Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1693
Peer-review started: January 24, 2021
First decision: April 6, 2021
Revised: April 20, 2021
Accepted: August 5, 2021
Article in press: August 5, 2021
Published online: October 15, 2021
Processing time: 262 Days and 5.6 Hours
The prevalence of diabetes has increased rapidly throughout the world in recent years. Currently, approximately 463 million people are living with diabetes, and the number has tripled over the last two decades. Here, we describe the global epidemiology of diabetes in 2019 and forecast the trends to 2030 and 2045 in China, India, USA, and the globally. The gut microbiota plays a major role in metabolic diseases, especially diabetes. In this review, we describe the interaction between diabetes and gut microbiota in three aspects: probiotics, antidiabetic medication, and diet. Recent findings indicate that probiotics, antidiabetic medications, or dietary interventions treat diabetes by shifting the gut microbiome, particularly by raising beneficial bacteria and reducing harmful bacteria. We conclude that targeting the gut microbiota is becoming a novel therapeutic strategy for diabetes.
Core Tip: The current review describes the global epidemiology of diabetes in 2019 and forecasted the trends to 2030 and 2045 in China, India, USA, and globally. This review also summarizes the interaction between diabetes and the gut microbiota in three aspects: probiotics, antidiabetic medications, and diet.
- Citation: Xi Y, Xu PF. Diabetes and gut microbiota. World J Diabetes 2021; 12(10): 1693-1703
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1693.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1693
The global prevalence of diabetes has grown rapidly in recent decades. Diabetes is becoming a serious global health threat, and is one of the top 10 leading causes of death among adults[1]. The etiology and progression of diabetes are commonly driven by genetic and environmental factors. The International Diabetes Federation (IDF) estimates that in 2019 there were 463 million cases of diabetes mellitus worldwide and approximately 4.2 million adults died from diabetes and its complications[2]. It is estimated that approximately 700 million adults will be diagnosed with diabetes by 2045. Diabetes mellitus is a group of metabolic diseases that cause high blood glucose, and primarily includes type 2 diabetes (T2D), type 1 diabetes, prediabetes, and gestational diabetes. T2D is the most common type of diabetes and represents approximately 90% of all diabetes patients worldwide[3].
The gut microbiota is a collective term for the intestinal microbial community, which plays a crucial role in maintaining health and disease pathogenesis. Recently, the gut microbiome has become an emerging research area for diabetes management, as gut dysbiosis directly or indirectly participates in diabetes by affecting host intestinal barrier functions and metabolic homeostasis[4]. Animal and human studies have identified related differences in the composition of the gut microbiota in patients with diabetes[5]. In this review, we describe global trends in diabetes in 2019, predict the trends to 2030 and 2045, and summarize the latest findings regarding the gut microbiota in diabetes.
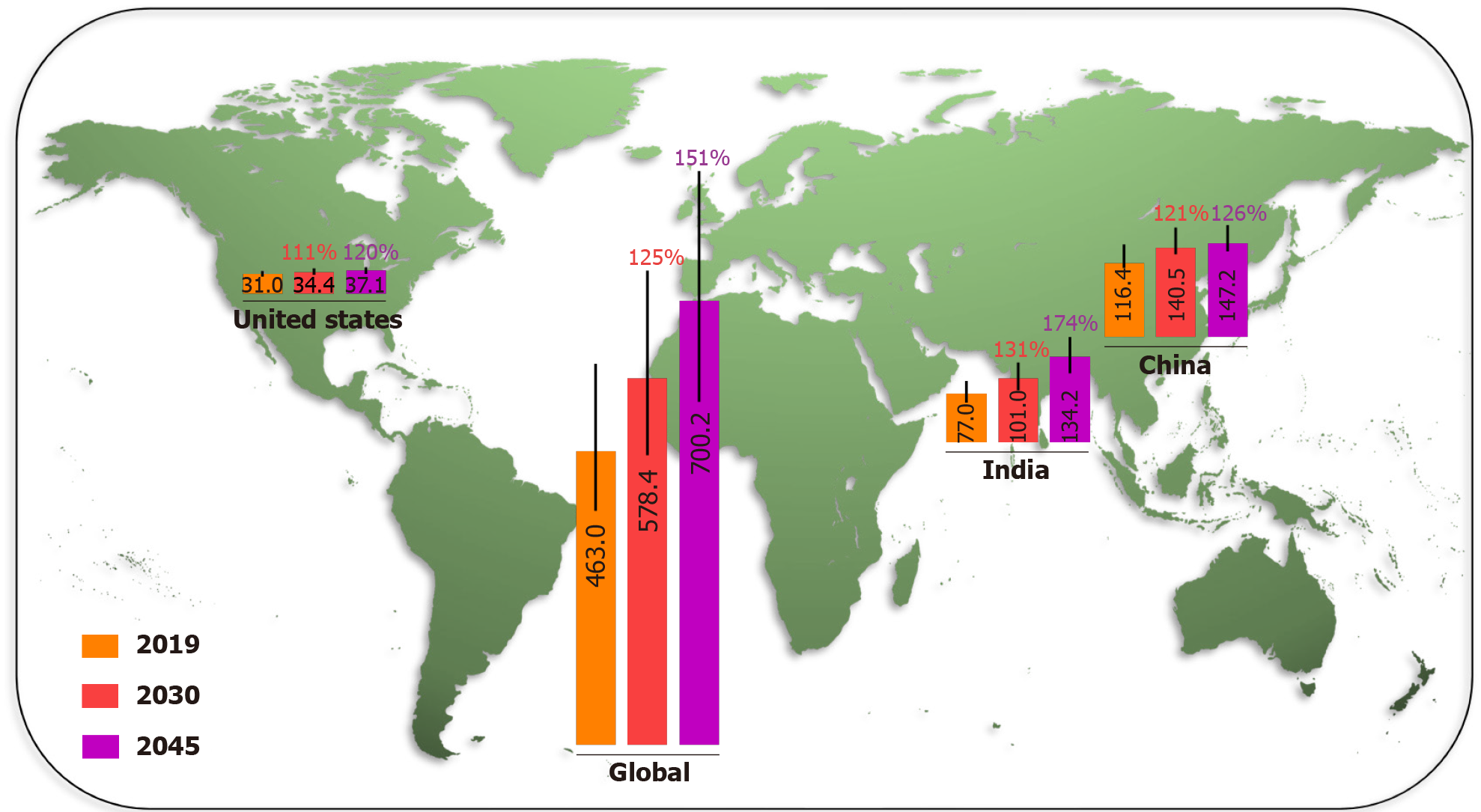
