©The Author(s) 2025.
World J Diabetes. Sep 15, 2025; 16(9): 110515
Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110515
Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110515
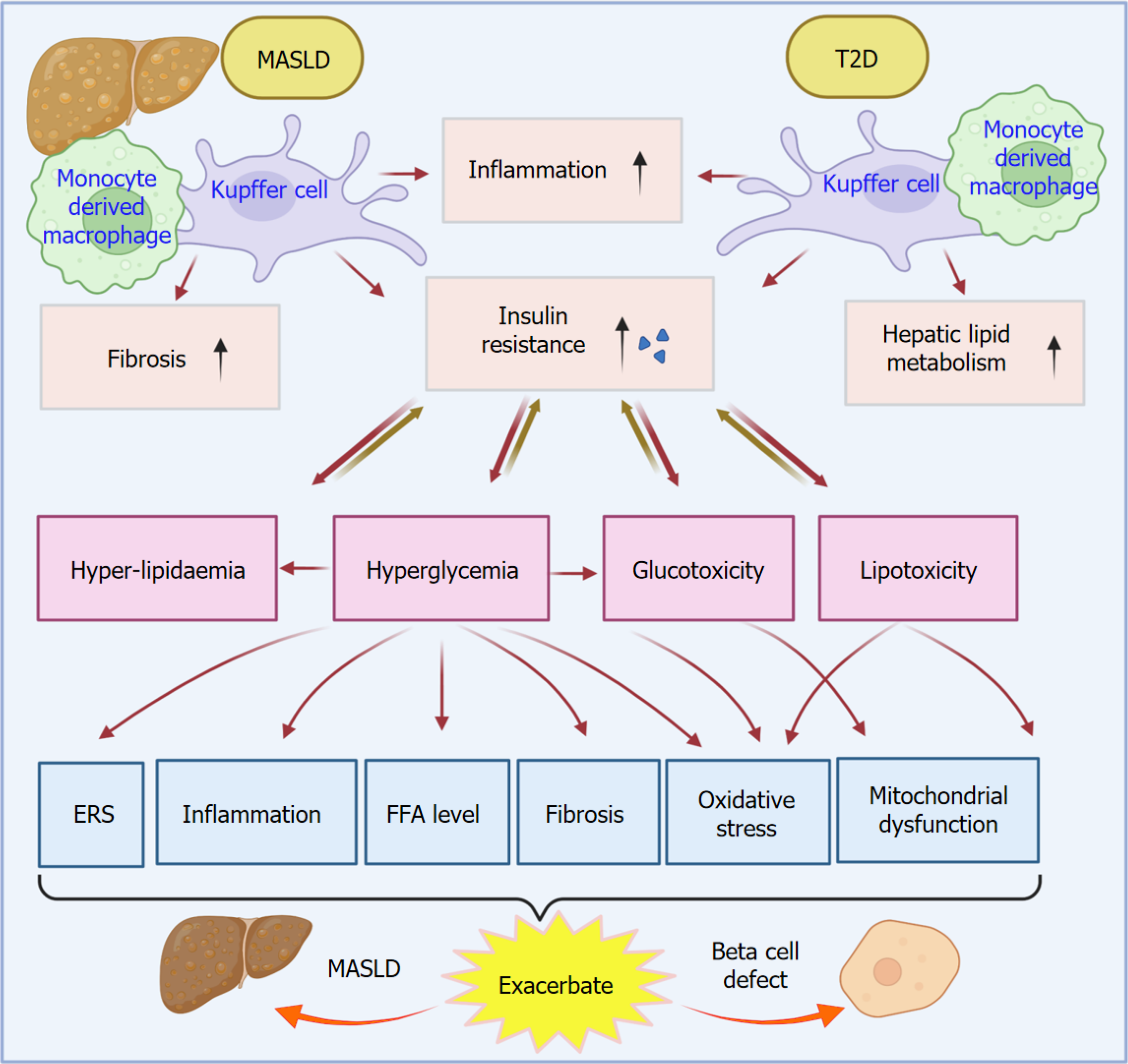
Figure 1 Liver resident macrophage, Kupffer cells, and monocyte-derived macrophages play an essential role in the crosslink of metabolic dysfunction-associated steatotic liver disease and type 2 diabetes.
