©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 111813
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.111813
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.111813
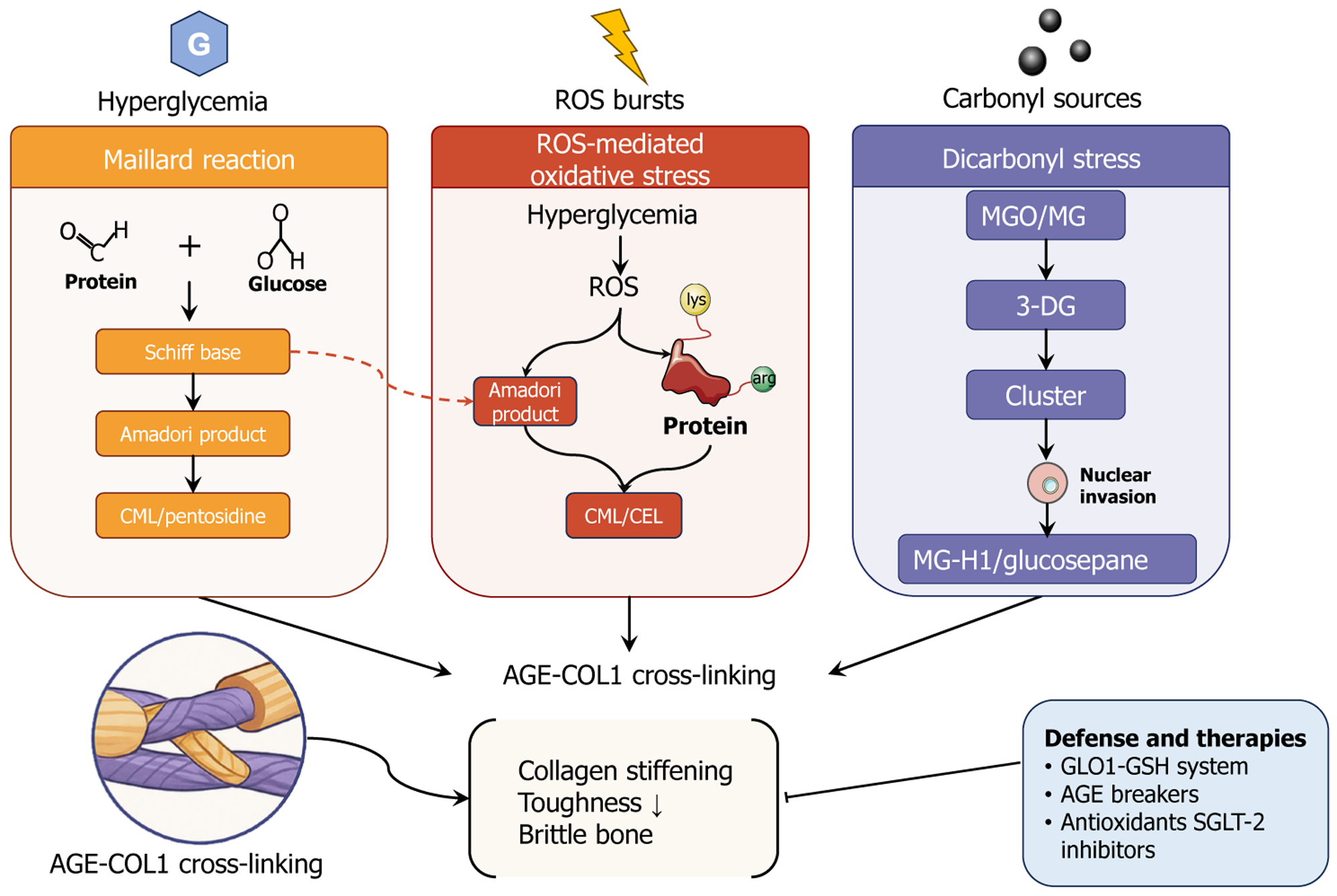
Figure 1 Hyperglycemia-induced biochemical routes to advanced glycation end product-type I collagen cross-linking and their skeletal consequences, together with representative defense strategies.
