dysfunction Associated With Viral Infection. Curr Diab Rep. 2015;15:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Rodriguez-Calvo T. Enterovirus infection and type 1 diabetes: unraveling the crime scene. Clin Exp Immunol. 2019;195:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Bose SK, Ray R. Hepatitis C virus infection and insulin resistance. World J Diabetes. 2014;5:52-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Desbois AC, Cacoub P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. World J Gastroenterol. 2017;23:1697-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 70. | Brault C, Levy PL, Bartosch B. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses. 2013;5:954-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Hum J, Jou JH. The link between hepatitis C virus and diabetes mellitus: Improvement in insulin resistance after eradication of hepatitis C virus. Clin Liver Dis (Hoboken). 2018;11:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 198] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (15)] |
| 73. | Jia Y, Qu B, Wang Z, Han X, Ren G. Effects of active and latent H. pylori infection coupled with chronic alcohol ingestion on cytokine profiles and markers of oxidative balance in men seropositive for H. pylori CagA Ab: An observational study. Medicine (Baltimore). 2018;97:e11991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 74. | Mladenova I. Helicobacter pylori and cardiovascular disease: update 2019. Minerva Cardioangiol. 2019;67:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Kivrak Salim D, Sahin M, Köksoy S, Adanir H, Süleymanlar I. Local Immune Response in Helicobacter pylori Infection. Medicine (Baltimore). 2016;95:e3713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Jamkhande PG, Gattani SG, Farhat SA. Helicobacter pylori and cardiovascular complications: a mechanism based review on role of Helicobacter pylori in cardiovascular diseases. Integr Med Res. 2016;5:244-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Chmiela M, Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J Gastroenterol. 2017;23:3964-3977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 78. | Hasni SA. Role of Helicobacter pylori infection in autoimmune diseases. Curr Opin Rheumatol. 2012;24:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (7)] |
| 80. | Li JZ, Li JY, Wu TF, Xu JH, Huang CZ, Cheng D, Chen QK, Yu T. Helicobacter pylori Infection Is Associated with Type 2 Diabetes, Not Type 1 Diabetes: An Updated Meta-Analysis. Gastroenterol Res Pract. 2017;2017:5715403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 81. | Mansori K, Moradi Y, Naderpour S, Rashti R, Moghaddam AB, Saed L, Mohammadi H. Helicobacter pylori infection as a risk factor for diabetes: a meta-analysis of case-control studies. BMC Gastroenterol. 2020;20:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 82. | He C, Yang Z, Lu NH. Helicobacter pylori infection and diabetes: is it a myth or fact? World J Gastroenterol. 2014;20:4607-4617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Hosseininasab Nodoushan SA, Nabavi A. The Interaction of Helicobacter pylori Infection and Type 2 Diabetes Mellitus. Adv Biomed Res. 2019;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Aydemir S, Bayraktaroglu T, Sert M, Sokmen C, Atmaca H, Mungan G, Gun BD, Borazan A, Ustundag Y. The effect of Helicobacter pylori on insulin resistance. Dig Dis Sci. 2005;50:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Polyzos SA, Kountouras J, Zavos C, Deretzi G. The association between Helicobacter pylori infection and insulin resistance: a systematic review. Helicobacter. 2011;16:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 86. | Chen LW, Chien CY, Yang KJ, Kuo SF, Chen CH, Chien RN. Helicobacter pylori Infection Increases Insulin Resistance and Metabolic Syndrome in Residents Younger than 50 Years Old: A Community-Based Study. PLoS One. 2015;10:e0128671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 87. | Kachuei A, Amini M, Sebghatollahi V, Feizi A, Hamedani P, Iraj B. Effect of Helicobacter pylori eradication on insulin resistance among prediabetic patients: A pilot study and single-blind randomized controlled clinical trial. J Res Med Sci. 2016;21:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol. 2005;11:1644-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Jeffery PL, McGuckin MA, Linden SK. Endocrine impact of Helicobacter pylori: focus on ghrelin and ghrelin o-acyltransferase. World J Gastroenterol. 2011;17:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Chabot F, Caron A, Laplante M, St-Pierre DH. Interrelationships between ghrelin, insulin and glucose homeostasis: Physiological relevance. World J Diabetes. 