INTRODUCTION
Based on the recommendations of the American Diabetes Association, type 1 diabetes mellitus (T1DM) includes two types: Idiopathic diabetes and immune-mediated diabetes[1]. The etiology of idiopathic diabetes is not known. The related patients bear perpetual insulinopenia and show a trend to ketoacidosis, however, autoimmunity is not observed. Resulting from T cell-mediated attack of insulin-producing β-cells of the pancreas, immune-mediated diabetes was previously termed T1DM, insulin-dependent diabetes, or juvenile-onset diabetes[1]. In this review, T1DM is used to refer to immune-mediated diabetes.
TYPE 1 DIABETES MELLITUS
As an autoimmune disorder, T1DM is marked by T cell-mediated destruction of β-cells of the pancreas, which leads to an almost complete loss of the ability to synthesize insulin[2]. This insulin deficiency results in loss of the ability to regulate blood sugar, therefore, exogenous insulin administration is necessary for patients to control blood sugar and to reduce the incidence of related chronic diabetic complications, such as the microvascular, macrovascular, and neuropathic complications[3]. The incidence and prevalence of T1DM have been growing all over the world and the relative annual increase in T1DM incidence is approximately 2%-3%[4,5]. The greatest increase is detected in children younger than 5 years[6]. T1DM can occur at any age[7] and therefore, the previous definition of T1DM as juvenile-onset diabetes may not be suitable.
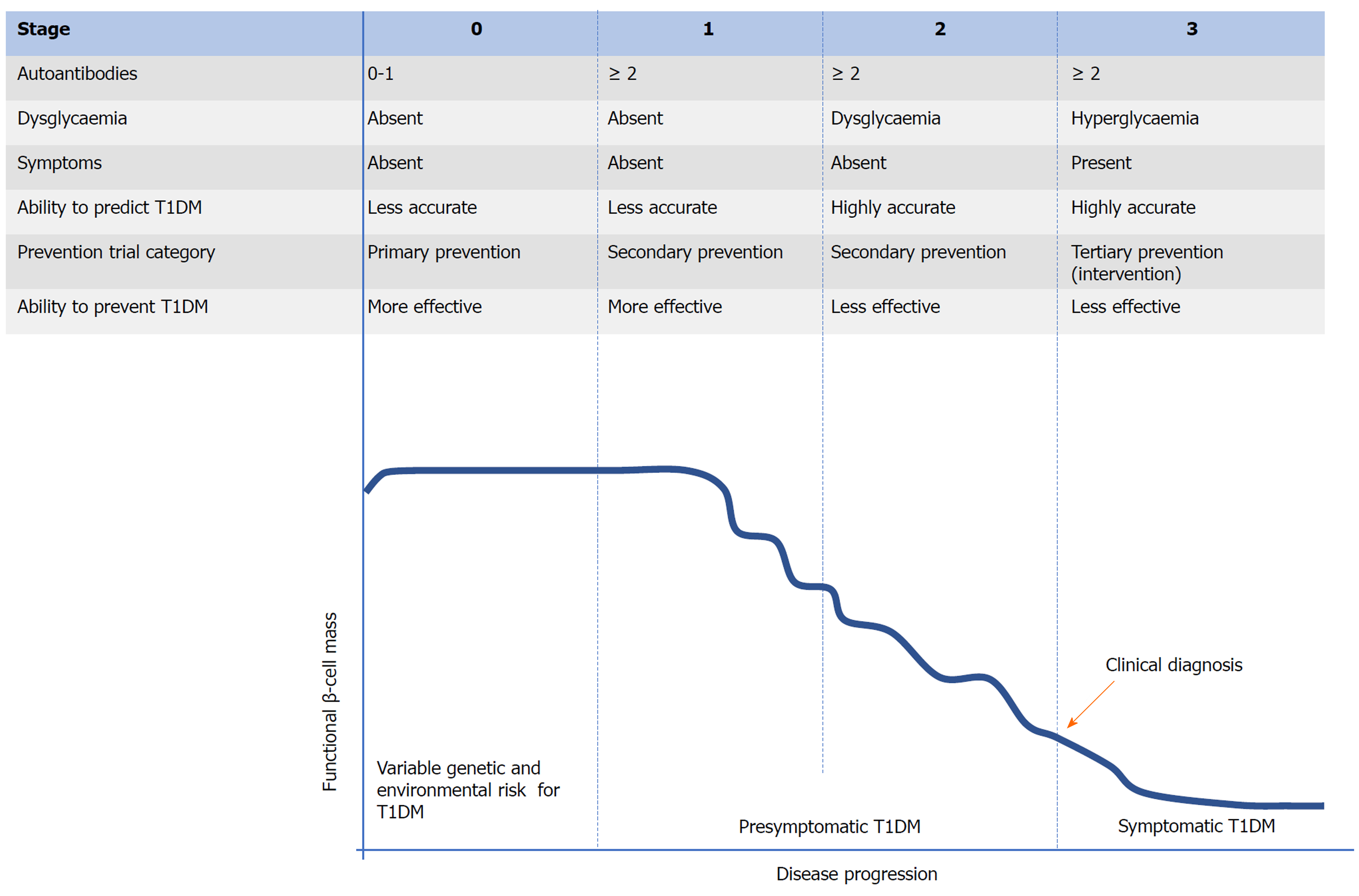
During the development of T1DM, the autoimmune attack of β-cells of the pancreas mediated by T cells is regarded as the final step[8]. Typical T1DM related autoantibodies target various proteins, including (pro) insulin, glutamic acid decarboxylase (GAD) 65, Hsp60, IA-2, ZNT8, and tetraspanin-7[9-11]. Various autoantibodies can be observed many months or years prior to clinical onset (Figure 1). These autoantibodies can be considered biomarkers of β-cell injury rather than its cause. The mechanism triggering this autoimmune process remains elusive and this is the most crucial challenge for preventing T1DM. Studies to date propose that interactions between genetic susceptibility and environmental exposures (Figure 1, stage 0) are necessary in the development of T1DM. There have been identified over 60 genetic loci related to susceptibility to T1DM[12]. These loci can be divided to two types: HLA and non-HLA genes[13,14]. Combined with familial risk analysis, HLA risk analysis can be applied for identifying people at a high risk for T1DM[15,16]. However, individual non-HLA loci could not be applied for predicting T1DM or distinguishing it from other types of diabetes. To well predict the risk of developing T1DM and distinguish it from T2DM (type 2 diabetes mellitus), genetic risk scores derived from combined analysis of HLA and non-HLA genes can be applied[17,18]. Compared to the relatively well-known genetic factors, environmental determinants remain poorly understood despite intensive research. Studies to date show that diet (maternal diet, cow’s milk protein, breastfeeding, gluten, and micro- and macro-nutrients), vitamin D sufficiency, virus infection, and the bacterial microbiome in the gastrointestinal tract (GIT) are associated with T1DM[2,16]. In addition, both genetic and environmental factors, particularly the latter, might influence the entire natural history of T1DM[13,19].
