Published online Jan 15, 2020. doi: 10.4239/wjd.v11.i1.13
Peer-review started: September 18, 2019
First decision: October 13, 2019
Revised: November 1, 2019
Accepted: November 25, 2019
Article in press: November 25, 2019
Published online: January 15, 2020
Processing time: 90 Days and 8.4 Hours
Type 1 diabetes (T1D) is an autoimmune disease that usually strikes early in life, but can affect individuals at almost any age. It is caused by autoreactive T cells that destroy insulin-producing beta cells in the pancreas. Epidemiological studies estimate a prevalence of 1 in 300 children in the United States with an increasing incidence of 2%-5% annually worldwide. The daily responsibility, clinical management, and vigilance required to maintain blood sugar levels within normal range and avoid acute complications (hypoglycemic episodes and diabetic ketoacidosis) and long term micro- and macro-vascular complications significantly affects quality of life and public health care costs. Given the expansive impact of T1D, research work has accelerated and T1D has been intensively investigated with the focus to better understand, manage and cure this condition. Many advances have been made in the past decades in this regard, but key questions remain as to why certain people develop T1D, but not others, with the glaring example of discordant disease incidence among monozygotic twins. In this review, we discuss the field’s current understanding of its pathophysiology and the role of genetics and environment on the development of T1D. We examine the potential implications of these findings with an emphasis on T1D inheritance patterns, twin studies, and disease prevention. Through a better understanding of this process, interventions can be developed to prevent or halt it at early stages.
Core tip: Type 1 diabetes (T1D) is one of the most common childhood chronic conditions with its incidence increasing annually. Its arduous management requires vigilance to avoid complications that impact quality of life and health care costs. A better understanding of how T1D develops can help find a way to prevent it from developing at all. In this review, we discuss the current understanding of the complex relationship between the roles of genetics and the environment in the development of T1D.
- Citation: Giwa AM, Ahmed R, Omidian Z, Majety N, Karakus KE, Omer SM, Donner T, Hamad ARA. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J Diabetes 2020; 11(1): 13-25
- URL: https://www.wjgnet.com/1948-9358/full/v11/i1/13.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i1.13
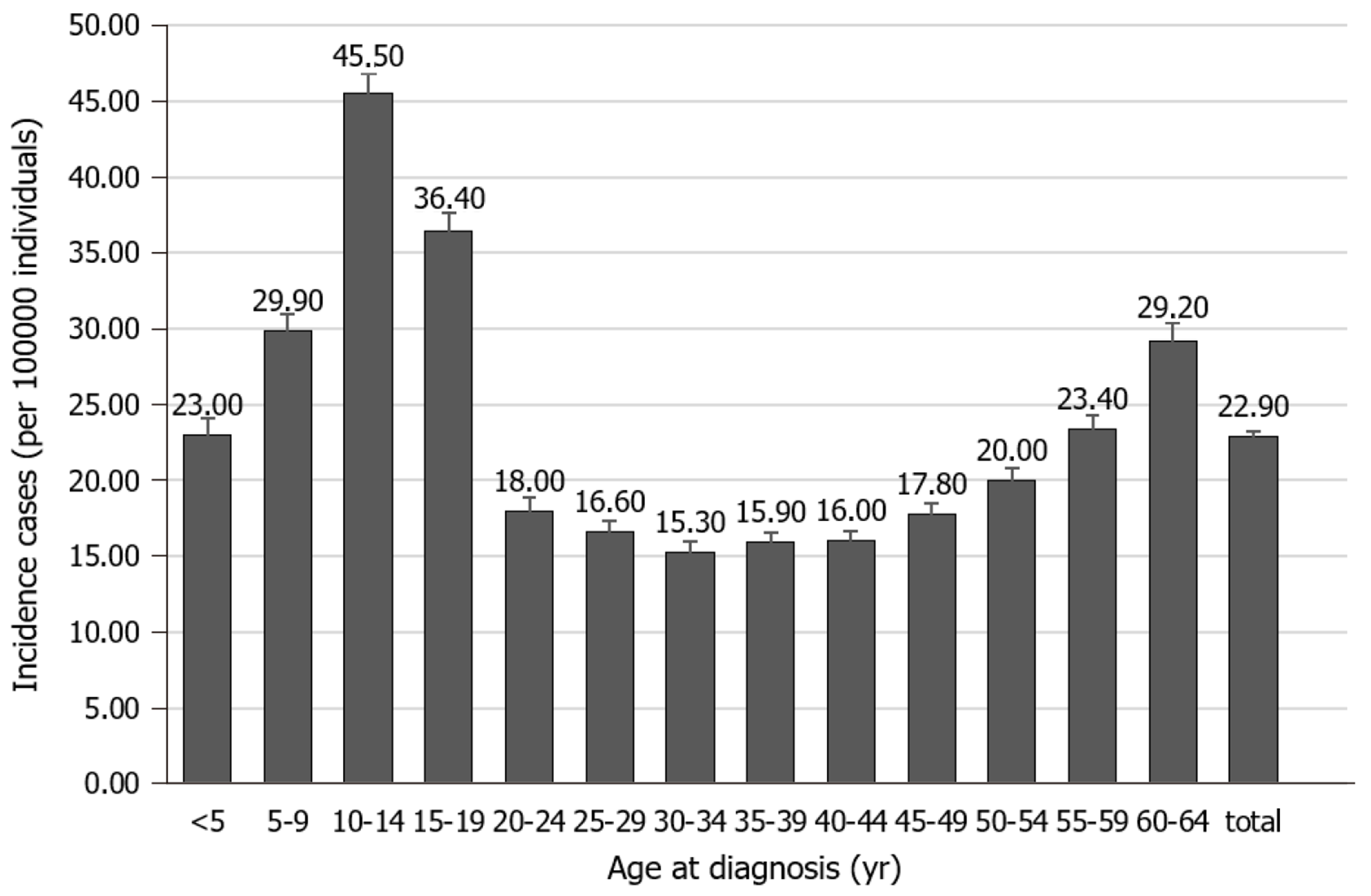
Type 1 diabetes (T1D) is one of the most common childhood chronic conditions. Though its peak incidence is between the ages of 10 and 14, its clinical presentation can occur at almost any age from early infancy to as late as the 9th decade of life[1-3]. See Figure 1[3], for the relative incidence at different age groups. Data from large epidemiologic studies worldwide have indicated that the incidence of T1D has been increasing by about 2%-5% worldwide annually and that the prevalence of T1D has increased to approximately 1 in 300 in the United States by 18 years of age[1]. It affects 2.28 in 1000 children as compared to the 1.24 of 1000 youths (< 20 years old) affected by cancer and 120 of 1000 youths suffering from asthma[1,4]. Additionally, T1D can significantly affect the quality of life of patients and families because they are required to vigilantly monitor their blood sugars with needled lancets or other monitoring devices and administer insulin with syringes, pens, or insulin pumps several times a day. Moreover, there is a significant public health impact with an estimated total cost of $132 billion in the United States in 2002[4,5].
