Published online Nov 15, 2020. doi: 10.4239/wjd.v11.i11.553
Peer-review started: July 18, 2020
First decision: August 9, 2020
Revised: August 22, 2020
Accepted: October 15, 2020
Article in press: October 15, 2020
Published online: November 15, 2020
Processing time: 117 Days and 20.8 Hours
Diabetic nephropathy (DN) is the main cause of chronic kidney disease and end-stage renal disease worldwide. Although available clinical trials have shown that endothelin receptor (ER) antagonists may be a novel and beneficial drug for DN, no consistent conclusions regarding their sufficient effectiveness and safety for patients with DN have been presented.
To assess the effectiveness and safety of ER antagonists among patients with DN.
The EMBASE, PubMed, MEDLINE, Cochrane, and ClinicalTrials.gov databases were searched without any language restrictions. Relative risks with 95% confidence intervals (CIs) for dichotomous data and mean differences or standardized mean difference with 95%CIs for continuous data were calculated using Review Manager 5.3 software. Publication bias was assessed using Egger’s test with Stata/SE software.
We enrolled seven studies with six data sets and 5271 participants. The ER antagonists group showed a significantly greater reduction in albuminuria and more patients with 40% reduction in urinary albumin-to-creatinine ratio than the control group (P < 0.0001 and P = 0.02, respectively). Subgroup analysis for reductions in estimated glomerular filtration rate (eGFR) showed that for the middle-dosage subgroup, the ER antagonists group exhibited lower eGFR reduction than the control group (P < 0.00001; mean difference, 0.70 95%CI: 0.66, 0.74). Moreover, significant reductions in systolic and diastolic blood pressure were observed in the invention group.
ER blockades combined with angiotensin converting enzyme inhibitor /angiotensin II type 1 receptor blockers may be an effective treatment to lower blood pressure and reduce proteinuria in DN with declined eGFR. However, attention should be given to adverse events, including cardiac failure, anemia, and hypoglycemia, as well as serious adverse events.
Core Tip: Patients with type 2 diabetes are at increased risk for vascular complications, such as nephropathy, coronary artery disease, and retinopathy. Endothelin receptor (ER) antagonists might be a novel and beneficial drug for diabetic nephropathy (DN). However, no consistent conclusions regarding their effectiveness and safety for patients with DN have been presented. We conducted this meta-analysis of available clinical data on ER antagonists aimed to assess the effectiveness and safety of ER antagonists among patients with DN.
- Citation: Zhang L, Xue S, Hou J, Chen G, Xu ZG. Endothelin receptor antagonists for the treatment of diabetic nephropathy: A meta-analysis and systematic review. World J Diabetes 2020; 11(11): 553-566
- URL: https://www.wjgnet.com/1948-9358/full/v11/i11/553.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i11.553
Studies show diabetic nephropathy (DN) has been the main cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide[1]. The activation of the renin–angiotensin–aldosterone system (RAAS) has been regarded as an important element for CKD progression. Current treatment approaches rely on angiotensin converting enzyme inhibitors or angiotensin II type 1 receptor blockers to control blood pressure (BP), reduce proteinuria, and delay CKD progression[2]. However, such approaches remain controversial given the evidence showing that RAAS blockade may increase the long-term risk for ESRD in patients with diabetes[3]. Thus, identifying novel, effective treatments for DN beyond RAAS blockades is imperative.
In the kidneys, endothelin-1 (ET-1) exerts several physiological effects, including control of water and sodium homeostasis. The ET system is complicated, consisting of a converting enzyme and two active receptors: The ETA (ETA-R) and ETB (ETB-R) receptors[4]. The activation of ETA-Rs mainly in vascular smooth muscles causes extremely potent vasoconstriction, endothelial dysfunction, insulin resistance, inflammation, and fibrosis. On the other hand, ETB-Rs, which are mainly expressed in the vascular endothelium, can induce vasodilatation via nitric oxide and prostanoid release[4,5]. Patients with type 2 diabetes have been found to have increased plasma ET-1 Levels, which contribute to endothelial dysfunction[6]. Several clinical trials have certified that endothelin receptor (ER) antagonists could reduce albuminuria in patients with DN[7,8] and that ETA-R blockers were safe at low dosages and might provide additional benefits to an already existing RAAS blockade[4]. This suggests that ER antagonists might be a novel and beneficial drug for DN[9]. However, no consistent conclusions regarding their effectiveness and safety for patients with DN have been presented. Therefore, this meta-analysis of available clinical data on ER antagonists aimed to assess the effectiveness and safety of ER antagonists among patients with DN. The detailed PICOS process were as below: P-population: Patients 18 to 85 years old and diagnosed with diabetes mellitus (DM) for at least 4 wk with albuminuria or/and declined estimated glomerular filtration rate (eGFR); I-inventions: ET receptor antagonists; C-comparison: Placebo; O-outcome: (1) The effect of endothelin receptor antagonists on proteinuria; (2) Effect of endothelin receptor antagonists on eGFR; and (3) Adverse events, severe adverse or common adverses were reported; S-study design: Randomized controlled trials (RCTs).
This meta-analysis has been registered in the International Prospective Register of Systematic Reviews with CRD42020164436 and was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions guidelines[10]. No funding has been received for this study.
The EMBASE, PubMed, MEDLINE, and Cochrane databases were searched from inception to the 10th of January 2020 without any language restrictions using Medical Subject Headings terms and the following corresponding key words: “ETA-selective antagonist*”, “ER antagonist*”, “ET receptor antagonist*”, “Endothelin-receptor antagonist*”, “Endothelin receptor antagonist*”, “Bosentan”, “Avosentan”, “Atrasentan”, “Sitaxsentan”, “Diabetic nephropathy”, “Diabetes mellitus”, “Diabetes”. We also searched ClinicalTrials.gov and manually identified other potentially appropriate trials by checking their bibliographies.
The inclusion criteria were as follows: (1) Patients 18 to 85 years old; (2) Diagnosed with DM for at least 4 wk; (3) Albuminuria: Urinary albumin ejection rate (UAER) > 0.2 mg/min or urinary albumin-to-creatinine ratio (UACR) > 3 mg/mmoL); and (4) Measured eGFR > 15 mL/min per 1.73 m2 or serum creatinine (sCr) < 3 mg/dL.
The exclusion criteria were as follows: (1) A diagnosis of myocardial infarction or unstable angina or previous hospital admission for heart failure, a history of severe peripheral or facial edema; (2) History of pulmonary hypertension, pulmonary fibrosis, or any lung diseases requiring oxygen therapy; (3) Diagnosis of known non-diabetic kidney disease; and (4) Any concomitant disease that could interfere with study compliance or completion.
