Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1674
Peer-review started: May 21, 2021
First decision: June 16, 2021
Revised: June 29, 2021
Accepted: August 31, 2021
Article in press: August 31, 2021
Published online: October 15, 2021
Processing time: 144 Days and 15.3 Hours
Diabetes, whether due to pancreatic beta cells insufficiency or peripheral resistance to insulin, has been suggested as a risk factor of developing severe acute respiratory disease coronavirus-2 (SARS-CoV-2) infections. Indeed, diabetes has been associated with a higher risk of infections and higher risk of developing severe forms of coronavirus disease 2019 (COVID-19) related pneumonia. Diabetic patients often present associated comorbidities such as obesity, hypertension and cardiovascular diseases, and complications of diabetes, including chronic kidney disease, vasculopathy and relative immune dysfunction, all of which make them more susceptible to infectious complications. Moreover, they often present low-grade inflammation with increased circulating interleukin levels, endothelial susceptibility to inflammation and dysfunction, and finally, hyperglycemia, which increases this risk. Additionally, corticosteroids, which count among the few medications which showed benefit on survival and mechanical ventilation requirement in COVID-19 pneumonia in large randomized controlled trials, are associated to new onsets of diabetes, and metabolic disorders in patients with previous history of diabetes. Finally, SARS-CoV-2 via the alternate effects of the renin-angiotensin system, mediated by the angiotensin-converting-enzyme 2, was also associated with insulin resistance in key tissues involved in glucose homeostasis, such as liver, skeletal muscles, and adipose tissue; and also, with impaired insulin secretion by pancreatic β-cells. In this work, we reviewed all elements which may help understand how diabetes affects patients with COVID-19, how treatments affect outcomes in patients with COVID-19, how they may cause new onsets of diabetes, and finally review how SARS-CoV-2 may inherently be a risk factor of developing diabetes, through immune-mediated diabetogenic mechanisms.
Core Tip: Diabetes features complex interactions with the severe acute respiratory disease coronavirus-2 (SARS-CoV-2). Diabetic patients are at higher risk of severe infections. They often present associated comorbidities such as obesity, hypertension and cardiovascular diseases, and complications of diabetes, including chronic kidney disease, vasculopathy and relative immune dysfunction. Additionally, corticosteroids, which count among the few medications which showed benefit on survival are associated to new onsets of diabetes, and metabolic disorders in patients with previous history of diabetes. Finally, SARS-CoV-2 via the alternate effects of the renin-angiotensin system, mediated by the angiotensin-converting-enzyme 2, was also associated with insulin resistance.
- Citation: Sabri S, Bourron O, Phan F, Nguyen LS. Interactions between diabetes and COVID-19: A narrative review. World J Diabetes 2021; 12(10): 1674-1692
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1674.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1674
Preexisting diabetes is associated with poor outcomes of coronavirus disease 2019: There is a set of arguments to say that patients with diabetes are at high risk of severe form of coronavirus disease 2019 (COVID-19) pneumonia. Quickly, at the start of the epidemic, reports from China and Italy suggested that diabetes rate ratio was two or three-fold in patients with more severe than in those with less severe infections[1-5]. Patients with diabetes and COVID-19 pneumonia were more likely to require intensive care, present organ failure, hypercoagulability state with increased levels of inflammatory factors[1,3,6]. In terms of absolute risk, three people with diabetes in every 1000 developed fatal or critical care unit-treated COVID-19[7]. In the French COVID CORONADO cohort, only 50% of the 2796 patients with diabetes were discharged and 20% died within 28 d after hospitalization, reflecting the severity of the disease in the diabetic population[8]. In other countries, mortality rate of patients with diabetes and COVID-19 during the first wave was between 11% and 33%[9-11]. In a retrospective study of 7337 cases of COVID-19 in China, 952 patients with pre-existing type 2 diabetes (T2D) required more medical interventions and presented multiple organ failure than non-diabetic individuals[6]. In this study, T2D was significantly associated with the incidence of acute respiratory distress syndrome (ARDS), septic shock, and acute kidney injury with respective adjusted hazard ratios (HR) of 1.44 (95%CI: 1.20-1.73), 1.95 (95%CI: 1.18-3.20) and 3.01 (95%CI: 1.94-4.68). These findings explain the high mortality rate observed in this population. In Italy, for example, a third of people who died from COVID during the first wave were diabetic[12]. In March 28th, 2020, 2112 deaths from confirmed COVID-19 cases were reported to CDC and diabetes was one of the more frequently reported conditions among all cases. Indeed, a third of the patients hospitalized in intensive care had diabetes[13]. On the other hand, the prevalence of diabetes in non-hospitalized patients among COVID-19 patients was 6%, less than the prevalence of diabetes among United States adults estimated to 10.1%[14]. This may suggest that diabetes is more a risk factor of severity in case of COVID-19 than a risk factor of COVID-19 itself. Data from New York hospitals also found a significantly increased risk of severe forms associated with diabetes, which however disappeared after adjusting for potential confounding factors[15-17]. All data converge to say that diabetes is associated with severe forms of COVID-19, but other factors may impact this association. Diabetic patients constitute a very heterogeneous population, in terms of type of diabetes, disease duration, quality of glycemic control, presence of diabetic complications, antidiabetic treatment used, presence of comorbidities such as obesity, hypertension, dyslipidemia, tobacco and cardiovascular diseases. Yet, only few studies accounted for these potential confounders.
Impact of age in morbi-mortality of COVID-19 in patients with diabetes: As T2D is more prevalent in elderly patients, whether it is a risk factor of COVID-19 independent from age is currently unknown. Cohort studies in COVID-19 patients which focused on patients with T2D, yielded a mean age between 62 (55-68) in China, and 69.8 ± 13.0 in the French nationwide multicenter CORONADO study[8,18]. The latter study aimed to determine predictors of discharge from hospital and death within 28-d after hospital admission in patients with diabetes and COVID-19. After multiple adjustments, older age and history of microvascular complications were associated were most associated with poor outcomes.
In a systematic review and meta-analysis which included observational studies and investigated risk phenotypes of diabetes and association with COVID-19 severity and deaths, older age (> 65 years) was associated with a 3.49 higher relative risk of COVID-19-related death (95%CI: 1.82-6.69)[19]. Several studies have also shown the absence of excess mortality in young or middle aged patients with type 1 diabetes (T1D)[8,20].
Impact of diabetic complications in morbi-mortality of COVID-19: As the organs affected by COVID-19 are the same affected by diabetes, a relevant question which quickly rose was whether diabetes-induced organ injuries impacted COVID-19 severity. In the French CORONADO study, microvascular complications were associated with a greater risk of death[8]. In a Scottish register study, microvascular complications such as retinopathy and nephropathy were also significantly associated with developing fatal or critical care unit-treated COVID-19[7]. In a population-based cohort study of people with diabetes, increased COVID-19-related mortality was associated with cardiovascular and renal complications of diabetes[21]. Diabetic patients with related chronic kidney disease were even more at risk, in a meta-analysis, with a relative risk of COVID-19-related mortality of 2.53 (95%CI: 0.93-6.88)[19].
Impact of type of diabetes and prognosis of COVID-19: Data about T1D are scarce and contradictory due to the heterogeneous study populations and the presence of many limitations. The French CORONADO study showed a low prevalence of T1D among patients with diabetes hospitalized for COVID-19, 2.1%, lower than that expected in the general population. Data also suggested a lower risk of severe prognosis in patients hospitalized with T1D and COVID-19 than in those with T2D, with half the risk of death by day 7. In a Belgian cohort study, risk of hospitalization was similar in 2336 patients with T1D, compared to 15239 normoglycemic individuals (0.21% vs 0.17%)[20]. In a large British population cohort study, including 61 million individuals, showed that patients with T1D (n = 263830; 0.4%) presented an increased risk of in-hospital death due to COVID-19 compared with those without known diabetes [OR: 3.50 (95%CI: 3.15–3.89)][22]. In this study, mortality was higher in T1D than in T2D patients. Even then, age represented a risk factor in T1D patients. In the Belgian cohort, those hospitalized for COVID-19 were older [66 years (58-80) vs 49 years (35-61), P = 0.010]. Moreover, similarly as in the British study, in the French CORONADO study, no deaths in young patients with T1D (less than 50-55 years old) was reported. In addition, children and adolescents with T1D showed similar risk of infection and subsequent mortality than those without T1D in several cohorts, which emphasize this age-related risk of being infected and developing severe forms of COVID-19 pneumonia in patients with T1D[23,24].