The high number of people affected by T1D and its health burden has spurned a robust growth of clinical research aimed at improving the quality of life of those affected and basic research looking at ways to better understand the pathogenesis of this disease in the quest for a cure. Though significant advances have been made, there is still much we do not understand about what triggers T1D and how to effectively control this disease, and thusly much research work is still needed. This review article will focus on our current understanding of the development of T1D from an immunologic, genetic, and environmental standpoint. We will highlight promising and interesting studies that seem closest to uncovering why and how T1D develops.
Diabetes mellitus (DM) is a condition characterized by a state of relatively insufficient or complete lack of insulin production. There are various types of DM that include: T1D, T2D, gestational, post-pancreatectomy, Mature Onset Diabetes of the Youth, neonatal diabetes, and medication-induced diabetes. T1D “contributes up to about 10% of the estimated 422 million diabetes cases worldwide”[6].
T1D results from the destruction of the insulin-producing cells in the pancreas called beta cells by the adaptive immune system. This process is promoted by an incompletely understood interaction between a person’s genetics and their environment. Genetic factors (i.e., individuals with an overexpression of human leukocyte antigen or HLA class molecules DR4, DQ8, and DQ2 increasing their susceptibility is present in approximately 90% of T1D patients) and one or more environmental factors lead to the recognition of beta cell components as autoantigens that the immune system erroneously recognizes as foreign leading to an autoimmune attack[1,7,8]. Identified autoantigens include insulin B chain peptide (11-23) and other components of beta cell secretory granules such as glutamic acid decarboxylase 65, protein phosphatase-like IA-2, and transmembrane Zn transporter. The presence of one known autoantibody confers a moderate risk of developing T1D while the presence of each additional autoantibody increases the risk exponentially[7].
These autoantigens are presented by HLA molecules major histocompatibility complexes (MHC) I and II on antigen presenting cell (APCs) to diabetogenic autoreactive T cells. Autoreactive CD4 T cells stimulate APCs, including B cells that produce high-affinity autoantibodies against beta cells. Autoreactive CD4 T Cells also help diabetogenic CD8+ T cells to acquire cytolytic activity and attack beta cells through the release of cytokines (including TNF-α and IFN-γ, Fas/FASL and perforin/granzyme). Released cytokines also stimulate macrophages and other innate immune cells to further damage beta cells yielding a positive feedback loop with the production of more toxic cytokines to propagate further beta cell destruction[7,8].
There has been much debate regarding the identity of the nidus that initiates the autoimmune destruction of beta cells. Other groups are currently studying potential players in the immune system such as FasL B cells and dual expresser (DE) cells that may play a role in T1D pathogenesis. Using Flow Cytometry, high-throughput sequencing, and transcriptional analysis utilizing RNA-seq, interesting subsets of CD5+ B Cells that include IL-10 producing B-reg cells, FasL-expressing B cells and DE cells that express both B cell receptor (surface immunoglobulin, Ig) and T cell receptor have been discovered[9]. Findings suggest that the CDR3 region of the heavy chain of surface Ig on the DE cell expresses an extremely potent diabetogenic T-cell autoantigen that is able to stimulate other T cells and may contribute to the development of T1D and similarly potent x-autoantigens with different CDR3 sequences could be involved in pathogenicity of other autoimmune diseases[10]. Another study also suggests that T1D could be caused by a paucity of anti-inflammatory IL-10-producing Breg cells by apoptosis mediated by FasL expressing B cells leading to the unchecked proliferation and expansion of diabetogenic T cells[11]. This is supported by the elevated levels of FasL-expressing CD5+ B cells in the splenocytes and lymph nodes of T1D patients compared to controls and the prevented development of T1D in non-obese diabetic (NOD) knockout mice or mice treated with FasL-neutralizing monoclonal antibody[11]. Further studies are being performed to determine the significance of blocking FasL in humans and targeting DE cells as a form of immunotherapy.
T1D is a multifactorial disease that is affected by a number of genes. Even in the case of high-risk conferring haplotypes that are present in 90%-95% of T1D children, only 5% or fewer with the haplotype actually develop overt T1D in the general population[1]. This supports the fact that there must be other factors, genetic, epigenetic, and/or environmental that play a role in ultimately determining disease manifestation. According to genome wide association studies, over 60 genes can affect the risk of the developing T1D especially those that affect the HLA loci[12,13]. Up to 30%-50% of the genetic risk in T1D is related to HLA class II alleles. Of the HLA class II genes, the mutations found on the HLA region of chromosome 6p21 have been shown to be a major susceptibility locus for T1D. There are also several non-MHC loci that have a lesser contribution to disease risk, such as IFIH1, IL2RA, PTPN22, and CTLA4[13-15]. See Table 1 for a list of prevalent T1D-associated genes associated with risk factors[16,17] and protective factors[18,19].
Interestingly, although the association of T1D with the HLA class II genes DQ and DR has been well validated, individuals who are heterozygous for HLA-DRB1*04 and HLA-DRB1* 03 type have been found to be at highest risk[14]. It has been suggested that almost 50% of children with the HLADR3/4-DQ8 heterozygous genotype will develop anti-islet autoimmunity before the age of 5[14,15]. Children with the DR3/4-DQ8 or DR4/DR4 homozygous genotype and a family history of T1D have more than a 1 in 5 risk to developing islet autoantibodies, whereas children with no family history and DR3/4-DQ8 or DR4/DR4 genotype have a 1 in 20 risk[15].