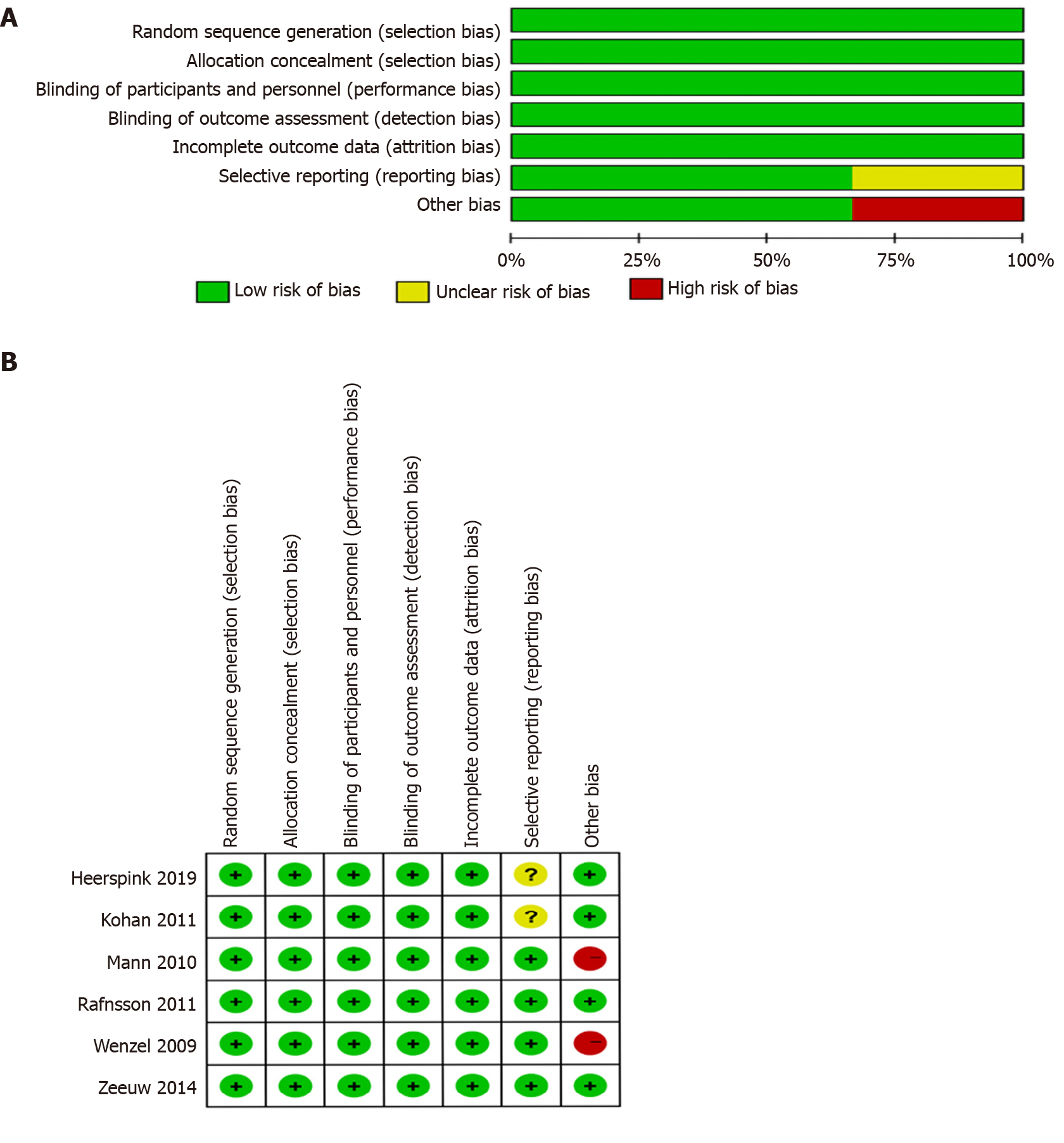
Zhang L assessed the search results according to relevance of information. Two reviewers (Zhang L and Chen G) then independently assessed the titles and abstracts of the remaining studies for relevance against the protocol criteria. Thereafter, the same reviewers browsed the full text to extract detailed information. Each study was selected according to the eligibility criteria denied herein. Any disagreements were resolved through consultation with a third reviewer (Xu ZG). Zhang L assessed the risk of bias in each included study using the relevant, validated tool for each study design. Hou J then checked the risk of bias. Risk of bias among included trials was assessed using the Cochrane RCTs risk-of-bias tool for RCTs.
Review Manager (RevMan) 5.3 software (Nordic Cochrane Centre) was used for all analyses. Relative risks with 95%CIs for dichotomous data and mean differences (MDs) with 95%CIs for continuous data were calculated. When applied scales differed, the standardized mean difference (SMD) was adopted instead of MDs. Heterogeneity test were conducted across studies using the Q-test and I2 statistic. If P value of Q-test was less than 0.1 and I2 value was less than 50%, statistically significant heterogeneity existed[11]. A random-effects model was used when obvious heterogeneity was present; otherwise, a fixed-effects model was chosen. Publication bias was assessed using the Egger’s test with Stata/SE software (version 15.1). P < 0.05 indicated a possibility for publication bias. Missing means were substituted with reported medians, while missing standard deviations were computed from confidence intervals, standard errors, t values, P values, or correlations evaluated from other enrolled studies[10]. All treatment dosages in the ER antagonist groups of each trial were integrated into one single group and compared to placebo if necessary. Combined data were analyzed using RevMan 5.3 software.
A total of 167 articles were initially identified through our search of the EMBASE, PubMed, MEDLINE, and Cochrane databases. After excluding duplicate studies and reviewing the abstracts, 26 articles remained. Ultimately, seven studies with six data sets were analyzed herein. The identification and selection of the studies are outlined in Figure 1.
The present meta-analysis included a total of 5271 participants (3331 and 1940 in the experimental and control groups, respectively). In particular, Heerspink et al[12] reported 4711 participants who completed the enrichment period (with open-label treatment of atrasentan 0.75 mg/d), among whom 2648 were responders and were randomly allocated to the atrasentan group or placebo group. The characteristics of the included selected studies are presented in Table 1. Three articles[7,9,12] studied atrasentan, one[13] bosentan, and two[8,14] avosentan. The primary endpoints of two articles[8,12] were doubling of sCr, ESRD, or death. The primary endpoints of three[7,9,14] articles were change in UAER/UACR from baseline. The primary endpoint of Rafnsson et al[13]’s trial was microvascular endothelium-dependent vasodilatation change. However, the secondary endpoints of the included trials were quite different. The secondary endpoint of Kohan et al’s trial[7] was the proportion of participants achieving at least a 25% and 40% reduction in UACR and mean eGFR change. The secondary endpoint of Mann et al[8]’s trial was changes in UACR and eGFR and cardiovascular outcomes. The secondary endpoint of Zeeuw et al[9]’s trial was the proportion of subjects achieving at least a 30%, 40%, and 50% reduction in UACR, and the mean eGFR change. The secondary endpoint of Heerspink et a[l[12]’s trial was 50% eGFR reduction or a cardiorenal composite endpoint. The secondary endpoint of Rafnsson et al[13]’s trial was brachial artery flow-mediated vasodilatation change. The secondary endpoint of Wenzel et al[14]’s trial was mean urinary protein excretion rate, sCr, creatinine clearance, systolic blood pressure (SBP) and diastolic blood pressure (DBP), hemoglobin A1c (HbA1c), and total cholesterol. Furthermore, no significant difference in baseline characteristics, including age, sex, eGFR, UACR, SBP, DBP, and HbA1c, were observed between the ER antagonist and control groups (Table 1). All participants of five studies[7-9,12,14] received standard angiotensin converting enzyme inhibitor (ACEI)/angiotensin II type 1 receptor blocker (ARB) treatment for at least 4 wk before screening, which they continued after randomization. In one trial[13], 19 of 24 participants in the control group and 20 of 22 participants in the invention group received ACEI/ARB treatment at baseline.