Obesity, an added risk factor of COVID-19 severity: Obesity is highly prevalent in patients hospitalized for COVID-19 and has also been identified as an independent risk factor for the severity of the disease[25]. Obesity and diabetes, especially T2D, are two commonly associated diseases that are supported by epidemiological as well as genetic studies. One study assessed the relationship between obesity classes and COVID-19 prognosis in patients with T2D. Among 1965 patients with T2D, intubation for mechanical ventilation and death were significantly and independently increased in overweight patients [OR: 1.65 (95%CI: 1.05-2.59)], in patients with class I obesity [OR: 1.93 (95%CI: 1.19-3.14)] and class II/III obesity [OR: 1.98 (95%CI: 1.11-3.52)]. In a prospective, community-based, cohort study among 6910695 individuals, a linear increase in risk of severe COVID-19 leading to admission to hospital and death was observed. This risk increase was superior to that expected to diabetes only. The relative risk due to increasing body mass index was greater in people younger than 40 years and African origins[26]. Hence, T2D combined with obesity may be a synergic risk factor of severe COVID-19 pneumonia. Yet it has to be noted that obesity does not impact mortality as much in in elderly patients[27].
Influence of glycemic control on the prognosis of COVID-19: A key question is the role of hyperglycemia in patients with diabetes and COVID-19. This question must be analyzed differently depending on whether one considers the time before hospitalization, on admission or during the hospitalization phase. Results on glycemic control before hospitalization are contradictory. A major result of the CORONADO study is that glycemic control prior to hospitalization, assessed by the dosage of glycated hemoglobin (HbA1c), does not seem to have a significant impact on the severity of COVID-19 in people with diabetes who are hospitalized. On the contrary, in a population-based cohort study of people with diagnosed diabetes in England, people with T2D had a higher COVID-19-related mortality when HbA1c was superior to 59 mmol/mol (76%) in comparison to an HbA1c in the range 48–53 mmol/mol (65%-70%). In patients with T2D, this between-group difference was more pronounced in those under 70 than in those over 70 years-old. No significant difference was observed for HbA1c and risk of severe form of COVID-19 in patients with T1D[8,21].
On the other hand, association between hyperglycemia at the time of admission and mortality due to COVID-19 is clearer. Sardu et al[28] found that a glycaemia > 7.7 mmol/L on admission was associated with outcome in 132 Italian hyperglycemic patients hospitalized for COVID-19 pneumonia. In a Chinese retrospective multicenter study including hospitalized patients with COVID-19, well-controlled glycaemia (defined as a glycemic variability between 3.9 to 10.0 mmol/L) was associated with markedly lower mortality compared to individuals with poorly controlled glycaemia (upper limit of glycemic variability exceeding 10.0 mmol/L) (adjusted HR: 0.14)[6]. In a retrospective study among patients with diabetes and COVID-19 with use of continuous glucose monitoring, both glucose levels of > 8.8 mmol/L and < 3.85 mmol/L were associated with a significantly high risk of composite adverse outcomes of COVID-19 [i.e., need for admission to the intensive care unit (ICU), for mechanical ventilation, for vasopressor-requiring hypotension, multiple organ dysfunction] as well as with a prolonged hospitalization. Higher glycemic variability on admission was also significantly associated with a poorer outcome of COVID-19[29,30]. In contrast, mean sensor glucose level was not significantly associated with morbi-mortality[30].
While hyperglycemia on admission was associated with severe outcomes, question of causality remains elusive. Although Sardu et al[28] showed that the greater the decrease in blood glucose the better the outcomes were, in their observational study, causal relationship between correction of hyperglycemia and better prognosis could not be ascertained. In other settings than COVID-19, glycaemia on admission may be used as a biomarker to identify patients at higher risk of severe pneumonia[31,32]. Hence, randomized controlled studies are still needed to definitely answer this causality question.
There are many hypotheses about the mechanism of how diabetes affects COVID-19 course.
Hyperglycaemia and diabetes are associated with higher risk of infectious diseases: Infectious diseases, including pneumonia are leading causes of death in people with diabetes[33-35]. Specifically, diabetes was previously proven a major risk factor of mortality related to other viruses than severe acute respiratory disease coronavirus-2 (SARS-CoV-2), such as influenza A (H1N1) influenzae or the Middle East respiratory syndrome-related coronavirus (MERS-CoV)[36,37]. Mechanisms of susceptibility towards severe viral infections include neutrophil dysfunction and disturbance in the adaptative immune response in hyperglycemic environment[38-40]. As a matter of fact, hyperglycemia in itself, was associated with immune system impairment, complement fixation or altered cytokines and chemokines production enhancing SARS-CoV-2 replication[38,41-43]. However, severe forms of COVID-19 pneumonia in patients with hyperglycemia do not appear to result from an impaired humoral response against SARS-CoV-2[44]. These elements may explain the observations of improved prognosis associated with better blood glucose control in hospitalized patients with COVID-19, mentioned before.
Altered immune response and hyperinflammation in patients with diabetes and COVID-19: Histopathologic analysis revealed in fatal COVID-19 patients the presence of massive inflammatory cell infiltration in many organs such as lung, myocardial, liver, brain and nerves, kidney and pancreas[45]. Indeed, cytokines are secreted in great abundance and increased levels of inflammatory cytokines were associated with increased mortality in patients hospitalized for severe COVID-19 pneumonia. Among them, interleukin-6 (IL-6), also associated with cytokine release syndrome, is usually associated with diabetes, and denotes low-grade inflammation with circulating IL-6 Levels higher than in population without diabetes. Interestingly, study cohorts in patients admitted for severe COVID-19 pneumonia, showed higher levels of inflammation biomarkers including circulating IL-6, in patients with diabetes than other patients[46]. In T2D patients suffering from coronavirus infection, monocytopenia, morphological anomaly of increased monocyte size and CD8+T cells specific lymphopenia were observed[47]. The deregulation of innate and adaptive immune responses could lead to the hyperinflammation observed in severe COVID-19, especially in T2D patients. Visceral adipose tissue that is increased in patients with diabetes could represent a reservoir of cytokines and therefore could also explain the disproportionate inflammatory response observed in patients with diabetes and COVID-19.
Obesity and related disorders associated with the risk of severe forms of COVID-19: COVID-19 pneumonia may deteriorate in obese patients with diabetes due to poor respiratory mechanics. Indeed, when combining obesity and diabetes, patients present weaker respiratory muscle strength, reduced lung volume, increased resistance to the airways, impaired gas exchange, dysregulation of ventilatory control and bronchial dysautonomia[48-50]. Thus, COVID-19 severity could be more severe in case of pre-existing lung damage associated with obesity. Furthermore, in case of T2D and obesity, nonalcoholic fatty liver disease (NAFLD) is highly prevalent, and some data suggest that liver steatosis and higher stages of NAFLD (i.e., non-alcoholic steatohepatitis and liver fibrosis) would be a risk marker for SARS-CoV-2 infection severity[51]. Insulin resistance, a common pathophysiologic trait between obesity and T2D, could explain the increased risk of COVID-19 mortality in diabetes and obesity. Indeed, triglycerides and glucose index was closely associated with the severity and morbidity in COVID-19 patients[52]. In addition, complex interactions can occur between adipose tissue and the immune system[53]. Among them, hyperleptinemia and leptin resistance commonly observed in obesity, are implicated in the increased secretion of pro-inflammatory cytokines that sustain and enhance the inflammatory responses. Interestingly, hyperleptinemia and leptin resistance may aggravate clinical outcomes in infectious diseases, including H1N1 influenzae but also COVID-19[54,55]. Adipose tissue can finally constitute a viral reservoir of SARS-CoV-2, exacerbating the severity of COVID-19 through amplification of immune and cytokine activation[56]. Indeed, SARS-CoV-2 has a high affinity to bind the angiotensin-converting enzyme 2 (ACE2) receptors, highly expressed in adipose tissue. Obese patients have more adipose tissue than lean individuals, resulting in more ACE2 receptors. However, association between obesity and viral load has not been confirmed[57]. In sum, it is difficult to date to distinguish the role of overweight or obesity on the severity of SARS-CoV-2 infections in patients with T2D mellitus (T2DM) and the diabetic state itself.
Thromboembolic risk is increased in people with diabetes and COVID-19: COVID-19 is associated with an increased risk of thromboembolic events. Although, as of yet, there is no evidence of increased risk of such events in patients with diabetes in the setting of COVID-19 infections, several publications reported an increased thromboembolic risk in patients with diabetes. In a large population-based study which included 56158 patients with T2D and 168474 control patients, T2D patients exhibited an increased risk of venous thromboembolism (HR: 1.44, 95%CI: 1.27-1.63). Furthermore, the risks of pulmonary embolism were greater in the patients with T2D than in the controls (HR: 1.52, 95%CI: 1.22-1.90)[58]. Interestingly, hyperglycemia potentiates coagulation, whereas hyperinsulinemia inhibits fibrinolysis, suggesting that T2D patients may be especially vulnerable to prothrombotic events during inflammatory states such as COVID-19[38,59].
