Published online Dec 15, 2017. doi: 10.4251/wjgo.v9.i12.497
Peer-review started: June 26, 2017
First decision: August 7, 2017
Revised: August 24, 2017
Accepted: November 3, 2017
Article in press: November 3, 2017
Published online: December 15, 2017
Processing time: 172 Days and 20.2 Hours
Solid pseudopapillary neoplasm (SPN), also known as Gruber-Frantz tumor, is a rare form of neoplasm that almost exclusively occurs in the pancreas and in young females. While the potential of malignancy is low for SPN, these tumors can mimic other diseases and require a meticulous investigation and a standard treatment by total surgical resection. We present an unusual case of SPN arising in the mesentery of a 40-year-old man with subsequent multiple metastases. Histopathological examination showed similar properties of the mesenteric neoplasm to those of SPN in pancreas. Although the mass was surgically removed, the patient died of recurrent disease 4 years after the initial presentation. We speculate that SPN originates from pancreatic progenitor cells. Further histopathological analyses are required for the prediction of SPN recurrence after resection.
Core tip: Solid pseudopapillary neoplasm (SPN) has been recognized by World Health Organization since 2010, and classified as a low malignant potential neoplasm. Such neoplasm is characterized by the presence of a mutation in the gene that encodes β-catenin. β-catenin is an important factor in the Wnt signaling pathway (β-catenin-dependent Wnt signaling). The identification of extrapancreatic SPN, especially in the mesentery, indicates a possible endoderm link between pancreatic progenitor cells and SPN cells.
- Citation: Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J Gastrointest Oncol 2017; 9(12): 497-501
- URL: https://www.wjgnet.com/1948-5204/full/v9/i12/497.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i12.497
Solid pseudopapillary neoplasm (SPN) is a rare and indolent type of neoplasm that occurs in pancreas; SPN forms 0.3% to 2.7% of all pancreatic exocrine tumors. A large body of SPN indices are found in young female patients, and well-circumscribed. A margin negative surgical resection shows curative result in majority of cases[1-3]; recurrence after surgical resection is reported in 2% to 10% of patients[4,5]. Patients with unresectable SPN may have a long-term survival (5 years), and require complex chemo- and radio-therapy treatments; the efficacy of adjuvant therapies in the SPN treatment remains largely unknown and a clinical challenge. Thus, it is important to differentiate the risk of recurrence in SPN patients. An extrapancreatic development of SPN is a rare incident; only 16 cases of extrapancreatic SPN have been reported so far worldwide (Table 1). In the present article, we report a patient, in whom SPN was found in the mesentery; no invasion or attachments to adjacent organs was observed. To the best of our knowledge, this article is the first to report a SPN case in the mesentery.
| Ref. | Age | Sex | Location | Size (cm) | Procedure | Follow-up |
| Miyazaki et al[19] | 22 | F | Retroperitoneum | 7 | Laparoscopy | 6 mo NED |
| Hibi et al[20] | 45 | M | Omentum | 15 | Laparoscopy | 96 mo DOD |