Figure 1 Proposed staging of type 1 diabetes mellitus.
T1DM: Type 1 diabetes mellitus.
The establishment of β-cell autoimmunity can be confirmed by the occurrence of one or more autoantibodies as described above. Compared to individuals without autoantibodies, individuals with a single autoantibody may be at higher risk for T1DM. However, among people with a single autoantibody, the incidence of T1DM over 10 years is less than 10%[20,21]. If there are two or more autoantibodies present, the individual will eventually progress to clinical onset of T1DM[22] and this progression may be divided into three stages (Figure 1)[23]. Once two or more autoantibodies appear, it means that the individual has stage 1 T1DM. Apart from the number of autoantibodies observed and their seroconversion age, the type and affinity of autoantibody appearing in this stage contribute to the rate of progression to symptomatic T1DM[23]. However, the individual is normoglycemic and bears no clinical symptom in this stage. As the functional β-cell mass decreases, the individual in stage 2 develops dysglycaemia or glucose intolerance. Similar to stage 1, there is no obvious clinical symptom in stage 2 and therefore, stages 1 and 2 can be termed “presymptomatic T1DM”. As the disease progresses, the functional β-cell mass has been severely impaired, leading to the inability to maintain normal metabolic function. Therefore, hyperglycaemia and typical clinical symptoms of diabetes (polyuria, polydipsia, and weight loss) occur and T1DM can be clinically diagnosed in stage 3 (symptomatic T1DM)[24]. At the time of diagnosis, there is approximately 80% to 90% of the original β-cell mass disrupted[25]. From a few weeks to up to 20 years will be needed to progress from the presence of autoantibodies to clinical onset and this prediabetic phase facilitates to predict and prevent or delay T1DM[16,26,27]. For prevention of T1DM, similar to other autoimmune diseases, it is more effective during the early stages (stage 0, primary prevention or stage 1, secondary prevention) of disease progression[24,28]. However, compared to stages 2 and 3, prediction strategies performed in stages 0 and 1 show a lower accuracy[26,28]. This imbalance between the predictive and preventive effect may lead to the fact that there has been a substantial loss of functional β-cell mass when T1DM is predicted accurately, which makes various therapies less effective in preventing, delaying, or reversing T1DM. Currently, few studies to successfully prevent the onset of T1DM have been reported[24,29].
ORAL TOLERANCE
Oral tolerance represents an adaptation, unresponsiveness, or hypo-responsiveness to an orally administered antigen by the immune system. Oral tolerance prevents inappropriate immune responses to innocuous antigens contained in food or commensal organisms. Thus, this process functions importantly in maintaining a balance between reactions against these exogenous antigens and self-components of the body in the gut mucosa[30]. Once this balance breaks down due to the disruption of oral tolerance, immunoglobulin E (IgE)-mediated food allergies, inflammatory bowel disorders, autoimmune disorders, or infections may occur[31]. As a physiological response to dietary antigens, oral tolerance mainly develops in the GIT. Although most of the orally delivered antigens are digested into short peptides and/or amino acids, a small amount of intact antigens can reach the intestinal epithelium where they are delivered in various manners, depending on their characters (e.g., size and solubility). The delivery manner affects the subsequent immune response including the induction of tolerance or immunity[32].