Therefore, it stands to reason that HLA typing could be a cost-effective method to screen for T1D susceptibility and identify high risk individuals at an early age if targeted immunotherapy is available to prevent disease onset. There are many ongoing international projects, including DIPP, TEDDY, TRIGR, and TrialNet that are monitoring high-risk individuals, their risk alleles and their progression to T1D to better elucidate the positive and negative predictive values of identifying the presence of high-risk alleles. Thus far, it is clear that the inheritance of T1D is complex and development of overt disease involves both a genetic predisposition and an environmental trigger(s) since the presence of high-risk genetic determinants does not yield a 100% positive predictive value.
The strong multifactorial genetic component of T1D is further supported by the effects of familial inheritance. Interestingly, the offspring of mothers with T1D have a reduced risk of developing T1D when compared to the offspring of fathers with T1D. Harjutsalo’s group’s study of at-risk (offspring of T1D patients) Finnish children found that the risk of developing T1D was 1.7 times higher if the father had T1D as compared to when the mother had T1D (incidence of 7.8% vs 5.3%, respectively)[20]. They also found that sons (more than daughters) of a parent with T1D had an increased risk of developing T1D. A younger age of the male parent (at diagnosis) was also found to be a significant predictor with fathers diagnosed at the age of ≤ 4 years being 2.66 times more likely to have children with T1D than fathers diagnosed at the age of 15-17 years[20].
The reason why mothers with T1D are less likely to pass on T1D to children than fathers with T1D remains unclear. Some hypothesize that it may be caused by selective loss of fetuses in mothers with T1D since the rate of miscarriage is higher (15%-30%) in T1D mothers than the general population usually as a result of hyperglycemia at conception and early pregnancy[21-23]. Others hypothesize that it may be due to upregulation of CD4+CD25+FOXP3+ Treg cells that have been found to be significantly elevated in the offspring of mothers with T1D in comparison to infants of healthy mothers[24]. In addition, studies have found that children born before maternal onset of T1D have a higher risk of T1D than infants born after maternal disease onset. Their studies also showed that in vitro insulin stimulation of CD4+CD25+ cells upregulated the expression of anti-inflammatory genes and FOXP3 + Treg cells suggesting that insulin treatment of T1D mothers during pregnancy increases the expansion of anti-inflammatory regulatory cells in their offspring thereby decreasing their risk of T1D development as compared to the offspring of fathers with T1D[24].
Studies in twins have demonstrated the important role of gene and gene modification in the development of T1D. Dizygotic (non-identical) twins and siblings share about 50% of their genes, while monozygotic or identical twins share 100% of their genes meaning differences found between monozygotic twin pairs are likely the result of environmental factors or post-translational modification of histones, the activation of microRNAs, the methylation of DNA, or acquired postconceptional genetic discordance. Multiple studies indicate a significantly higher concordance rate for T1D in monozygotic twins than dizygotic twins (23%-61% vs 0-27% probandwise, respectively). Table 2 compares the concordance rates of a co-twin developing T1D when there is a proband twin diagnosed with T1D. In addition, it was noted that “more monozygotic twins were positive for > 1 autoantibodies that dizygotic twin siblings”[25]. A study followed siblings for 3 years after screening for autoantibodies to see how many would develop T1D and showed that the T1D rate increases with the increasing number of autoantibodies present in the co-twin when the proband twin had been diagnosed with T1D[26]. It also showed that a larger proportion of monozygotic twins are likely to have positive antibodies than dizygotic twins or full siblings[26], see Table 3. Interestingly, a North American study also found that the relative risk of developing TID increased if the proband was diagnosed at an earlier age. It noted that “if the proband is diagnosed before 15 years of age, the long-term risk to the co-twin is estimated at 44% (monozygotic) and 19% (dizygotic); it reaches 65% for the co-twin of a monozygotic proband diagnosed before 5 years of age with time after the proband’s diagnosis”[27]. Additionally, the discordance time among the concordant could range from 1-36 years[25,28] with the mean time being 3.3 (+/- 0.6) years for monozygotic twins and 6.1 (+/- 1.5) years in dizygotic twins[27]. This suggests that while genetics play an important role in the ultimate development of T1D, there must be other factors that contribute since the concordance rate is not 100%.
| Study population | Relation | No. of twin pairs | No. of concordant pairs | Probandwise concordance rate (%) |
| Australia[95] | Monozygotic | 14 | 6 | 61 |
| Dizygotic | 32 | 2 | 12 | |
| Finland[28] | Monozygotic | 44 | 12 | 42.90 |
| Dizygotic | 183 | 7 | 7.40 | |
| Japan[96] | Monozygotic | 19 | 7 | 53.81 |
| Dizygotic | 13 | 1 | 14.31 | |
| United States[25] | Monozygotic | 53 | 12 | 36.91 |
| Dizygotic | 30 | 0 | 0 | |
| Denmark[97] | Monozygotic | 26 | 10 | 53 |
| Dizygotic | 69 | 4 | 11 | |
| Finland[98] | Monozygotic | 26 | 3 | 23.10 |
| Dizygotic | 83 | 2 | 4.80 | |
| North America[27] | Monozygotic | 132 | 38 | 45 |
| Dizygotic | 92 | 13 | 25 | |
| United Kingdom[99] | Monozygotic | 49 | 15 | 25 (1 yr)1 |
| 40 (5 yr)1 | ||||
| 50.7 (10 yr)1 |
| Relation | 0 Autoantibodies | 1 Autoantibody | ≥ 2 Autoantibodies | |||
| No. of individuals | Progressed to T1D | No. of individuals | Progressed to T1D | No. of individuals | Progressed to T1D | |
| Monozygotic Twins | 89 | 1.50% | 25 | 69% | 29 | 69% |
| Dizygotic Twins | 231 | 0% | 22 | 13% | 17 | 72% |
| Full Siblings | 13944 | 0.50% | 1456 | 12% | 900 | 47% |
To address this issue, some have looked to epigenetic factors such as DNA methylation, which is important in gene expression and transcriptional regulation. Rakyan’s group performed an epigenomic-wide association study looking at DNA methylation profiles from T1D monozygotic discordant twin pairs and diabetes-associated antibodies from longitudinally sampled pre-and post-diagnosis T1D singletons to identify CpG sites with T1D-MVPs (T1D-associated methylation variable positions), genetic differences, and epigenetic variations. In this manner, they identified a number of genes including INS-IGF2, SH2B3, MEG3 and ORMDL3, that are known to be correlated with T1D and had differential CpG methylation when they compared T1D-affected to non-affected twins[29]. This demonstrates that in addition to inheritance and the presence of risk genes/alleles, epigenetic factors (such as DNA methylation or exposure to insulin in utero) that regulate gene expression or upregulated anti-inflammatory cells could determine whether T1D would develop.
