Published online Sep 15, 2025. doi: 10.4239/wjd.v16.i9.109768
Revised: June 14, 2025
Accepted: August 6, 2025
Published online: September 15, 2025
Processing time: 113 Days and 18.5 Hours
This editorial highlighted the central role of pancreatic β-cell dysfunction in the pathogenesis of diabetes mellitus and discussed the emerging significance of Ras homolog enriched in brain 1 (Rheb1) as a key regulator of β-cell mass and insulin-secretory capacity. While molecular mechanisms governing β-cell homeostasis remain incompletely defined, Yang et al have recently demonstrated that Rheb1 could promote β-cell proliferation through dual activation of mechanistic target of rapamycin complex 1 and AMP-activated protein kinase signaling pathways, rather than relying solely on mechanistic target of rapamycin complex 1. Notably, Rheb1 expression is higher in pancreatic islets from younger individuals and upregulates hepatocyte nuclear factor 4 alpha, which is recognized as a tran
Core Tip: Dysregulation of β-cell mass and function contributes to the development and progression of diabetes mellitus. In a recent study, Yang et al identified Ras homolog enriched in brain 1 (Rheb1) as a critical regulator of β-cell proliferation via both mechanistic target of rapamycin complex 1 and AMP-activated protein kinase signaling pathways. Rheb1 also enhances hepatocyte nuclear factor 4 alpha expression, further supporting its role in maintaining β-cell functionality. These results reveal an intricate signaling network through by Rheb1 supports β-cell growth and survival, highlighting its potential as a therapeutic target for diabetes management. Further research is warranted to explore the translational applications of these findings.