| Deshpande et al[21] | 17 | F | Left ovary | 25.5 | Open surgery | 72 mo NED |
| 57 | F | Right ovary | 3 | Open surgery | NA | |
| 21 | F | Left ovary | 14 | Open surgery | NA | |
| He et al[22] | 39 | F | Right ovary | 6 | Laparoscopy | 36 mo NED |
| Fukunaga et al[23] | 46 | F | Omentum | 5 | Laparoscopy | 3 mo NED |
| Ishikawa et al[24] | 13 | F | Mesocolon | 4 | Open surgery | 36 mo NED |
| Guo et al[25] | 47 | F | Retroperitoneum | 16 | Open surgery | 14 mo NED |
| Geng et al[26] | 37 | F | Retroperitoneum | 8 | Open surgery | NA |
| Zhu et al[27] | 22 | F | Retroperitoneum | 6 | Laparoscopy | 14 mo NED |
| Chen et al[28] | 47 | F | Left ovary | 6 | Open surgery | 18 mo NED |
| Cheuk et al[29] | 25 | F | Right ovary | 16.5 | Open surgery | 144 mo NED |
| Walter et al[30] | 32 | F | Stomach | 10 | Open surgery | 24 mo LWD |
| 73 | M | Duodenum | 14 | Open surgery | 3 mo DOD | |
| Stoll et al[31] | 48 | F | Left ovary | 8 | Open surgery | 9 mo NED |
| Present case | 40 | M | Mesentery | 28 | Open surgery | 48 mo DOD |
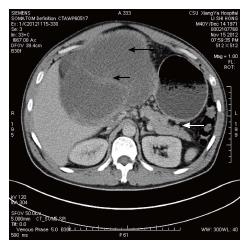
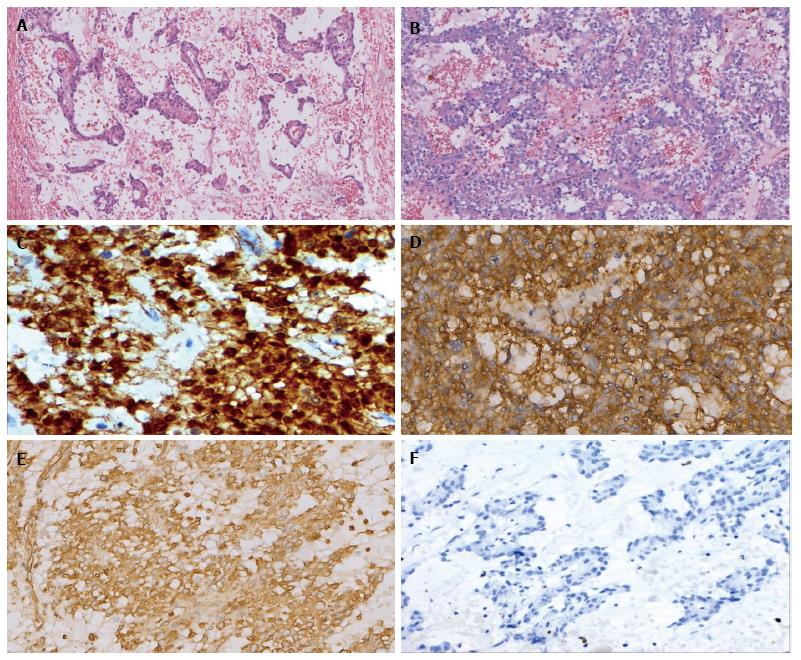
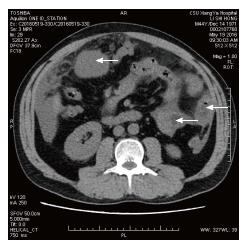
A 40-year-old Chinese male came to hospital on November 15, 2012. His main complaint was abdominal distention that lasted over 6 mo. His physical examination revealed a 30 cm soft mass in the abdomen. An abdominal computed tomography (CT) scan exhibited solid and mixed cystic lesions, measuring > 28 cm diameter (Figure 1). Patient’s blood test results were unremarkable. On November 22, 2012, the patient underwent an exploratory laparotomy, and the tumor protruding from the mesentery was completely excised. At that time, no invasion or attachments to adjacent organs was observed. In addition, the postoperative course was uneventful. The resected specimen of the mesenteric tumor was 25 cm × 15 cm × 28 cm, and showed a multilobulated structure with rich microvasculature. Microscopic characterization of the tumor showed that the tumor formation was a mix of solid and pseudopapillary areas. There was no evidence of pancreatic tissue in the analyzed sample. Further, the specimen was positive for alpha-1-antitrypsin, vimentin, CD56 and β-catenin immunostaining, whereas negative for S-100, neuron-specific enolase, E-cadherin, calretinin, progesterone receptor, chromogranin, and pancytokeration (Figure 2). Such results led to the diagnosis of SPN in the mesentery. Following 3.5 years, the patient continued to complain about abdominal distention and occasional polypnea. An abdominal CT scan exhibited multiple tumors in peritoneum, greater omentum, and colonic wall (Figure 3). Meanwhile, cells in the pleural effusion were found positive for alpha-1-antitrypsin, vimentin, CD56 and β-catenin. It was clear that the patient was suffering from recurrence of the disease. Before the surgical operation to clean the recurrent tumors, the patient received the treatment of 60 mg cisplatin by hyperthermic intraperitoneal chemotherapy (HIPEC). Unfortunately, there was no response to the treatment, and the patient was transferred to the palliative care unit. Soon after the patient’s physical conditions worsened, we lost the patient on November 2016, 4 years after the initial surgery.