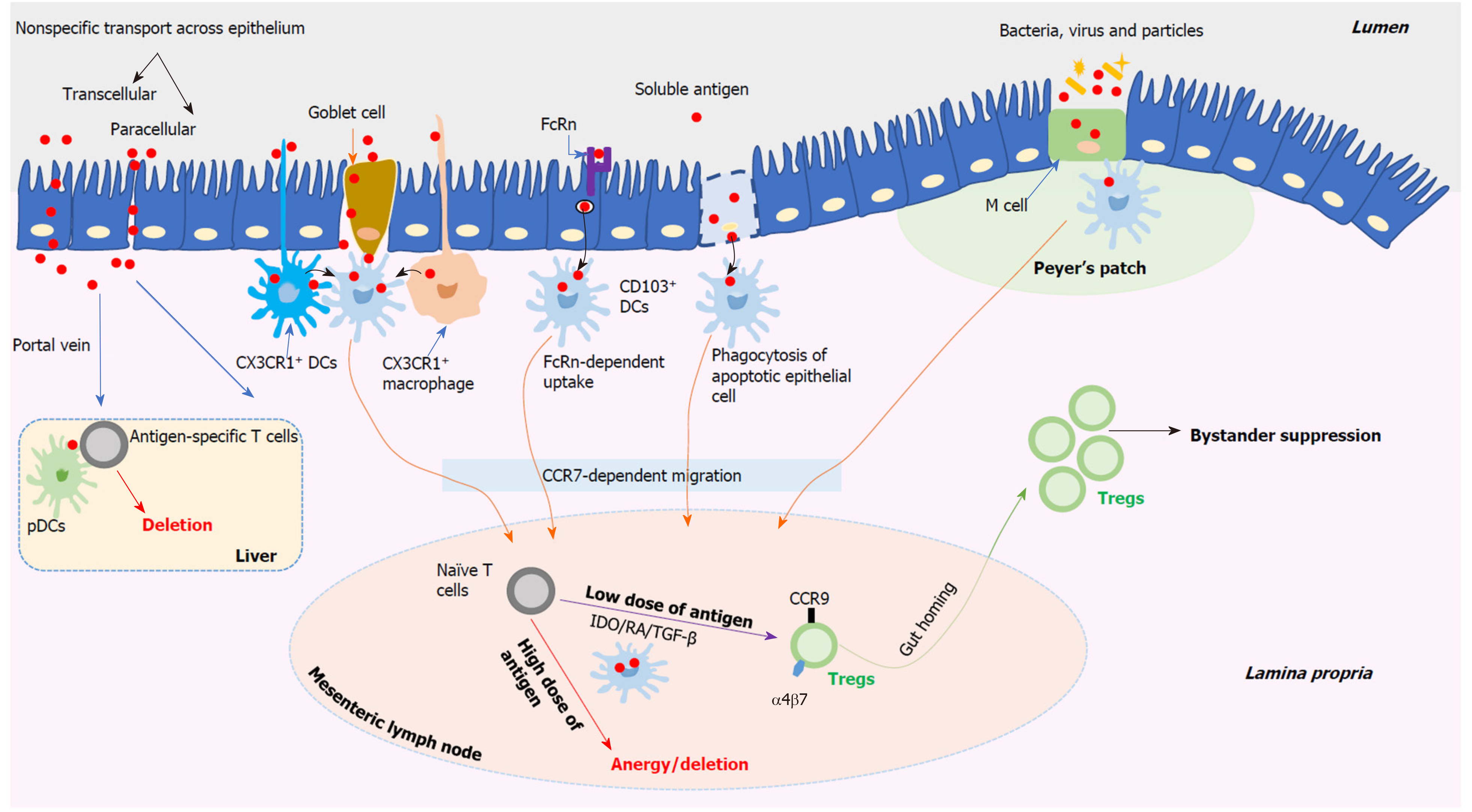
The delivery of antigens contained in the gut lumen involves complex processes and there are various possible routes for antigens passing through the intestinal epithelium as shown in Figure 2. Soluble antigens might be delivered by enterocytes in two nonspecific manners, including the transcellular manner and the paracellular manner[33]. For the transcellular manner, antigens contained in vesicles may be degraded and however, some intact antigens can reach the basolateral space. For the paracellular manner, it may be suppressed under homeostatic conditions as a result of the tight junctions between enterocytes. The neonatal IgG receptor (FcRn), which locates on the surface of enterocytes, may be used to capture and internalize antigens by forming the antigen-antibody complexes[34]. And then, antigens are delivered across the epithelium to lamina propria dendritic cells (DCs) bearing FcRn as well as other Fc receptors. Antigens contained in the apoptotic enterocytes can be captured by neighbouring DCs. Both CX3CR1+ macrophages and CX3CR1+ DCs can sample luminal antigens efficiently by means of extending their dendrites between enterocytes without disrupting the integrity of the epithelium[35,36]. The above DCs or macrophages have no capacity to transport antigens directly to naïve T cells that locate in the mesenteric lymph nodes (MLN). However, they can transport these antigens to neighbouring CD103+ DCs, which subsequently enter the MLN, via a CC-chemokine receptor 7-dependent mechanism, and present antigens to naïve T cells[36,37]. In addition, mucus-secreting goblet cells perform an important role in delivering soluble antigens via goblet cell-associated antigen passages, which provide a conduit for delivering soluble antigens exclusively to CD103+ DCs and therefore, this process may perform a critical role in oral tolerance induction[38]. Located in the epithelial layer covering Peyer’s patches (PPs), microfold cells (M cells) can specially deliver antigens including particles, bacteria, and viruses to CD103+ DCs that reside within the M cell pocket[39]. However, M cells can also be found outside PPs to deliver antigens[40]. Thus, the uptake of antigens through the intestinal epithelium is a complicated process. Currently, various routes as descried above have been suggested, however, this still is a disputed topic and needs to be further investigated.
Figure 2 Schematic overview of the stepwise mechanisms of oral tolerance induction.
DCs: Dendritic cells; M cell: Microfold cell; CCR: CC-chemokine receptor; pDCs: Plasmacytoid DCs; IDO: Indoleamine 2,3-dioxygenase; RA: Retinoic acid; TGF-β: Transforming growth factor-beta.