- Citation: Peng Y, Zhang DD, Gan L, Zhang JQ. Targeting Ras homolog enriched in brain 1 to restore β-cell mass and function: A potential therapeutic strategy for diabetes. World J Diabetes 2025; 16(9): 109768
- URL: https://www.wjgnet.com/1948-9358/full/v16/i9/109768.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i9.109768
Diabetes mellitus represents one of the most pressing global health challenges, with rising prevalence and substantial socioeconomic burdens. A central feature in the pathogenesis of both type 1 and type 2 diabetes is the progressive loss of functional pancreatic β-cells, driven by mechanisms such as autoimmune destruction, metabolic stress-induced dys
To overcome this limitation, regenerative strategies targeting β cell preservation, proliferation, or functional restoration have emerged as a promising therapeutic frontier. Multiple molecular regulators, such as pancreatic and duodenal homeobox 1, which is essential for β-cell development and maintenance, and neurogenin 3 (a key inducer of endocrine differentiation) have been investigated for their capacity to expand β-cell mass[3,4]. However, these targets mainly face limitations, including limited proliferative efficacy and difficulties in achieving precise modulation without unintended off-target effects.
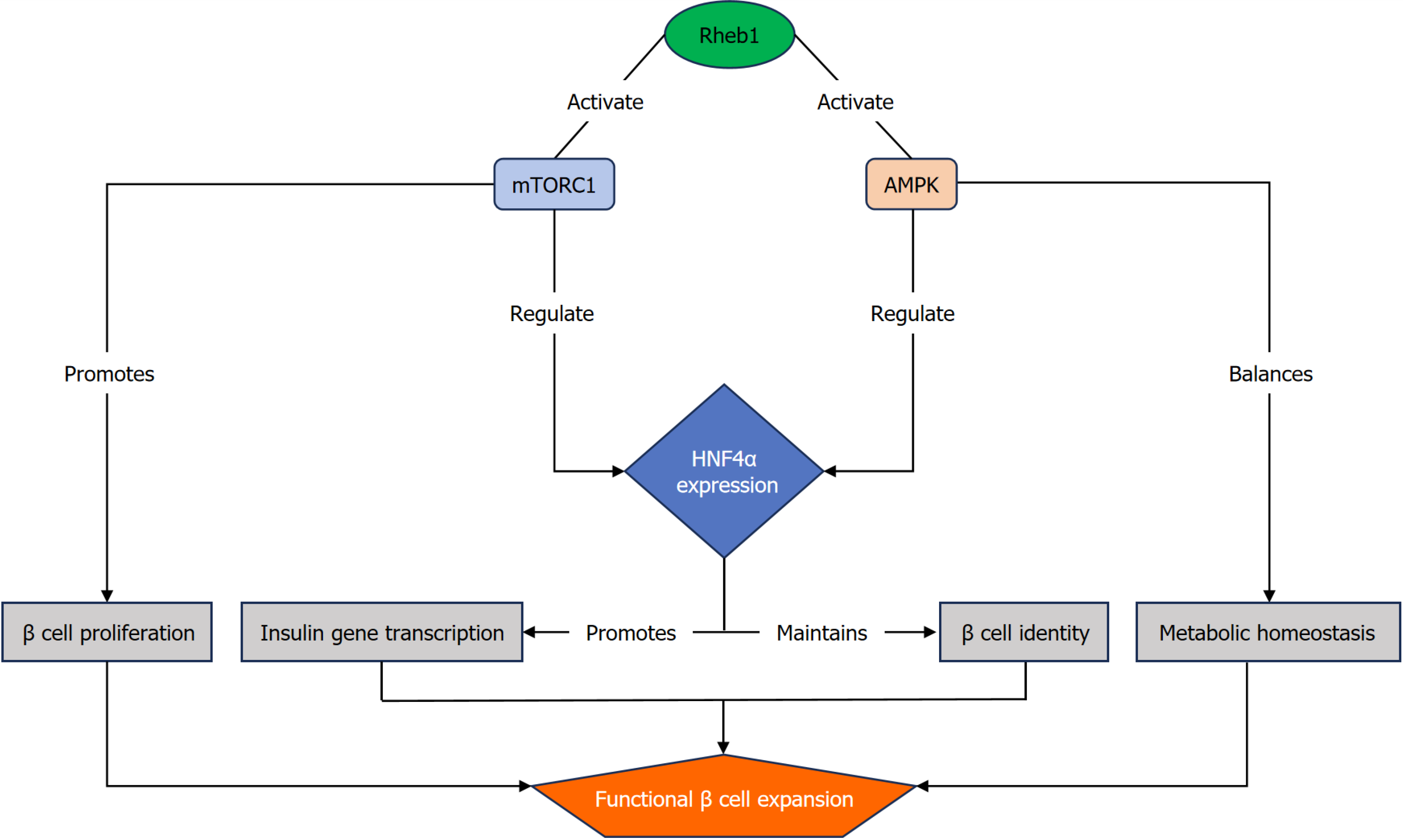
In this context, Yang et al[5] introduced Ras homolog enriched in brain 1 (Rheb1) as a novel and multifaceted regulator of β-cell expansion and function. In contrast to single-pathway targets, Rheb1 uniquely engages both mechanistic target of rapamycin complex 1 (mTORC1) and AMP-activated protein kinase (AMPK) signaling pathways, coupling nutrient-sensing mechanisms with energy homeostasis to promote β-cell proliferation. Furthermore, Rheb1 upregulates he