| Ref. | Patients No. | Interventions (dose/d) | Treatment period (wk) | Age mean (SD) | Sex, n (%) female | eGFR [mean (SD) mL/min/1.73 m2] | UACR, mg/g creatinine median (Q1 to Q3) or mean (SD) | SBP, mmHg mean (SD) | DBP, mmHg mean (SD) | Hemoglobin A1c, % mean (SD) | Study type | NCT number |
| Heerspink et al[12], 2019 | 2648 | Atrasentan 0.75 mg | 53 mo? (follow-up 2.2 years) | I: 64.8 (8.6) | 25% | 44.0 (13.7) | 792 (462-1480) | 136.5 (15.2) | 75.0 (9.9) | 7.8 (1.5) | RCT | NCT01858532 |
| C: 64.7 (8.7) | 26.60% | 43.7 (13.7) | 805 (444-1451) | 136.2 (14.8) | 74.8 (10.0) | 7.8 (1.5) | ||||||
| Kohan et al[7], 2011 | 89 | Atrasentan 0.25 mg, 0.75 mg, 1.75 mg | 8 | I: 63 (12) 67 (9) 64 (13) | 41% 36% 27% | 31 (4) 34 (6) 33 (5) | 350 (194-1226) 360 (209-726) 433 (157-998) | 134 (14) 137 (15) 135 (11) | 75 (8) 74 (8) 75 (9) | 7.6 (1.0) 7.6 (1.2) 7.3 (1.1) | RCT | N/A |
| C: 61 (8) | 17% | 34 (5) | 515 (170-1477) | 138 (14) | 78 (8) | 7.4 (0.9) | ||||||
| Mann et al[8], 2010 | 1392 | Avosentan 25, 50 mg | 48 | I: 61.2 (8.8) 61.0 (9.1) | 30.8% 32.8% | 29.9 (6.2) 30.4 (6.5) | 1422 (728.9-2425.3) 1472 (758.5-2515) | 137.1 (13.8) 137.0 (14.3) | 77.9 (9.2) 77.5 (8.6) | 8.0 (1.5) 8.1 (1.6) | RCT | NCT00120328 |
| C: 60.8 (8.9) | 33.80% | 30.1 (6.2) | 1531 (794.3-2823.9) | 135.4 (15.1) | 77.2 (9.5) | 8.0 (1.5) | ||||||
| Rafnsson et al[13], 2011 | 28 | Bosentan 250 mg | 4 | I: 62 (8) | 18.00% | 28.9 (7.4) | 415 (681.6) | 149 (24) | 81 (10) | 7.4 (1.1) | RCT | NCT01357109 |
| C: 63 (9) | 20.80% | 31.5 (4.0) | 409 (512.7) | 151 (25) | 78 (9) | 8.0 (1.4) | ||||||
| Wenzel et al[14], 2009 | 286 | Avosentan 5, 10, 25, and 50 mg | 12 | I: 60.8 (10.0) 58.4 (10.0) | 34% 30% | 31.3 (7.0) 32.2 (5.0) | N/A | N/A | N/A | N/A | RCT | N/A |
| C: 58.4 (10.0) | 13% | 30.5 (5.0) | N/A | N/A | N/A | N/A | ||||||
| Zeeuw et al[9], 2014 | 211 | Atrasentan 0.75 mg or 1.25 mg | 12 | I: 65.0 (9.8) 64.5 (8.8) | N/A | N/A | 878 (515-1682) 826 (481-1389) | 138 (14) 136 (15) | 75 (10) 74 (9) | 7.5 (1.5) 7.7 (1.4) | RCT | NCT01356849 NCT01424319 |
| C: 64.3 (9.0) | N/A | N/A | 671 (410-1536) | 136 (14) | 72 (10) | 7.4 (1.3) |
A summary of the risk of bias assessment for the included studies is presented in Figure 2. The quality of selected studies, all of which were RCTs, was generally good. However, the study by Mann et al[8] 2010 had high bias given that it was terminated early.
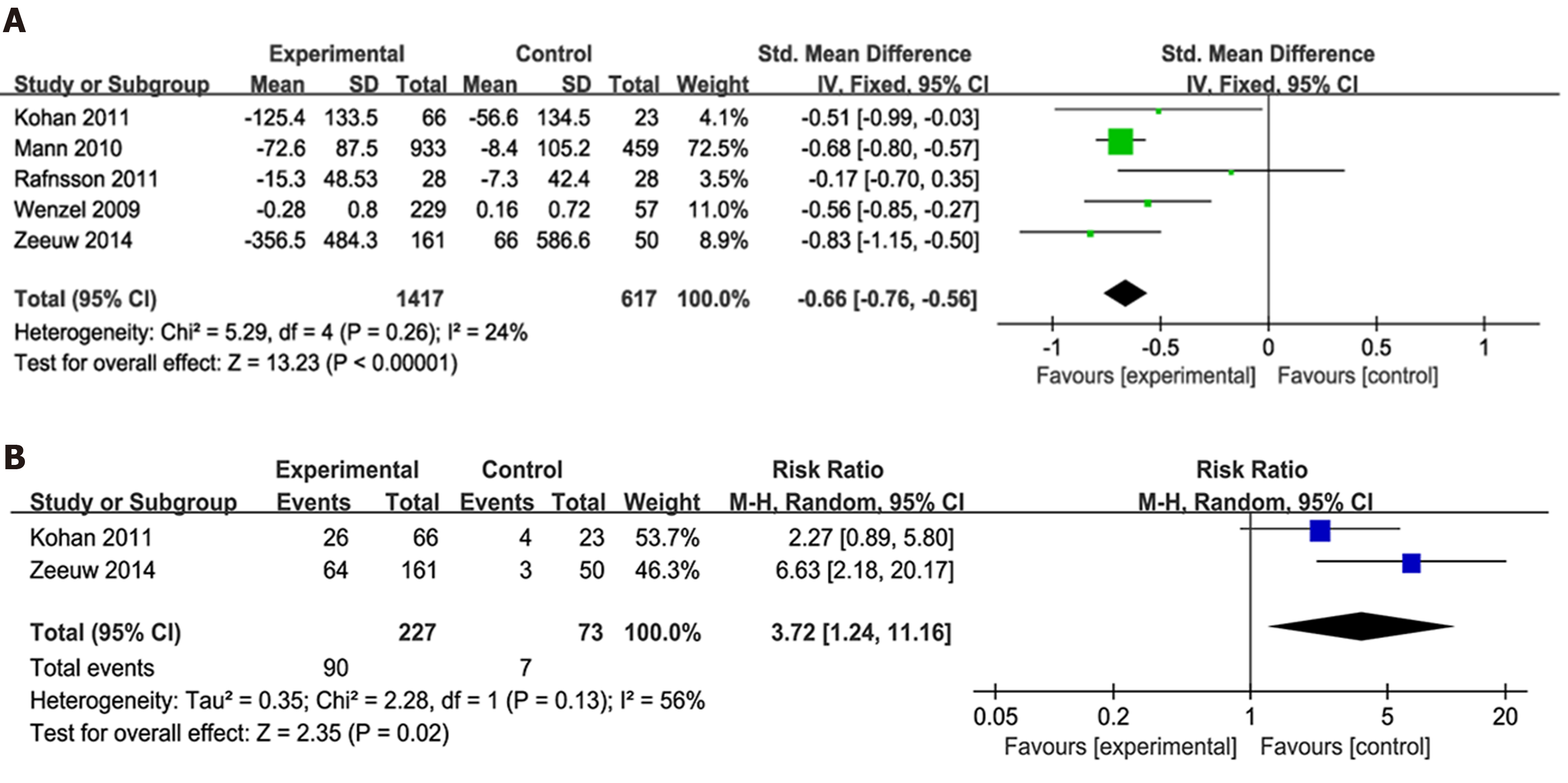
The effect of endothelin receptor antagonists on proteinuria: Five articles[7-9,13,14] had reported on UACR or UAER changes from baseline. Considering that UACR and UAER data had different scales, the SMD was used as a summary statistic in the analysis of the effect of ER antagonists on proteinuria. Accordingly, our analysis showed that the ER antagonists group had significantly higher albuminuria reduction values than the control group (P < 0.0001; SMD -0.66; 95%CI: -0.76, -0.56) with no significant heterogeneity (Figure 3A).