2014;5:328-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 91. | Zhang CS, Wang LX, Wang R, Liu Y, Song LM, Yuan JH, Wang B, Dong J. The Correlation Between Circulating Ghrelin and Insulin Resistance in Obesity: A Meta-Analysis. Front Physiol. 2018;9:1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 92. | Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Prioreschi A, Munthali RJ, Soepnel L, Goldstein JA, Micklesfield LK, Aronoff DM, Norris SA. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open. 2017;7:e013953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 94. | Lee MR, Huang YP, Kuo YT, Luo CH, Shih YJ, Shu CC, Wang JY, Ko JC, Yu CJ, Lin HH. Diabetes Mellitus and Latent Tuberculosis Infection: A Systematic Review and Metaanalysis. Clin Infect Dis. 2017;64:719-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 95. | McKane CK, Marmarelis M, Mendu ML, Moromizato T, Gibbons FK, Christopher KB. Diabetes mellitus and community-acquired bloodstream infections in the critically ill. J Crit Care. 2014;29:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 96. | Wang Z, Ren J, Wang G, Liu Q, Guo K, Li J. Association Between Diabetes Mellitus and Outcomes of Patients with Sepsis: A Meta-Analysis. Med Sci Monit. 2017;23:3546-3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | Taganna J, de Boer AR, Wuhrer M, Bouckaert J. Glycosylation changes as important factors for the susceptibility to urinary tract infection. Biochem Soc Trans. 2011;39:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Xiao E, Mattos M, Vieira GHA, Chen S, Corrêa JD, Wu Y, Albiero ML, Bittinger K, Graves DT. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe. 2017;22:120-128.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 287] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 99. | Garnett JP, Baker EH, Naik S, Lindsay JA, Knight GM, Gill S, Tregoning JS, Baines DL. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax. 2013;68:835-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 100. | Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications. 2012;26:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 101. | Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, Usiskin K. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 102. | Zhang XL, Zhu QQ, Chen YH, Li XL, Chen F, Huang JA, Xu B. Cardiovascular Safety, Long-Term Noncardiovascular Safety, and Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis With Trial Sequential Analysis. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 103. | Mikamo H, Yamagishi Y, Sugiyama H, Sadakata H, Miyazaki S, Sano T, Tomita T. High glucose-mediated overexpression of ICAM-1 in human vaginal epithelial cells increases adhesion of Candida albicans. J Obstet Gynaecol. 2018;38:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, Laffitte E. Cutaneous Granulomatosis: a Comprehensive Review. Clin Rev Allergy Immunol. 2018;54:131-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 105. | CEYHAN AM, YILDIRIM M, BASAK PY, AKKAYA VB. Unusual multifocal cutaneous leishmaniasis in a diabetic patient. Eur J Dermatol. 2009;19:514-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Papagianni M, Metallidis S, Tziomalos K. Herpes Zoster and Diabetes Mellitus: A Review. Diabetes Ther. 2018;9:545-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 107. | Chen HH, Lin IC, Chen HJ, Yeh SY, Kao CH. Association of Herpes Zoster and Type 1 Diabetes Mellitus. PLoS One. 2016;11:e0155175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Hegazy WAH, Henawy MA. Hepatitis C virus pathogenesis: Serum IL-33 Level indicates liver damage. Afr J Micro Res. 2015;1386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 110. | Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 111. | Rao S, Rahim M, Iqbal K, Haroon F, Hasan Z. Impact of diabetes on mechanisms of immunity against Mycobacterium tuberculosis. J Pak Med Assoc. 2019;69:94-98. [PubMed] |
| 112. | Kajiwara C, Kusaka Y, Kimura S, Yamaguchi T, Nanjo Y, Ishii Y, Udono H, Standiford TJ, Tateda K. Metformin Mediates Protection against Legionella Pneumonia through Activation of AMPK and Mitochondrial Reactive Oxygen Species. J Immunol. 2018;200:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 113. | Wallet SM, Puri V, Gibson FC. Linkage of Infection to Adverse Systemic Complications: Periodontal Disease, Toll-Like Receptors, and Other Pattern Recognition Systems. Vaccines (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | Roilides E, Kontoyiannis DP, Walsh TJ. Host defenses against zygomycetes. Clin Infect Dis. 2012;54 Suppl 1:S61-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Baig Mirza AM, Fida M, Murtaza G, Niazi R, Hanif A, Irfan K, Masud F. Association of metabolic factors with dengue viral infection on admission triage which predict its clinical course during Lahore dengue epidemic. J Pak Med Assoc. 2016;66:1102-1106. [PubMed] |