Diabetes is one of the fastest growing global health challenges in the last 40 years, with the number of adults living with diabetes rising from 108 million in 1980 to 463.0 million (368.7–600.6 million) in 2019. This number is projected to reach 578.4 million (456.5–747.6 million) in 2030 and 700.2 million (540.7–904.6 million) in 2045. The global prevalence of adult diabetes increased from 4.7% in 1980 to 8.3% (6.2%–11.8%) in 2019, and is projected to reach 9.2% (6.8%–12.9%) in 2030 and 9.6% (7.1%–13.4%) in 2045[1]. Although the common long-term complications in diabetic patients develop gradually, they could be disabling or even life-threatening over time[6]. Diabetes is a major cause of many diseases, such as eye damage, kidney failure, heart and blood vessel disease, neuropathy, Alzheimer’s disease, and lower limb amputation. Global diabetes-related health spending continues to grow rapidly as well. It was 760 billion US dollars in 2019, approximately 10% of total global health spending, and is expected to reach 825 billion US dollars in 2030 and 845 billion in 2045[7].
China and India were the two countries with the highest number of adult diabetic patients in 2019 and are projected to remain so in 2030 and 2045, due to the demographic and socioeconomic status factors. The IDF Diabetes Atlas (9th edition 2019) estimated the number of people with diabetes in China, India, USA, and the world in 2019, and projected that by 2030 and 2045 (Figure 1), the number of adults living with diabetes in China will increase from 116.4 million (108.6–145.7 million) in 2019 to 140.5 million (130.3–172.3 million) in 2030, and 147.2 million (134.7–176.2 million) in 2045. In India, the number of diabetes cases is projected to grow from 77.0 million (62.4–96.4 million) in 2019 to 101.0 million (81.6–125.6 million) in 2030, and 134.2 million (108.5–165.7 million) in 2045. The number of adult diabetes cases in the USA will increase from 31.0 million (26.7–35.8 million) in 2019, to a projected 34.4 million (29.7–39.8 million) in 2030 and 36.0 million (31.0–41.6 million) in 2045. Over the last 40 years, the number of people with diabetes has quadrupled throughout the world. The prevalence of diabetes will increase more rapidly in low-income than in high-income countries in the near future[1]. Unmet medical needs related to diabetes are a growing global public health problem.
Observational findings from recent epidemiological, physiological and metabolomic studies, complemented by cellular and animal experiments and clinical trials, it appears that microbial communities may contribute to the pathogenesis of a variety of common metabolic disorders, including obesity and diabetes, and their complications[3,8]. Although accumulative evidence suggests that the gut microbiota is a factor influencing diabetes, the underlying mechanisms remain unclear. Due to the crosstalk between the gut microbiota and host homeostasis, the gut microbiome is thought to play a crucial role in obesity and associated metabolic dysfunction[9,10]. The gut microbiome has been shown to affect host metabolism, food consumption, body weight, and glucose and lipid homeostasis. Gut dysbiosis or altered microbiota composition has been detected in obesity and diabetes in human and murine models[11]. Treatment with probiotics, antidiabetic medications, or dietary interventions can orchestrate the gut microbiome, leading to increased probiotic bacteria and decreased harmful bacteria, and these changes subsequently contribute to bodyweight loss, suppression of inflammation, and maintenance of glucose homeostasis in the host[12]. Targeting the gut microbiota is developing into a possible therapeutic strategy for diabetes.
