©The Author(s) 2025.
World J Diabetes. Sep 15, 2025; 16(9): 110053
Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110053
Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.110053
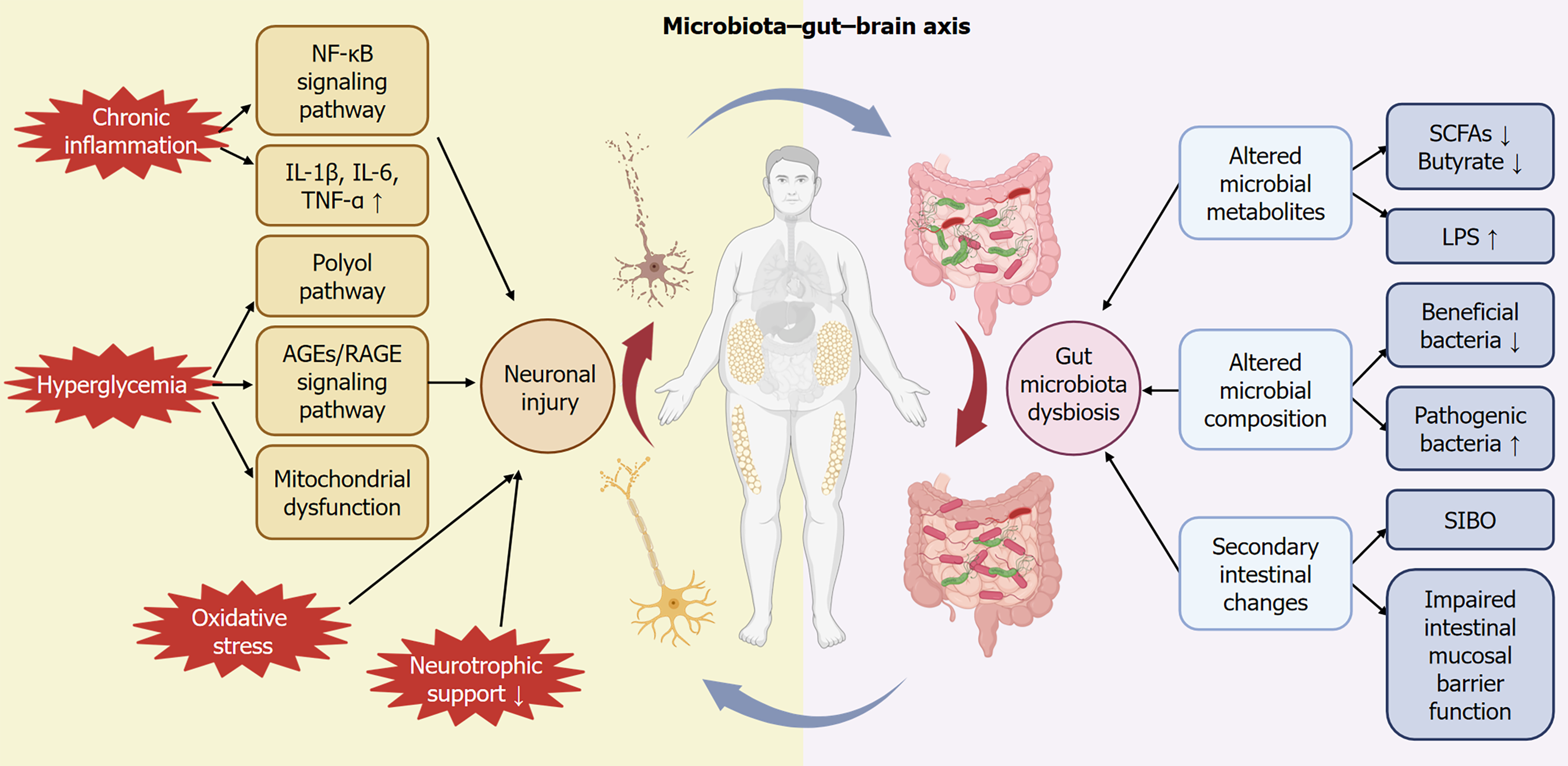
Figure 1 Metabolism-gut microbiota-enteric autonomic nervous system crosstalk.
Hyperglycemia-induced direct injury includes activation of the polyol pathway, accumulation of advanced glycation end-products, and mitochondrial dysfunction. Inflammation and immune-mediated injury, involving activation of the nuclear factor kappa B (NF-kB) signaling pathway and increased release of pro-inflammatory cytokines exacerbate neuronal and glial cell apoptosis. Reduced neurotrophic support contributes significantly to neuronal degeneration. These neuronal injuries interact bidirectionally with gut microbiota dysbiosis, which manifests as decreased beneficial bacteria and increased pathogenic bacteria. Altered microbial metabolites, notably reduced short-chain fatty acids (SCFAs) and elevated lipopolysaccharides (LPS), further disrupt the intestinal mucosal barrier and exacerbate inflammation, creating a vicious cycle with neuronal injury. Clinically, these mechanisms collectively lead to gastrointestinal autonomic dysfunction, such as gastroparesis, intestinal dysmotility, and altered nutrient absorption. SIBO: Small intestinal bacterial overgrowth.