Persistent hyperglycemia initiates three chemically distinct yet convergent pathways that accelerate the accumulation of advanced glycation end products (AGEs) on type I collagen within bone: (1) Maillard reaction (orange panel): The non-enzymatic condensation of reducing sugars with protein carbonyl groups generates reversible Schiff bases, which rearrange into Amadori products and subsequently oxidize them into stable AGEs such as Nepsilon-(carboxymethyl)lysine and pentosidine; (2) Reactive oxygen species-mediated oxidative stress (red panel): Hyperglycemia-driven bursts of reactive oxygen species further oxidize Amadori products or directly modify lysine and arginine side-chains, yielding Nepsilon-(carboxymethyl)lysine and Nepsilon (carboxyethyl)lysine; and (3) Dicarbonyl stress (purple panel): Excess reactive carbonyl species (methylglyoxal, glyoxal) and their downstream 3-deoxyglucosone intermediates form intracellular clusters that can invade the nucleus and react with proteins to produce methylglyoxal-derived hydroimidazolone-1 and glucosepane cross-links. These three routes collectively intensify AGE-type I collagen crosslinking (lower left inset), which stiffens the collagen network, diminishes tissue toughness, and culminates in brittle bone (center). Endogenous and pharmacological counter-measures include the glyoxalase 1-glutathione detoxification system, chemical AGE breakers, antioxidants, and sodium-glucose cotransporter-2 inhibitors (lower right inset). CML: Nepsilon-(carboxymethyl)lysine; AGE: Advanced glycation end-product; COL1: Type I collagen; ROS: Reactive oxygen species; CEL: Nepsilon-(carboxyethyl)lysine; MGO: Methylglyoxal; MG: Glyoxal; 3-DG: 3-deoxyglucosone; MG-H1: Methylglyoxal-derived hydroimidazolone-1; GLO1: Glyoxalase 1; GSH: Glutathione; SGLT-2: Sodium-glucose cotran
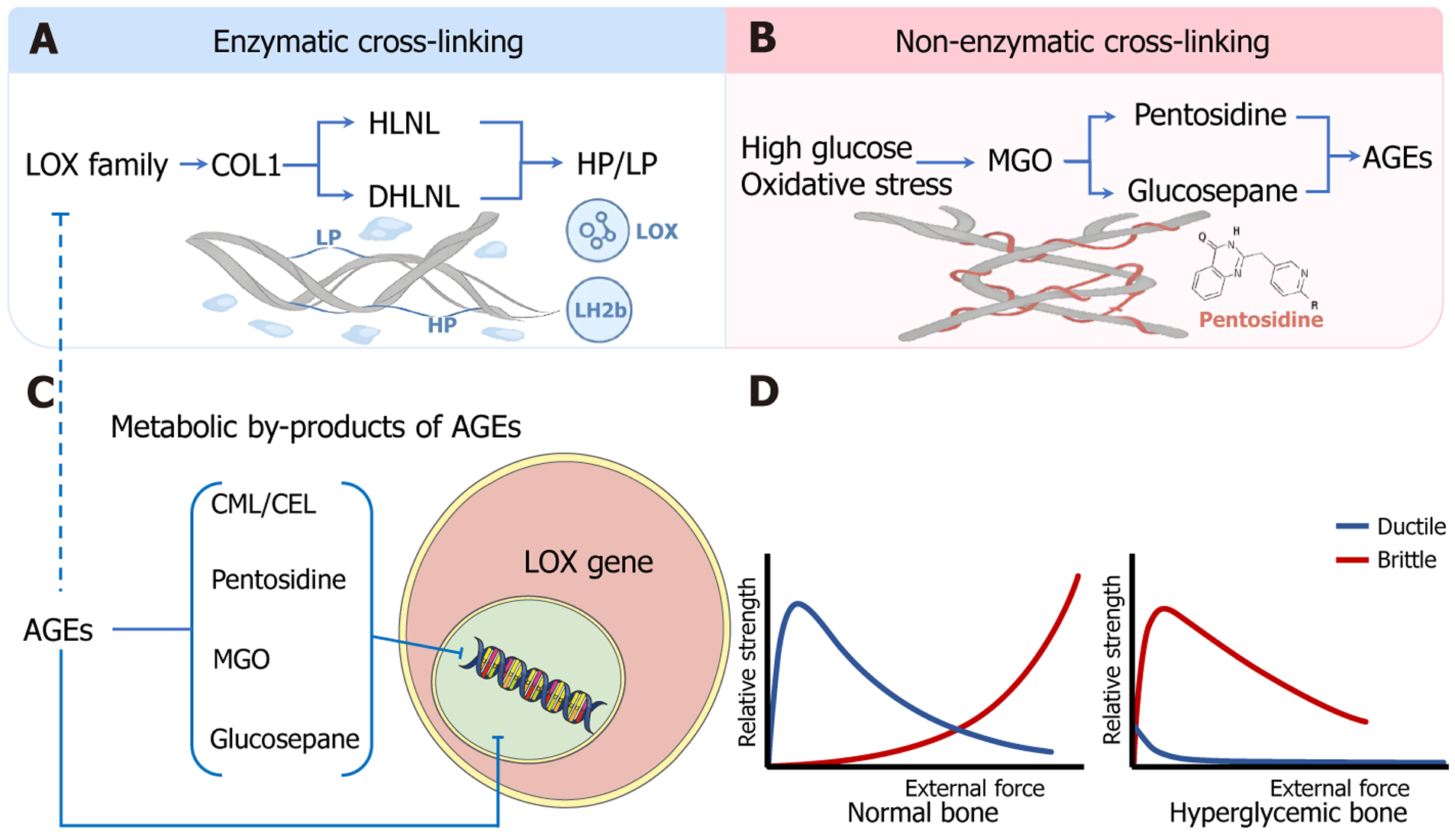
Figure 2 Dual-pathway schematic of collagen type I crosslinking shift from “tough-ductile” to “stiff-brittle”.