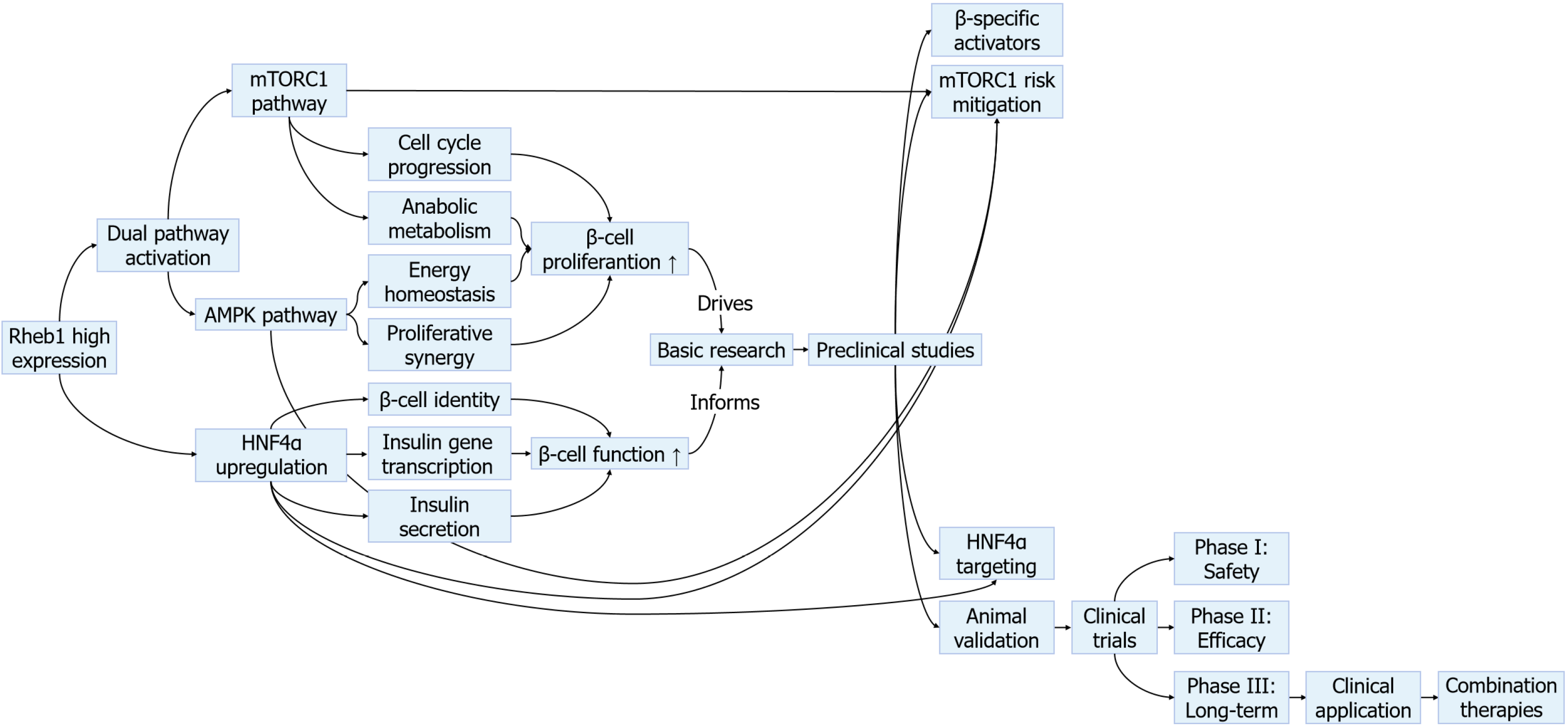
Nevertheless, translating these insights into clinical applications remains challenging due to obstacles, such as ensuring tissue-specific delivery, mitigating oncogenic risks associated with sustained mTORC1 activation, and navigating the delicate balance between promoting proliferation and preserving β-cell functional maturation. This editorial critically examined Rheb1’s role in β-cell biology within the larger landscape of regenerative strategies, while addressing the challenges and opportunities for its therapeutic development (Figure 2).
Over the past decade, research into Rheb1’s role in β cell biology has significantly advanced. Initially characterized in 2005 as a potent activator of mTORC1[6], Rheb1 was primarily studied in the context of cancer and nutrient sensing before assessing its potential role in β cells. Early studies (2010-2015) examining mTORC1 activation in β cells reported conflicting results, while some studies demonstrated enhanced proliferation, others found that chronic mTORC1 activation could lead to β-cell exhaustion and impaired function[7]. These discrepancies highlighted the need to understand mTORC1 regulation in a more robust context. Rheb1, as a member of the Ras superfamily of GTPases, gained particular attention when transcriptomic analyses revealed its differential expression patterns in human islets across developmental stages. This temporal expression profile indicates a potential role of Rheb1 in β cell growth regulation. Yang et al[5] evidenced that Rheb1 is highly expressed in islets from younger individuals and promotes β-cell proliferation through a novel dual-pathway mechanism.