| 116. | Reading PC, Allison J, Crouch EC, Anders EM. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defense of the lung by glucose? J Virol. 1998;72:6884-6887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Muñoz-Quiles C, López-Lacort M, Ampudia-Blasco FJ, Díez-Domingo J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009-2014. Hum Vaccin Immunother. 2017;13:2606-2611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Kawai K, Yawn BP. Risk Factors for Herpes Zoster: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2017;92:1806-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 119. | van Crevel R, van de Vijver S, Moore DAJ. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2017;5:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 120. | Hegazy WAH. Diclofenac inhibits virulence of Proteus mirabilis isolated from diabetic foot ulcer. Afr J Micro Res. 2016;733. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 121. | Gan YH. Host susceptibility factors to bacterial infections in type 2 diabetes. PLoS Pathog. 2013;9:e1003794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Vardakas KZ, Horianopoulou M, Falagas ME. Factors associated with treatment failure in patients with diabetic foot infections: An analysis of data from randomized controlled trials. Diabetes Res Clin Pract. 2008;80:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, Jarlier V, Nathwani D, Goossens H; Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6:e619-e629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 465] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 124. | Bongiorno D, Mongelli G, Stefani S, Campanile F. Burden of Rifampicin- and Methicillin-Resistant Staphylococcus aureus in Italy. Microb Drug Resist. 2018;24:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Abd El-Hamid MI, Y El-Naenaeey ES, M Kandeel T, Hegazy WAH, Mosbah RA, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 126. | Bendary MM, Ibrahim D, Mosbah RA, Mosallam F, Hegazy WAH, Awad NFS, Alshareef WA, Alomar SY, Zaitone SA, Abd El-Hamid MI. Thymol Nanoemulsion: A New Therapeutic Option for Extensively Drug Resistant Foodborne Pathogens. Antibiotics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 127. | Askoura M, Hegazy WAH. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog Dis. 2020;78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 128. | Askoura M, Youns M, Halim Hegazy WA. Investigating the influence of iron on Campylobacter jejuni transcriptome in response to acid stress. Microb Pathog. 2020;138:103777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27-S36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 539] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 130. | Horikawa C, Kodama S, Fujihara K, Hirasawa R, Yachi Y, Suzuki A, Hanyu O, Shimano H, Sone H. High risk of failing eradication of Helicobacter pylori in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2014;106:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Te Brake LHM, Yunivita V, Livia R, Soetedjo N, van Ewijk-Beneken Kolmer E, Koenderink JB, Burger DM, Santoso P, van Crevel R, Alisjahbana B, Aarnoutse RE, Ruslami R; TANDEM Consortium. Rifampicin Alters Metformin Plasma Exposure but Not Blood Glucose Levels in Diabetic Tuberculosis Patients. Clin Pharmacol Ther. 2019;105:730-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Khamaisi M, Leitersdorf E. Severe hypoglycemia from clarithromycin-repaglinide drug interaction. Pharmacotherapy. 2008;28:682-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 133. | Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 134. | Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med. 2014;174:1605-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 135. | Fujisaka S, Ussar S, Clish C, Devkota S, Dreyfuss JM, Sakaguchi M, Soto M, Konishi M, Softic S, Altindis E, Li N, Gerber G, Bry L, Kahn CR. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest. 2016;126:4430-4443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 136. | Cabral DJ, Penumutchu S, Reinhart EM, Zhang C, Korry BJ, Wurster JI, Nilson R, Guang A, Sano WH, Rowan-Nash AD, Li H, Belenky P. Microbial Metabolism Modulates Antibiotic Susceptibility within the Murine Gut Microbiome. Cell Metab. 2019;30:800-823.