Two studies[7,9], including 300 participants, had reported on 40% reduction in final UACR. Accordingly, our results showed that more patients in the invention group achieved a 40% reduction in UACR than the control group [P = 0.02; risk ratio (RR) 3.72; 95%CI: 1.24, 11.16] (Figure 3B).
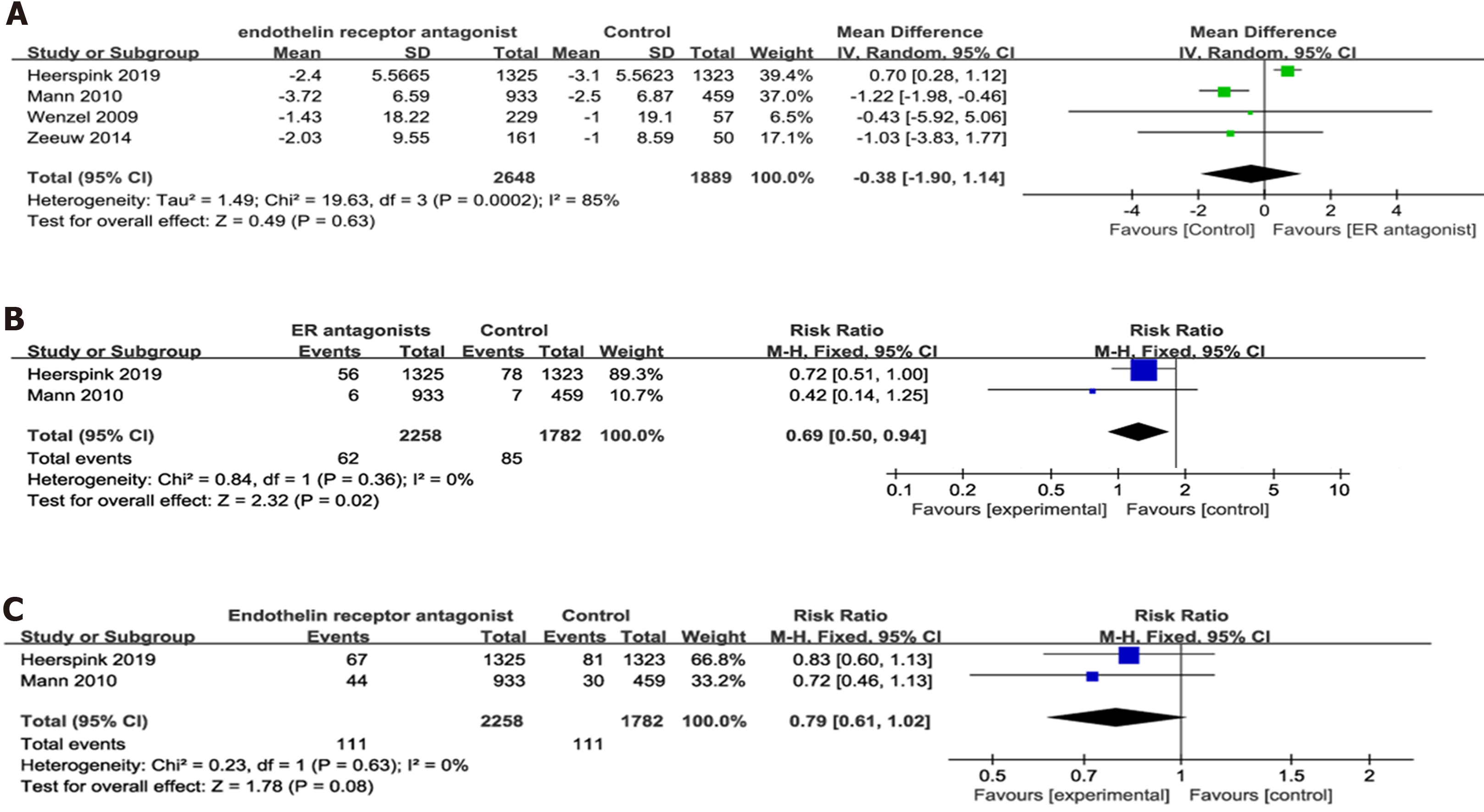
Four RCTs[8,9,12,14], including 4537 participants, had reported on eGFR outcome after ER antagonists therapy. Accordingly, our findings showed no significant difference in eGFR change from baseline between the experimental and control groups (P = 0.63) with significant heterogeneity (P = 0.0002; I2 = 85%) (Figure 4A).
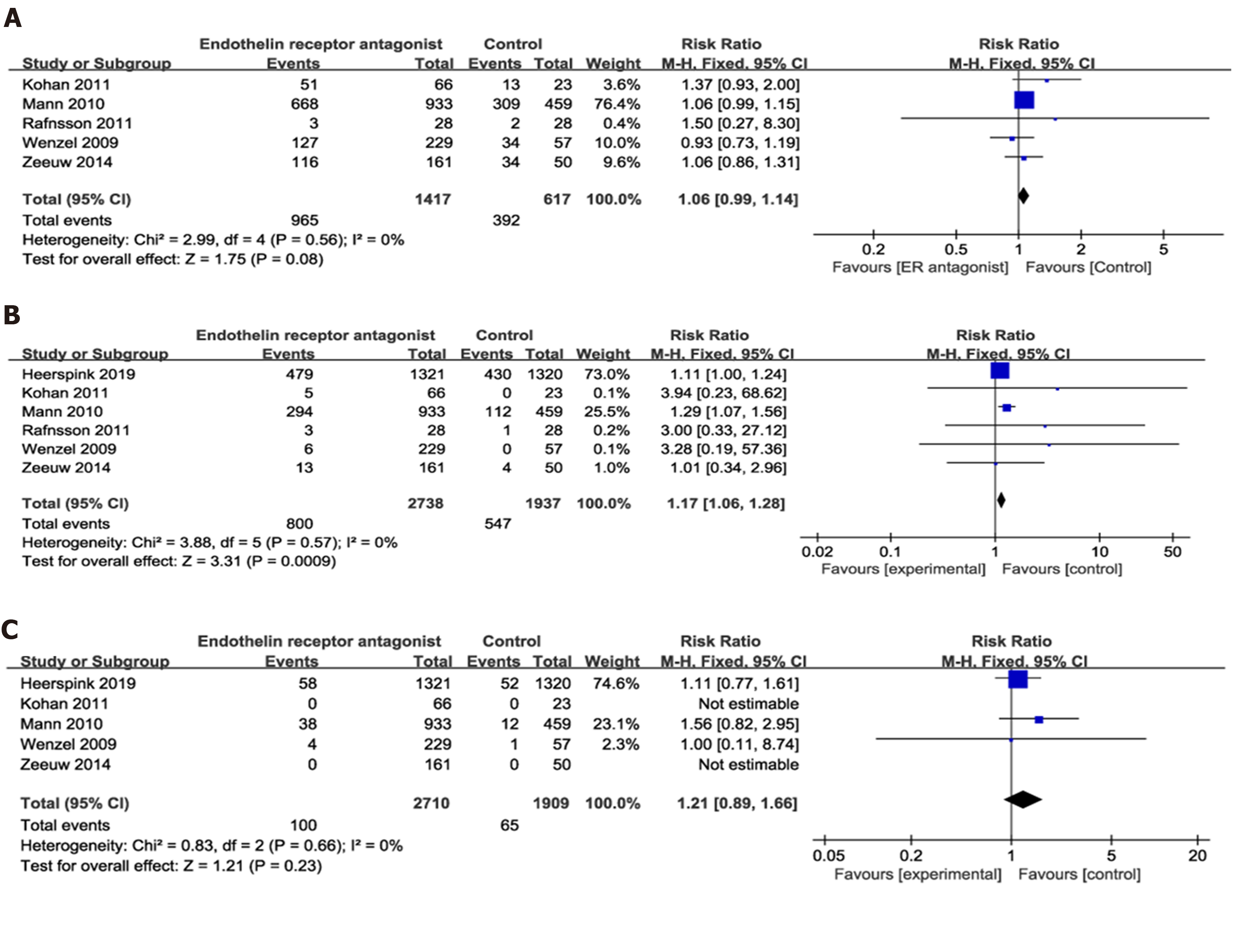
Two studies[8,12] had reported on the ratio of sCr doubling and the onset of ESRD. The onset of ESRD was defined as chronic dialysis for > 90 d, eGFR < 15 mL/min per 1.73 m2 confirmed by a second measurement ≥ 90 d later, kidney transplantation, or death from kidney failure. No heterogeneity was present in both comparisons. Accordingly, our analysis showed that the invention group had significantly lower sCr doubling ratio than the control group (P = 0.02). However, no significant difference in the onset of ESRD was observed between both groups (Figure 4B and C).