Probiotics are living microorganisms that provide health benefits to their host, particularly the digestive system. Probiotics, such as Akkermansia, Bacteroides, Bifidobacterium and Lactobacillus, are currently suggested as novel and potential biotherapeutics in the prevention and management of diabetes[13,14]. Oxidative stress is a key player in the development of diabetes and diabetes-related complications[15]. Supplementation with probiotics and also synbiotics could be beneficial for patients diagnosed with diabetes also because these products lower oxidative stress levels[16,17]. Cumulative studies have proven the efficacy of probiotics in the treatment of diabetes by decreasing fasting glucose and insulin levels in animal models and clinical trials[18].
Akkermansia muciniphila is a species of mucin-degrading bacteria recently found in the human gut, and its abundance has been reported to be inversely correlated with obesity, T2D and inflammation[19-22]. Administration of A. muciniphila protected against high fat diet (HFD)-induced obesity and insulin resistance by suppressing inflammation and improving gut barrier function. In addition, a purified protein in the outer membrane of A. muciniphila called Amuc-1100 could improve metabolic syndrome in obese and diabetic mice through the Toll-like receptor 2 signaling pathway[23]. In human clinical trials, supplementation with A. muciniphila compared to the placebo improved insulin sensitivity, reduced insulinemia and plasma total cholesterol, and decreased body weight in overweight/obese insulin-resistant volunteers[24]. In our recent studies, we found that melatonin, a probiotic agent, partially improved insulin resistance by increasing the abundance of A. muciniphila in HFD-fed mice[25]. A. muciniphila is considered a promising probiotic to improve diabetes and obesity-associated metabolic disorders.
Bacteroides is a common genus associated with the risk of T2D in patients. However, the role of Bacteroides in diabetes is controversial. Some studies have shown that the abundance of Bacteroides is inversely associated with diabetes risk[26-30], while others have reported a positive association in different species[31-33]. This inconsistency may be explained by the underlying feedback mechanism of the gut microbiome at different stages of the disease or in different animal models. The ratio of Bacteroidetes to Firmicutes, previously identified as a marker for metabolic diseases, does not seem to be consistently associated with diabetes risk[14]. In animal studies, treatment with Bacteroides acidifaciens and Bacteroides uniformis prevents obesity and improves insulin susceptibility in diabetic mice[34,35]. These studies suggest that Bacteroides may have a beneficial effect on diabetes.
Bifidobacterium, also known as Lactobacillus bifidus, is frequently reported in T2D protection studies. Bifidobacterium strains are crucial probiotics in the dairy industry, due to their unique function of fermenting carbohydrates via the fructose-6-phosphate phosphoketolase pathway[36]. Numerous studies have shown that Bifidobacterium has beneficial effects on glucose tolerance in individuals with T2D and diabetic murine models[37-39]. Oral administration of Bifidobacterium decreases blood glucose concentration and glycosylated hemoglobin levels, and improves lipid profiles, insulin resistance, and antioxidant indexes, through insulin receptor substrate/phosphoinositide 3-kinase/protein kinase B and kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling pathway in murine diabetic models[40]. Bifidobacterium may be a promising probiotic to treat diabetes.
Lactobacillus is the most commonly used probiotic in industry to control food fermentation, such as yogurt, cheese, wine, and other fermented foods. Studies of the composition of gut microbiota showed some species in this genus were increased in T2D patients, such as Lactobacillus acidophilus, Lactobacillus gasseri and Lactobacillus salivarius, whereas Lactobacillus amylovorus was decreased in patients with diabetes[41-43]. Oral supplementation of Lactobacillus, such as Lactobacillus casei, Lactobacillus curvatus, L. gasseri, Lactobacillus paracasei, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus rhamnosus and Lactobacillus sakei, exhibited beneficial effects in diabetic mice and individuals with diabetes[44-54]. The antidiabetic mechanism of Lactobacillus by inhibiting endotoxin secretion and activating G-protein-coupled receptor 43 pathway has been reported[55]. The combination of Lactobacillus and Bifidobacterium is widely used in clinical practice to synergistically maintain a healthy digestive tract. Growing evidence supports that probiotics are a safe and effective treatment strategy under certain clinical conditions of diabetes.
Diet is an essential regulator of the gut microbiome[56]. Interactions between diet and gut microbiota have been reported to affect obesity, insulin resistance, and the chronic inflammatory response of the host[57]. Here, we mainly summarize the roles of diet in the gut microbiome and diabetes.