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 137. | Youns M, Askoura M, Abbas HA, Attia GH, Khayyat AN, Goda RM, Almalki AJ, Khafagy ES, Hegazy WAH. Celastrol Modulates Multiple Signaling Pathways to Inhibit Proliferation of Pancreatic Cancer via DDIT3 and ATF3 Up-Regulation and RRM2 and MCM4 Down-Regulation. Onco Targets Ther. 2021;14:3849-3860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 138. | Supuran CT. Special Issue: Sulfonamides. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, Corlianò F, Fra GP, Bartoli E, Derosa G. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 140. | White JR Jr. A Brief History of the Development of Diabetes Medications. Diabetes Spectr. 2014;27:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 141. | Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 142. | Rossio R, Arcudi S, Peyvandi F, Piconi S. Persistent and severe hypoglycemia associated with trimethoprim-sulfamethoxazole in a frail diabetic man on polypharmacy: A case report and literature review . Int J Clin Pharmacol Ther. 2018;56:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 143. | Roustit M, Blondel E, Villier C, Fonrose X, Mallaret MP. Symptomatic hypoglycemia associated with trimethoprim/sulfamethoxazole and repaglinide in a diabetic patient. Ann Pharmacother. 2010;44:764-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Marchant J. When antibiotics turn toxic. Nature. 2018;555:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 145. | Vishwa B, Moin A, Gowda DV, Rizvi SMD, Hegazy WAH, Abu Lila AS, Khafagy ES, Allam AN. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 146. | Saraya A, Yokokura M, Gonoi T, Seino S. Effects of fluoroquinolones on insulin secretion and beta-cell ATP-sensitive K+ channels. Eur J Pharmacol. 2004;497:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 147. | Aspinall SL, Good CB, Jiang R, McCarren M, Dong D, Cunningham FE. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 148. | Dirim AB, Demir E, Safak S, Garayeva N, Ucar AR, Oto OA, Yazici H, Medetalibeyoglu A, Kose M, Esen F, Simsek Yavuz S, Turkmen A. Hydroxychloroquine-Associated Hypoglycemia in Hemodialysis Patients With COVID-19. Kidney Int Rep. 2020;5:1811-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 149. | Hage MP, Al-Badri MR, Azar ST. A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther Adv Endocrinol Metab. 2014;5:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 150. | Infante M, Ricordi C, Fabbri A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: Risks and benefits at the time of COVID-19 pandemic. J Diabetes. 2020;12:659-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 151. | Phadatare PD, Chandrashekhar VM. Influence of esomeprazole on hypoglycemic activity of oral antidiabetic agents in rats and rabbits. Mol Cell Biochem. 2011;354:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 152. | Ostrovskaya RU, Ivanov SV, Voronin MV, Ozerova IV, Zolotov NN, Seredenin SB. Antidiabetic Activity of Afobazole in Wistar Rats. Bull Exp Biol Med. 2018;165:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 153. | Tallón de Lara P, Himschoot T, Frossard JL, Negro F. Does telaprevir possess a direct antidiabetic effect? Liver Int. 2014;34:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 154. | Doyle MA, Cooper C. Successful Hepatitis C Antiviral Therapy Induces Remission of Type 2 Diabetes: A Case Report. Am J Case Rep. 2015;16:745-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Davis TME, Davis WA, Jeffrey G. Successful Withdrawal of Insulin Therapy After Post-Treatment Clearance of Hepatitis C Virus in a Man with Type 2 Diabetes. Am J Case Rep. 2017;18:414-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 156. | Abdel Alem S, Elsharkawy A, Fouad R, Adel E, Abdellatif Z, Musa S, Nagy A, Hussein MS, Yosry A, Esmat G. Improvement of glycemic state among responders to Sofosbuvir-based treatment regimens: Single center experience. J Med Virol. 2017;89:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 157. | Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin Pharmacokinet. 2003;42:819-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 158. | Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 3022] [Article Influence: 377.8] [Reference Citation Analysis (0)] |