Endothelial cell dysfunction is observed in patients with diabetes and COVID-19: Histopathologic studies in patients with COVID-19 revealed evidence of viral presence in endothelial cells, and endothelitis was found in vascular beds of multiple organs such as heart, kidney, lungs, small intestine, and liver[60]. Vascular endothelial dysfunction seem to contribute to the pathophysiology of SARS-CoV-2 infection, by causing inflammatory cell infiltration, endothelial cell apoptosis and microvascular prothrombotic effects[61]. Interestingly endothelial dysfunction also features in patients with diabetes[62]. Hence, infection of dysfunctional endothelial cell by SARS-CoV-2 may be additive to that of diabetes.
The question of the benefit/risk balance of anti-diabetic treatments in patients suffering from COVID-19 quickly arose during the first half of 2020. The benefits and risks of each antidiabetic treatment as well as the recommendation for their use during COVID-19 are summarized in Table 1.
| Insulin | Sulfonylurea | Metformin | DPP-4 inhibitors | SGLT-2is | GLP-1a | |
| Benefits | Guarantee of achieving glycemic control; Cardiovascular neutrality; Possible use in multivisceral failure | Cardiovascular neutrality (demonstrated only with Glimepiride) | Probable cardiovascular benefit; No risk of hypoglycemia improvement of inflammation and endothelial dysfunction | Cardiovascular neutrality; Possible use in severe renal impairment and hypoxia; Possible inhibitory role on the entry of the virus into the cell; No risk of hypoglycemia | Proved cardiovascular and renal protective benefits; No risk of hypoglycemia Improvement of inflammation | Proved cardiovascular and renal protective benefits; Possibility of use up to the stage of severe renal failure; No risk of hypoglycemia; Improvement of inflammation and endothelial dysfunction |
| Risks | Increased glycaemic variability hypoglycaemic risks | Hypoglycaemic risk, contraindication in case of severe liver and renal failure | Multiple contraindications (hypoxia, severe renal failure, severe heart failure, severe liver failure); Risk of lactic acidosis especially in severe renal failure | Possible dysregulation of T cell function and T cell mediated inflammatory and immune responses | Reduced efficacy in moderate to severe renal impairment; Risk of ketoacidosis, especially in severe sepsis Risk of dehydration | Risk of digestive side effects; Risk of worsening undernutrition |
| Association with severe form of COVID-19 in observational studies | Conflicting results | Lower risk of severe form of COVID-19 or neutral association | Lower risk of severe form of COVID-19 or neutral association | Conflicting results | Lower risk of severe form of COVID-19 or neutral association | Neutral association |
| Medication use and severity of COVID-19 infection | Possibility of use at all stages of the disease and particularly in severe forms, especially recommended if blood sugar level is over 10-11 mM | Possible use up to moderate forms in the absence of severe renal and liver failure | Recommended use up to moderate forms in the absence of contraindications | Possible use up to moderate forms | Possible use up to moderate forms in the absence of moderate to severe renal failure | Possible use up to moderate forms |
Insulin: Insulin is often the recommended first-line anti-diabetic treatment in severe sepsis, especially if there is associated organ failure. Insulin infusion may be effective to achieve glycemic targets and improve outcomes in patients with COVID-19[28]. Although study design precluded causality analyses, patients with hyperglycemia treated with insulin infusion showed lower risk of severe disease than patients without insulin infusion. Meanwhile, other studies showed opposite results, with significant association between insulin treatment and a poorer prognosis of COVID-19[8,63,64]. Remarkably, the association between insulin use and mortality was independent of patients’ age. Nevertheless, the worse outcome in patients under insulin may be related to a more severe and complicated overall state rather than a treatment effect. This treatment is also more frequently associated with glycemic variability, which has been associated with severe forms of COVID-19. Insulin treatment remains, until now, the standard treatment in diabetic patients with severe forms of COVID-19.
Dipeptidyl peptidase-4 inhibitors: Dipeptidyl peptidase-4 (DPP4) inhibitors modify the biological activity of substrates involved in the immune response to the infection and therefore could have potential benefit or harm in COVID-19 course. However, evidence from clinical trials on the association between the use of DPP4 inhibitors and the risk of community-acquired pneumonia in T2D patients did not show any increased risk[65]. Although ACE2 represents the main receptor, DPP4 might also bind to SARS-CoV-2[66]. Hence, DPP4 inhibition may play a role in antagonizing the DPP4/CD26, which interacts with the S1 domain of the viral spike glycoprotein, protein by which SARS-CoV-2 attaches to the ACE-2 receptor expressed on the cells surface[67]. Some studies showed better outcome in COVID-19 patients taking DDP-4 inhibitors, with less severe pneumonia and lower mortality risk[64]. However, in a large register study, covering almost the entire population of patients with type 2 diabetes and COVID in England, DPP-4 inhibitors had a higher risk of COVID-19 related mortality[63]. However, here again the existence of confounding bias doesn’t allow to conclude to a causal effect. Finally, a propensity score analysis from the CORONADO study concluded that use of DPP-4 inhibitors during the COVID-19 pandemic was safe and that they should not be discontinued[68].
Sodium/glucose cotransporter 2 inhibitors: Given the risk of ketoacidosis, especially in severe sepsis, some have recommended not to prescribe sodium/glucose cotransporter 2 inhibitors (SGLT-2is) in patients with COVID-19, since SGLT-2is are associated with an increased risk of ketoacidosis[69]. However, SGLT-2is could impact many processes dysregulated during COVID-19. For example, SGLT-2is reduced, in T2D patients, infiltration of inflammatory cells into arterial plaques and decreased the mRNA expression levels of some cytokines and chemokines, such as TNF, IL-6 and monocyte chemoattractant protein 1[70,71]. This pharmacological class also features significant cardiovascular and reno-protective benefits in cardiometabolic disease, and may provide similar organ protection in COVID-19. Khunti et al[63] showed, in a register study, that SGLT-2is are associated with a significant 18% mortality reduction due to COVID-19. DARE-19, a randomized, double-blind, placebo-controlled trial in 1250 patients is aiming to evaluate the safety and efficacy of dapagliflozin in addition to standard of care therapy in hospitalized patients with COVID-19 and high risk of severe form including T2D[72]. The full DARE-19 trial results will be presented shortly and could answer to the question of the usefulness of iSGLT-2 in COVID-19.
GLP-1 analogues: GLP-1 analogues (GLP-1a) could represent a good therapeutic alternative to treat T2D patients. First, targeting GLP-1 axis could improve many pathways dysregulated during COVID-19. Exendin-4 can reduce inflammation, macrophages activation and monocyte adhesion to endothelial cells and improve endothelial function[73,74]. Second, GLP-1a could ameliorate lung injury in animal models[75]. Furthermore, GLP-1a have cardiovascular and reno-protective properties that could be beneficial during SARS-CoV-2 infection. In the other hand, GLP-1a have been associated with increased ACE2 expression in lungs and heart tissue suggesting of possible helpful and harmful effects in COVID-19[76]. Khunti et al[63] have shown, in a register study, that GLP-1a had a neutral effect for COVID-19-related death, as observed in the CORONADO study[8]. Although there is insufficient data to support the use of GLP-1a instead of insulin in patients with T2D and COVID-19, there is no evidence to discontinue them.
Sulfonylureas: Sulfonylureas are not associated with higher risk of COVID-19 mortality or are even sometimes associated with a lower risk[63]. However, no conclusion can be drawn since several biases exist in these studies. Indeed, use of sulfonylureas is limited in older and frailty people, in view of an increased risk of hypoglycemia.
Metformin: Many studies indicate that chronic metformin usage may have beneficial effects on COVID-19 with pre-existing T2D[19,63,77,78]. The findings of these studies could be related at least in part to confounding biases. Indeed, metformin is used early in the disease course of T2D, whereas other treatments such as insulin are initiated later or in case of contraindication to metformin such as renal failure. But surprisingly, benefits of metformin on COVID-19 outcomes occurred in spite of an apparently greater severity on admission compared with non-users[77]. Metformin could have beneficial effects on COVID-19 prognosis because of their protective properties on many cell types (e.g., endothelial cells, neurons and glial and cells, cardiomyocytes, hepatocytes, macrophages)[79]. Activation of adenosine monophosphate-activated protein kinase pathways by metformin could also affect the expression of ACE2, the receptor for SARS-CoV-2[80]. Interestingly, one study have shown that metformin benefits would be greater in female patients with diabetes and COVID-19[81]. Besides COVID-19 setting, metformin use is also associated with a reduction in mortality from sepsis in diabetic patients hospitalized in intensive care units[82]. Although there is no direct evidence for a protective role of metformin in SARS-CoV-2 infection, some elements plead in favor of maintaining this treatment in the absence of contraindication and in particular acute renal failure or hypoxia.