Diet composition is vital in diabetes development. Diabetes was considered a disease of the rich, because of its high prevalence among the rich who access food more easily, including flour, sugar, fat and meat[58]. It has been shown that diets with high levels of sugar, fat and cholesterol increase the risk of diabetes. These diets cause gut dysbiosis and damage the intestinal mucosal barrier that facilitates the development of diabetes[59,60]. High-fiber diet is a well-known healthy diet with various benefits, such as improving bowel movements, lowering cholesterol, achieving a healthy weight, and controlling blood sugar levels. Dietary fibers consist of cellulose, resistant starch and dextrin, inulin, lignin, pectin, -glucan, and oligosaccharides. They are abundant in whole-grain bread and cereals, legumes, rice, vegetables and fruits, and cannot be completely digested or absorbed by the human digestive system[61,62]. Dietary fibers play an essential role in maintaining the gut microbiota and gut health, as they can be catalyzed and fermented by certain gut microbes and produce beneficial metabolites, such as short-chain fatty acids (SCFAs)[63]. In the gut, approximately 95% of SCFAs are acetate (C2), propionate (C3), and butyrate (C4)[64]. Studies have shown that acetate is mainly produced by bacteria, such as A. muciniphila, Bifidobacterium spp., Bacteroides spp., Lactobacillus spp., Prevotella spp., Ruminococcus spp. and Streptococcus spp. through the acetyl-coenzyme A pathway[65,66]. Propionate is mainly produced by Bacteroides spp., Coprococcus catus, Dialister spp., Megasphaera elsdenii, Phascolarctobacterium succinatutens, Roseburia inulinivorans, Ruminococcus obeum, Salmonella spp. and Veillonella spp. through three known pathways, i.e., succinate pathway, acrylate pathway, and propanediol pathway[66,67]. Butyrate is produced primarily in Anaerostipes caccae, Clostridium leptum, Coprococcus catus, Coprococcus eutactus, Eubacterium hallii, Eubacterium rectale, Faecalibacterium prausnitzii, and Roseburia spp., by enzymatic catalysis, such as butyryl-CoA dehydrogenase, butyryl-CoA transferase, and phosphotransbutyrylase or butyrate kinase[66,68]. SCFAs are critical modulators in patho
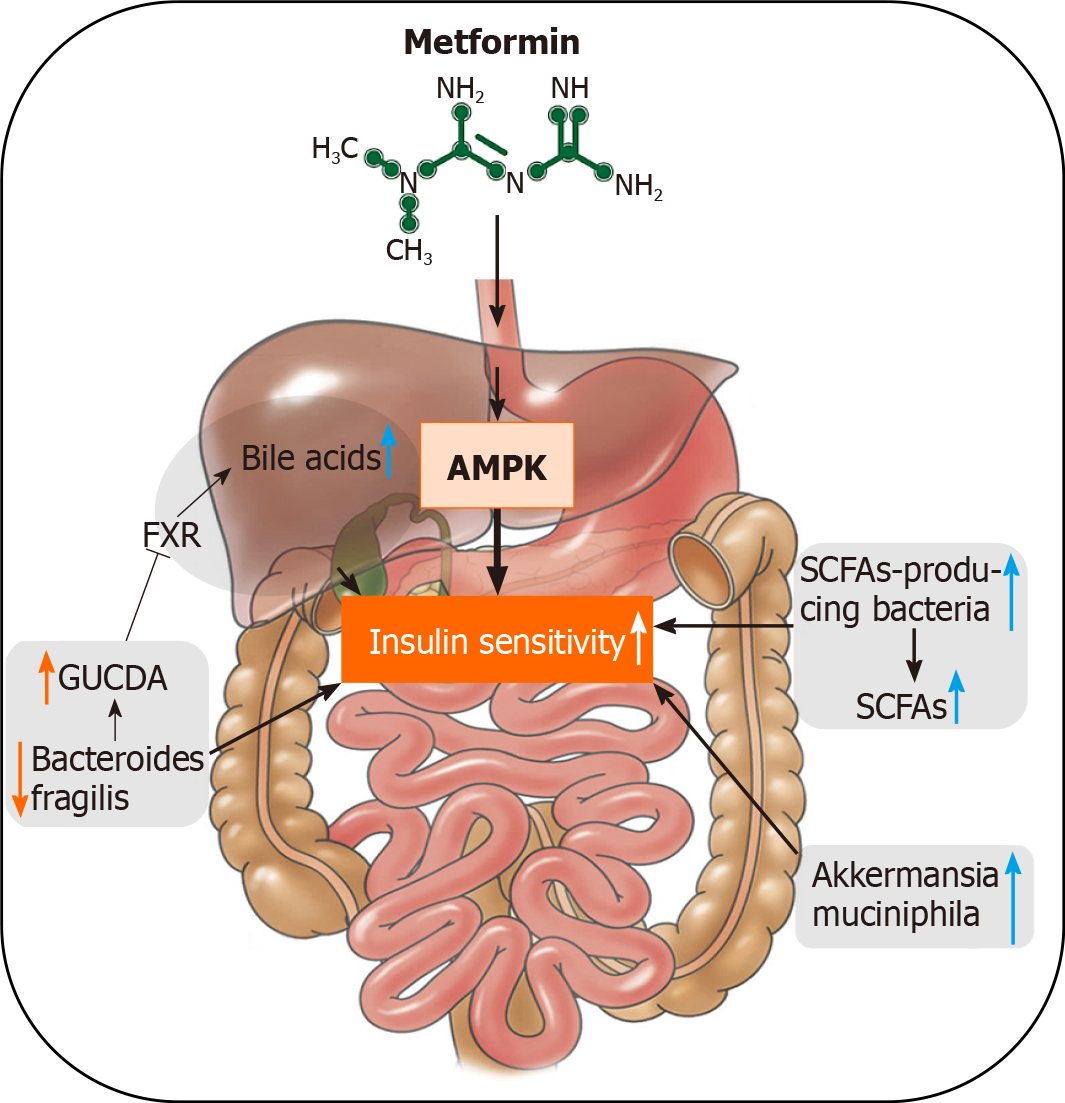
Metformin is an oral antidiabetic medication. It has been used in the treatment of T2D for > 60 years due to its distinct effects on decreasing glucose production and increasing insulin sensitivity, as well as its safety profile. Metformin originates from Galega officinalis, a natural source of galegine[73]. Traditionally, activation of the AMP-activated protein kinase signaling pathway in the liver is thought to be the mechanism of its antidiabetic effects[74]. Recent findings indicate that metformin also orchestrates gut