| 159. | Khayyat AN, Abbas HA, Mohamed MFA, Asfour HZ, Khayat MT, Ibrahim TS, Youns M, Khafagy E-S, Abu Lila AS, Safo MK, Hegazy WAH. Not Only Antimicrobial: Metronidazole Mitigates the Virulence of Proteus mirabilis Isolated from Macerated Diabetic Foot Ulcer. App Sci. 2021;6847. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 160. | Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care. 2016;39 Suppl 2:S244-S252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 161. | Thevis M, Thomas A, Schänzer W. Insulin. Handb Exp Pharmacol. 2010;209-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 162. | Yano H, Kinoshita M, Fujino K, Nakashima M, Yamamoto Y, Miyazaki H, Hamada K, Ono S, Iwaya K, Saitoh D, Seki S, Tanaka Y. Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect Immun. 2012;80:4409-4416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 163. | Bollenback CH, Fox SW. The Antibacterial Activity of Protamine Zinc Insulin. Science. 1946;103:445-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 164. | Schade DS, Eaton RP. Bactericidal properties of commercial U.S.P. formulated insulin. Diabetes. 1982;31:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 165. | Sun M, Zheng S, Gao X, Lin Z. The role of immune cells in obesogenic memory. Cell Mol Immunol. 2020;17:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 166. | Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 167. | Kulkarni AS, Gubbi S, Barzilai N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020;32:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 534] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 168. | Ursini F, Russo E, Pellino G, D'Angelo S, Chiaravalloti A, De Sarro G, Manfredini R, De Giorgio R. Metformin and Autoimmunity: A "New Deal" of an Old Drug. Front Immunol. 2018;9:1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 169. | Allam AN, Mehanna MM. Formulation, physicochemical characterization and in-vivo evaluation of ion-sensitive metformin loaded-biopolymeric beads. Drug Dev Ind Pharm. 2016;42:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 170. | Liu Y, Jia Y, Yang K, Li R, Xiao X, Zhu K, Wang Z. Metformin Restores Tetracyclines Susceptibility against Multidrug Resistant Bacteria. Adv Sci (Weinh). 2020;7:1902227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 171. | Kuryłowicz A, Koźniewski K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 172. | Afrasiabi S, Pourhajibagher M, Bahador A. The Photomodulation Activity of Metformin Against Oral Microbiome. J Lasers Med Sci. 2019;10:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 173. | Oglesby W, Kara AM, Granados H, Cervantes JL. Metformin in tuberculosis: beyond control of hyperglycemia. Infection. 2019;47:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 174. | Yu X, Li L, Xia L, Feng X, Chen F, Cao S, Wei X. Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: a systematic review. BMC Infect Dis. 2019;19:859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 175. | Zhang M, He JQ. Impacts of metformin on tuberculosis incidence and clinical outcomes in patients with diabetes: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 176. | Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front-line antibiotics for Tuberculosis. J Transl Med. 2015;13:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 177. | Frenkel JDH, Ackart DF, Todd AK, DiLisio JE, Hoffman S, Tanner S, Kiran D, Murray M, Chicco A, Obregón-Henao A, Podell BK, Basaraba RJ. Metformin enhances protection in guinea pigs chronically infected with Mycobacterium tuberculosis. Sci Rep. 2020;10:16257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 178. | Rodriguez-Carlos A, Valdez-Miramontes C, Marin-Luevano P, González-Curiel I, Enciso-Moreno JA, Rivas-Santiago B. Metformin promotes Mycobacterium tuberculosis killing and increases the production of human β-defensins in lung epithelial cells and macrophages. Microbes Infect. 2020;22:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 179. | Montandon SA, Jornayvaz FR. Effects of Antidiabetic Drugs on Gut Microbiota Composition. Genes (Basel). 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 180. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1908] [Article Influence: 318.0] [Reference Citation Analysis (2)] |
| 181. | Abbas HA, Elsherbini AM, Shaldam MA. Repurposing metformin as a quorum sensing inhibitor in Pseudomonas aeruginosa. Afr Health Sci. 2017;17:808-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 182. | Abbas HA, Hegazy WAH. Targeting the virulence factors of serratia marcescens by ambroxol. Roum Arch Microbiol Immunol. 2017;27. |