Though many genetic factors have been implicated in the development of T1D, it’s widely believed that environmental factors must be involved. Genetic susceptibility persists for a lifetime. Individuals can develop T1D at any time from age 1 to 100 years old suggesting that “T1D precipitates in genetically susceptible individuals, very likely as a result of an environmental trigger”[1,14]. This is also supported by the temporally discordant times of T1D development (if at all) in monozygotic twins. Several environmental factors have been suggested as potential risk and protective factors[30,31] affecting T1D incidence such as viruses, anthropometric development[32-34], ethnicity, maternal age and weight[35], microbiota[36-38], number of siblings[35], season, diet, and geographic location[18,31] among other factors. See Table 4 for a list of environmental factors likely contributing to T1D development.
| Risk factor | Protective factors |
| Group B Coxsackieviruses[42,44,45] | Longer breastfeeding duration[78-80] |
| Early introduction of cow’s milk duration[76,77] | Vitamin D intake supplements[68,69] |
| Early cereal introduction[72,87] | Polyunsaturated fatty acids[89,90] |
| High latitudes[18,31] | Intestinal microbiome[36-38] |
| Cold seasons[55-57] | Multiple living siblings[35] |
| Acceleated linear growth and weight gain[32-34] | |
| White ethnicity[35] | |
| Maternal age > 35 yr[35] |
Of the external factors, viruses have long been suggested as a potential environmental trigger for the disease and has some of the strongest evidence as a potential factor. Though there has been strong evidence to suggest the association of viruses with T1D, evidence of a definitive causative relationship leading to the initiation or acceleration of islet autoimmunity is circumstantial[39-41]. Viral infection by enteroviruses such as coxsackieviruses (CVB) or echoviruses have been strongly implicated as potential disease triggers. Several epidemiological studies have linked the association of CVB infection with T1D onset. One study performed between 1964-1984, found that among children diagnosed with T1D, 67% of them had positive CVB IgM serology[42]. Similarly, among 14 children presenting with new-onset T1D, 64% of them were found to have serologic evidence of enteroviral infection, which were mainly CVB3 and CVB4[43]. CVB1 was found to increase the risk of developing T1D in studies looking at children who were at high risk or recently developed T1D[44,45].
The exact mechanism by which viral infections contribute to the destruction of islet cells is still unknown. However, various mechanisms have been suggested such as molecular mimicry, inflammation, endoplasmic reticulum (ER) stress, and bystander activation or suppression of T cells, which are detrimental to beta cell function and survival[30,31]. Some studies have reported cross-reactivity between islet cell autoantigens and certain viral proteins. However, there is no proof that this cross-reactivity leads to T1D autoimmunity[30]. Interestingly, at-risk children with insulin autoantibodies have a reduced ability to produce antibodies to VP1 (markers of viral infections), possibly facilitating enterovirus infection and persistence[46]. It was found that inflammation and ER stress induced by viruses can cause beta cell dysfunction and protein misfolding, which could lead to an abnormal presentation of autoantigens and thus to autoimmunity[47,48]. Viral infection can also stress the ER causing beta cells to release exosomes loaded with autoantigens and immuno-stimulatory chaperones, which are then taken-up by APCs[30,49]. These can then be presented to the adaptive immune system and cause beta cell destruction.
Such associations have also been found in experimental mouse models. One study was able to induce diabetes in mice using a coxsackie virus (CVB4E2) isolated from a patient with diabetic ketoacidosis[50,51]. Additionally, coxsackievirus infection has been found to escalate the progression of T1D in mice engrafted with human islets[52]. Transfer of CVB-specific antibodies from mothers to their offspring in NOD mouse models decreases the risk in the offspring for CVB infection and protects them from T1D development[53,54]. However, a conclusive link between viral infection and the onset of T1D in humans is still lacking. While mouse models have shown a potentially causative relationship between viruses and T1D development, there have mostly been epidemiological studies in humans and no conclusive trials to establish a causative link. However, if a viral link were confirmed, it would garner support for research to develop an enterovirus vaccine to help prevent T1D.
Interestingly, studies have revealed that migrants tend to develop the same level of risk of developing T1D as the population in the area of their new residence, despite their origin being from a low incidence region of the world. This supports the theory of the environmental effects of T1D development, which could be influenced by factors such as seasonality or geographic location.
Numerous reasons have been proposed for the apparent link between seasons and the onset of T1D. Some of these theories include that there is a seasonal variation in the blood glucose and insulin levels possibly due to a typically reduced level of activity in young people in the cold winter months and seasonal viral infections. Several studies suggest a seasonality that conforms to a sinusoidal model that peaks in the winter and troughs in the summer[55,56]. Epidemiological studies following birth cohorts found that many people (79%) with high risk genotypes seroconverted during the fall/winter months[57]. A large international study found that 42 out of 105 centers exhibited this seasonal trend with 28 centers having peaks of diagnosis in the winter and 33 had troughs in the summer[58].