Composed of the MLN and PPs, gut-associated lymphoid tissue (GALT) functions heavily in inducing oral tolerance successfully. Based on those proposed delivery routes as described above, CD103+ DCs harboring antigens derived from the gut lumen move into the MLN[41], which has been identified as an important site of oral tolerance induction[42]. However, compared to the MLN, PPs may not be necessary for oral tolerance induction[43,44]. In the MLN, CD103+ DCs harboring antigens meet naïve CD4+ and CD8+ T cells and oral tolerance induction occurs in an antigen dose-dependent manner, including low- and high-dose tolerance[33,45]. With regard to repeated feeding of a low dose of antigens (Figure 2), the generation of regulatory T cells (Tregs) helps to induce oral tolerance. Derived from CD103+ DCs harboring antigens in the MLN, indoleamine 2,3-dioxygenase, retinoic acid, and transforming growth factor-beta (TGF-β) appear to cooperate to facilitate naïve T cells differentiation towards Tregs which bear the gut-homing-associated adhesion molecules, such as chemokine receptor CCR9 and α4β7 integrin[33,39]. Therefore, these induced antigen-specific Tregs, which perform suppressor activity, can return into the lamina propria in which they are expanded with help of interleukin-10 (IL-10) secreted by CX3CR1+ macrophages. Both the induced Tregs’ gut-homing and expansion are essential to achieve oral tolerance successfully[46,47]. The best-described Tregs are CD4+CD25+Foxp3+ Tregs which function a lot in oral tolerance induction, and this can be supported by the observation that specific depletion of Foxp3+ cells appears to abrogate oral tolerance[47]. Apart from CD4+CD25+Foxp3+ Tregs, other peripherally induced Tregs, such as T helper (Th) 3 cells and type 1 regulatory T (Tr1) cells, are also related to oral tolerance induction[33,37]. All these peripherally induced Tregs contribute to inducing systemic immune tolerance by means of cytokine-mediated bystander suppression which is induced by oral feeding with one antigen and then works in a non-antigen-specific manner[30,48,49]. However, the potential function of Tr1 cells in oral tolerance induction and the potential relationship or interaction between these different Treg subsets are not clear and need to be further studied. Transferring T cells from tolerized animals obtained by feeding a low dose of antigens can transfer antigen-specific tolerance to naïve animals[45,50,51].
In respect to single feeding of a high dose of antigens (Figure 2), anergy and/or deletion of antigen-specific T cells can be induced in GALT, which contributes to oral tolerance[30]. However, transferring T cells from tolerized animals obtained by feeding a high dose of antigens fails to transfer this tolerance to naïve animals[45,52]. Apart from the MLN, the liver provides a complementary effect in oral tolerance induction (Figure 2). The portal vein offers an access for the gut-derived antigens, which are not processed in the gut, to the liver in which the tolerogenic environment contributes to the induction of oral tolerance[53]. Mediated by plasmacytoid DCs, the deletion of antigen-specific T cells occurs and this induces systemic tolerance to these antigens[54]. In addition, the tonsils and sublingual mucosa, especially their contained DCs, may also contribute to developing oral tolerance[55,56]. An important role for antibodies has been suggested in oral tolerance induction[33,45]. IgE-mediated hypersensitivity can be suppressed by administration of IgG antibodies[57]. Peanut tolerance can be induced among high-risk children by early introduction of peanut, which is mediated by the production of peanut-specific IgG4[58]. However, the mechanisms how antibodies benefit for oral tolerance induction remains to be determined. Additionally, it has been proposed that the intestinal microbiota and diet, such as vitamin D[59], may perform a positive effect for oral tolerance induction[45]. Among those oral tolerance studies performed during the past decades, most have been designed and applied to prevent and/or treat food allergy and autoimmune diseases, including T1DM[37,39].
ORAL TOLERANCE IN T1DM
As described above, individuals at risk for T1DM may be identified years before clinical onset. This makes oral tolerance, which acts as an antigen-specific immunotherapy, an attractive treatment for preventing and/or delaying T1DM[60,61]. Since its first application in the non-obese diabetic (NOD) mouse[62], which shares various immunological and pathological similarities to human T1DM and has been used as the primary model for investigating T1DM[63], many attempts to induce antigen-specific tolerance in NOD mice by feeding various autoantigens have been reported. Apart from the NOD mouse, other animal models including BioBreeding (BB) rats and a genetically modified mouse model induced by the lymphocytic choriomeningitis virus (LCMV) were also applied in oral tolerance induction for autoimmune diabetes. In addition, oral tolerance trials have been applied in human T1DM based on the results in animals. In order to get a comprehensive understanding of the progress that has been made so far in oral tolerance induction for T1DM, reports related to these valuable trials in both animals and human are collected, classified, and reviewed.
Oral tolerance trials in animals
Based on the formula used, these trials can be divided into two types, direct oral administration of a single autoantigen and combinatorial therapy. The latter may be further divided into the following types: A combination of different autoantigens and a combination of autoantigen(s) with various delivery vehicles and/or immune adjuvants and/or immunomodulatory agents.
Direct oral administration of a single autoantigen
As described above, six autoantigens related to T1DM have been identified, however, only two of them have been applied for oral tolerance induction in animals by direct oral administration, including insulin (proinsulin) and GAD65. Furthermore, among these two autoantigens, insulin was the most often used one.