The unique capacity of Rheb1 to concurrently activate both mTORC1 and AMPK signaling pathways challenges the traditional view of these pathways as mutually antagonistic. While mTORC1 primarily promotes cell cycle progression and anabolic processes, AMPK modulates catabolic responses under energy-deprived conditions[8]. This dual-pathway coordination overcomes a fundamental limitation of interventions, targeting a single signaling pathway, as evidenced by the constrained proliferative outcomes observed with rapamycin-mediated mTORC1 suppression and the transient effects of AMPK-specific activation.
The identification of Rheb1’s dual activation of growth-promoting and homeostatic pathways provides a potential explanation for previous therapeutic limitations. By concurrently stimulating mTORC1 and AMPK signaling pathways, Rheb1 may sustain a metabolic balance, promoting β-cell proliferation without compromising function[9]. This delicate balance is particularly crucial for β cells, which must constantly adapt to fluctuating nutrient levels while maintaining precise insulin secretion capacity.
However, some studies have reported minimal effects of Rheb1 manipulation in certain β cell models, demonstrating that its regulatory role may be context-dependent. These contrasting findings emphasize the need for further research into the specific conditions under which Rheb1 exerts its proliferative effects. Nevertheless, the unique signaling convergence mediated by Rheb1 positions it as a multifunctional regulator capable of coordinating proliferative expansion while minimizing detrimental stress responses or dedifferentiation[10].
Beyond increasing β-cell numbers, preserving functional maturity and identity is essential for any regenerative approach to be clinically effective. Immature or dedifferentiated β cells mainly exhibit impaired insulin secretion, leading to glycemic instability[11]. Current therapies, such as GLP-1 analogs, mainly enhance the function of residual β cells without expanding their population, whereas stem cell-derived islet transplantation remains limited by donor scarcity and immune rejection. Yang et al[5] found that Rheb1 upregulates HNF4α a nuclear transcription factor critical for ma
The ability of Rheb1 to simultaneously enhance proliferation while maintaining functional gene expression programs marks it as a particularly valuable therapeutic target[12]. It addresses a fundamental limitation of many regenerative approaches, which may promote proliferation at the cost of cellular identity and insulin secretory function. Nevertheless, the potential tumorigenic risk associated with sustained mTORC1 activation via Rheb1 warrants cautious evaluation, particularly given the oncogenic potential of other Ras family members.
Targeting Rheb1 provides a novel and multifaceted strategy for diabetes therapy. In contrast to traditional pharmacologic agents that aim to reduce blood glucose level or enhance insulin action, Rheb1-based interventions could potentially restore intrinsic β-cell mass and functionality, reducing or eliminating the need for lifelong insulin therapy[13]. This represents a fundamental shift from current standard-of-care treatments to a potentially curative approach.
Moreover, the molecular accessibility of Rheb1-related signaling pathways presents multiple pharmacologic targets. Both mTORC1 and AMPK are well-characterized targets with existing small-molecule modulators and biologics already in clinical use or under active development[14-16]. For instance, rapamycin analogs that selectively inhibit mTORC1 and metformin, a widely used AMPK activator, may serve as foundational components for Rheb1-targeted strategies. These agents can promote more rapid clinical translation compared with the development of entirely novel therapeutic classes. Modulating Rheb1 itself, or downstream effectors, such as HNF4α, may thus be feasible within a relatively short translational window. However, the field must draw lessons from the limitations of prior regenerative strategies, such as β-cell exhaustion resulting from sustained GLP-1 receptor stimulation and the inconsistent functional maturation found in stem cell-derived β cells[17].
Additional attention must be given to the context-specific effects of Rheb1 activation. Overactivation of mTORC1 has been linked to β-cell exhaustion and endoplasmic reticulum stress, while long-term AMPK stimulation may impair anabolic capacity essential for insulin production[18,19]. Additionally, the proliferative effects of Rheb1 activation could theoretically increase cancer risk, particularly in tissues with high basal mTORC1 activity. Thus, future therapies will require precise tuning of Rheb1 activity, potentially through tissue-specific gene modulation, small interfering RNAs, or targeted delivery systems, in order to optimize efficacy while minimizing systemic risks. Combination approaches involving transient Rheb1 activation alongside differentiation-promoting factors may provide a means to balance β-cell proliferation with the preservation of functional maturity.