COVID-19 pneumonia may various presentations, from the most benign cough to the most severe ARDS requiring venovenous extracorporeal membranous oxygenation. The pathophysiological features of severe cases of COVID-19 are damages of the alveolii, inflammatory infiltrates, and microvascular circulation disorder leading to thrombosis. As previously stated, inflammatory organ injury may also, and multiples therapeutics have been suggested to decrease inflammatory organ injury but the effect of glucocorticoids has been debated.
Since the publication of results of the RECOVERY trial[83], all guidelines concur towards the use of corticosteroids in COVID-19 pneumonia: In patients hospitalized with COVID-19, use of dexamethasone resulted in lower 28-d mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization. Study protocol specified 6 mg of intravenous dexamethasone per day for 10 d. However, many patients are now treated at home, and French high council for public health recommended as alternative the use of methylprednisolone, 32 mg per day, or prednisone, 40 mg per day, or at very least the hydrocortisone at the dose of 160 mg for days (with decrease in 3 or 4 d), based on glucocorticoid equivalence, although results were less conclusive by oral route.
In a large meta-analysis, patients randomized to receive systemic dexamethasone, hydrocortisone, or methylprednisolone were compared to those who received usual care or placebo[84]. Primary outcome was all-cause mortality at 28 d. This analysis included 1703 patients, with 5 trials which reported mortality at 28 d, 1 trial at 21 d, and 1 trial at 30 d. There were 222 deaths among 678 patients randomized to corticosteroids and 425 deaths among 1025 patients randomized to usual care or placebo [odds-ratio = 0.66 (95%CI: 0.53-0.82), P < 0.001], in agreement with RECOVERY. Hereafter, medical community acknowledged the usefulness of corticosteroids in treating COVID-19 patients. Yet, corticosteroids are associated with well-known side effects, one of which being steroid-induced diabetes, for which other risk factors may increase this risk of adverse reaction. Meanwhile, means to prevent steroid-induced diabetes also exist.
This entity is defined as an abnormal increase in blood glucose due to the treatment, in patients with or without previous history of diabetes. Thresholds are for 8-h fasting glucose: Above 7.0 mmol/L; after 2-h post-75 g oral glucose tolerance test: Above 11.1 mmol/L (2 g/L); HbA1c above 6.5%; or in symptomatic patients, a random plasma glucose above 11.1 mmol/L (2 g/L)[85]. Prevalence is estimated to between 18.6% and 25% of patients who use of corticosteroids daily[86]. In a meta-analysis which aggregated 13 studies and included 34907 non-diabetic patients treated with glucocorticoids, the incidence of hyperglycemia was 32.3% and that of diabetes was 18.6%[87].
Mechanisms are plural and mostly feature beta cell dysfunction and insulin resistance. Beta cell dysfunction participates to insulin insufficiency due to decreased systemic release, and decreased sensitivity to glucose. Insulin resistance occurs in the liver, skeletal muscle, and fat cells, leading to decreased intracellular signals mediated by insulin. From a molecular point of view, glucocorticoids use schematically leads to a decrease in phosphorylation of the protein kinase B (PKB), which in turn leads to a decrease in activity of the glucose transporters GLUT4, which are less translocated to the cell surface, hence, lead to insulin resistance and decrease in glucose uptake, particularly at the muscle and adipose tissue level[88]. Besides, glucocorticoids induce an upregulation of the enzyme phosphoenolpyruvate carboxykinase (PEPCK) activity in the liver, while simultaneously downregulating PEPCK activity in adipose tissue. Circulating free fatty acids increase, which leads to insulin resistance and gluconeogenesis.
While the effects of glucocorticoids widely differ based on patients who use them, even in healthy individuals, they may have a significant impact on metabolism, in animal models[89], and even in healthy people[90]. In a study which focused on metabolomic profiling in 20 healthy men, 214 plasmatic metabolites were analyzed before and after the administration of dexamethasone 4 mg. Overall, 150 of 214 metabolites were significantly altered even after a single dose of dexamethasone. All main energy pathways, including glycolysis, Krebs cycle, urea cycle and lipids, fatty acids and amino acids were altered with an expected inter-individual variability.
Given that glucocorticoids may have that much of an impact even in healthy individuals, patients more at risk of developing diabetes such as elderly patients, overweight or already insulin resistant patients may be even more impacted by the smallest dosages of glucocorticoids. In addition to usual risk factors of developing diabetes, other comorbidities are at risk of developing subsequent steroid-induced diabetes. Specifically, rheumatic disorders and patients afflicted with chronic kidney disease, treated by glucocorticoids may be at higher risk. In a retrospective study which included 128 non-diabetic patients with either rheumatic or renal disease who started glucocorticoids, 84 (65%) developed diabetes, much higher that the incidence observed in the overall population[91]. Independent variables associated with incidence of diabetes included age 65 years, HbA1c level 6% and glomerular filtration rate below 40 mL/min/1.73m². Interestingly, dosage did not influence risk of developing diabetes.
While dexamethasone treatment indicated during COVID-19 treatment is relatively short (10 d), adverse effects may occur. In-vitro, beta cell function was studied under three regimens of dexamethasone (0.1, 0.5, and 1.0 mg/kg) for 5 d. In the first group (0.1 mg/kg) beta cell function increased to satisfy insulin demand. In the second group (0.5 mg/kg), beta cell proliferation increased, associated with hyperinsulinemia but not hyperglycemia. Finally, the last group (1.0 mg/kg) presented hyperglycemia and hyperinsulinemia, and a major increase in beta-cell proliferation and size[92]. In-vivo, in 6 healthy men, a single-dose of 75 mg prednisolone did not change fasting plasma glucose or insulin, but decreased oral glucose insulin sensitivity[93]. On day 2 beta cells recovered, as evidenced by an increase in fasting insulin secretion. The same study also looked at the impact of a 2-wk exposure of prednisolone in 33 healthy men, the treatment increased the fasting plasma glucose, decreased the index of insulin resistance, and decreased the index of insulin sensitivity. These elements showed that prednisolone impairs beta cell function in healthy subjects, both in acute and 2-wk exposure, meaning that steroids induce insulin resistance but also beta cell dysfunction, even for these short periods of administration.
Interestingly, in RECOVERY trial, only few severe adverse events related to dexamethasone administration were reported, yet, among 4 severe adverse events reported, 2 (50%) included severe hyperglycemia[83]. Only time will allow to assess the incidence of diabetes in patients treated with dexamethasone, as compared to control treatment. Moreover, the influence of COVID-19 itself on the risk of developing diabetes needs to be accounted for.
Infections, including COVID-19, may induce hyperglycemia in people without a previous diagnosis of diabetes. In fact, patients may present with stress hyperglycemia and thus, surpass the threshold only in the context of SARS-CoV-2 infection. Thus, hyperglycemia per se is not specific to COVID-19, especially since acute diabetes was commonly observed during SARS-CoV-1 epidemic among patients without prior history of diabetes and before the use of glucocorticoids[37]. However, the question has been raised as to whether SARS-CoV-2 can cause diabetes since new diabetes onset have been reported in numerous case reports simultaneously with acute SARS-CoV-2 infection. Although a meta-analysis of 8 studies including more than 3700 patients hospitalized for COVID-19 revealed a pooled incidence of 14.4% for new onset diabetes[94], mechanisms by which SARS-CoV-2 may induce diabetes remain unclear. Traditionally, 2 components are known to feature in diabetes pathogenesis: (1) Insulin resistance in the key tissues involved in glucose homeostasis, i.e., liver, skeletal muscles, and adipose tissue; and (2) Impaired insulin secretion by pancreatic β-cells.
Structural evidence reported that ACE2 was the receptor of SARS-CoV-2. Viral infection of host cells occurs through viral spike protein and ACE2 receptor. SARS-CoV-2 entrance into host cells then induces ACE2 internalization and shedding, leading to a down-regulation of ACE2.