Insulin: In 1991, the first trial in oral tolerance therapy for T1DM in NOD mice was applied by feeding porcine insulin[62]. At 5 wk of age, NOD mice were administered porcine insulin orally two times weekly for 5 wk and then weekly until one year old. Compared to animals administered phosphate buffer saline, or 10 μg or 100 μg of porcine insulin orally, animals fed 1 mg of porcine insulin showed a delayed onset and a reduced incidence of T1DM. In following years, this research group continued to apply modified trials for oral tolerance induction in animal models and to explore the responsible mechanisms. Based on their research and reports, they have made an important contribution to clarifying the mechanisms of oral tolerance as described above[30,46,49,64]. In respect to oral tolerance induction for T1DM, equine insulin[65,66], the B-chain of bovine insulin (30 amino acids)[67,68], and human insulin and its B-chain peptide (10-24)[69,70] have been applied by this research group. All these trials showed a positive effect in delaying and/or suppressing the process of insulitis and T1DM in NOD mice. Furthermore, cell culture, cytokine assays, and adoptive transfer studies suggested that this protective effect was related to a T cell dependent mechanism in which Tregs secreting IL-4, IL-10, and TGF-β played a critical role. In this context, there was a decrease in Th1 responses (downregulation of IL-2 and interferon-gamma) and an increase in Th2 responses (upregulation of IL-4 and IL-10) to the antigen administered orally. These non-antigen-specific cytokines mediated bystander suppression to different autoantigens[64]. Also, they found that feeding insulin or its B-chain peptide to one-day-old neonatal NOD mice performed effectively in preventing T1DM rather than accelerating the progression of diabetes[69]. Apart from these reports from the above research group, many trials applied by other groups supported the positive effect of tolerance derived from oral administration of insulin towards suppressing autoimmune diabetes in animals. Diabetes can be actively suppressed in NOD mice by feeding 20 U of insulin every 2-3 d for a month beginning at 6 wk of age and this protective effect was related to insulin-specific regulatory CD4+ T cells which were induced in the gut and subsequently homed to the islets[71]. The same research group illuminated that insulin-reactive Tregs can be induced in the spleen by oral insulin and the induced Tregs preferentially migrate to the pancreas and pancreatic lymph nodes[72,73]. No matter applied before or after the induced autoimmune response, 1 mg of insulin administered orally twice weekly can suppress the disruption of β-cells and prevent diabetes in the LCMV induced mice, and the change of the profile of pancreatic cytokine expression from interferon-gamma to immunosuppressive ones (IL-4, IL-10, and TGF-β) was responsible for this protective effect[74]. However, one amino acid substitution in insulin (B30A to T) abrogated this protective effect in both NOD and LCMV induced mice, indicating that the structure and/or sequence of the applied antigens may significantly affect the efficiency of oral tolerance induction[75]. Simultaneously, this report indicated that oral tolerance induction was not dependent on hormonal activity of insulin and accordingly, β-cell rest was not the reason why oral insulin may prevent or delay the onset of T1DM.
In addition to these above trials bearing a positive effect by direct oral administration of insulin, there were reports indicating no effect in preventing T1DM by feeding insulin or its related peptides to animal models. Oral bovine insulin at all applied doses (0.5, 1.0, and 2.0 mg/wk, 3 wk) had no effect in preventing or delaying autoimmune diabetes in BB rats and the species/strain-specific traits of disease progression may be responsible for this failure to tolerance induction in BB rats compared to NOD mice[76]. Beginning at 30 d of age, NOD mice received 1 mg oral human recombinant insulin twice weekly. The protective effect obtained from this treatment showed no significant difference from the control group although a delay of the onset of disease was detected[77]. An oral tolerance study repeated in 2016 showed that feeding NOD mice of 5 or 9 wk of age with various dosages of different types of insulin for 5 wk had no ability to prevent T1DM[78]. The source and purity of these used insulin, the efficiency of insulin digestion, or the variability in disease penetrance in NOD mouse colonies caused by geographical differences may be responsible for this inability to prevent T1DM, which is in stark contrast to those above trials bearing positive effects in preventing T1DM in animals by direct oral feeding of insulin.
GAD65: In addition to insulin, GAD65 was the other autoantigen applied for oral tolerance induction in animal models by direct oral administration and however, there was only one related report as described below. Oral administration of 0.5 mg porcine GAD65 twice per week beginning at 30 d of age showed disease preventive effects during a long research period (> 400 d), however, compared to the control group, no difference in the final disease incidence was observed (P = 0.226)[77].
Combinatorial therapy
On the basis of the effects of direct oral administration of single autoantigen on tolerance induction in trials described above, combinatorial therapy has been suggested by various reports to improve the tolerance induction efficiency and the preventive effect towards autoimmune diabetes in animals. In addition to combination of different autoantigens by direct oral administration, various combinatorial strategies have been applied. To protect autoantigens from being degraded when passing through the GIT and/or reduce the required amounts of the used autoantigens, plant-, microbe-, or nanoparticles (NPs)-based delivery vehicles have been developed. In addition, immune adjuvants and immunomodulatory agents have been applied to combination therapy with autoantigens.
Direct oral administration of different autoantigens: Compared to oral admini-stration of porcine GAD65 or human recombinant insulin alone, combined therapy with both autoantigens performed significant anti-diabetic effects (P = 0.011). Splenocytes from mice treated with the combined therapy showed a reduced response to both porcine GAD65 and insulin, indicating that antigen-specific inhibition was generated[77]. In addition to the above report, there was no more report applying direct oral feeding with various autoantigens for tolerance induction in animals, however, oral feeding with different autoantigens has been applied in combination with various delivery vehicles and/or immune adjuvants, and this will be reviewed in the following sections.