Translating Rheb1 into clinical applications requires developing targeted delivery systems that can safely modulate its activity in pancreatic β cells. Promising approaches include β cell-specific nanoparticles, engineered viral vectors, and modified small molecules that can precisely regulate Rheb1-mTORC1-AMPK signaling[20]. These technologies must overcome key challenges in tissue specificity, dosage control, and long-term safety before clinical use. Rheb1-based therapies could revolutionize diabetes prevention by enabling early intervention. Identifying at-risk individuals through molecular profiling of Rheb1 pathway activity may allow treatment during the critical β cell compensation phase. Developing reliable biomarkers from blood samples and imaging techniques will be essential for implementing this precision medicine approach.
Several important scientific questions remain to be addressed to advance Rheb1-based therapeutics. Fundamental priorities include elucidating how various diabetic states modulate Rheb1 signaling and identifying optimal therapeutic windows during disease progression. Ongoing safety studies are essential to evaluate strategies to mitigate risks, such as β-cell over-proliferation, including the use of drug-inducible expression systems and combination regimens to enhance precision and control.
Cutting-edge research platforms are rapidly advancing the development of Rheb1-based therapeutics. Complicated animal models, human islet-on-chip systems, and single-cell technologies are yielding deeper insights into Rheb1’s role in β-cell biology[21-23]. Computational modeling and artificial intelligence are employed to optimize drug design and predict therapeutic responses. Visual tools, such as molecular network diagrams and pathway schematics, are increasingly utilized to clarify Rheb1’s regulatory role and promote communication of these complex concepts.
The identification of Rheb1 as a multifunctional regulator of β-cell proliferation, metabolic function, and identity maintenance has provided important insights for the therapy of diabetes. In contrast to single-pathway targets, Rheb1’s unique capacity to coordinately activate both mTORC1 and AMPK signaling pathways, while simultaneously upregulating HNF4α, positions it as a particularly promising molecular target for β-cell restoration. These dual mechanisms address the critical need for therapies that can simultaneously expand β-cell mass while preserving functional maturity. However, key translational challenges remain, including the development of tissue-specific delivery systems, mitigation of potential oncogenic risks from chronic pathway activation, and establishment of optimal treatment windows. As the field progresses from symptomatic management to regenerative approaches, Rheb1-targeted therapies provide the potential for transformative, disease-modifying interventions that directly address the fundamental pathophysiology of diabetes, including the loss of functional β-cell mass. Future research should concentrate on overcoming these translational barriers while further elucidating Rheb1’s context-dependent regulatory role across diabetes subtypes.