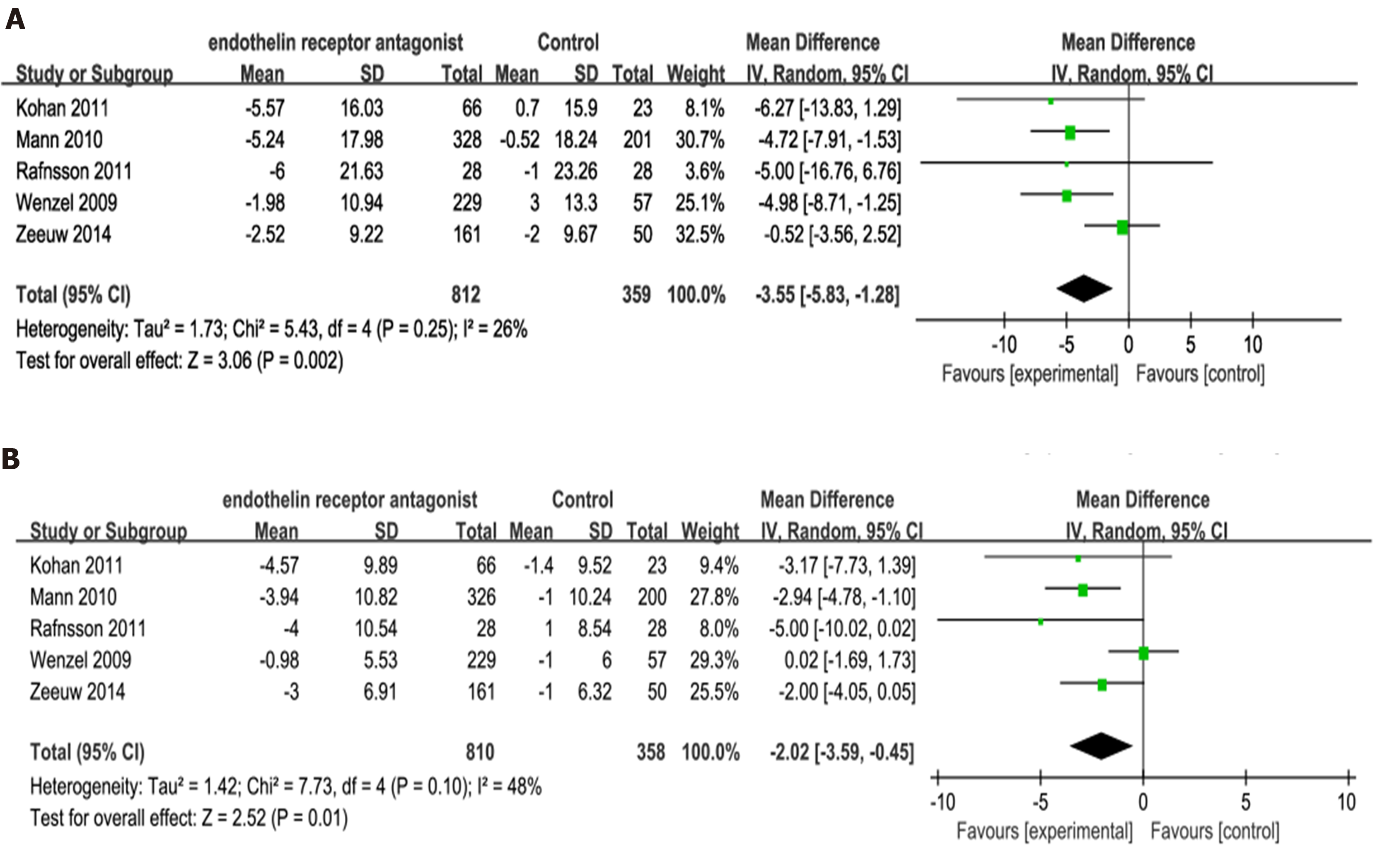
Five trials[7-9,13,14] had reported on changes in SBP and DBP. Our analysis showed that the invention group exhibited significantly greater reductions in SBP and DBP compared to placebo (SBP: P = 0.002; MD -3.55; 95%CI: -5.83, -1.28; Figure 5A; DBP: P = 0.01; MD -2.02; 95%CI: -3.59, -0.45; Figure 5B).
Adverse events, serious adverse events, and mortality: Six articles[7-9,12-14] had reported on the adverse events (AE), six on severe SAE, and five on mortality. Accordingly, our analysis showed no significant difference in AEs (P = 0.08; heterogeneity I2 = 0%) and mortality (P = 0.23; I2 = 0%) between the ER antagonists and control groups (Figure 6A and C). However, the intervention group had a higher incidence of SAEs than the control group (P = 0.0009; RR 1.17; 95%CI: 1.06, 1.28; I2 = 0%; Figure 6B).
The most common AEs reported were cardiovascular outcomes, cardiac failure, anemia, hypoglycemia, headache, edema, hyperkalemia, hypotension, and fluid retention in all included articles (Supplementary Figure 1). Accordingly, our findings showed that the ER antagonist group had a higher incidence of cardiac failure, anemia, and hypoglycemia compared to the control group (P = 0.03, 0.003, 0.03, respectively). However, no differences in the incidence of cardiovascular outcomes, headache, edema, hyperkalemia, hypotension, and fluid retention were observed between both groups.