Various vehicles applied for delivery of autoantigen(s) to induce oral tolerance: In 1997, GAD67, a GAD isoform predominating in mouse islets, was expressed in transgenic plants. Oral administration of these GAD-containing plants (approximately 1-1.5 mg of GAD daily) from 5 wk to 8 mo of age suppressed the development of T1DM in NOD mice[79]. However, oral therapy with tobacco expressing 0.5 mg human GAD65, once a week for 10 wk beginning at 5 wk of age, was not sufficient to protect the NOD mice from autoimmune diabetes[80]. Compared to the above study applying GAD67-expressing transgenic plants for oral tolerance induction, the low dosage and the short treatment course applied in this study may be responsible for the failure to prevent T1DM in NOD mice. Various strains of lactic acid bacteria (LAB), generally regarded as safe, have been tested as mucosal delivery vehicles for protein/DNA-based vaccines[81] and for tolerogenic immunotherapy[82]. Some T1DM autoantigens or their related peptides, such as proinsulin[83], HSP60[84], GAD65[85], and IA-2[86], have been expressed in recombinant Lactococcus lactis (L. lactis) and applied for antigen-specific tolerance induction in combination with some immunomodulatory agents to prevent or reverse T1DM in animal models. This will be discussed in the next section. In respect to its two-chain structure, the expression of insulin is challenging for L. lactis and therefore, the expression of single-chain insulin (SCI) analogue may be an alternative strategy[87]. Containing a short peptide linker between two chains of insulin, SCI-57[88] and SCI-59[89] were successfully secreted in L. lactis, respectively, retaining their insulin receptor-binding ability. Apart from the genetically modified LAB, non-living LAB bacterium-like particles (BLPs), which perform less anti-carrier response when administrated orally, have been applied as immunostimulants and/or mucosal vaccine delivery vehicles[90]. Oral feeding with BLPs-SCI-59, in which the above SCI-59 is bound to LAB BLPs in a non-covalent manner, could suppress the process of autoimmune diabetes in NOD mice by restoring the Th1/Th2 imbalance and enhancing Tregs proportions[91].
Autoantigen(s) in combination with immune adjuvants and/or immunomodulatory agents: Based on the preventive effect towards T1DM by direct oral feeding of autoantigen(s) as described above, its therapeutic potential may be restricted by requiring repeated administrations of a large amount of autoantigens. To overcome such restrictions, the nontoxic cholera toxin B (CTB) subunit has been applied as a mucosal carrier molecule for the conjugated autoantigens to induce oral tolerance[92]. The proposed mechanism for applying CTB as an effective mucosal carrier may be associated with its high GM1 binding affinity, which facilitates the conjugated protein delivery and presentation into the GALT. In addition, CTB’s immunomodulatory properties may also be responsible[92,93]. The incidence of disease can be reduced significantly in NOD mice even by feeding a single dose of minute amounts (2-20 μg) of CTB-insulin (CTB-INS) conjugate at 8 wk of age and a more durable effect can be obtained by applying five consecutive dosages of this CTB-INS conjugate[94]. This protective mechanisms by feeding microgram amounts of CTB-INS depend on the upregulation of Tregs which selectively home to the pancreas and its draining lymph nodes[95] and oral delivery of CTB-INS conjugates makes the precise sources of the applied insulin less important and reduces the required antigenic dosages[96]. At 5 wk of age, NOD mice were orally administered with transgenic potato harboring approximately 20 μg of CTB-INS conjugate (once per week for 5 consecutive weeks)[93]. Compared to administration of potato harboring insulin or CTB alone, feeding of the above tissues containing CTB-INS had significant suppression of autoimmune diabetes[93]. Beginning at 5 wk of age, oral feeding of transgenic plant tissues containing approximately 14 μg of CTB-proinsulin (CTB-pINS) protein (once a week for 7 consecutive weeks) alleviated pancreatic insulitis and significantly preserved pancreatic β-cells. And Th2 lymphocyte-mediated oral tolerance contributed to this beneficial effect[97]. However, beginning at 5 wk of age, feeding of transplastomic tobacco leaf material containing 25 μg, or 250 μg, or 500 μg CTB-human proinsulin (CTB-hpINS) once per week for 10 wk was not sufficient to prevent disease in NOD mice. Furthermore, no synergism was detected by oral feeding of tobacco tissues containing CTB-hpINS and GAD[80].
Using the silkworm as a bioreactor, edible vaccines containing CTB-INS, CTB-INS B chain, CTB-INS bearing a green fluorescent protein tag (CTB-INS-GFP), or CTB-INS fused to three copies of GAD65 peptide 531-545 (CTB-INS-GAD) were produced and applied to induce oral tolerance in NOD mice by one research group from Zhejiang University. Beginning at 5 wk of age, oral administration of 30 μg of CTB-INS or CTB-INS B chain (3-4 times one week for 5 consecutive weeks) can effectively prevent autoimmune diabetes in NOD mice[92,98]. Beginning at 5 wk of age, oral administration of 50 μg of CTB-INS-GFP (every other day for 5 consecutive weeks) induced specific immune tolerance and suppressed T1DM onset in NOD mice. This treatment increased Tregs proportions in the peripheral lymphocytes and influenced the bioactivity of spleen lymphocytes[99]. Beginning at 5 wk of age, oral administration of 10 μg of CTB-INS-GAD contained in silkworm pupae (every other day for 5 consecutive weeks) suppressed T1DM more effectively than insulin or GAD65 treatment alone in NOD mice. The synergistic beneficial effect of this combined dual antigen therapy was associated with restored Th1/Th2 imbalance by increasing Tregs proportions[100].
In addition to coupling T1DM autoantigens to CTB as described above, whether a nonconjugated form of CTB can improve oral tolerance induction was investigated. Mixing with CTB significantly enhanced the ability of orally administered insulin to induce tolerance. Admixtures of insulin and CTB at an optimal insulin:CTB ratio of 100:1 prevented diabetes in both LCMV induced mice and old NOD mice with established insulitis[101]. Apart from CTB, several other bacterial and plant enterotoxin B subunits were coupled to proinsulin or GAD. Beginning at 5 wk of age, oral feeding of 50 μg of these purified adjuvant-autoantigen proteins (once a week for 4 wk) reduced pancreatic insulitis levels significantly and induced a Th2 immune response[102].