microbiome in mice and humans[43]. Sun et al[33] reported that metformin improves hyperglycemia through the gut microbiota-bile acid-intestinal farnesoid X receptor (FXR) axis in T2D patients. FXR is an important target in regulating glucose and lipid homeostasis. Metformin reduces the level of Bacteroides fragilis in the gut, leading to an increase in the FXR antagonist, glycoursodeoxycholic acid. Treatment with metformin also increased the abundance of probiotics A. muciniphila and SCFA-producing microbiota, such as Butyrivibrio, B. bifidum, and Megasphaera in murine and human studies[31]. Here, we summarize the role of the gut microbiome in the antidiabetic effects of metformin (Figure 2).
Acarbose, an α-glucosidase inhibitor, is an oral prescription medication used to control blood glucose in T2D treatment. Acarbose has been reported to alter the composition of gut microbiota in patients with T2D, in particular increasing the abundance of Bifidobacterium longum and decreasing the level of lipopolysaccharides[75]. Vildagliptin, a dipeptidyl peptidase 4 inhibitor, is an oral antihyperglycemic agent that enhances insulin secretion and suppresses glucagon release. Vildagliptin supplementation decreases the level of Oscillibacter and increases the proportion of Lactobacillus in HFD-induced mouse models[76]. Sitagliptin, another DPP-4 inhibitor, appears to exhibit antidiabetic functions during pregnancy in rats by reducing Lactobacillus spp. and increasing Bifidobacterium spp.[9,77]. Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, is a medication used to treat T2D. Treatment with dapagliflozin decreases the ratio of Firmicutes to Bacteroidetes and the abundance of Oscillospira, and increases the abundance of A. muciniphila in diabetic murine models[78,79]. Thiazolidinediones (TZDs) are a class of oral hypoglycemic agents for the treatment of T2D[80,81]. TZDs function through the activation of the peroxisome proliferator-activated receptor (PPAR) signaling pathway[82,83]. Pioglitazone, a member of TZDs, is widely used to treat T2D. It has been reported that treatment with pioglitazone reduces the α-diversity of the gut microbiota in murine T2D models, which may be one of the mechanisms mediating its antidiabetic function[79]. In our previous studies, Danshensu Bingpian Zhi, a synthetic derivative of danshensu and borneol, is a PPARγ agonist that prevents HFD-induced atherosclerosis, obesity, and insulin resistance in mice in part by reversing intestinal microbiota dysbiosis, such as increasing the ratio of Bacteroidetes to Firmicutes, increasing the level of Akkermansia, and reducing the level of the harmful bacterium Helicobacter marmotae[84]. These results suggest that gut microbiome is a potential target of many anti-diabetic medications clinically.