| 183. | Jiang Q, Chen J, Yang C, Yin Y, Yao K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed Res Int. 2019;2019:2015978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 184. | Solis-Herrera C, Triplitt C, Garduno-Garcia Jde J, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 185. | Liao X, Song L, Zeng B, Liu B, Qiu Y, Qu H, Zheng Y, Long M, Zhou H, Wang Y, Du Y, Xu J, Shen R, Tong Q, Cai L, Li X, Guo S, Yang G, Zhu Z, Pu X, Wei H, Zheng H. Alteration of gut microbiota induced by DPP-4i treatment improves glucose homeostasis. EBioMedicine. 2019;44:665-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 186. | Hegazy WAH, Khayat MT, Ibrahim TS, Youns M, Mosbah R, Soliman WE. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz J Microbiol. 2021;52:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 187. | De A, Pompilio A, Francis J, Sutcliffe IC, Black GW, Lupidi G, Petrelli D, Vitali LA. Antidiabetic "gliptins" affect biofilm formation by Streptococcus mutans. Microbiol Res. 2018;209:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 188. | Anwar A, Siddiqui R, Raza Shah M, Khan NA. Antidiabetic Drugs and Their Nanoconjugates Repurposed as Novel Antimicrobial Agents against Acanthamoeba castellanii. J Microbiol Biotechnol. 2019;29:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 189. | Solerte SB, D'Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, Dell'Acqua M, Ippolito E, Scaranna C, Bellante R, Galliani S, Dodesini AR, Lepore G, Geni F, Fiorina RM, Catena E, Corsico A, Colombo R, Mirani M, De Riva C, Oleandri SE, Abdi R, Bonventre JV, Rusconi S, Folli F, Di Sabatino A, Zuccotti G, Galli M, Fiorina P. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care. 2020;43:2999-3006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 190. | Mozafari N, Azadi S, Mehdi-Alamdarlou S, Ashrafi H, Azadi A. Inflammation: A bridge between diabetes and COVID-19, and possible management with sitagliptin. Med Hypotheses. 2020;143:110111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 191. | Dastan F, Abedini A, Shahabi S, Kiani A, Saffaei A, Zare A. Sitagliptin Repositioning in SARS-CoV-2: Effects on ACE-2, CD-26, and Inflammatory Cytokine Storms in the Lung. Iran J Allergy Asthma Immunol. 2020;19:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 192. | Kumar A, Loharch S, Kumar S, Ringe RP, Parkesh R. Exploiting cheminformatic and machine learning to navigate the available chemical space of potential small molecule inhibitors of SARS-CoV-2. Comput Struct Biotechnol J. 2021;19:424-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 193. | Bardaweel SK, Hajjo R, Sabbah DA. Sitagliptin: a potential drug for the treatment of COVID-19? Acta Pharm. 2021;71:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 194. | Nar H, Schnapp G, Hucke O, Hardman TC, Klein T. Action of Dipeptidyl Peptidase-4 Inhibitors on SARS-CoV-2 Main Protease. ChemMedChem. 2021;16:1425-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 195. | Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001;13-18. [PubMed] |
| 196. | Katsiki N, Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: A "promised land" in the COVID-19 era? J Diabetes Complications. 2020;34:107723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 197. | Nakashima M, Kinoshita M, Nakashima H, Kotani A, Ishikiriyama T, Kato S, Hiroi S, Seki S. Pioglitazone improves phagocytic activity of liver recruited macrophages in elderly mice possibly by promoting glucose catabolism. Innate Immun. 2019;25:356-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 198. | Masadeh MM, Mhaidat NM, Al-Azzam SI, Alzoubi KH. Investigation of the antibacterial activity of pioglitazone. Drug Des Devel Ther. 2011;5:421-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 199. | Ribeiro NQ, Santos APN, Emídio ECP, Costa MC, Freitas GJC, Carmo PHF, Silva MF, de Brito CB, de Souza DG, Paixão TA, Santos DA. Pioglitazone as an adjuvant of amphotericin B for the treatment of cryptococcosis. Int J Antimicrob Agents. 2019;54:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 200. | Sucheta, Tahlan S, Verma PK. Biological potential of thiazolidinedione derivatives of synthetic origin. Chem Cent J. 2017;11:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 201. | Lv W, Wang X, Xu Q, Lu W. Mechanisms and Characteristics of Sulfonylureas and Glinides. Curr Top Med Chem. 2020;20:37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 202. | Krajacić MB, Kujundzić N, Dumić M, Cindrić M, Brajsa K, Metelko B, Novak P. Synthesis, characterization and in vitro antimicrobial activity of novel sulfonylureas of 15-membered azalides. J Antibiot (Tokyo). 2005;58:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 203. | Kreisberg JF, Ong NT, Krishna A, Joseph TL, Wang J, Ong C, Ooi HA, Sung JC, Siew CC, Chang GC, Biot F, Cuccui J, Wren BW, Chan J, Sivalingam SP, Zhang LH, Verma C, Tan P. Growth inhibition of pathogenic bacteria by sulfonylurea herbicides. Antimicrob Agents Chemother. 2013;57:1513-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 204. | Lee YT, Cui CJ, Chow EW, Pue N, Lonhienne T, Wang JG, Fraser JA, Guddat LW. Sulfonylureas have antifungal activity and are potent inhibitors of Candida albicans acetohydroxyacid synthase. J Med Chem. 2013;56:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 205. | Ramudu DBJ, Babu PH, Venkateswarlu N, Vijaya T, Rasheed S, Raju CN, Chalapathi PV. Sulfonylurea derivatives of tolbutamide analogues: Synthesis and evaluation of antimicrobial and antioxidant activities. Indian J Chem. 2018;127. |