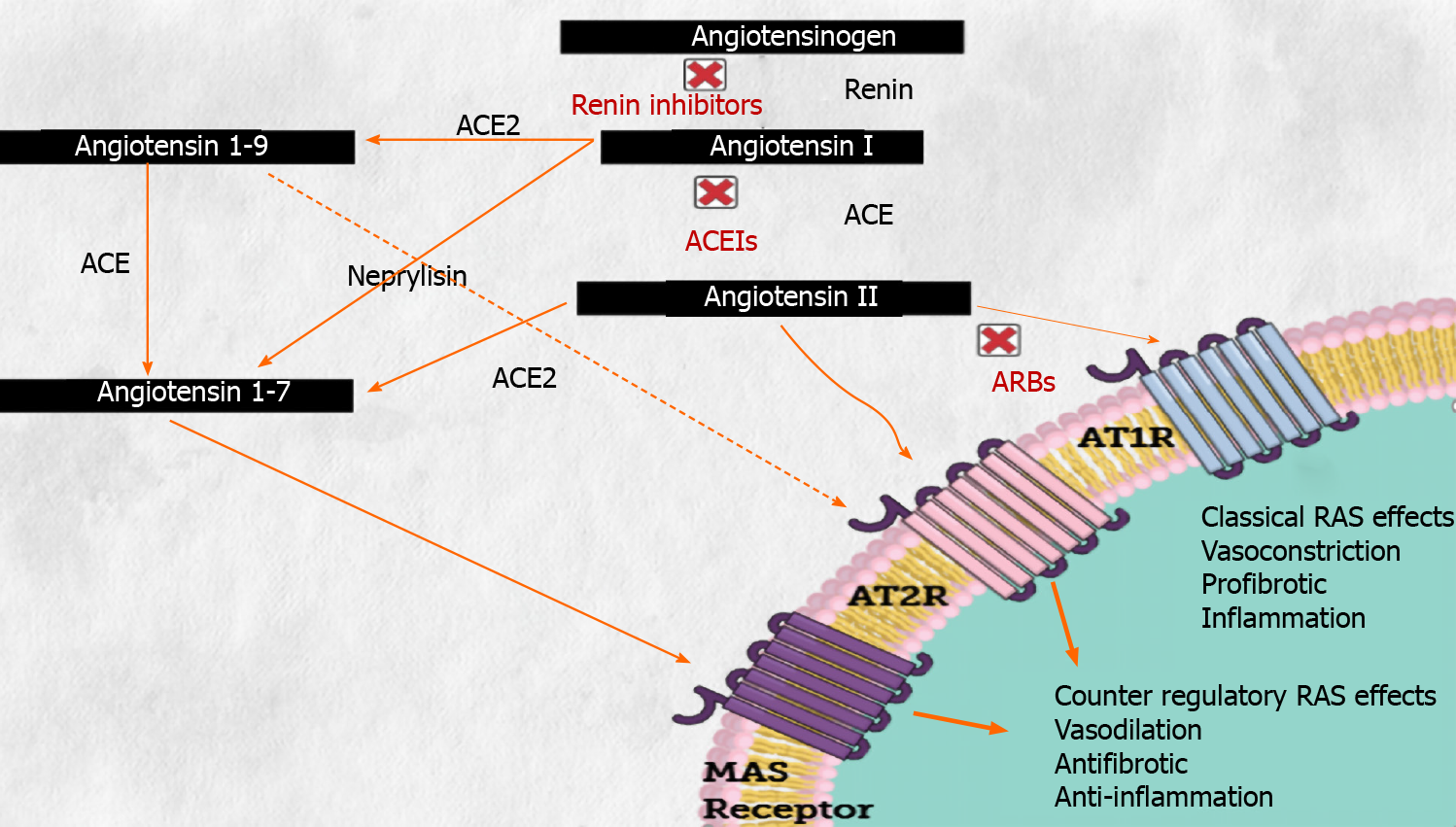
Of note, ACE2 belongs to the renin-angiotensin system (RAS). Briefly, angiotensinogen, produced by the liver, is cleaved by renin to form angiotensin-I which is then catalyzed by ACE to produce angiotensin-II (Ang II). ACE2 is a homologue of ACE that can hydrolyze Ang II to Ang-(1-7), whose reported effects include vasodilatation, anti-fibrosis, and anti-inflammation (these elements are summarized in Figure 1). As Ang II is associated with insulin resistance[95], a major component of T2DM, disturbance of ACE2 activity by SARS-CoV-2 in glucose homeostasis key-tissues may induce acute hyperglycemia. In addition, ACE2 expression was also reported in the endocrine pancreas suggesting that SARS-CoV-2 may cause beta cell damage inducing diabetes through insulin secretion deficiency[96], but also in exocrine pancreatic cells[97]. Therefore, dysregulation of ACE2 activity caused by COVID-19 infection could lead to diabetes through several mechanisms.
One of the most obvious mechanism by which SARS-CoV-2 induces insulin resistance is through systemic inflammation. Indeed, any inflammatory state can cause an insulin resistance leading to an increase hepatic glucose production through increased counter-regulatory hormones, cytokine and lipid release, and direct hepatocyte injury, irrespective of specific potential effects of SARS-CoV-2 on ACE2 activity and its repercussions on glucose homeostasis.
Then, since ACE2 is expressed in liver, skeletal muscles and adipose tissue, a disturbance in ACE2/Ang-(1-7) activity could lead to a glucose homeostasis disorder. Further support to this hypothesis comes from the fact that SARS-CoV-2 viral particles were not only identified in the cytoplasm of hepatocytes inducing a massive hepatic apoptosis[98], but were also involved in myositis occurrence[99]. Moreover, the loss of ACE2 gene in mice leads to hepatic fibrosis and impaired glucose homeostasis through an elevated hepatic reactive oxygen species level, an increased oxidative stress and inflammation in liver leading to an impairment of insulin signaling[100]. In adipose tissue, ACE2 deficiency worsens inflammation in response to a diet-induced obesity in mice[101]. Conversely, overexpression of ACE2 or Ang-(1-7) administration improves these metabolic disorders i.e., glycemic control and insulin sensitivity[102-104]. Indeed, mechanistically, Ang-(1-7) rescues insulin signaling pathway by stimulating PKB phosphorylation, a main mediator of insulin signaling pathway, what will then activate the downstream glycogen synthase kinase-3β in liver and skeletal muscles resulting in a decrease of glycaemia through glycogen storage[105], in several murine models of diet-induced insulin resistance such as high-fat diet fed mice or in fructose-fed rats[106,107]. In adipose tissue, activation of ACE2/Ang-(1-7) prevents inflammation and oxidative stress induced by a high-fat diet and increases glucose uptake and adiponectin level[108-110], while its disturbance results in a lower insulin-dependent glucose uptake and adiponectin secretion[111].
Besides the effects of the inhibition of the above mentioned alternate effects of the RAS, in the context of metabolic diseases such as obesity, T2DM or nonalcoholic fatty liver disease, plasmatic Ang II is positively correlated with body weight and is associated with insulin resistance, suggesting that ACE/Ang II activity is upregulated in those metabolic disorders[95]. Besides, on a tissue scale, Ang II was associated with increased insulin resistance through oxidative stress leading to a hepatic fibrosis and cirrhosis, provoking an impairment of insulin signaling[112]. In skeletal muscles, Ang II also induces a decreased glucose uptake and impairs insulin sensitivity[113], while in adipose tissue, it inhibits adiponectin secretion and insulin signaling still through an increased oxidative stress[114]. These elements emphasize the pro-diabetogenic effects of the classical RAS effects.
In a few COVID-19 human pancreas postmortem examinations, SARS-CoV-2 nucleocapsid protein were detected in pancreatic exocrine cells as well as in endocrine β-cells[115]. Furthermore, the RAS, including ACE2 was also found involved in the pancreatic insulin-producing tissue[116]. However, ACE2 expression by β cells responsible for insulin secretion remains controversial. Indeed, analyses from transcriptional datasets of human islet cells find a very weak expression of ACE2 in beta cells. These analyses are supported by immunohistochemistry of human pancreatic tissues that do not identify ACE2 expression on β cells but rather on ducts and microvascular structures[97], whereas double immunofluorescence labelling in rat pancreas indicates that insulin-containing beta cells abundantly express ACE2[117]. The latter observations suggest that ACE2 would play a role in insulin-containing beta cells and are supported by experiments in ACE2-deficient mice indicating that ACE2 loss aggravates beta cell dedifferentiation and impairs their proliferation, leading to a significant reduction of beta cell mass[118]. Similarly, in a genetic murine model of obesity and diabetes, ACE2 overexpression in pancreas, improved glycemic control through Ang-(1-7), inducing both β-cell proliferation and apoptosis reduction[102]. As expected, similarly as a loss of ACE2, Ang II supplementation in beta cells significantly increased endoplasmic reticulum (ER) stress and inflammation leading to reduced insulin secretion whereas Ang II receptor blockade in beta cells reduced significantly ER stress and rescues insulin secretion[119].