| 1. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1337] [Article Influence: 89.1] [Reference Citation Analysis (1)] |
| 2. | Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull Exp Biol Med. 2021;171:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 3. | Weidemann BJ, Marcheva B, Kobayashi M, Omura C, Newman MV, Kobayashi Y, Waldeck NJ, Perelis M, Lantier L, McGuinness OP, Ramsey KM, Stein RW, Bass J. Repression of latent NF-κB enhancers by PDX1 regulates β cell functional heterogeneity. Cell Metab. 2024;36:90-102.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Gribben C, Lambert C, Messal HA, Hubber EL, Rackham C, Evans I, Heimberg H, Jones P, Sancho R, Behrens A. Ductal Ngn3-expressing progenitors contribute to adult β cell neogenesis in the pancreas. Cell Stem Cell. 2023;30:498-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Song WJ, Zhang JJ. Ras homolog enriched in brain 1 regulates β cell mass and β cell function via mTORC1/AMPK/Notch1 pathways. World J Diabetes. 2025;16:104973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (5)] |
| 6. | Wang X, Li M, Gao Y, Gao J, Yang W, Liang H, Ji Q, Li Y, Liu H, Huang J, Cheng T, Yuan W. Rheb1-mTORC1 maintains macrophage differentiation and phagocytosis in mice. Exp Cell Res. 2016;344:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Goul C, Peruzzo R, Zoncu R. The molecular basis of nutrient sensing and signalling by mTORC1 in metabolism regulation and disease. Nat Rev Mol Cell Biol. 2023;24:857-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 8. | Smiles WJ, Ovens AJ, Kemp BE, Galic S, Petersen J, Oakhill JS. New developments in AMPK and mTORC1 cross-talk. Essays Biochem. 2024;68:321-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Yang Y, Cai Z, Pan Z, Liu F, Li D, Ji Y, Zhong J, Luo H, Hu S, Song L, Yu S, Li T, Li J, Ma X, Zhang W, Zhou Z, Liu F, Zhang J. Rheb1 promotes glucose-stimulated insulin secretion in human and mouse β-cells by upregulating GLUT expression. Metabolism. 2021;123:154863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Wang X, Gao Y, Gao J, Li M, Zhou M, Wang J, Pang Y, Cheng H, Yuan C, Chu Y, Jiang Y, Zhou J, Luo HR, Ju Z, Cheng T, Yuan W. Rheb1 loss leads to increased hematopoietic stem cell proliferation and myeloid-biased differentiation in vivo. Haematologica. 2019;104:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Memon B, Abdelalim EM. Stem Cell Therapy for Diabetes: Beta Cells versus Pancreatic Progenitors. Cells. 2020;9:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Rahman M, Nguyen TM, Lee GJ, Kim B, Park MK, Lee CH. Unraveling the Role of Ras Homolog Enriched in Brain (Rheb1 and Rheb2): Bridging Neuronal Dynamics and Cancer Pathogenesis through Mechanistic Target of Rapamycin Signaling. Int J Mol Sci. 2024;25:1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Yang W, Jiang W, Luo L, Bu J, Pang D, Wei J, Du C, Xia X, Cui Y, Liu S, Mao Q, Chen M. Genetic deletion of Rheb1 in the brain reduces food intake and causes hypoglycemia with altered peripheral metabolism. Int J Mol Sci. 2014;15:1499-1510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Jiao L, Liu Y, Yu XY, Pan X, Zhang Y, Tu J, Song YH, Li Y. Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduct Target Ther. 2023;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 189] [Reference Citation Analysis (0)] |
| 15. | Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, Sengupta S, Kumar D, Garg M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023;8:375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 559] [Article Influence: 186.3] [Reference Citation Analysis (13)] |
| 16. | Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 607] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 17. | Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 1009] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 18. | Guillén C, Benito M. mTORC1 Overactivation as a Key Aging Factor in the Progression to Type 2 Diabetes Mellitus. Front Endocrinol (Lausanne). 2018;9:621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Liu B, Xu J, Lu L, Gao L, Zhu S, Sui Y, Cao T, Yang T. Metformin induces pyroptosis in leptin receptor-defective hepatocytes via overactivation of the AMPK axis. Cell Death Dis. 2023;14:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Li K, Lin H, Yu Y, Liu Y, Yang W, Chen S, Xu L, Huang W, Wang H, Meng C, Shao Z, Wei Y, Zhao L, Peng Y. Nucleus pulposus cell-mimicking nanoparticles for cell-specific HIF1A editing to modulate SASP-mediated disc inflammation via autophagy activation. Acta Biomater. 2025;197:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Vandereyken K, Sifrim A, Thienpont B, Voet T. Methods and applications for single-cell and spatial multi-omics. Nat Rev Genet. 2023;24:494-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 695] [Article Influence: 231.7] [Reference Citation Analysis (0)] |
| 22. | Triolo TM, Bellin MD. Lessons from Human Islet Transplantation Inform Stem Cell-Based Approaches in the Treatment of Diabetes. Front Endocrinol (Lausanne). 2021;12:636824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Pandey S, Dvorakova MC. Future Perspective of Diabetic Animal Models. Endocr Metab Immune Disord Drug Targets. 2020;20:25-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/