Traditional Chinese medicines (TCMs) have a long history of treating diabetes, but their mechanisms are not fully understood. Several studies have suggested that TCMs have multiple therapeutic effects on diabetes, including antioxidation, suppression of inflammation, protection of intestinal mucosal barrier, and inhibition of lipotoxicity, mainly by remodeling the gut microbiota[85]. Berberine, a well-known bioactive alkaloid extracted from TCM Coptis chinensis, has been used for the treatment of diarrhea and diabetes. Berberine is useful in diabetes management because its administration is associated with a decrease of obesity indices, such as body mass index and waist circumference[86]. Berberine maintains gut health in rats and humans with diabetes by increasing the abundance of Bifidobacterium and Lactobacillus, and decreasing the abundance of Escherichia coli[87,88]. Gegen Qinlian Decoction can relieve T2D in clinical trials, which is associated with an increase in the level of beneficial bacteria, such as Faecalibacterium spp.[89]. In addition, Banxia Xiexin Decoction, Huanglian Jiedu Decoction, and Qijian mixture also have beneficial effects by regulating gut microbiota[85,90,91]. These results suggest that gut microbiota is likely a new direction in elucidating the antidiabetic mechanism of TCMs.
Diabetes has become an urgent public health threat, and the growing trend of diabetes cases is expected to continue for the next two decades and beyond. Gut microbiome plays a critical role in health maintenance, and the dysregulation of gut microbiome can contribute to the development and progression of the disease. Here, we summarized the interaction between diabetes and the gut microbiota. Gut dysbiosis is increasingly recognized as a mechanism that induces metabolic diseases. Accumulating studies have shown that the gut microbiome is a key factor in the pathophysiology of diabetes, but research in this area is still in the early stages. Most of the studies have only shown that changes in the composition of the gut microbiota are associated with the progression of metabolic diseases. The exact causal relationship between a specific intestinal bacterium and phenotypic exposure is still not well understood. Further experiments using fecal or bacterial transplantation in germ-free mice and clinical studies are required to obtain a deeper understanding of the roles of individual bacteria in metabolic diseases. The use of metabolomics and transcriptomics to study the gut microbiome is a more effective strategy to understand the role of microbiota in the progression of host disease.
Traditionally, most pharmacological agents used for treatment of diabetes directly regulate the signaling pathways involved in glucose and insulin homeostasis. However, the gut microbiota is becoming an emerging therapeutic target for diabetes. In view of the good performance of herbal agents, particularly TCMs, in regulating gut microbiota, more consideration should be given to the use of medicinal herbs for the treatment of diabetes.
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 6420] [Article Influence: 917.1] [Reference Citation Analysis (12)] |
| 2. | Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, Unwin N, Wild SH, Williams R. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (4)] |
| 3. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3697] [Article Influence: 462.1] [Reference Citation Analysis (0)] |
| 4. | Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, Ma J, Gu X, Xue Y, Huang S, Yang J, Chen L, Chen G, Qu S, Liang J, Qin L, Huang Q, Peng Y, Li Q, Wang X, Kong P, Hou G, Gao M, Shi Z, Li X, Qiu Y, Zou Y, Yang H, Wang J, Xu G, Lai S, Li J, Ning G, Wang W. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (2)] |
| 6. | Nathan DM, Bennett PH, Crandall JP, Edelstein SL, Goldberg RB, Kahn SE, Knowler WC, Mather KJ, Mudaliar S, Orchard TJ, Temprosa M, White NH; Research Group. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia. 2019;62:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 7. | Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 553] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 8. | Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 3093] [Article Influence: 515.5] [Reference Citation Analysis (1)] |
| 9. | Kyriachenko Y, Falalyeyeva T, Korotkyi O, Molochek N, Kobyliak N. Crosstalk between gut microbiota and antidiabetic drug action. World J Diabetes. 2019;10:154-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 10. | Ye P, Xi Y, Huang Z, Xu P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 11. | Sircana A, Framarin L, Leone N, Berrutti M, Castellino F, Parente R, De Michieli F, Paschetta E, Musso G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr Diab Rep. 2018;18:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 12. | He C, Shan Y, Song W. Targeting gut microbiota as a possible therapy for diabetes. Nutr Res. 2015;35:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Panwar H, Rashmi HM, Batish VK, Grover S. Probiotics as potential biotherapeutics in the management of type 2 diabetes - prospects and perspectives. Diabetes Metab Res Rev. 2013;29:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1386] [Cited by in RCA: 1187] [Article Influence: 197.8] [Reference Citation Analysis (1)] |
| 15. | Găman MA, Epîngeac ME, Diaconu CC, Găman AM. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J Diabetes. 2020;11:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Sohouli MH, Fatahi S, Sharifi-Zahabi E, Santos HO, Tripathi N, Lari A, Pourrajab B, Kord-Varkaneh H, Găman MA, Shidfar F. The Impact of Low Advanced Glycation End Products Diet on Metabolic Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2021;12:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Pourrajab B, Fatahi S, Sohouli MH, Găman MA, Shidfar F. The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. 2017;60:943-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 19. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3484] [Article Influence: 268.0] [Reference Citation Analysis (1)] |