Moreover, ACE2 was also found on ductal structures and microvasculature of the pancreas, new diabetes onset may be secondary to pancreatitis as SARS-CoV-2 has been isolated in a pancreatic pseudocyst from a patient with acute pancreatitis. However, acute pancreatitis seems to be a very infrequent complication of SARS-CoV-2 infection. Two cohort studies which included 11000 and 63822 patients with COVID-19 respectively, acute pancreatitis prevalence was estimated at 0.27% and 0.07%[120]. Therefore, acute pancreatitis occurrence in patients with COVID-19 is exceedingly rare and its putative mechanism related to direct viral damage of pancreatic cells still need investigations.
Therefore, these findings suggest that ACE2 may play a role in the beta cell insulin secretory response to hyperglycemia and imply that SARS-CoV-2 could penetrate then destroy the insulin-containing beta cells, causing subsequently acute diabetes through insulin secretion deficiency.
Viral infections, in particular by enteroviruses and coronaviruses, have been widely associated with T1DM pathogenesis[121]. T1DM is characterized by an autoimmune pancreatic β-cells progressive destruction leading to insulin deficiency. Therefore, SARS-CoV-2 could also act as an infectious trigger decompensating and precipitating diabetic ketoacidosis in patients with no history of diabetes as reported in few case reports[122-124], and arising evidence highlight the ability of SARS-CoV-2 to trigger autoimmune disorders[125]. Nonetheless, data remain conflictual on this point. Evidence from an italian cross-sectional study revealed 23% fewer new-onset cases, with more children with new-onset disease presenting in diabetic ketoacidosis during early months of pandemia compared to the same period in 2019 while a multicenter study from the United Kingdom described an 80% increase in new-onset T1DM in children[126]. From a German Diabetes-Prospective Follow-up registry, the rate of new-onset T1DM from March to May 2020 did not differ significantly from rates observed over the previous decade[127]. However, when this study was done, COVID-19 infection incidence rate was relatively low in Germany, and weak effect cannot be excluded. Thus, from these studies, no compelling evidence emerge for a causal role of SARS-CoV-2 in a change of T1DM incidence. Furthermore, it was difficult to differentiate a viral secondary diabetes from a real T1DM as no assay for type 1 diabetes antibodies (GAD, IA2, ZNT8, ICA antibodies) has been performed in those series. More complexly, a few cases of insulin-dependent diabetes with negative antibodies start to emerge suggesting a T1bDM[128]. However, such form of diabetes is particularly widespread among Sub-Saharan African, African-Americans and Hispanic descendants and case reports concern Caucasian and Asian ethnicities suggesting a viral secondary diabetes more than a T1DM or T1bDM. Follow-up studies on the evolution of anti-diabetic therapy are needed to understand the pathophysiology of SARS-CoV-2-induced diabetes.
In the end, the potential diabetogenic role of SARS-CoV-2 may be more complex than the simple beta cell hosts destruction by the virus through ACE2 expression. New-onset diabetes can result from several pathogenic processes involving pancreatic cell destruction (including exocrine and endocrine cells) through viral or autoimmunity destruction and/or insulin resistance in liver, skeletal muscles, and adipose tissue through disturbance of ACE2/Ang-(1-7) activity.
Diabetic patients are heavily impacted by the effects of COVID-19, as they are more at risk of developing severe forms and are more at risk of mortality. While antidiabetic treatments are still under investigation, data do not warrant discontinuation of these treatment in diabetic patients. While corticosteroids count among the few validated medications in severe COVID-19 pneumonia, they expose patients to a hypothetical risk of new onset of diabetes or diabetes deterioration, even though treatment duration is short. Finally, risk of developing diabetes after COVID-19, due to interactions with the angiotensin-converting-enzyme 2 needs to be accounted for when assessing risk of subsequent diabetes in treated patients.
| 1. | Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 339] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 2. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2137] [Article Influence: 356.2] [Reference Citation Analysis (0)] |
| 3. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14870] [Article Influence: 2478.3] [Reference Citation Analysis (1)] |
| 4. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5579] [Article Influence: 929.8] [Reference Citation Analysis (1)] |
| 5. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18404] [Article Influence: 3067.3] [Reference Citation Analysis (13)] |
| 6. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1123] [Article Influence: 187.2] [Reference Citation Analysis (0)] |
| 7. | McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, Hutchinson S, Caparrotta TM, Mellor J, Jeyam A, O'Reilly JE, Wild SH, Hatam S, Höhn A, Colombo M, Robertson C, Lone N, Murray J, Butterly E, Petrie J, Kennon B, McCrimmon R, Lindsay R, Pearson E, Sattar N, McKnight J, Philip S, Collier A, McMenamin J, Smith-Palmer A, Goldberg D, McKeigue PM, Colhoun HM; Public Health Scotland COVID-19 Health Protection Study Group; Scottish Diabetes Research Network Epidemiology Group. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 8. | Wargny M, Potier L, Gourdy P, Pichelin M, Amadou C, Benhamou PY, Bonnet JB, Bordier L, Bourron O, Chaumeil C, Chevalier N, Darmon P, Delenne B, Demarsy D, Dumas M, Dupuy O, Flaus-Furmaniuk A, Gautier JF, Guedj AM, Jeandidier N, Larger E, Le Berre JP, Lungo M, Montanier N, Moulin P, Plat F, Rigalleau V, Robert R, Seret-Bégué D, Sérusclat P, Smati S, Thébaut JF, Tramunt B, Vatier C, Velayoudom FL, Vergès B, Winiszewski P, Zabulon A, Gourraud PA, Roussel R, Cariou B, Hadjadj S; CORONADO investigators. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64:778-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | Agarwal S, Schechter C, Southern W, Crandall JP, Tomer Y. Preadmission Diabetes-Specific Risk Factors for Mortality in Hospitalized Patients With Diabetes and Coronavirus Disease 2019. Diabetes Care. 2020;43:2339-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2061] [Cited by in RCA: 2092] [Article Influence: 348.7] [Reference Citation Analysis (0)] |
| 11. | Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30:1236-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 12. | Istituto Superiore di Sanita. Report of characteristics of patients died positive for COVID-19 in Italy. [cited 20 May 2021]. Available from: https://www.epicentro.iss. |
| 13. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6580] [Article Influence: 1096.7] [Reference Citation Analysis (0)] |
| 14. | National Health Interview Survey. Early release of selected estimates based on data from the 2018 National Health Interview Survey. [cited 20 May 2021]. Available from: https://www.cdc.gov/nchs/nhis/releases/released201905.htm#14. |
| 15. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1629] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 16. | Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1847] [Article Influence: 307.8] [Reference Citation Analysis (1)] |
| 17. | Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, Chaudhry Z, Vahia A, Abreu Lanfranco O, Ramesh M, Zervos MJ, Alangaden G, Miller J, Brar I. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 450] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 18. | Shi Q, Zhang X, Jiang F, Hu N, Bimu C, Feng J, Yan S, Guan Y, Xu D, He G, Chen C, Xiong X, Liu L, Li H, Tao J, Peng Z, Wang W. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 19. | Schlesinger S, Neuenschwander M, Lang A, Pafili K, Kuss O, Herder C, Roden M. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia. 2021;64:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Vangoitsenhoven R, Martens PJ, van Nes F, Moyson C, Nobels F, Van Crombrugge P, Wierckx K, van Pottelbergh I, Van Huffel L, Gillard P, Mathieu C. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients With Type 1 Diabetes. Diabetes Care. 2020;43:e118-e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 620] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 22. | Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 678] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 23. | d'Annunzio G, Maffeis C, Cherubini V, Rabbone I, Scaramuzza A, Schiaffini R, Minuto N, Piccolo G, Maghnie M. Caring for children and adolescents with type 1 diabetes mellitus: Italian Society for Pediatric Endocrinology and Diabetology (ISPED) statements during COVID-19 pandemia. Diabetes Res Clin Pract. 2020;168:108372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Verma A, Rajput R, Verma S, Balania VKB, Jangra B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 Diabetes Mellitus. Diabetes Metab Syndr. 2020;14:1213-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 25. | Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, Mathieu D, Subtil F, Frobert E, Alligier M, Delaunay D, Vanhems P, Laville M, Jourdain M, Disse E; COVID Outcomes HCL Consortium and Lille COVID–Obesity Study Group. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 26. | Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O'Rahilly S, Aveyard P, Jebb SA. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9:350-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 370] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 27. | Smati S, Tramunt B, Wargny M, Caussy C, Gaborit B, Vatier C, Vergès B, Ancelle D, Amadou C, Bachir LA, Bourron O, Coffin-Boutreux C, Barraud S, Dorange A, Fremy B, Gautier JF, Germain N, Larger E, Laugier-Robiolle S, Meyer L, Monier A, Moura I, Potier L, Sabbah N, Seret-Bégué D, Winiszewski P, Pichelin M, Saulnier PJ, Hadjadj S, Cariou B, Gourdy P; CORONADO investigators. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes Obes Metab. 