A: Enzymatic crosslinking pathway: The lysyl oxidase family catalyzes the oxidation of lysine/hydroxylysine residues on type I collagen, generating aldehydes that form initial divalent crosslinks, hydroxylysine and dihydroxylysinonorleucine, which gradually mature into trivalent pyridinoline crosslinks, including hydroxylysylpyridinoline and lysylpyridinoline, endowing the bone matrix with ductility and postyield toughness; B: Nonenzymatic crosslinking pathway: Hyperglycemia and oxidative stress promote the formation of reactive dicarbonyls such as methylglyoxal, which in turn induce advanced glycation end products (AGEs) to crosslink, such as pentosidine and glucosepane. These AGEs stiffen collagen fibrils and predispose the bone matrix to brittle failure; C: AGEs and their metabolic derivatives (e.g., Nepsilon-(carboxymethyl)lysine, Nepsilon-(carboxyethyl)lysine, pentosidine, methylglyoxal, and glucosepane) further suppress lysyl oxidase gene expression, thereby inhibiting enzymatic crosslink formation and contributing to a maladaptive “gain-loss” imbalance between the two pathways; D: In healthy bone, the enzymatic crosslinking-dominated “tough-ductile” model enables resilience under high load and strain. In contrast, diabetic bone, owing to AGE accumulation and impaired enzymatic crosslinking, presents a “stiff-brittle” mechanical profile characterized by early strength decline and insufficient energy absorption. LOX: Lysyl oxidase; COL1: Type I collagen; HLNL: Hydroxylysine; DHLNL: Dihydroxylysinonorleucine; HP: Hydroxylysylpyridinoline; LP: Lysylpyridinoline; LH2: Lysyl hydroxylase 2; MGO: Methylglyoxal; AGEs: Advanced glycation end products; CEL: Nepsilon-(carboxyethyl)lysine; CML: Nepsilon-(carboxymethyl)lysine.
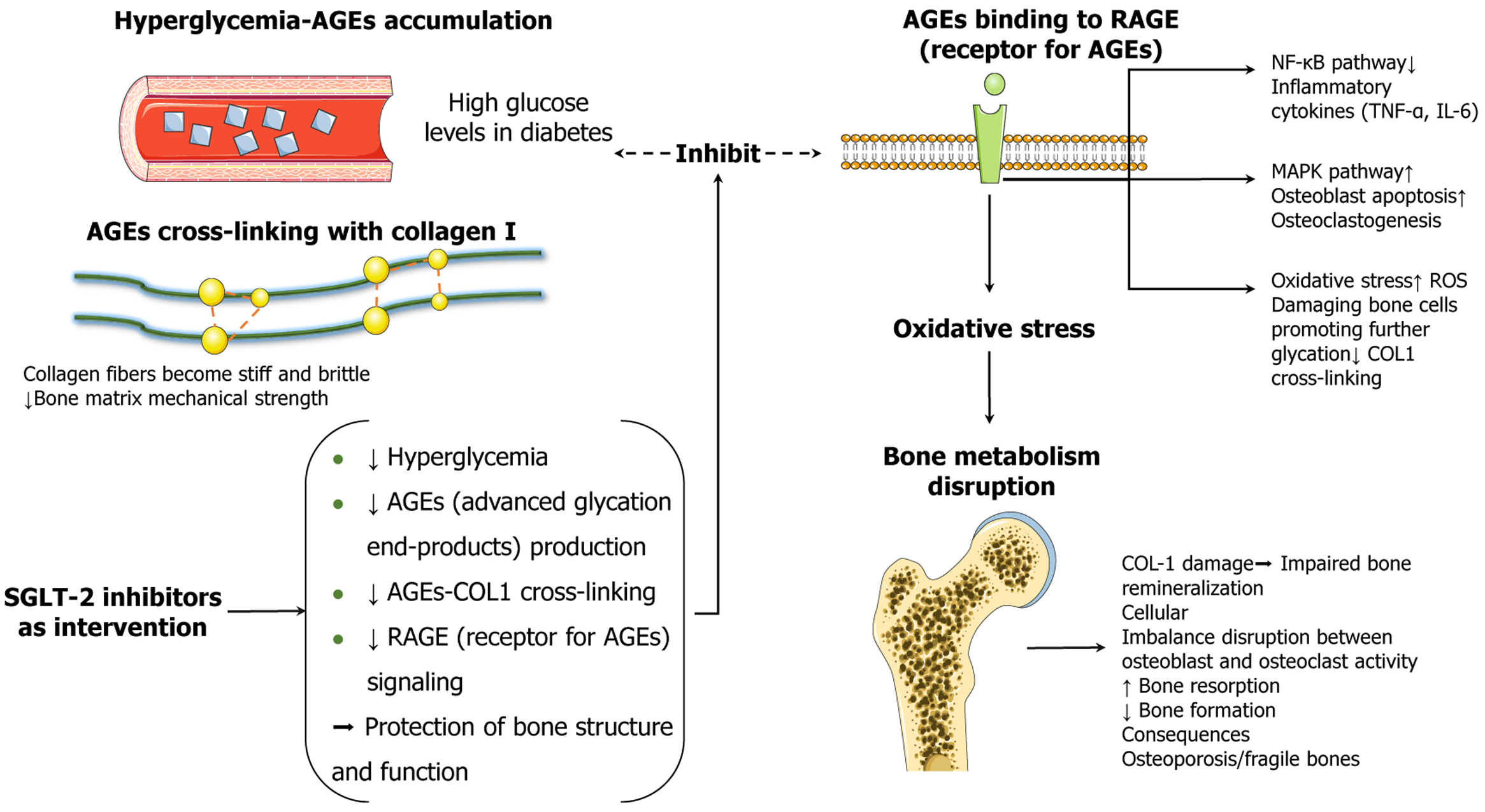
Figure 3 Schematic illustration of the mechanisms by which sodium-glucose cotransporter 2 inhibitors alleviate diabetic bone fragility via multitarget modulation of the advanced glycation end product-type I collagen-receptor for advanced glycation end products axis.