As mentioned above, recombinant L. lactis has been applied to deliver T1DM autoantigens in combination with various immunomodulatory agents. Derived from human HSP60, P277 or DiaPep277 possesses potential roles to modulate immunological attack on β-cells in NOD mice[103], and serial clinical tests have been finished or are ongoing[104,105]. HSP65 from Mycobacterium tuberculosis may serve as an immunogenic vehicle for P277-based vaccines in NOD mice by nasal delivery of the fusion protein HSP65-6×P277[106]. This fusion protein was expressed in L. lactis and direct feeding of the recombinant strain (beginning at 4 wk of age and one time a day during the first week and then one time a week for 36 consecutive weeks) induced antigen-specific immunological tolerance and reduced the incidence of T1DM in NOD mice[84]. Based on the similar strategy, IA2P2, containing ten amino acids of B cell epitopes of autoantigen IA-2 and thirteen amino acids of human HSP60 (448-460), was expressed by L. lactis in form of HSP65-6×IA2P2. Direct feeding of the recombinant L. lactis prevented T1DM onset in NOD mice by formation of antigen-specific tolerance[86]. In addition, this research group developed the gut DCs-targeting NPs to deliver the above fusion protein HSP65-6×P277. The used NPs protected HSP65-6×P277 from degradation when passing through the GIT and significantly enhanced its uptake by DCs in the gut PPs. T1DM was prevented in all immunized mice by oral feeding of HSP65-6×P277-loaded targeting NPs and oral tolerance induction was achieved by repairing Th1/Th2 imbalance and increasing the functional CD4+Foxp3+CD25+ Tregs proportions[107].
The research group of Chantal Mathieu has also applied recombinant L. lactis to reverse diabetes in NOD mice. Combined with therapy with low-dose systemic anti-CD3 antibodies, oral feeding of L. lactis concurrently secreting human proinsulin and the immunomodulatory cytokine IL-10, which possesses the potential to improve the protective effect of oral insulin by mucosal administration[108], can stably reverse T1DM and autoantigen-specific long-term tolerance was induced in NOD mice[83]. Based on the similar strategy, a clinical-grade self-containing L. lactis formulation performed similar therapeutic efficacy in T1DM remission in NOD mice compared to the above plasmid-containing L. lactis strain and the therapy-induced tolerance depended on the emergence of functional Foxp3+ T cells[109]. Combined with short-course low-dose anti-CD3 antibodies, oral feeding of recombinant L. lactis secreting GAD65370-575 and IL-10 can reverse diabetes in NOD mice by increasing the proportions of functional CD4+Foxp3+CD25+ Tregs[85]. Using preexisting anti-insulin antibodies as biomarkers, the research group of Matthias von Herrath showed that anti-CD3/oral insulin combination therapy induced long-term protection by increasing insulin-specific Tregs numbers in NOD mice[110]. As described above, anti-CD3 antibody has been used widely to modify the course of T1DM in animals or in clinical trials[111]. However, combination therapy with anti-CD6[112] or anti-CD20[113] and oral insulin failed to stimulate lasting tolerance and to prevent or reverse T1DM in mice.
In addition, compared to feeding insulin B-chain alone, coadministration of Schistosome egg antigens worked in a synergistic way to enhance Th2 type responses in NOD mice[68]. The immunoregulatory cytokine IL-4 and human GAD65 were expressed in transgenic tobacco plants, respectively. No protective effect was detected in NOD mice if either was fed alone, however, combined therapy by feeding plant-derived IL-4 and human GAD65 protected NOD mice from insulitis and T1DM by inducing oral tolerance[114].
Oral tolerance in clinical trials
Based on the effects obtained in animal models, clinical trials towards preventing or delaying T1DM in human by oral tolerance induction have been performed or are underway. Compared to oral tolerance trials in animals, in human clinical trials, insulin is the only used T1DM autoantigen. In 2000, there were two reports investigating whether tolerance can be induced in recent-onset T1DM individuals by oral delivery of insulin and both trials can be regarded as secondary prevention (Figure 2). Combined with intensive subcutaneous insulin treatment, oral administration of insulin (5 mg/d) was performed in patients with clinical T1DM (< 4 wk duration); however, this treatment made no sense to modify the disease course in the following year and no effect towards residual β-cell function was detected[115]. In the other report, oral insulin (2.5 or 7.5 mg/d) was administrated for 1 year and similarly, this treatment was useless to prevent deterioration in the function of residual β-cells in the first year after clinical diagnosis[116]. Therefore, the opinion that oral tolerance could be induced even when the immune process is initiated was challenged at that time and more experimental work may be required[117]. Two-dose (1 or 10 mg/d) oral insulin tolerance test in newly diagnosed (< 2 years) T1DM individuals was reported in 2004 and no clinical benefits were detected. However, a chemical benefit was detected that 1 mg daily doses of oral insulin delayed progression of β-cell failure in patients diagnosed at ages above 20 years[118]. Whether oral tolerance can be induced by oral insulin was tested in the Diabetes Prevention Trial-Type 1 (DPT-1), which was designed as a secondary prevention trial (Figure 2). It showed that oral insulin (7.5 mg/d) had no effect in delaying or preventing T1DM in people who were autoantibody positive; however, the beneficial effect of delaying T1DM onset in patients with confirmed insulin autoantibody (IAA) levels ≥ 80 nU/mL was observed by subgroup analyses[119]. Longitudinal data analysis of IAA levels in the DPT-1 study showed that oral insulin (7.5 mg/d) did not affect IAA levels in individuals already present of IAA at onset of therapy[120]. The 13-year follow-up study indicated that the above beneficial effect observed in patients with IAA ≥ 80 nU/mL may be maintained as long as treatment continues[121]. These results suggested that IAA levels may be a key recruitment criterion for such studies. Promoted by the DPT-1 study, a randomized clinical trial (ClinicalTrials.gov Identifier: NCT00419562) showed that oral insulin (7.5 mg/d) exhibited no effect to delay or prevent T1DM over 2.7 years in autoantibody-positive relatives of patients with T1DM and oral insulin formulation applied by this study cannot be used for diabetes prevention[122]. As a primary prevention trial (Figure 2), the Pre-POINT Randomized Clinical Trial (ISRCTN76104595) fed T1DM-high-risk children who had no signs of islet autoimmunity with high doses of insulin and compared with placebo, oral insulin (67.5 mg/d) led to a regulatory immune response without hypoglycemia. The enhanced saliva IgG towards insulin and regulatory profiles of T-cells responding to insulin among treated individuals indicated the successful induction of oral tolerance[123]. Benefited from these promising findings observed in this small pilot study, a larger phase 2 trial (ClinicalTrials.gov Identifier: NCT02547519) is underway. In addition, there are some clinical trials that are underway, such as Frida insulin intervention (ClinicalTrials.gov Identifier: NCT02620072) titled “Mechanistic Study Using Oral Insulin for Immune Efficacy in Secondary Prevention of Type 1 Diabetes” containing two insulin dose strengths (7.5 mg/d and 67.5 mg/d) and oral insulin therapy for prevention of autoimmune diabetes in infants (4 mo to 7 mo) with high genetic risk: the GPPAD-POInT (Global Platform of Autoimmune Diabetes-Primary Oral Insulin Trial, ClinicalTrials.gov Identifier: NCT03364868)[124].