2021;23:391-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Sardu C, D'Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, Maggi P, Coppola N, Paolisso G, Marfella R. Outcomes in Patients With Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care. 2020;43:1408-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 29. | Chao WC, Tseng CH, Wu CL, Shih SJ, Yi CY, Chan MC. Higher glycemic variability within the first day of ICU admission is associated with increased 30-day mortality in ICU patients with sepsis. Ann Intensive Care. 2020;10:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Shen Y, Fan X, Zhang L, Wang Y, Li C, Lu J, Zha B, Wu Y, Chen X, Zhou J, Jia W. Thresholds of Glycemia and the Outcomes of COVID-19 Complicated With Diabetes: A Retrospective Exploratory Study Using Continuous Glucose Monitoring. Diabetes Care. 2021;44:976-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Lepper PM, Ott S, Nüesch E, von Eynatten M, Schumann C, Pletz MW, Mealing NM, Welte T, Bauer TT, Suttorp N, Jüni P, Bals R, Rohde G; German Community Acquired Pneumonia Competence Network. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Lepper PM, Bals R, Jüni P, von Eynatten M. Blood glucose, diabetes and metabolic control in patients with community-acquired pneumonia. Diabetologia. 2020;63:2488-2490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Harding JL, Benoit SR, Gregg EW, Pavkov ME, Perreault L. Trends in Rates of Infections Requiring Hospitalization Among Adults With Versus Without Diabetes in the U.S., 2000-2015. Diabetes Care. 2020;43:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Magliano DJ, Harding JL, Cohen K, Huxley RR, Davis WA, Shaw JE. Excess Risk of Dying From Infectious Causes in Those With Type 1 and Type 2 Diabetes. Diabetes Care. 2015;38:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Alqahtani FY, Aleanizy FS, Ali El Hadi Mohamed R, Alanazi MS, Mohamed N, Alrasheed MM, Abanmy N, Alhawassi T. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2018;147:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, Sun GZ, Yang GR, Zhang XL, Wang L, Xu X, Xu XP, Chan JC. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 502] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 38. | Stegenga ME, van der Crabben SN, Blümer RM, Levi M, Meijers JC, Serlie MJ, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (4)] |
| 39. | Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29-34. [PubMed] [DOI] [Full Text] |
| 40. | Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic Control and Risk of Infections Among People With Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care. 2018;41:2127-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 41. | Hair PS, Echague CG, Rohn RD, Krishna NK, Nyalwidhe JO, Cunnion KM. Hyperglycemic conditions inhibit C3-mediated immunologic control of Staphylococcus aureus. J Transl Med. 2012;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, van den Pangaart PS, de Vos AF, Tanck MW, Roos D, Sauerwein HP, van der Poll T. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med. 2008;25:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de Biagi Junior CAO, Crunfli F, Jimenez Restrepo JL, Vendramini PH, Reis-de-Oliveira G, Bispo Dos Santos K, Toledo-Teixeira DA, Parise PL, Martini MC, Marques RE, Carmo HR, Borin A, Coimbra LD, Boldrini VO, Brunetti NS, Vieira AS, Mansour E, Ulaf RG, Bernardes AF, Nunes TA, Ribeiro LC, Palma AC, Agrela MV, Moretti ML, Sposito AC, Pereira FB, Velloso LA, Vinolo MAR, Damasio A, Proença-Módena JL, Carvalho RF, Mori MA, Martins-de-Souza D, Nakaya HI, Farias AS, Moraes-Vieira PM. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:437-446.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 583] [Article Influence: 97.2] [Reference Citation Analysis (3)] |
| 44. | Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, Davalli A, Caretto A, Laurenzi A, Martinenghi S, Molinari C, Vitali G, Di Filippo L, Mercalli A, Melzi R, Tresoldi C, Rovere-Querini P, Landoni G, Ciceri F, Bosi E, Piemonti L. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020;63:2548-2558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 45. | Eketunde AO, Mellacheruvu SP, Oreoluwa P. A Review of Postmortem Findings in Patients With COVID-19. Cureus. 2020;12:e9438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 359] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 47. | Alzaid F, Julla JB, Diedisheim M, Potier C, Potier L, Velho G, Gaborit B, Manivet P, Germain S, Vidal-Trecan T, Roussel R, Riveline JP, Dalmas E, Venteclef N, Gautier JF. Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes. EMBO Mol Med. 2020;12:e13038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Fuso L, Pitocco D, Antonelli-Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metab Res Rev. 2019;35:e3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Schubert L, Laroche S, Hartemann A, Bourron O, Phan F. Impaired hypoxic ventilatory drive induced by diabetic autonomic neuropathy, a cause of misdiagnosed severe cardiac events: brief report of two cases. BMC Cardiovasc Disord. 2021;21:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 52. | Ren H, Yang Y, Wang F, Yan Y, Shi X, Dong K, Yu X, Zhang S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 53. | De Bandt JP, Monin C. Obesity, Nutrients and the Immune System in the Era of COVID-19. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, Yuen KY. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Karampela I, Christodoulatos GS, Dalamaga M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr Obes Rep. 2019;8:434-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 56. | Ryan PM, Caplice NM. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity (Silver Spring). 2020;28:1191-1194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 57. | Argyropoulos KV, Serrano A, Hu J, Black M, Feng X, Shen G, Call M, Kim MJ, Lytle A, Belovarac B, Vougiouklakis T, Lin LH, Moran U, Heguy A, Troxel A, Snuderl M, Osman I, Cotzia P, Jour G. Association of Initial Viral Load in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patients with Outcome and Symptoms. Am J Pathol. 2020;190:1881-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost. 2015;114:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;142:184-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 877] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 60. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2113] [Article Influence: 352.2] [Reference Citation Analysis (7)] |
| 61. | Oxford AE, Halla F, Robertson EB, Morrison BE. Endothelial Cell Contributions to COVID-19. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 850] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 63. | Khunti K, Knighton P, Zaccardi F, Bakhai C, Barron E, Holman N, Kar P, Meace C, Sattar N, Sharp S, Wareham NJ, Weaver A, Woch E, Young B, Valabhji J. Prescription of glucose-lowering therapies and risk of COVID-19 mortality in people with type 2 diabetes: a nationwide observational study in England. Lancet Diabetes Endocrinol. 2021;9:293-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 64. | Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, Mazziotti G, Lania AG. Impact of Comorbidities and Glycemia at Admission and Dipeptidyl Peptidase 4 Inhibitors in Patients With Type 2 Diabetes With COVID-19: A Case Series From an Academic Hospital in Lombardy, Italy. Diabetes Care. 2020;43:3042-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 65. | Gorricho J, Garjón J, Alonso A, Celaya MC, Saiz LC, Erviti J, López A. Use of oral antidiabetic agents and risk of community-acquired pneumonia: a nested case-control study. Br J Clin Pharmacol. 2017;83:2034-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Li Y, Zhang Z, Yang L, Lian X, Xie Y, Li S, Xin S, Cao P, Lu J. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. iScience. 2020;23:101160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 67. | Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 454] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 68. | Roussel R, Darmon P, Pichelin M, Goronflot T, Abouleka Y, Ait Bachir L, Allix I, Ancelle D, Barraud S, Bordier L, Carlier A, Chevalier N, Coffin-Boutreux C, Cosson E, Dorange A, Dupuy O, Fontaine P, Fremy B, Galtier F, Germain N, Guedj AM, Larger E, Laugier-Robiolle S, Laviolle B, Ludwig L, Monier A, Montanier N, Moulin P, Moura I, Prevost G, Reznik Y, Sabbah N, Saulnier PJ, Serusclat P, Vatier C, Wargny M, Hadjadj S, Gourdy P, Cariou B; CORONADO investigators. Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: A propensity score analysis from the CORONADO study. Diabetes Obes Metab. 2021;23:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Scheen AJ. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46:423-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 70. | Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE -/- mice fed a western diet. Diabetologia. 2017;60:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 71. | Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 72. | Kosiborod M, Berwanger O, Koch GG, Martinez F, Mukhtar O, Verma S, Chopra V, Javaheri A, Ambery P, Gasparyan SB, Buenconsejo J, Sjöström CD, Langkilde AM, Oscarsson J, Esterline R. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID-19: Design and rationale for the DARE-19 study. Diabetes Obes Metab. 2021;23:886-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 73. | Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 74. | Ceriello A, Kilpatrick ES. Glycemic variability: both sides of the story. Diabetes Care. 2013;36 Suppl 2:S272-S275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154:4503-4511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 76. | Dambha-Miller H, Albasri A, Hodgson S, Wilcox CR, Khan S, Islam N, Little P, Griffin SJ. Currently prescribed drugs in the UK that could upregulate or downregulate ACE2 in COVID-19 disease: a systematic review. BMJ Open. 2020;10:e040644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Lalau JD, Al-Salameh A, Hadjadj S, Goronflot T, Wiernsperger N, Pichelin M, Allix I, Amadou C, Bourron O, Duriez T, Gautier JF, Dutour A, Gonfroy C, Gouet D, Joubert M, Julier I, Larger E, Marchand L, Marre M, Meyer L, Olivier F, Prevost G, Quiniou P, Raffaitin-Cardin C, Roussel R, Saulnier PJ, Seret-Begue D, Thivolet C, Vatier C, Desailloud R, Wargny M, Gourdy P, Cariou B; CORONADO investigators. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab. 2020;47:101216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 78. | Cheng X, Xin S, Chen Y, Li L, Chen W, Li W, Zhou B, Li C, Gong Y, Li F, Duan P, Zhou X. Effects of metformin, insulin on COVID-19 patients with pre-existed type 2 diabetes: A multicentral retrospective study. Life Sci. 2021;275:119371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Wiernsperger N. Metformin as a cellular protector; a synoptic view of modern evidences. J Nephropharmacol. 2015;4:31-36. [PubMed] |