Persistent hyperglycemia accelerates the accumulation of advanced glycation end product (AGEs) in both the vasculature and bone matrix, promoting nonenzymatic type I collagen (COL1). This process increases collagen fibril stiffness and brittleness, thereby compromising the mechanical integrity of the bone matrix. Excess AGEs bind to their receptor, triggering downstream inflammatory and apoptotic pathways, including nuclear factor-κB and mitogen-activated protein kinase signaling, and amplifying reactive oxygen species-mediated oxidative stress. These cascades impair osteoblast function, promote osteoclast differentiation, and lead to reduced bone formation, enhanced bone resorption, and degradation of the COL1 structure, ultimately resulting in disrupted bone remodeling and osteoporosis. Sodium-glucose cotransporter 2 inhibitors not only reduce blood glucose levels but also suppress AGE formation, inhibit AGE-COL1 crosslinking, and downregulate receptor for advanced glycation end products signaling. Collectively, these effects help preserve bone structure and function in diabetic conditions. AGEs: Advanced glycation end products; SGLT-2: Sodium-glucose cotransporter 2; COL1: Type I collagen; RAGE: Receptor for advanced glycation end products; NF-κB: Nuclear factor-κB; MAPK: Mitogen-activated protein kinase; IL-6: Interleukin 6; TNF-α: Tumor necrosis factor α; ROS: Reactive oxygen species.
- Citation: Li ZP, Luo C, Yu XM, Ye LY, Sun D, Duan CZ, Xu SY, Zeng MQ, Xu H, Peng ZY, Wang P, Wang YB, Ruan WJ, Xue ME, Zhang CJ, He DJ. Diabetic bone fragility through advanced glycation end product-collagen axis: Mechanisms and therapy of sodium glucose cotransporter 2 inhibitors. World J Diabetes 2025; 16(10): 111813
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/111813.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.111813