CONCLUSION
More detailed pathophysiological mechanisms responsible for the development of T1DM have been clarified by various studies performed during the past decades. However, the true cause of this disease remains unclear and translation of those above findings to an effective treatment has not been achieved. Working as an antigen-specific immunotherapy, oral tolerance therapy, if applied properly, exhibits the ability to restore the immune system towards durable tolerance to disease-related autoantigen(s). Various T cell subsets, such as Th1/2 and Tregs, and the related cytokines contribute to inducing oral tolerance, however, the potential signaling pathways controlling such immune responses contained in the development of oral tolerance are unclear and these processes as well as many aspects of the involved mechanisms need to be further studied to offer new targets for regulating tolerance.
As reviewed above, oral tolerance therapy for T1DM has been widely investigated in animal models, especially in NOD mice. Most previous studies indicate that oral administration of T1DM related autoantigens exhibits a positive effect to prevent, delay, or reverse the disease in NOD mice. However, these preclinical reports are somewhat conflicting in respect to the timing of therapy initiation, frequency and dosage of autoantigen administration, treatment duration, type of autoantigen applied, demand for combinational reagents and, perhaps most importantly, degree of efficacy. In addition, there are some reports indicating that oral autoantigen therapy exhibits no effect in delaying or preventing the onset of T1DM in animal models, possibly because antigens passing through the GIT are degraded, which leads to little autoantigen reaching the mucosal immune surface and subsequently influences tolerance induction. Therefore, a high amount of autoantigen may be needed and in order to circumvent this problem, some plant-, microbe-, or NPs-based delivery vehicles have been applied and shown promising outcomes. Based on the results obtained from oral autoantigen alone therapy, combinatorial therapy has been proposed by researchers to enhance tolerance induction. In addition to direct oral administration of different autoantigens (insulin and GAD65), various immune adjuvants, such as CTB and HSP65, and/or immunomodulatory agents, such as anti-CD3 antibody, IL-10 and IL-4, have been applied in combination with oral administration of autoantigen(s). These combination therapies show synergy and perform potential as effective treatment options to enhance the protective effect of oral administration of autoantigen(s) by inducing tolerance in NOD mice.
Compared to trials applied in animals in which various autoantigens or combinational strategies have been performed, oral tolerance trials in human clinical trials mainly focus on the direct oral administration of various dosages of insulin. Although some oral insulin therapies for T1DM have been shown to be effective in animals, their clinical translation has not been achieved. Some important differences related to the progression of T1DM in animal models and humans may be responsible for this translation failure, such as the time course of disease, the speed and degree of destruction or recovery of β-cell, cytokine profile and function, male or female preference, and genetic polymorphisms. Furthermore, compared to the mouse models, humans exhibit more complicated regulation in the immune system, which may result in a lower immune response to the similar dosage of autoantigens. In addition, it should be noted that efficacy rather than safety is more addressed during the design and implementation of animal studies and the potential treatment-associated complications are less investigated or documented. However, the safety of oral tolerance induction should be considered when designing clinical trials and therefore, among the performed clinical trials, a lower dose of orally administered autoantigens is applied, and some combinational therapies using various delivery vehicles, immune adjuvants, or immunomodulatory agents have not been introduced.
As an antigen-based immunotherapy, we believe that oral tolerance therapy shows promise for restoring immune tolerance to autoantigen(s) and subsequently preventing, delaying, or reversing T1DM. To achieve this aim in human clinical trials, the following factors need to be carefully considered. First, immune biomarkers with improved sensitivity and specificity are needed to identify individuals at risk of T1DM as early as possible. Appropriate combination of immune biomarkers plays an absolutely important role in including the most appropriate target population into the right trials and evaluating treatment effects early. Second, since T1DM is a heterogeneous disease, all patients might not exhibit a similar response to a similar oral tolerance therapy. Therefore, more personalized strategies for tolerance induction may be needed to develop. Third, the results obtained from animal and human tests have shown that it may be far easier to prevent T1DM by oral tolerance therapy than to arrest or reverse its effects after clinical onset. Therefore, assuming that it is safe, oral tolerance therapy should be introduced in at-risk subjects, who are often children, as early as possible prior to the clinical onset of T1DM. Finally, animal studies have indicated that combinational therapies consisting of autoantigen administration with immune adjuvants and/or immunomodulatory agents possess the potential to safely prevent or stably reverse disease processes. Such combination studies in humans, which may be difficult to conduct, have not been published; however, these strategies still exhibit high therapeutic potential.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