| 80. | Zangiabadian M, Nejadghaderi SA, Zahmatkesh MM, Hajikhani B, Mirsaeidi M, Nasiri MJ. The Efficacy and Potential Mechanisms of Metformin in the Treatment of COVID-19 in the Diabetics: A Systematic Review. Front Endocrinol (Lausanne). 2021;12:645194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 81. | Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J, McNeil C, Feng R, Guzman G, Abdelwahab N, King S, Tamariz L, Meehan T, Pendleton KM, Benson B, Vojta D, Tignanelli CJ. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34-e41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 82. | Liang H, Ding X, Li L, Wang T, Kan Q, Wang L, Sun T. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. 2019;23:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 83. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7583] [Article Influence: 1516.6] [Reference Citation Analysis (7)] |
| 84. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1709] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 85. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1214] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 86. | Hwang JL, Weiss RE. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 87. | Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 88. | van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009;39:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 89. | Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 1997;99:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 294] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 90. | Bordag N, Klie S, Jürchott K, Vierheller J, Schiewe H, Albrecht V, Tonn JC, Schwartz C, Schichor C, Selbig J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci Rep. 2015;5:15954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 91. | Katsuyama T, Sada KE, Namba S, Watanabe H, Katsuyama E, Yamanari T, Wada J, Makino H. Risk factors for the development of glucocorticoid-induced diabetes mellitus. Diabetes Res Clin Pract. 2015;108:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab. 2009;296:E681-E689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | van Raalte DH, Nofrate V, Bunck MC, van Iersel T, Elassaiss Schaap J, Nässander UK, Heine RJ, Mari A, Dokter WH, Diamant M. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes Metab. 2021;23:870-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 95. | Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Miyashita Y, Shirai K. Circulating angiotensin II is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 787] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 97. | Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G; HPAP Consortium, Brissova M, Powers AC. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020;32:1028-1040.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 98. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 99. | Beydon M, Chevalier K, Al Tabaa O, Hamroun S, Delettre AS, Thomas M, Herrou J, Riviere E, Mariette X. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 100. | Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 101. | Montaigne D, Coisne A, Marechal X, Staels B. Comment on Patel et al. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 2016;65:85-95. Diabetes. 2016;65:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540-2548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 103. | Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 104. | Cao X, Yang F, Shi T, Yuan M, Xin Z, Xie R, Li S, Li H, Yang JK. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci Rep. 2016;6:21592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 105. | Muñoz MC, Giani JF, Dominici FP. Angiotensin-(1-7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept. 2010;161:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Feltenberger JD, Andrade JM, Paraíso A, Barros LO, Filho AB, Sinisterra RD, Sousa FB, Guimarães AL, de Paula AM, Campagnole-Santos MJ, Qureshi M, dos Santos RA, Santos SH. Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension. 2013;62:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Santos SH, Andrade JM, Fernandes LR, Sinisterra RD, Sousa FB, Feltenberger JD, Alvarez-Leite JI, Santos RA. Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides. 2013;46:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Santos SH, Fernandes LR, Pereira CS, Guimarães AL, de Paula AM, Campagnole-Santos MJ, Alvarez-Leite JI, Bader M, Santos RA. Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept. 2012;178:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Liu C, Lv XH, Li HX, Cao X, Zhang F, Wang L, Yu M, Yang JK. Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol. 2012;49:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 110. | Santos SH, Braga JF, Mario EG, Pôrto LC, Rodrigues-Machado Mda G, Murari A, Botion LM, Alenina N, Bader M, Santos RA. Improved lipid and glucose metabolism in transgenic rats with increased circulating angiotensin-(1-7). Arterioscler Thromb Vasc Biol. 2010;30:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 111. | Santos SH, Fernandes LR, Mario EG, Ferreira AV, Pôrto LC, Alvarez-Leite JI, Botion LM, Bader M, Alenina N, Santos RA. Mas deficiency in FVB/N mice produces marked changes in lipid and glycemic metabolism. Diabetes. 2008;57:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 112. | Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. 2008;49:417-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137-35146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 114. | Chou CL, Lin H, Chen JS, Fang TC. Renin inhibition improves metabolic syndrome, and reduces angiotensin II levels and oxidative stress in visceral fat tissues in fructose-fed rats. PLoS One. 2017;12:e0180712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C, Fois G, Eiseler T, Gehrmann J, van Vuuren J, Wessbecher IM, Frick M, Costa IG, Breunig M, Grüner B, Peters L, Schuster M, Liebau S, Seufferlein T, Stenger S, Stenzinger A, MacDonald PE, Kirchhoff F, Sparrer KMJ, Walther P, Lickert H, Barth TFE, Wagner M, Münch J, Heller S, Kleger A. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 381] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 116. | Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol. 2007;580:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 117. | Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res. 2010;38:558-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Shoemaker R, Yiannikouris F, Thatcher S, Cassis L. ACE2 deficiency reduces β-cell mass and impairs β-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab. 2015;309:E621-E631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 119. | Ramalingam L, Sopontammarak B, Menikdiwela KR, Moustaid-Moussa N. Endoplasmic Reticulum (ER) Stress in Part Mediates Effects of Angiotensin II in Pancreatic Beta Cells. Diabetes Metab Syndr Obes. 2020;13:2843-2853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Inamdar S, Benias PC, Liu Y, Sejpal DV, Satapathy SK, Trindade AJ; Northwell COVID-19 Research Consortium. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients With Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology. 2020;159:2226-2228.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 121. | Lönnrot M, Lynch KF, Elding Larsson H, Lernmark Å, Rewers MJ, Törn C, Burkhardt BR, Briese T, Hagopian WA, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Hyöty H; TEDDY Study Group. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 122. | Benyakhlef S, Abdellaoui W, Tahri A, Rouf S, Latrech H. Diabetic Ketoacidosis at Onset of Pediatric Type-1 Diabetes Triggered by Covid-19: An Original Case Report. Cureus. 2021;13:e13958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Rabizadeh S, Hajmiri M, Rajab A, Emadi Kouchak H, Nakhjavani M. Severe diabetic ketoacidosis and coronavirus disease 2019 (COVID-19) infection in a teenage patient with newly diagnosed diabetes. J Pediatr Endocrinol Metab. 2020;33:1241-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 124. | Eskandarani RM, Sawan S. Diabetic Ketoacidosis on Hospitalization with COVID-19 in a Previously Nondiabetic Patient: A Review of Pathophysiology. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420984125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 125. | Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19:102695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 126. | Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, Kwong RMW, Kumar P, Logan KM. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care. 2020;43:e170-e171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 127. | Tittel SR, Rosenbauer J, Kamrath C, Ziegler J, Reschke F, Hammersen J, Mönkemöller K, Pappa A, Kapellen T, Holl RW; DPV Initiative. Did the COVID-19 Lockdown Affect the Incidence of Pediatric Type 1 Diabetes in Germany? Diabetes Care. 2020;43:e172-e173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 128. | Hollstein T, Schulte DM, Schulz J, Glück A, Ziegler AG, Bonifacio E, Wendorff M, Franke A, Schreiber S, Bornstein SR, Laudes M. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020;2:1021-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Azarbakhsh H, Papadopoulos KI S-Editor: Yan JP L-Editor: A P-Editor: Ma YJ