| 20. | Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22:658-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 645] [Cited by in RCA: 753] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 21. | Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1391] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 22. | Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 730] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 23. | Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1583] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 24. | Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 1584] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 25. | Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, Jia L, Zhai Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 26. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 655] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 27. | Yamaguchi Y, Adachi K, Sugiyama T, Shimozato A, Ebi M, Ogasawara N, Funaki Y, Goto C, Sasaki M, Kasugai K. Association of Intestinal Microbiota with Metabolic Markers and Dietary Habits in Patients with Type 2 Diabetes. Digestion. 2016;94:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Munukka E, Wiklund P, Pekkala S, Völgyi E, Xu L, Cheng S, Lyytikäinen A, Marjomäki V, Alen M, Vaahtovuo J, Keinänen-Kiukaanniemi S. Women with and without metabolic disorder differ in their gut microbiota composition. Obesity (Silver Spring). 2012;20:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, Kautzky-Willer A, Paulweber B, Hackl E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 30. | Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, Peano C, Turroni S, Rampelli S, Pozzilli P, Pianesi M, Fallucca F, Brigidi P. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 31. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1207] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 32. | Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017;27:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 33. | Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X, Liu J, Deng Y, Xia J, Chen B, Zhang S, Yun C, Lian G, Zhang X, Zhang H, Bisson WH, Shi J, Gao X, Ge P, Liu C, Krausz KW, Nichols RG, Cai J, Rimal B, Patterson AD, Gonzalez FJ, Jiang C. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 773] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 34. | Gauffin Cano P, Santacruz A, Moya Á, Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One. 2012;7:e41079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 35. | Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, Kim HM, Ahn SH, Kwon BE, Ko HJ, Kweon MN. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 344] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 36. | Manome A, Abiko Y, Kawashima J, Washio J, Fukumoto S, Takahashi N. Acidogenic Potential of Oral Bifidobacterium and Its High Fluoride Tolerance. Front Microbiol. 2019;10:1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M, Shen J. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 545] [Article Influence: 45.4] [Reference Citation Analysis (1)] |
| 38. | Le TK, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le TH, Pham XD. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res. 2015;36:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Zhang JL, Wang SB, Zeng Z, Qin YX, Shen Q, Li PL. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J Funct Foods. 2020;67:103843. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2241] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 42. | Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, Bornstein SR. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 43. | Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J; MetaHIT consortium, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1208] [Cited by in RCA: 1597] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 44. | Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R, Zhuang K, Zhang W, Li T, Man C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018;9:3630-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 46. | Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe-/- mice. PLoS One. 2012;7:e46837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI. Lactobacillus plantarum Strain Ln4 Attenuates Diet-Induced Obesity, Insulin Resistance, and Changes in Hepatic mRNA Levels Associated with Glucose and Lipid Metabolism. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 48. | Lim SM, Jeong JJ, Woo KH, Han MJ, Kim DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 49. | Martinic A, Barouei J, Bendiks Z, Mishchuk D, Heeney DD, Martin R, Marco ML, Slupsky CM. Supplementation of Lactobacillus plantarum Improves Markers of Metabolic Dysfunction Induced by a High Fat Diet. J Proteome Res. 2018;17:2790-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 52. | Park KY, Kim B, Hyun CK. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J Clin Biochem Nutr. 2015;56:240-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Sabico S, Al-Mashharawi A, Al-Daghri NM, Yakout S, Alnaami AM, Alokail MS, McTernan PG. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. J Transl Med. 2017;15:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 54. | Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Li KK, Tian PJ, Wang SD, Lei P, Qu L, Huang JP, Shan YJ, Li BL. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J Funct Foods. 2017;38:561-570. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Li WZ, Stirling K, Yang JJ, Zhang L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J Diabetes. 2020;11:293-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (5)] |