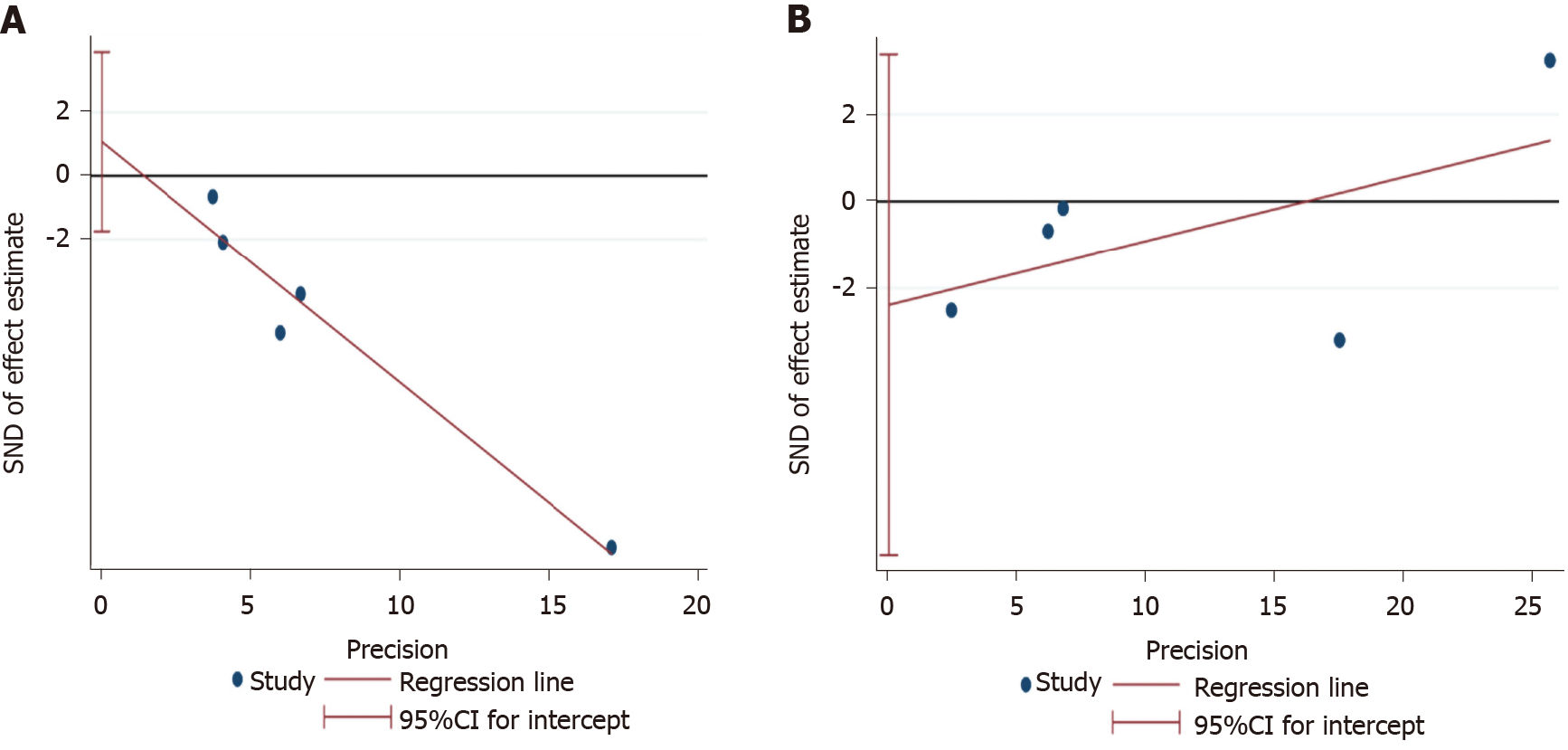
Egger’s test was used to assess for publication bias in changes in albuminuria and eGFR outcomes. Accordingly, our results showed no significant publication bias for UACR/UAER change outcomes (P = 0.313; 95%CI: -1.75, 3.90). Similarly, Egger’s test result showed no significant evidence of publication bias for eGFR outcomes (P = 0.279; 95%CI: -8.14, 3.37) (Figure 7).
Given that significant heterogeneity was present during comparison of eGFR outcomes and that differences in the dosage of ER antagonists within selected studies were observed, subgroup analysis was performed according to low, middle, and high drug dosages (Supplementary Figure 2). No significant heterogeneity was observed between the three subgroups (I2 = 0%). In the low-dosage subgroup, no significant difference had been observed between both groups. However, in the middle-dosage subgroup, the ER antagonists group exhibited lesser eGFR reduction than the control group (P < 0.00001; MD 0.70; 95%CI: 0.66, 0.74). This indicates that middle-dosage ER antagonists can protect renal function. However, in the high-dosage subgroup, the invention group displayed greater eGFR reduction than the control group (P = 0.0001; MD -1.63; 95%CI: -2.48, -0.79).
The main findings of the present meta-analysis were that ER antagonists significantly reduced albuminuria and increased the proportion of subjects who achieved a 40% reduction in UACR, which indicated its renoprotective effects among patients with DN. These findings were consistent with those presented in the systematic review by Yuan et al[15]. Several authors have attested that ER antagonists can reduce albuminuria in vitro by reducing podocyte loss, restoring the glomerular endothelial glycocalyx barrier, normalizing renal matrix protein expression, anti-inflammation, and preventing early glomerular hyperfiltration and hyperperfusion. Moreover, some articles[16-19] reported ER antagonists could preclude renal vasoconstriction, renal hypertrophy, and renal structural injury. Thus, ER antagonists may lower proteinuria in the subjects with DN through such mechanisms.
Additionally, no significant differences had been observed in pooled comparisons of eGFR reduction. However, for obvious heterogeneity (I2 = 85%), subgroup analysis had been performed. Accordingly, our subgroup analysis showed that no heterogeneity existed among all subgroups and that only the middle dosage ER antagonists were able to more effectively prevent eGFR reduction compared to placebo. Moreover, ER antagonist group had a significantly decreased incidence of sCr doubling. Both these results indicated that ER antagonists of appropriate dosages could effectively protect renal function. In the present meta-analysis, 0.25 mg of atrasentan and 25 mg daily of avosentan were regarded as low dosage, 1.75 mg daily of atrasentan and 50 mg daily of avosentan were regarded as high dosage, and 0.75 mg daily of atrasentan was considered as middle dosage. Therefore, our results suggest that 0.75 mg daily is the appropriate dosage for atrasentan. This finding differed from that presented in Yuan et al[15]’s systematic review[15] in which subgroup analysis of different dosages was not conducted. Notably, the present study found that a high ER antagonists dosage significantly worsened renal function. As such, we surmise that a high ER antagonist dosage may be harmful to renal function despite its effect on reducing proteinuria. However, more comprehensive clinical trials are needed to further corroborate our deduction.
Furthermore, the current study found that ER antagonists could effectively decrease the blood pressure of patients with DN, a result consistent with that provided in reviews by Yuan et al[15] and Burnier[20]. Burnie[20] reported that ER antagonists promoted significantly lower sitting office SBP and DPB, as well as 24h SBP and DPB, compared to placebo. However, his cohort did not exclusively consist of patients with diabetes or kidney disease. Several articles had reported that ER antagonists had considerable SBP and DBP lowering capabilities in patients with treatment-resistant hypertension[21-23]. Therefore, ER blockade may have high potential as a treatment option for hypertension and prevention of DN progression beyond calcium antagonists and renin–angiotensin system blockers[20].