However, other studies contradicted this correlation, stating that their findings indicated that there was no seasonality in the month of onset, rather that there was significant seasonality in the month of birth (peaking in November to January)[59]. In addition, the seasonality pattern appears to be dependent on the geographical position, in regards to the northern/southern hemisphere dichotomy. The correlation of seasonality appeared to disappear after adjustment was made for latitude, where centers furthest away from the equator were more likely to exhibit significant seasonality[58]. However, most of the data from the study came from the northern hemisphere, and there was inadequate information from Asia and Africa. Therefore, this correlation was deemed inconclusive, as more data on the population living below the 30th parallel north was needed for the information to be accurate[58]. Though still inconclusive, this data likely indicates that there is an increased susceptibility to developing T1D during times when people are most vulnerable to viral infections. This is generally during cold (viral infection) seasons, which may vary based on geographic location and could explain why regions in colder climates or with temperate seasons have higher T1D incidence rates than regions with warmer climates and tropical seasons.
There is increasing evidence that demonstrates a strong association between vitamin D signaling and many biological processes involved in regulating immune responses. A deficiency in vitamin D is associated with increased risk of autoimmune diseases such as multiple sclerosis, systemic lupus erythematosus, and T1D[60]. The vitamin D receptor (VDR) is expressed on several immune cells (B cells, T cells and antigen-presenting cells) and affects “cell proliferation and differentiation and immunologic effects resulting in an ability to maintain tolerance and promote protective immunity”[61].
FokI (rs10735810) has been identified as one of four polymorphisms in the VDR gene. Its presence has been suggested to cause a dysfunctional structural change on the VDR protein[62]. Studies demonstrated that mononuclear cells from subjects with the dysfunctional VDR genotype had a lower ED50 of 1,25 (OH)2D when compared with the normal VDR genotype[63]. Studies also reported that monocytes and dendritic cells from subjects with the dysfunctional genotype had a higher expression of interleukin-12 (pro-inflammatory cytokine) and proliferated more significantly after stimulation than those with the normal genotype[64].
A causal relationship between FokI polymorphism and vitamin D status in T1D has not been reported, but several studies have suggested a correlation. Vitamin D deficiency has been found to be higher in children with T1D in comparison to controls[65-67]. A birth cohort following over 12000 live births found that children receiving regular Vitamin D supplementation had a reduced risk of developing T1D with a RR of 0.22 (0.05-0.89), while children suspected of rickets (related to vitamin D deficiency) had a RR of 3.0 (1.0 -9.0) of developing T1D[68]. Another study involving patients with latent auto-immune diabetes found that c-peptide levels and beta cell function was better preserved in the group who received vitamin D supplements[69]. Some aspects regarding the relationship of islet autoimmunity and development of T1D remain controversial. Though still controversial, vitamin D appears to have a beneficial effect in preventing T1D, likely due to its role in modulating potentially pathogenic inflammatory processes.
Epidemiological studies have noted that a majority of children that develop T1D before the age of 10 years old, seroconvert within the 1st two years of life[57]. This suggests that early life exposures could play an important role in environmental factors leading to T1D such as nutrition and diet.
Dietary factors that have been explored include infant food exposures such breastfeeding, cow’s milk formula, and cereal. Their role in the development of T1D is controversial with a number of studies having conflicting conclusions and insufficient evidence to prove a causal effect[70-74]. However, a majority of birth cohort studies investigating high-risk infants indicate that short durations of breastfeeding (earlier than 2-4 mo)[75] and early introduction of cow’s milk (earlier than 4 mo)[76,77] may have a predisposing factor with an overall odds ratio of 1.43 (95%CI: 1.15-1.77) and 1.63 (95%CI: 1.22-2.17), respectively[78]. While a longer duration of exclusive breastfeeding (longer than 4 months) has a protective factor with an odds ratio of 0.17, 95%CI: 0.03-0.86[75]. The exact mechanism for these predispositions are still speculative. It is proposed that breastfeeding provides the benefit of additional immune protection from the passing of maternal antibodies that may help decrease the frequency of early enteroviral infections[79,80] and decreased intestinal permeability, which may protect from potentially T1D inciting exposures[81]. The predisposition caused by early exposure to cow proteins is thought to be mediated by inflammation of the intestinal mucosa[82], increased intestinal permeability[81] and a dysregulated immune response to the milk proteins[83-85]. Intertwined with this concept is the role of intestinal microbiome. Intestinal “microbes influence lipid and glucose metabolism, as well as immunity and systemic inflammation outside of the intestine”[86]. Available studies on this subject are currently inadequate to confirm a definitive connection, but there appears to be a trend of lower microbial diversity in people with T1D with a dominance of a microbiome less conducive to maintaining gut integrity[86,87].
Another study looking at exposure to cereal found that initial cereal exposure before 4 mo of age[72] and after 6 mo of age was associated with an increased risk of T1D or islet autoimmunity with a HR = 5.55 (95%CI: 1.92-16.03) and 12.53 (95%CI: 3.19-49.23), respectively[88].
Another dietary factor that has been investigated is the effect of polyunsaturated fatty acids diets, given its association in modulating the immune system and curtailing inflammatory responses[89,90]. An observational study in the United States found that omega-3 fatty acid intake was inversely associated with the risk of developing T1D or islet autoimmunity[91]. Another study noted linoleic acid had an inverse, marginal association with the development of T1D[92]. Conversely, other studies have found no correlation between fatty acids and islet autoimmunity. It is also interesting that some regions (i.e., India or Africa) that have relatively low polyunsaturated fatty acids in their diet do not necessarily have high T1D incidence rates. This could be because these fatty acids do not play a major role as an environmental factor, increase intake confers a protective factor, but its lack does not yield a risk factor. It could also be possible that other factors such as geography offset these possible risk factors.
Some of the first cases ascribed to T1D were discovered by the ancient Indians around 1500 BC when patients were noted to have excessive urination that would attract ants due to its sweetness[93]. This condition had been recognized later as a disease and eventually given the name T1D. Early on, T1D was a death sentence because no one understood the pathogenesis or could manage it. Those affected would suffer from insatiable hunger and slowly waste away and die from acute complications. After the seminal discoveries by Joseph von Mering and Oskar Minkowski in 1889 of the pancreas’ role in T1D and Frederick Banting and Charles Best ability to purify insulin (in the 1920s) from animal pancreas for the use in treatment of T1D patients, life with diabetes has become an issue of management and not certain death[94].