| 57. | Ponzo V, Fedele D, Goitre I, Leone F, Lezo A, Monzeglio C, Finocchiaro C, Ghigo E, Bo S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 58. | Lazar V, Ditu LM, Pircalabioru GG, Picu A, Petcu L, Cucu N, Chifiriuc MC. Gut Microbiota, Host Organism, and Diet Trialogue in Diabetes and Obesity. Front Nutr. 2019;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 59. | Serino M, Luche E, Gres S, Baylac A, Bergé M, Cenac C, Waget A, Klopp P, Iacovoni J, Klopp C, Mariette J, Bouchez O, Lluch J, Ouarné F, Monsan P, Valet P, Roques C, Amar J, Bouloumié A, Théodorou V, Burcelin R. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 60. | Fallucca F, Fontana L, Fallucca S, Pianesi M. Gut microbiota and Ma-Pi 2 macrobiotic diet in the treatment of type 2 diabetes. World J Diabetes. 2015;6:403-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 61. | Ganesan K, Chung SK, Vanamala J, Xu B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int J Mol Sci. 2018;19:3720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 62. | Wu WC, Inui A, Chen CY. Weight loss induced by whole grain-rich diet is through a gut microbiota-independent mechanism. World J Diabetes. 2020;11:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (5)] |
| 63. | Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients. 2020;12:859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 64. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 3528] [Article Influence: 271.4] [Reference Citation Analysis (3)] |
| 65. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 2060] [Article Influence: 171.7] [Reference Citation Analysis (1)] |
| 66. | Feng W, Ao H, Peng C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front Pharmacol. 2018;9:1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 67. | Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 68. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2466] [Article Influence: 352.3] [Reference Citation Analysis (0)] |
| 69. | Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014;2014:162021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 70. | Xu P, Hong F, Wang J, Cong Y, Dai S, Wang S, Jin X, Wang F, Liu J, Zhai Y. Microbiome Remodeling via the Montmorillonite Adsorption-Excretion Axis Prevents Obesity-related Metabolic Disorders. EBioMedicine. 2017;16:251-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 71. | Xu P, Dai S, Wang J, Zhang J, Liu J, Wang F, Zhai Y. Preventive obesity agent montmorillonite adsorbs dietary lipids and enhances lipid excretion from the digestive tract. Sci Rep. 2016;6:19659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients. 2019;11:2862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 551] [Article Influence: 78.7] [Reference Citation Analysis (1)] |
| 73. | Guo GL, Xie W. Metformin action through the microbiome and bile acids. Nat Med. 2018;24:1789-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2774] [Cited by in RCA: 3586] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 75. | Su B, Liu H, Li J, Sunli Y, Liu B, Liu D, Zhang P, Meng X. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 76. | Olivares M, Neyrinck AM, Pötgens SA, Beaumont M, Salazar N, Cani PD, Bindels LB, Delzenne NM. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018;61:1838-1848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 77. | Paul HA, Bomhof MR, Vogel HJ, Reimer RA. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci Rep. 2016;6:20683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 78. | Lee DM, Battson ML, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 79. | Yang M, Shi FH, Liu W, Zhang MC, Feng RL, Qian C, Ma J. Dapagliflozin Modulates the Fecal Microbiota in a Type 2 Diabetic Rat Model. Front Endocrinol (Lausanne). 2020;11:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Hong F, Xu P, Zhai Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int J Mol Sci. 2018;19:2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 81. | Xu P, Zhai Y, Wang J. The Role of PPAR and Its Cross-Talk with CAR and LXR in Obesity and Atherosclerosis. Int J Mol Sci. 2018;19:1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 82. | Xi Y, Zhang Y, Zhu S, Luo Y, Xu P, Huang Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells. 2020;9:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 83. | Hong F, Pan S, Guo Y, Xu P, Zhai Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules. 2019;24:2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 84. | Xu P, Hong F, Wang J, Zhao X, Wang S, Xue T, Xu J, Zheng X, Zhai Y. DBZ is a putative PPARγ agonist that prevents high fat diet-induced obesity, insulin resistance and gut dysbiosis. Biochim Biophys Acta Gen Subj. 2017;1861:2690-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Zhang B, Yue R, Chen Y, Yang M, Huang X, Shui J, Peng Y, Chin J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid Based Complement Alternat Med. 2019;2019:2634898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Xiong P, Niu L, Talaei S, Kord-Varkaneh H, Clark CCT, Găman MA, Rahmani J, Dorosti M, Mousavi SM, Zarezadeh M, Taghizade-Bilondi H, Zhang J. The effect of berberine supplementation on obesity indices: A dose- response meta-analysis and systematic review of randomized controlled trials. Complement Ther Clin Pract. 2020;39:101113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 87. | Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H, Zhao T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp Clin Endocrinol Diabetes. 2018;126:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 88. | Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 491] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 89. | Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, Guo Y, Zhang C, Zhou Q, Xue Z, Pang X, Tong X. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015;9:552-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 90. | Gao K, Yang R, Zhang J, Wang Z, Jia C, Zhang F, Li S, Wang J, Murtaza G, Xie H, Zhao H, Wang W, Chen J. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol Res. 2018;130:93-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 91. | Chen M, Liao Z, Lu B, Wang M, Lin L, Zhang S, Li Y, Liu D, Liao Q, Xie Z. Huang-Lian-Jie-Du-Decoction Ameliorates Hyperglycemia and Insulin Resistant in Association With Gut Microbiota Modulation. Front Microbiol. 2018;9:2380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA, Lee YL S-Editor: Liu M L-Editor: Kerr C P-Editor: Wang LYT