| 206. | Lowes DJ, Hevener KE, Peters BM. Second-Generation Antidiabetic Sulfonylureas Inhibit Candida albicans and Candidalysin-Mediated Activation of the NLRP3 Inflammasome. Antimicrob Agents Chemother. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 207. | Koh GC, Weehuizen TA, Breitbach K, Krause K, de Jong HK, Kager LM, Hoogendijk AJ, Bast A, Peacock SJ, van der Poll T, Steinmetz I, Wiersinga WJ. Glyburide reduces bacterial dissemination in a mouse model of melioidosis. PLoS Negl Trop Dis. 2013;7:e2500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 208. | Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W. A Protective Role of Glibenclamide in Inflammation-Associated Injury. Mediators Inflamm. 2017;2017:3578702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 209. | Grant JS, Graven LJ. Progressing From Metformin to Sulfonylureas or Meglitinides. Workplace Health Saf. 2016;64:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 210. | Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27:740-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 1318] [Article Influence: 164.8] [Reference Citation Analysis (1)] |
| 211. | Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, Liberti EA, Saad M, Carvalho C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 212. | Culha MG, Inkaya AC, Yildirim E, Unal S, Serefoglu EC. Glucagon like peptide-1 receptor agonists may ameliorate the metabolic adverse effect associated with antiretroviral therapy. Med Hypotheses. 2016;94:151-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 213. | Lee W, Park EJ, Kwak S, Lee KC, Na DH, Bae JS. Trimeric PEG-Conjugated Exendin-4 for the Treatment of Sepsis. Biomacromolecules. 2016;17:1160-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 214. | Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 1048] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 215. | Devineni D, Vaccaro N, Murphy J, Curtin C, Mamidi RN, Weiner S, Wang SS, Ariyawansa J, Stieltjes H, Wajs E, Di Prospero NA, Rothenberg P. Effects of rifampin, cyclosporine A, and probenecid on the pharmacokinetic profile of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in healthy participants. Int J Clin Pharmacol Ther. 2015;53:115-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 216. | Mohamed DI, Khairy E, Saad SST, Habib EK, Hamouda MA. Potential protective effects of Dapagliflozin in gentamicin induced nephrotoxicity rat model via modulation of apoptosis associated miRNAs. Gene. 2019;707:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 217. | Marques LPJ, Mendonça NA, Müller L, André ACPD, Madeira EPQ, Vieira LMSF. Impact of sodium-glucose cotransporter-2 inhibitors-induced glucosuria in the incidence of urogenital infection on postmenopausal women with diabetes. Postgrad Med. 2020;132:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 218. | Teng H, Chen L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit Rev Food Sci Nutr. 2017;57:3438-3448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 219. | Ling W, Huang YM, Qiao YC, Zhang XX, Zhao HL. Human Amylin: From Pathology to Physiology and Pharmacology. Curr Protein Pept Sci. 2019;20:944-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 220. | Tan K, Tesar C, Wilton R, Jedrzejczak RP, Joachimiak A. Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase. Protein Sci. 2018;27:1498-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 221. | Williams SJ, Goddard-Borger ED. α-glucosidase inhibitors as host-directed antiviral agents with potential for the treatment of COVID-19. Biochem Soc Trans. 2020;48:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 222. | Kaur J, Sharma P, Kaur R, Kaur S, Kaur A. Assessment of alpha glucosidase inhibitors produced from endophytic fungus Alternaria destruens as antimicrobial and antibiofilm agents. Mol Biol Rep. 2020;47:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 223. | Kaur J, Sharma A, Sharma M, Kumari Manhas R, Kaur S, Kaur A. Effect of α-glycosidase inhibitors from endophytic fungus Alternaria destruens on survival and development of insect pest Spodoptera litura Fab. and fungal phytopathogens. Sci Rep. 2019;9:11400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belančić A, Primadhi RA, Shi J S-Editor: Fan JR L-Editor: A P-Editor: Fan JR