Liver macrophages, including both liver-resident Kupffer cells and monocyte-derived macrophages, serve as a cellular link between metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes (T2D) through a variety of factors such as inflammation, insulin resistance, fibrosis, and hepatic lipid metabolism. The increased insulin resistance resulting from MASLD and T2D leads to hyperlipidemia, hyperglycemia, glucotoxicity, and lipotoxicity. These factors subsequently exacerbate the MASLD and beta cell defect by increasing endoplasmic reticulum stress, oxidative stress, inflammation, increased free fatty acid level, fibrosis, and mitochondrial dysfunction. T2D: Type 2 diabetes; MASLD: Metabolic dysfunction-associated steatotic liver disease; ERS: Endoplasmic reticulum stress; FFA: Free fatty acid.
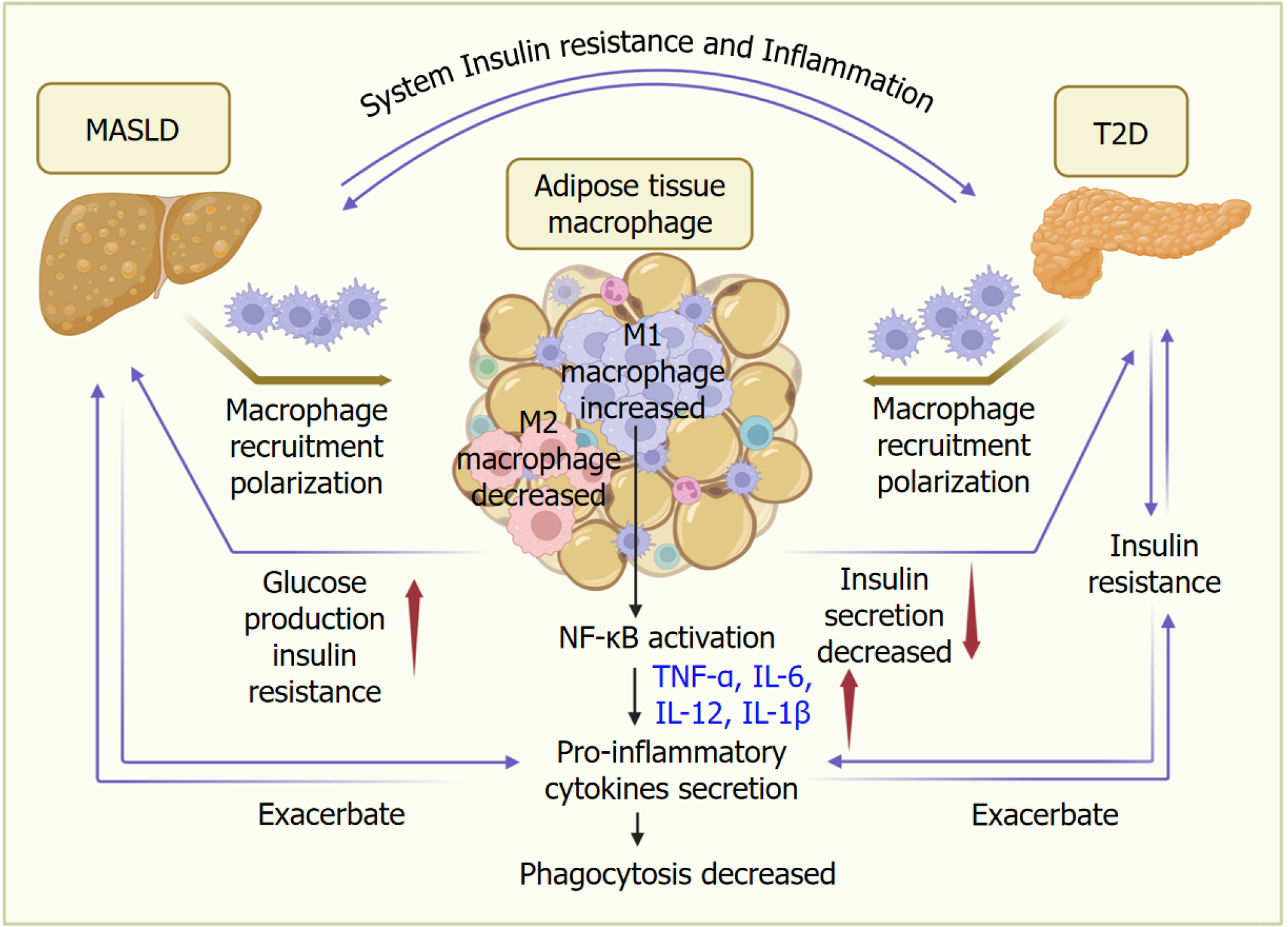
Figure 2 Adipose tissue macrophages play an essential role in the crosslink between metabolic dysfunction-associated steatotic liver disease and type 2 diabetes.