Since then, significant progress has been made in understanding T1D as a disease with a multifactorial etiology, including genetic factors, primarily affecting the adaptive immune system, and environmental triggers. However, our understanding of T1D pathogenesis remains incomplete. There are still many questions such as why particular genetic variants predispose to the disease, how epigenetics and environmental factors contribute to the disease onset (especially given the 40%-60% disconcordance rate in identical twins), and whether other unrecognized genetics and/or immunologic factors play a pivotal role in T1D development and progression (i.e., DE cells or FasL)[10,11].
Answering these questions is urgently needed as patients still suffer from reduced quality of life given the large treatment burden, the risk of life-threatening hypoglycemia and potentially debilitating end organ complications. Current research focusing on these parameters to clarify the pathogenesis of this disease are expected to help in the development of novel treatments that could prevent disease onset or slow progression by identifying new targets for immunotherapy or vaccines to prevent its initiation. Overall, while the battle against T1D is still ongoing, research has provided us the tools to gain an upper hand and the knowledge to hopefully win the battle in the early stages or before it has begun.
| 1. | Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 836] [Cited by in RCA: 762] [Article Influence: 47.6] [Reference Citation Analysis (5)] |
| 2. | Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, Edvardsson S, Landin-Olsson M. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 4. | SEARCH for Diabetes in Youth Study Group. Liese AD, D'Agostino RB Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, Standiford D, Williams DE. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 545] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Hamman RF, Bell RA, Dabelea D, D'Agostino RB, Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Pihoker C, Rodriguez BL, Saydah S; SEARCH for Diabetes in Youth Study Group. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336-3344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Cito M, Pellegrini S, Piemonti L, Sordi V. The potential and challenges of alternative sources of β cells for the cure of type 1 diabetes. Endocr Connect. 2018;7:R114-R125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 7. | Burrack AL, Martinov T, Fife BT. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front Endocrinol (Lausanne). 2017;8:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 8. | Aghazadeh Y, Nostro MC. Cell Therapy for Type 1 Diabetes: Current and Future Strategies. Curr Diab Rep. 2017;17:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Ahmed R, Omidian Z, Donner T, Hamad ARA. Hiding in plain sight: time to unlock autoimmune clues in human CD5+ B cells by using nextgen technology. Discov Med. 2018;26:79-83. [PubMed] |
| 10. | Ahmed R, Omidian Z, Giwa A, Cornwell B, Majety N, Bell DR, Lee S, Zhang H, Michels A, Desiderio S, Sadegh-Nasseri S, Rabb H, Gritsch S, Suva ML, Cahan P, Zhou R, Jie C, Donner T, Hamad ARA. A Public BCR Present in a Unique Dual-Receptor-Expressing Lymphocyte from Type 1 Diabetes Patients Encodes a Potent T Cell Autoantigen. Cell. 2019;177:1583-1599.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Xiao Z, Mohamood AS, Uddin S, Gutfreund R, Nakata C, Marshall A, Kimura H, Caturegli P, Womer KL, Huang Y, Jie C, Chakravarti S, Schneck JP, Yagita H, Hamad AR. Inhibition of Fas ligand in NOD mice unmasks a protective role for IL-10 against insulitis development. Am J Pathol. 2011;179:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Pociot F. Type 1 diabetes genome-wide association studies: not to be lost in translation. Clin Transl Immunology. 2017;6:e162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Morahan G. Insights into type 1 diabetes provided by genetic analyses. Curr Opin Endocrinol Diabetes Obes. 2012;19:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 698] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 15. | Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Delli AJ, Lindblad B, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, Marcus C, Lernmark A; Better Diabetes Diagnosis (BDD) Study Group. Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr Diabetes. 2010;11:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Fourlanos S, Varney MD, Tait BD, Morahan G, Honeyman MC, Colman PG, Harrison LC. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Eringsmark Regnéll S, Lernmark A. The environment and the origins of islet autoimmunity and Type 1 diabetes. Diabet Med. 2013;30:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Pugliese A, Boulware D, Yu L, Babu S, Steck AK, Becker D, Rodriguez H, DiMeglio L, Evans-Molina C, Harrison LC, Schatz D, Palmer JP, Greenbaum C, Eisenbarth GS, Sosenko JM; Type 1 Diabetes TrialNet Study Group. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 Haplotype Protects Autoantibody-Positive Relatives From Type 1 Diabetes Throughout the Stages of Disease Progression. Diabetes. 2016;65:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Harjutsalo V, Reunanen A, Tuomilehto J. Differential transmission of type 1 diabetes from diabetic fathers and mothers to their offspring. Diabetes. 2006;55:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Miodovnik M, Lavin JP, Knowles HC, Holroyde J, Stys SJ. Spontaneous abortion among insulin-dependent diabetic women. Am J Obstet Gynecol. 1984;150:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Rosenn B, Miodovnik M, Combs CA, Khoury J, Siddiqi TA. Glycemic thresholds for spontaneous abortion and congenital malformations in insulin-dependent diabetes mellitus. Obstet Gynecol. 1994;84:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Dorman JS, Burke JP, McCarthy BJ, Norris JM, Steenkiste AR, Aarons JH, Schmeltz R, Cruickshanks KJ. Temporal trends in spontaneous abortion associated with Type 1 diabetes. Diabetes Res Clin Pract. 1999;43:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Luopajärvi K, Nieminen JK, Ilonen J, Akerblom HK, Knip M, Vaarala O. Expansion of CD4+CD25+FOXP3+ regulatory T cells in infants of mothers with type 1 diabetes. Pediatr Diabetes. 2012;13:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Redondo MJ, Rewers M, Yu L, Garg S, Pilcher CC, Elliott RB, Eisenbarth GS. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: prospective twin study. BMJ. 1999;318:698-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Triolo TM, Fouts A, Pyle L, Yu L, Gottlieb PA, Steck AK; Type 1 Diabetes TrialNet Study Group. Identical and Nonidentical Twins: Risk and Factors Involved in Development of Islet Autoimmunity and Type 1 Diabetes. Diabetes Care. 2019;42:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (19)] |