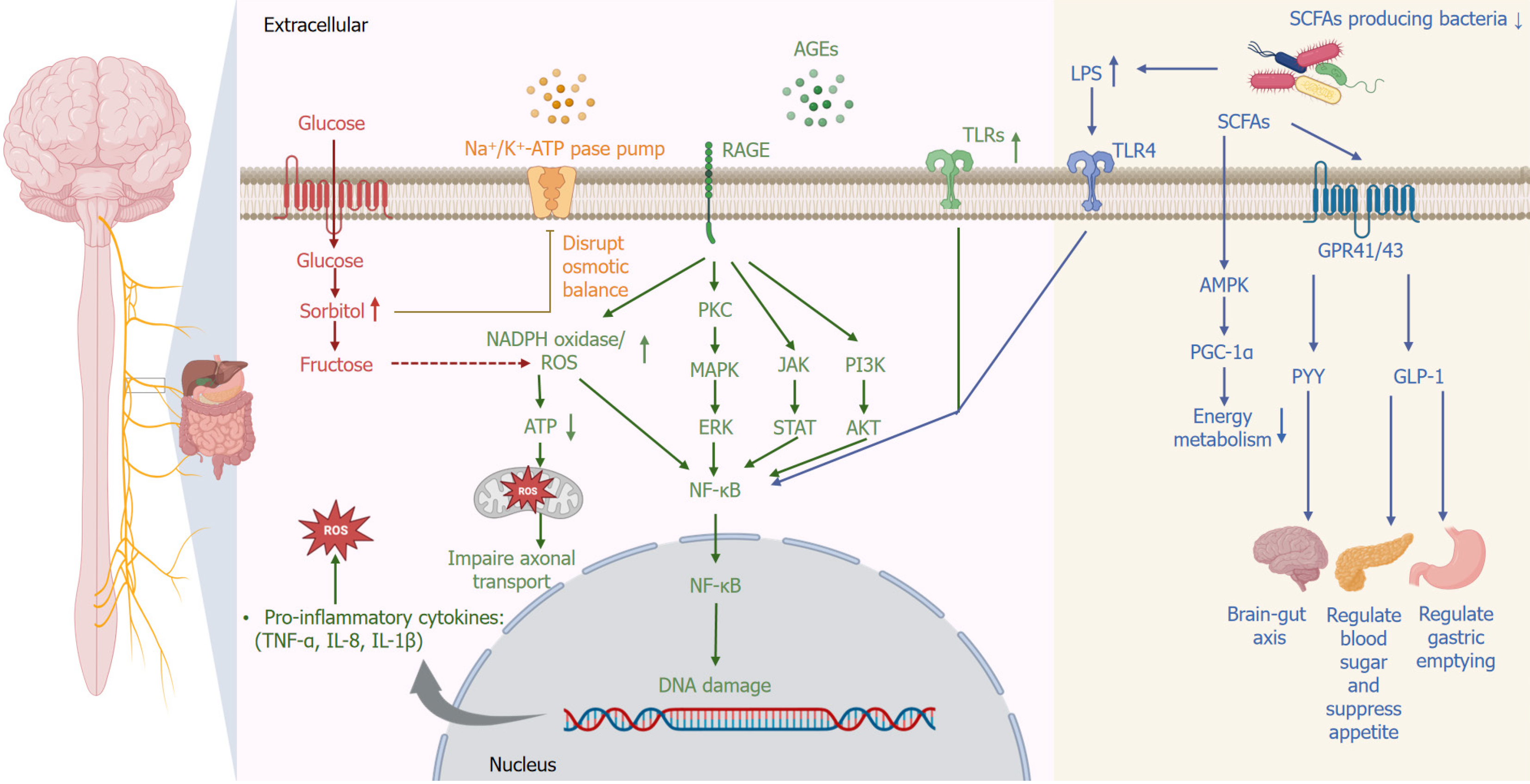
Figure 2 Molecular pathways underlying the pathophysiology of diabetic enteric autonomic neuropathy.
Persistent hyperglycemia activates multiple pathogenic signaling pathways leading to autonomic neuronal damage. Increased glucose influx activates the polyol pathway, causing intracellular accumulation of sorbitol and fructose, disrupting osmotic balance. Advanced glycation end (AGE)-products accumulate and interact with their receptors, activating downstream signaling pathways such as protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/Akt, leading to the activation of nuclear factor kappa B (NF-κB). Additionally, Toll-like receptors (TLRs), particularly TLR4, are activated by lipopolysaccharide (LPS), derived from gut microbiota dysbiosis, further promoting NF-κB activation. NF-κB translocates into the nucleus, triggering DNA damage and promoting transcription of pro-inflammatory cytokines (tumor necrosis factor alpha [TNF-α], interleukin 8 [IL-8], IL-1β). Increased oxidative stress, mediated by NADPH oxidase and impaired mitochondrial function, exacerbates axonal transport dysfunction. Concurrently, gut microbial dysbiosis reduces beneficial bacteria and short-chain fatty acid (SCFA) production, decreasing activation of AMP-activated protein kinase (AMPK) and proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), thus impairing neuronal energy metabolism. Reduced activation of SCFA receptors (G-protein-coupled receptor 41 [GPR41]/GPR43) also diminishes release of gut-derived neuroendocrine factors (glucagon-like peptide 1 [GLP-1], peptide YY [PYY]), impairing gut-brain signaling, appetite regulation, glucose homeostasis, and gastric motility. RAGE: Receptor for advanced glycation end-product; ROS: Reactive oxygen species.
- Citation: Zhou MX, Zhao Y, Chu CL, Behera TR, Shen QQ, Xing YB. Diabetic gastrointestinal autonomic neuropathy: Integrating neuronal degeneration and gut microbial dysbiosis. World J Diabetes 2025; 16(9): 110053
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/110053.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.110053