Adipose tissue macrophages play an essential role in connecting metabolic dysfunction-associated steatotic liver disease (MASLD) and type 2 diabetes (T2D). MASLD and T2D promote the recruitment and polarization of macrophages in adipose tissues. Upon polarization and activation of nuclear factor kappa-B, M1 macrophages increase the expression of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-12, IL-6, and IL-1β, which results in a decreased phagocytosis function of macrophages. Proinflammatory response in adipose tissues further exacerbates insulin resistance to promote T2D and MASLD development. Adipose tissue macrophages also contribute to the aggregation of T2D and MASLD by increasing glucose production and insulin resistance, as well as reducing insulin secretion. Through systemic inflammation and insulin resistance, MASLD and T2D interact with each other in a bidirectional way. T2D: Type 2 diabetes; MASLD: Metabolic dysfunction-associated steatotic liver disease; NF-κB: Nuclear factor kappa-B; IL: Interleukin; TNF: Tumor necrosis factor.
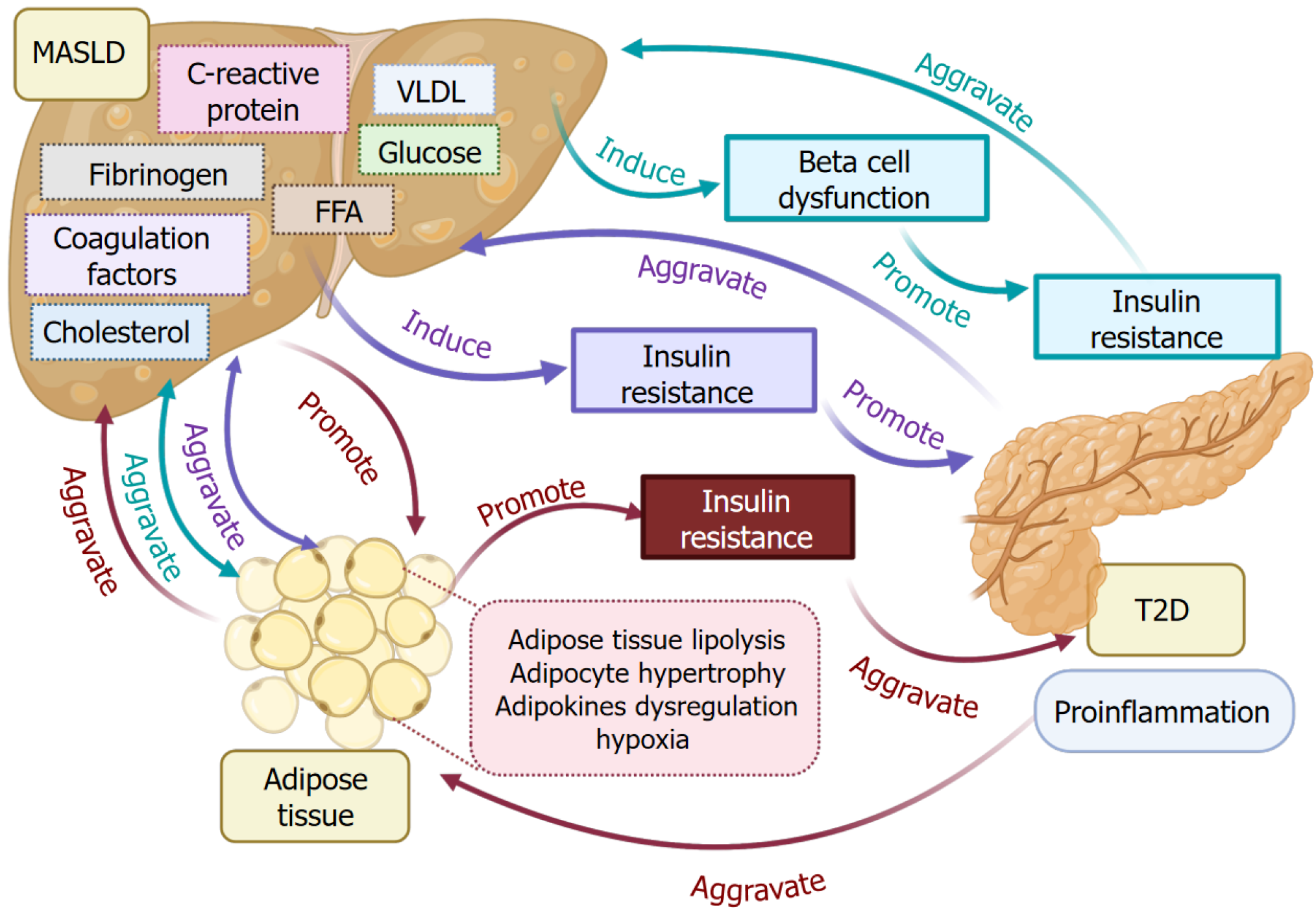
Figure 3 Metabolism function crosslinks among metabolic dysfunction-associated steatotic liver disease, type 2 diabetes, and adipose tissues.
Metabolic disorder induces alterations of energy metabolism, such as elevated levels of glucose, free fatty acid, very-low-density lipoprotein and cholesterol, C-reactive protein, coagulation factors, and fibrinogen in metabolic dysfunction-associated steatotic liver disease (MASLD) livers, beta cell dysfunction, and insulin resistance, causing type 2 diabetes (T2D) progression. T2D aggravates MASLD reciprocally. Meanwhile, the elevated metabolic factors also promote adipose tissue lipolysis, adipocyte hypertrophy, adipokine dysregulation, and hypoxia, which promote insulin resistance and aggregate MASLD. T2D-associated insulin resistance and inflammation aggravate adipose tissue metabolic dysfunction. T2D: Type 2 diabetes; MASLD: Metabolic dysfunction-associated steatotic liver disease; FFA: Free fatty acid; VLDL: Very-low-density lipoprotein.