| 27. | Kumar D, Gemayel NS, Deapen D, Kapadia D, Yamashita PH, Lee M, Dwyer JH, Roy-Burman P, Bray GA, Mack TM. North-American twins with IDDM. Genetic, etiological, and clinical significance of disease concordance according to age, zygosity, and the interval after diagnosis in first twin. Diabetes. 1993;42:1351-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52:1052-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 260] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Rakyan VK, Beyan H, Down TA, Hawa MI, Maslau S, Aden D, Daunay A, Busato F, Mein CA, Manfras B, Dias KR, Bell CG, Tost J, Boehm BO, Beck S, Leslie RD. Identification of type 1 diabetes-associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet. 2011;7:e1002300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 30. | Dunne JL, Richardson SJ, Atkinson MA, Craig ME, Dahl-Jørgensen K, Flodström-Tullberg M, Hyöty H, Insel RA, Lernmark Å, Lloyd RE, Morgan NG, Pugliese A. Rationale for enteroviral vaccination and antiviral therapies in human type 1 diabetes. Diabetologia. 2019;62:744-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Xia Y, Xie Z, Huang G, Zhou Z. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab Res Rev. 2019;35:e3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54 Suppl 2:S125-S136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 33. | Lammi N, Moltchanova E, Blomstedt PA, Tuomilehto J, Eriksson JG, Karvonen M. Childhood BMI trajectories and the risk of developing young adult-onset diabetes. Diabetologia. 2009;52:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia. 2001;44:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 418] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | D'Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypotheses. Arch Pediatr Adolesc Med. 2010;164:732-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2969] [Article Influence: 185.6] [Reference Citation Analysis (0)] |
| 37. | Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM, Peet A, Tillmann V, Pöhö P, Mattila I, Lähdesmäki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Orešič M, Huttenhower C, Knip M; DIABIMMUNE Study Group, Xavier RJ. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 892] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 38. | Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 1514] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 39. | Jakobsen OAJ, Szereday L. The "Three Amigos" lurking behind type 1 diabetes: Hygiene, gut microbiota and viruses. Acta Microbiol Immunol Hung. 2018;65:421-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Coppieters KT, Wiberg A, von Herrath MG. Viral infections and molecular mimicry in type 1 diabetes. APMIS. 2012;120:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Shamriz O, Shoenfeld Y. Infections: a double-edge sword in autoimmunity. Curr Opin Rheumatol. 2018;30:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 42. | Friman G, Fohlman J, Frisk G, Diderholm H, Ewald U, Kobbah M, Tuvemo T. An incidence peak of juvenile diabetes. Relation to Coxsackie B virus immune response. Acta Paediatr Scand Suppl. 1985;320:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 177] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 44. | Laitinen OH, Honkanen H, Pakkanen O, Oikarinen S, Hankaniemi MM, Huhtala H, Ruokoranta T, Lecouturier V, André P, Harju R, Virtanen SM, Lehtonen J, Almond JW, Simell T, Simell O, Ilonen J, Veijola R, Knip M, Hyöty H. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 45. | Oikarinen S, Tauriainen S, Hober D, Lucas B, Vazeou A, Sioofy-Khojine A, Bozas E, Muir P, Honkanen H, Ilonen J, Knip M, Keskinen P, Saha MT, Huhtala H, Stanway G, Bartsocas C, Ludvigsson J, Taylor K, Hyöty H; VirDiab Study Group. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Ashton MP, Eugster A, Walther D, Daehling N, Riethausen S, Kuehn D, Klingel K, Beyerlein A, Zillmer S, Ziegler AG, Bonifacio E. Incomplete immune response to coxsackie B viruses associates with early autoimmunity against insulin. Sci Rep. 2016;6:32899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Marré ML, James EA, Piganelli JD. β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol. 2015;3:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Phelps EA, Cianciaruso C, Michael IP, Pasquier M, Kanaani J, Nano R, Lavallard V, Billestrup N, Hubbell JA, Baekkeskov S. Aberrant Accumulation of the Diabetes Autoantigen GAD65 in Golgi Membranes in Conditions of ER Stress and Autoimmunity. Diabetes. 2016;65:2686-2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, Piemonti L, Hirosue S, Swartz MA, De Palma M, Hubbell JA, Baekkeskov S. Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes. 2017;66:460-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 50. | Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 620] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 51. | Gerling I, Nejman C, Chatterjee NK. Effect of coxsackievirus B4 infection in mice on expression of 64,000-Mr autoantigen and glucose sensitivity of islets before development of hyperglycemia. Diabetes. 1988;37:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Gallagher GR, Brehm MA, Finberg RW, Barton BA, Shultz LD, Greiner DL, Bortell R, Wang JP. Viral infection of engrafted human islets leads to diabetes. Diabetes. 2015;64:1358-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Oshima M, Knoch KP, Diedisheim M, Petzold A, Cattan P, Bugliani M, Marchetti P, Choudhary P, Huang GC, Bornstein SR, Solimena M, Albagli-Curiel O, Scharfmann R. Virus-like infection induces human β cell dedifferentiation. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Larsson PG, Lakshmikanth T, Svedin E, King C, Flodström-Tullberg M. Previous maternal infection protects offspring from enterovirus infection and prevents experimental diabetes development in mice. Diabetologia. 2013;56:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Lévy-Marchal C, Patterson C, Green A. Variation by age group and seasonality at diagnosis of childhood IDDM in Europe. The EURODIAB ACE Study Group. Diabetologia. 1995;38:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Willis JA, Scott RS, Darlow BA, Lewy H, Ashkenazi I, Laron Z. Seasonality of birth and onset of clinical disease in children and adolescents (0-19 years) with type 1 diabetes mellitus in Canterbury, New Zealand. J Pediatr Endocrinol Metab. 2002;15:645-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Kimpimäki T, Kupila A, Hämäläinen AM, Kukko M, Kulmala P, Savola K, Simell T, Keskinen P, Ilonen J, Simell O, Knip M. The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab. 2001;86:4782-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Moltchanova EV, Schreier N, Lammi N, Karvonen M. Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med. 2009;26:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 59. | Ye J, Chen RG, Ashkenazi I, Laron Z. Lack of seasonality in the month of onset of childhood IDDM (0.7-15 years) in Shanghai, China. J Pediatr Endocrinol Metab. 