After a detailed investigation on AEs, the present study suggests that ER antagonists do not induce greater AEs and mortality compared to placebo. However, the ER antagonists group did have a higher incidence of SAEs compared to the placebo group. Nearly 29.2% (n = 2738) and 28.2% (n = 1937) of patients in the intervention and placebo group reported at least one SAE, respectively, which is quite close to the figure reported in Yuan et al[15]’s review. Moreover, the present study found that cardiac failure, anemia, and hypoglycemia were the most common AEs in the ER antagonists group. Cardiac failure may result from fluid retention. One possible reason for fluid retention was ER antagonists could block ETB-R. Although most of clinical ER antagonists were highly selective ETA-R blockers, high dosages of such medicine could still block ETB-Rs[9]. Inhibition of the ETB-R in tubular cells also causes activity of Na+ and water transport[4]. Anemia may ascribe to hemodilution due to fluid retention[8,24]. Similarly, Yuan et al[15]’s report showed that the treatment group had significantly elevated incidence of anemia. However, our findings showed an anemia incidence of 14.9% for the ER antagonists group (vs 8.4% for placebo), which was slightly higher than that presented in the work of Yuan (8.5% for the treatment group vs 3.5% for the placebo group). The possible reason for this could be that Heerspink et al[12]’s trial was not included in his review. Given that Heerspink et al[12]’s trial involved a much longer follow-up compared to other trials, the proportion of the patients with anemia could have increased along with CKD progression over the duration of the trial. Furthermore, the incidence of hypoglycemia was 2.5% in ER antagonists group (vs 1.13% for placebo) in the present study. Said et al[25]’s experiments suggested ER antagonists may reduce insulin resistance when ineffective endogenous insulin was existed[25]. Thus ER antagonists may have a hypoglycemic effect in subjects with Type 2 DM[25,26]. However, this mechanism needs to be further certified. In addition, publication bias had not been observed for the UACR or eGFR change outcomes according to Egger’s test.
ET-1 receptors include the ETA-R and ETB-R. Avosentan and atrasentan are both ETA-R with approximately 500-fold and 1800-fold selectivity for ETA over ETB receptors, respectively[5,27]. Bosentan, a dual receptor antagonist[27], induced no significant changes in UACR, while avosentan and atrasentan promoted significant changes therein (Supplementary Figure 1). Maguire et al[28] also suggested that ETA-selective antagonists could be therapeutically superior to mixed antagonists in renal diseases. The possible reason would be that ETA receptors are predominant in all vessels, including renal vessels, whereas ETB receptors are only predominant in the kidneys and brain. ETA-R activation produces a vasoconstrictive response and maintains the basal vascular tone. By contrast, the activation of vascular ETB-R induces a vasodilative response due to the release of endothelial factors[20]. Moreover, the vasoconstrictive, anti-diuretic, and anti-natriuretic actions of exogenous ET-1 seemed to be induced by ETA receptor mediation in the human kidney[29]. Thus, highly-selective ETA receptor blockade should have more potential therapeutic function for DN.
In addition, almost all the participants of the included trials received ACEI/ARB treatment. This shows that ER antagonists might have an additive effect on top of ACE inhibition. Notably, a post-hoc analysis on atrasentan, in which subjects were dichotomized according to whether they received maximal dosages of RAS inhibitors, demonstrated no significant difference in UACR[30]. This confirms that the treatment effects of ER antagonists were present regardless of RAS inhibitor dosage. Moreover, an in vitro experiment showed that only ETA receptor blockade, but not ACE inhibition, completely restored the number of glomeruli in each kidney to expected normal levels and maintained the number of podocytes per glomerulus[31], suggesting that ER blockade has a specific glomeruloprotective effect that is separate from ACE inhibition. Furthermore, Balsiger et al[26] suggested that ER blockade could improve insulin sensitivity and decrease hyperglycemia severity[26]. Generally, the optimal dosage for highly-selective ETA receptor blockade combined with ACEI/ARB may be an effective treatment for DN. Therefore, there are several ongoing clinical trials of a dual ER and Angiotensin Receptor Blocker (Sparsentan) for IgA nephropathy or focal segmental glomerulosclerosis (FSGS) at present[32,33]. Although there is no trials conducted on the patients with DN, we surmise that such drugs should be more potential for DN. Nevertheless, except for ER antagonists the inhibitors of glucagon-like peptide-1 (GLP-1) receptor antagonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors also were considered as effective drugs of lowering renal end points and cardiovascular mortality in DM[34,35]. Furthermore, whether it can become a new strategy for DM that combined ER antagonists with GLP-1 receptor antagonists or SGLT2 inhibitors deserves to be further investigated.
The present meta-analysis included the latest clinical trials with much more participants and longer durations compared to previous meta-analyses. All the included studies were RCT. Among these studies, the SONAR study was the largest one in terms of the number of participants and duration of follow-up. In the SONAR study, before randomization all the participants received a diuretic, either an ACEI or ARB and open-label treatment with atrasentan 0.75 mg once per day for 6 wk. Then all responders (defined as patients with at least a 30% reduction in UACR, no more than 0.5 mg/dL or 20% from baseline increase in sCr and without substantial fluid retention) were randomly assigned to atrasentan 0.75 mg daily or turn into placebo[12]. That was different from other included studies. This design was intended to present a renoprotection function and unlikely develop into heart failure. Furthermore, we had characterized the more common AEs associated with treatment, which had never been reported before. Furthermore, heterogeneity was not obvious among the most pooled analyses in this study, while the selected trials were all of high quality. Moreover, this has been the first study to show that only optimal dosages of ER antagonists can delay renal function progression in DN. However, some limitations as to our study are worth noting. Firstly, the sample size was small, while some critical data were not presented in the publications. Thus, we may have overlooked some important information. For example, Heerspink et al[12] did not present the mean and SD values for UACR and BP changes, which may have unnoticeably impacted our results. Secondly, the duration of follow-up varied from 30 d to more than 2 years, while the dosage and types of drugs varied in all included trials. Most drugs in the trials included herein investigated selective ETA-R blockade, while only one trial investigated dual receptor antagonists. These may contribute to heterogeneity and consequently affect the reliability of our results. Finally, Mann’s study was terminated prematurely after a median follow-up of 4 mo (up to 16 mo) due to excess of cardiovascular events[8]. That may result in shortening follow-up time and have some unknown influence on observation of ER antagonists’ long-term effectiveness. Therefore, more long-term and high-quality trials need to be included to investigate the long-term effectiveness and safety of ER antagonists for patients with DN.
Taken together, ER blockades (optimal dosage) combined with ACEI/ARB may be an effective treatment for lowering BP, reducing proteinuria and delaying renal function progression in DN with declined eGFR. However, attention should be given to AEs and SAE, such as cardiac failure, anemia, and hypoglycemia. Nonetheless, more long-term RCTs are still needed to validate the long-term effectiveness and safety of ER antagonists.
Diabetic nephropathy (DN) is the main cause of chronic kidney disease and end-stage renal disease worldwide. Current treatment approaches for DN rely on angiotensin converting enzyme inhibitors or angiotensin II type 1 receptor blockers (ARBs) to control blood pressure (BP), reduce proteinuria, and delay chronic kidney disease progression. However, renin–angiotensin–aldosterone system (RAAS) blockade may increase the long-term risk for end-stage renal disease in patients with diabetes. Thus, identifying novel, effective treatments for DN beyond RAAS blockade is imperative.