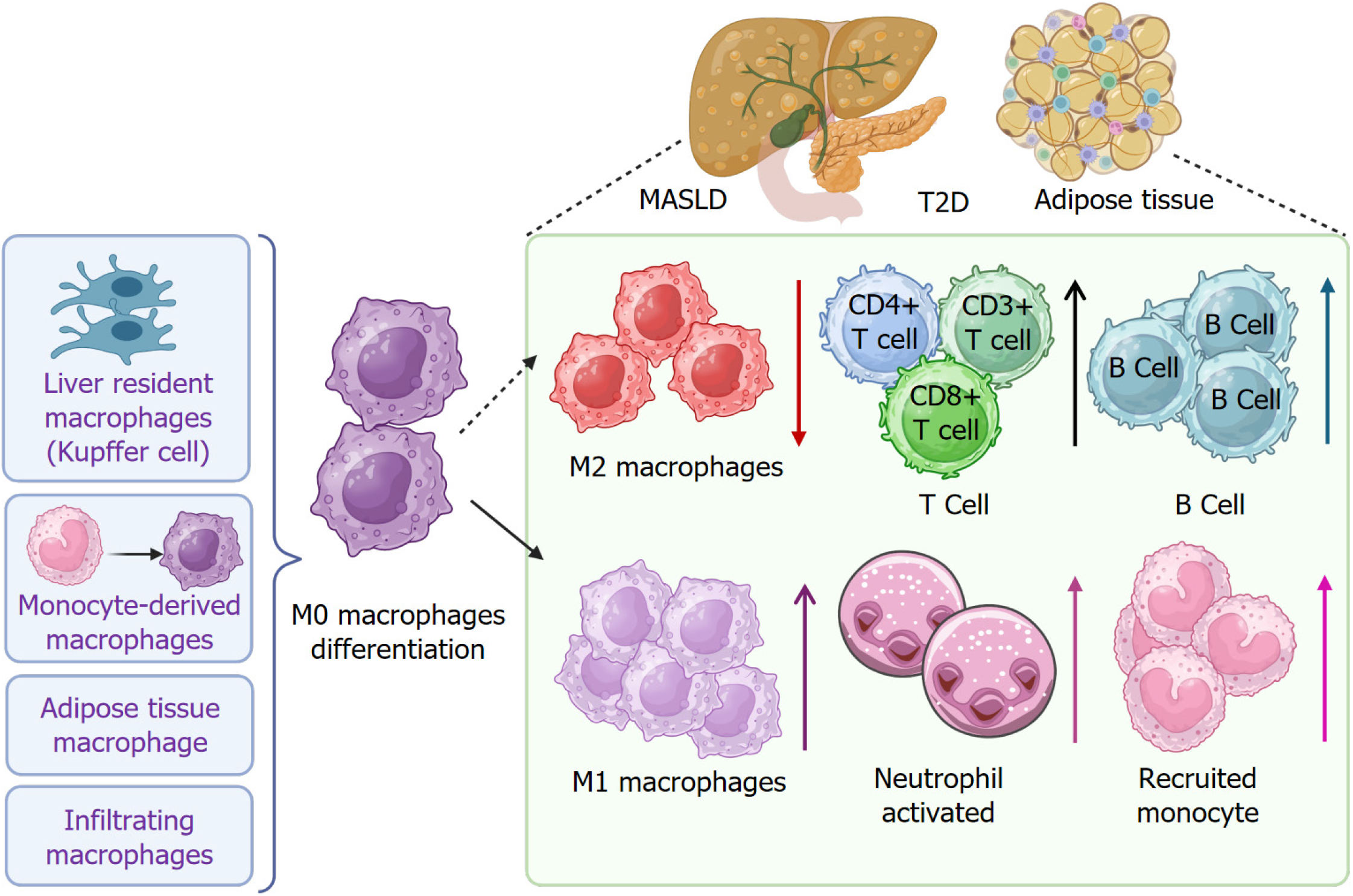
Figure 4 Immune cell infiltration in metabolic dysfunction-associated steatotic liver disease, type 2 diabetes, and adipose tissues.
Immune cell infiltration occurs in patients with metabolic dysfunction-associated steatotic liver disease and type 2 diabetes, as well as in adipose tissues. Liver resident macrophages (Kupffer cells and monocyte-derived macrophages), adipose tissue macrophages, and infiltrated macrophages are polarized into M1 macrophages under the pathologic condition. In contrast, M2 macrophage population is decreased. Infiltration of B cells and cluster of differentiation (CD) 3+, CD4+, and CD8+ T cells is increased. Neutrophil activation and monocyte recruitment are increased. T2D: Type 2 diabetes; MASLD: Metabolic dysfunction-associated steatotic liver disease; CD: Cluster of differentiation.
- Citation: Zhang CY, Liu S, Yang M. Macrophage and inflammation in diabetes and metabolic dysfunction-associated steatotic liver disease: From mechanisms to therapeutic strategies. World J Diabetes 2025; 16(9): 110515
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/110515.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.110515