1998;11:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 61. | Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 788] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 62. | Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1087] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 63. | Colin EM, Weel AE, Uitterlinden AG, Buurman CJ, Birkenhäger JC, Pols HA, van Leeuwen JP. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. Clin Endocrinol (Oxf). 2000;52:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | van Etten E, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB, Overbergh L, Verstuyf A, Bouillon R, Roep BO, Badenhoop K, Mathieu C. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, Guiter G, Mian M. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Borkar VV, Devidayal, Verma S, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2010;11:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Littorin B, Blom P, Schölin A, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjörnsdottir S, Nyström L, Ostman J, Sundkvist G. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2006;49:2847-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1163] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 69. | Li X, Liao L, Yan X, Huang G, Lin J, Lei M, Wang X, Zhou Z. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab Res Rev. 2009;25:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Gerstein HC, VanderMeulen J. The relationship between cow's milk exposure and type 1 diabetes. Diabet Med. 1996;13:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 71. | Norris JM, Beaty B, Klingensmith G, Yu LP, Hoffman M, Chase HP, Erlich HA, Eisenbarth GS, Rewers M. Lack of association between early exposure to cow's milk protein and beta-cell autoimmunity. Diabetes Autoimmunity Study in the Young (DAISY). JAMA. 1996;276:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 73. | Virtanen SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg-Kippilä C, Hakulinen T, Ahonen S, Uusitalo L, Niinistö S, Veijola R, Simell O, Ilonen J, Knip M. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Hakola L, Takkinen HM, Niinistö S, Ahonen S, Nevalainen J, Veijola R, Ilonen J, Toppari J, Knip M, Virtanen SM. Infant Feeding in Relation to the Risk of Advanced Islet Autoimmunity and Type 1 Diabetes in Children With Increased Genetic Susceptibility: A Cohort Study. Am J Epidemiol. 2018;187:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Kimpimäki T, Erkkola M, Korhonen S, Kupila A, Virtanen SM, Ilonen J, Simell O, Knip M. Short-term exclusive breastfeeding predisposes young children with increased genetic risk of Type I diabetes to progressive beta-cell autoimmunity. Diabetologia. 2001;44:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Holmberg H, Wahlberg J, Vaarala O, Ludvigsson J; ABIS Study Group. Short duration of breast-feeding as a risk-factor for beta-cell autoantibodies in 5-year-old children from the general population. Br J Nutr. 2007;97:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Dahlquist G, Savilahti E, Landin-Olsson M. An increased level of antibodies to beta-lactoglobulin is a risk determinant for early-onset type 1 (insulin-dependent) diabetes mellitus independent of islet cell antibodies and early introduction of cow's milk. Diabetologia. 1992;35:980-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Gerstein HC. Cow's milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994;17:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 218] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Jenista JA, Powell KR, Menegus MA. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 111] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Sadeharju K, Knip M, Virtanen SM, Savilahti E, Tauriainen S, Koskela P, Akerblom HK, Hyöty H; Finnish TRIGR Study Group. Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics. 2007;119:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 81. | Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Savilahti E, Akerblom HK, Tainio VM, Koskimies S. Children with newly diagnosed insulin dependent diabetes mellitus have increased levels of cow's milk antibodies. Diabetes Res. 1988;7:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Luopajärvi K, Savilahti E, Virtanen SM, Ilonen J, Knip M, Akerblom HK, Vaarala O. Enhanced levels of cow's milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr Diabetes. 2008;9:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Saukkonen T, Savilahti E, Vaarala O, Virtala ET, Tuomilehto J, Akerblom HK. Children with newly diagnosed IDDM have increased levels of antibodies to bovine serum albumin but not to ovalbumin. Childhood Diabetes in Finland Study Group. Diabetes Care. 1994;17:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 87. | Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6:e25792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 598] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 88. | Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 89. | Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 213] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 90. | Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 92. | Virtanen SM, Niinistö S, Nevalainen J, Salminen I, Takkinen HM, Kääriä S, Uusitalo L, Alfthan G, Kenward MG, Veijola R, Simell O, Ilonen J, Knip M. Serum fatty acids and risk of advanced beta-cell autoimmunity: a nested case-control study among children with HLA-conferred susceptibility to type I diabetes. Eur J Clin Nutr. 2010;64:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Ariyachet C, Tovaglieri A, Xiang G, Lu J, Shah MS, Richmond CA, Verbeke C, Melton DA, Stanger BZ, Mooney D, Shivdasani RA, Mahony S, Xia Q, Breault DT, Zhou Q. Reprogrammed Stomach Tissue as a Renewable Source of Functional β Cells for Blood Glucose Regulation. Cell Stem Cell. 2016;18:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 94. | Rosenfeld L. Insulin: discovery and controversy. Clin Chem. 2002;48:2270-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Condon J, Shaw JE, Luciano M, Kyvik KO, Martin NG, Duffy DL. A study of diabetes mellitus within a large sample of Australian twins. Twin Res Hum Genet. 2008;11:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Matsuda A, Kuzuya T. Diabetic twins in Japan. Diabetes Res Clin Pract. 1994;24 Suppl:S63-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 97. | Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. BMJ. 1995;311:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 206] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 98. | Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, Stengård J, Kesäniemi YA. Concordance for type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 376] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 99. | Olmos P, A'Hern R, Heaton DA, Millward BA, Risley D, Pyke DA, Leslie RD. The significance of the concordance rate for type 1 (insulin-dependent) diabetes in identical twins. Diabetologia. 1988;31:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Panchu P, Sahoo J S-Editor: Yan JP L-Editor: A E-Editor: Wu YXJ