Although available clinical trials have shown that endothelin receptor (ER) antagonists may be a novel and beneficial drug for DN, no consistent conclusions regarding their sufficient effectiveness and safety for patients with DN have been presented.
This meta-analysis aimed to assess the effectiveness and safety of ER antagonists among patients with DN.
We enrolled seven studies with six data sets and 5271 participants. The ER antagonists group showed a significantly greater reduction in albuminuria and more patients with 40% reduction in urinary albumin-to-creatinine ratio than the control group (P < 0.0001 and P = 0.02, respectively). Subgroup analysis for reductions in estimated glomerular filtration rate (eGFR) showed that for the middle-dosage subgroup, the ER antagonists group exhibited lower eGFR reduction than the control group (P < 0.00001; mean difference, 0.70 95%CI: 0.66, 0.74). Moreover, significant reductions in systolic blood pressure (SBP) and diastolic blood pressure (DBP) were observed in the invention group.
We enrolled seven studies with six data sets and 5271 participants. The ER antagonists group showed a significantly greater reduction in albuminuria and more patients with 40% reduction in urinary albumin-to-creatinine ratio than the control group (P < 0.0001 and P = 0.02, respectively). Subgroup analysis for reductions in eGFR showed that for the middle-dosage subgroup, the ER antagonists group exhibited lower eGFR reduction than the control group (P < 0.00001; mean difference, 0.70 95%CI: 0.66, 0.74). Moreover, significant reductions in SBP and DBP were observed in the invention group.
ER blockades combined with ACEI/ARBs may be an effective treatment to lower BP and reduce proteinuria in DN with declined eGFR. However, attention should be given to adverse events, including cardiac failure, anemia, and hypoglycemia, as well as serious adverse events.
ER blockade (optimal dosage) combined with ACEI/ARB may be an effective treatment for lowering BP, reducing proteinuria and delaying renal function progression in DN with declined eGFR. However, more long-term randomized controlled trials are still needed to validate the long-term effectiveness and safety of ER antagonists.
| 1. | Egido J, Rojas-Rivera J, Mas S, Ruiz-Ortega M, Sanz AB, Gonzalez Parra E, Gomez-Guerrero C. Atrasentan for the treatment of diabetic nephropathy. Expert Opin Investig Drugs. 2017;26:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol. 2014;10:325-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Turgut F, Bolton WK. Potential new therapeutic agents for diabetic kidney disease. Am J Kidney Dis. 2010;55:928-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Raina R, Chauvin A, Chakraborty R, Nair N, Shah H, Krishnappa V, Kusumi K. The Role of Endothelin and Endothelin Antagonists in Chronic Kidney Disease. Kidney Dis (Basel). 2020;6:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Kohan DE, Pollock DM. Endothelin antagonists for diabetic and non-diabetic chronic kidney disease. Br J Clin Pharmacol. 2013;76:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Jung C, Rafnsson A, Brismar K, Pernow J. Endothelial progenitor cells in relation to endothelin-1 and endothelin receptor blockade: a randomized, controlled trial. Int J Cardiol. 2013;168:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G; ASCEND Study Group. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Higgins J, Thomas J, Chandler J, Cumpston M, Li TJ, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev, 2020. Available from: https://training.cochrane.org/handbook/current. |
| 11. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48567] [Article Influence: 2111.6] [Reference Citation Analysis (4)] |
| 12. | Heerspink HJL, Parving HH, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 485] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 13. | Rafnsson A, Böhm F, Settergren M, Gonon A, Brismar K, Pernow J. The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia. 2012;55:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A; SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Yuan W, Li Y, Wang J, Li J, Gou S, Fu P. Endothelin-receptor antagonists for diabetic nephropathy: A meta-analysis. Nephrology (Carlton). 2015;20:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Hocher B, Schwarz A, Reinbacher D, Jacobi J, Lun A, Priem F, Bauer C, Neumayer HH, Raschack M. Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron. 2001;87:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Ding SS, Qiu C, Hess P, Xi JF, Zheng N, Clozel M. Chronic endothelin receptor blockade prevents both early hyperfiltration and late overt diabetic nephropathy in the rat. J Cardiovasc Pharmacol. 2003;42:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, Koster AJ, van Zonneveld AJ, van Faassen E, Gröne HJ, van den Berg BM, Rabelink TJ. Atrasentan Reduces Albuminuria by Restoring the Glomerular Endothelial Glycocalyx Barrier in Diabetic Nephropathy. Diabetes. 2016;65:2429-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Burnier M. Update on Endothelin Receptor Antagonists in Hypertension. Curr Hypertens Rep. 2018;20:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 22. | Webb DJ. DORADO: opportunity postponed: lessons from studies of endothelin receptor antagonists in treatment-resistant hypertension. Hypertension. 2010;56:806-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Nakov R, Pfarr E, Eberle S; HEAT Investigators. Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens. 2002;15:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Epstein BJ. Efficacy and safety of darusentan: a novel endothelin receptor antagonist. Ann Pharmacother. 2008;42:1060-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Said SA, Ammar el SM, Suddek GM. Effect of bosentan (ETA/ETB receptor antagonist) on metabolic changes during stress and diabetes. Pharmacol Res. 2005;51:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Balsiger B, Rickenbacher A, Boden PJ, Biecker E, Tsui J, Dashwood M, Reichen J, Shaw SG. Endothelin A-receptor blockade in experimental diabetes improves glucose balance and gastrointestinal function. Clin Sci (Lond). 2002;103 Suppl 48:430S-433S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Anguiano L, Riera M, Pascual J, Soler MJ. Endothelin Blockade in Diabetic Kidney Disease. J Clin Med. 2015;4:1171-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Maguire JJ, Davenport AP. Endothelin receptors and their antagonists. Semin Nephrol. 2015;35:125-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Honing ML, Hijmering ML, Ballard DE, Yang YP, Padley RJ, Morrison PJ, Rabelink TJ. Selective ET(A) receptor antagonism with ABT-627 attenuates all renal effects of endothelin in humans. J Am Soc Nephrol. 2000;11:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Andress DL, Coll B, Pritchett Y, Brennan J, Molitch M, Kohan DE. Clinical efficacy of the selective endothelin A receptor antagonist, atrasentan, in patients with diabetes and chronic kidney disease (CKD). Life Sci. 2012;91:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Benz K, Amann K. Endothelin in diabetic renal disease. Contrib Nephrol. 2011;172:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Trachtman H, Nelson P, Adler S, Campbell KN, Chaudhuri A, Derebail VK, Gambaro G, Gesualdo L, Gipson DS, Hogan J, Lieberman K, Marder B, Meyers KE, Mustafa E, Radhakrishnan J, Srivastava T, Stepanians M, Tesar V, Zhdanova O, Komers R; DUET Study Group. DUET: A Phase 2 Study Evaluating the Efficacy and Safety of Sparsentan in Patients with FSGS. J Am Soc Nephrol. 2018;29:2745-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Enevoldsen FC, Sahana J, Wehland M, Grimm D, Infanger M, Krüger M. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Sarafidis PA, Tsapas A. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374:1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 35. | Álvarez-Villalobos NA, Treviño-Alvarez AM, González-González JG. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:1797-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barzilay J, Eleftheriadis T, Klimontov VV S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