SPN has been recognized by the WHO classification as a low malignant potential neoplasm in 2010[3]. It was first named as Gruber-Frantz tumor and after that it had been called the pancreatic solid papillary epithelial neoplasm, pancreatic papillary cystic neoplasm, pancreatic solid cystic tumor and solid pseudopapillary tumor. The differential diagnosis of SPN may include: pseudocyst, pancreatic mucinous neoplasms, well-differentiated ductal adenocarcinoma, pancreatic endocrine neoplasm, and acinic cell carcinoma. The pathogenesis of SPN remains unclear. Likewise, genetic events that contribute to the development of SPN are yet to be discovered. There are two basic proposals for the SPN origin: (1) genital ridge-related cells and (2) pancreatic progenitor cells[1,6]. To note, an important proportion of SPN cases show mutations in the somatic β-catenin coding gene (CTNNB1)[7-9]. Such mutations can affect Wnt signaling pathways as well as self-renewal capability of stem cells[10]. SPN cells were reported to be positive for β-catenin, vimentin, alpha-1-anti-trypsin, CD10, CD56, and progesterone receptors by immunohistochemical analysis[11]; however this staining pattern fails to reveal a clear phenotypic relationship between SPN and any of the defined cell lineages of the pancreas. Thereby, it can be speculated as SPN cells show multipotential differentiation. According to the study concerned with the embryonic development of the human pancreas, dorsal and ventral pancreatic buds were reported to proliferate from gut epithelium of endoderm during the 4th week of gestation. Dorsal pancreas fuses with ventral pancreas at the 7th week of gestation due to the rotation of the stomach and duodenum development[12]. Identification of extrapancreatic SPN in the ovary, retroperitoneum and the omentum, as listed in Table 1, indicates a possible endoderm link, substantiated by the migration of pancreas during embryogenesis. We therefore believe that extrapancreatic SPN originates from pancreatic progenitor cells.
In SPN patients, tumor resection confers an 8 year survival rate in 85% of cases; nevertheless, local recurrence or distant metastases can occur in some patients[13]. Histological and clinical parameters for prediction of disease recurrence after the initial surgical operation remain a challenge as there is still no consensus in the medical community. Many clinicians and researchers have been working to determine such criteria. For example, Kang et al[14] listed: (1) a tumor size larger than 8 cm; (2) cellular atypia; (3) vascular invasion; (4) perineural invasion; (5) systemic metastasis; and (6) peritoneal seeding as significant prognostic factors for tumor recurrence in a multicenter study. A case series study conducted by Yang et al[15] showed that vascular invasion, extra-pancreatic invasion, lymph node metastasis, and Ki-67 index ≥ 4% are associated with SPN recurrence. It is important to note that a rupture of the tumor or laparoscopic biopsy may seed the tumor cells into the peritoneal cavity, and could be an etiological factor responsible for the peritoneal recurrence[16]. Nonetheless, a recurrence prediction scoring model require more investigation. Such model will help clinicians to distinguish a high-risk group from low-risk group. Likewise, there is still no consensus on the treatment strategy in patients with SPN recurrence. A previous report described a 35 years old woman relapsing 8 mo after the resection of an SPN, which ruptured preoperatively. The patient firstly underwent a complete cytoreductive surgery, but relapsed within 8 mo, and received another cytoreductive surgery combined with HIPEC (oxaliplatin and irinotecan). At 31 mo of follow-up, the patient showed no evidence of disease recurrence[17]. Thus, a complete cytoreductive surgery combined with HIPEC stands as an important treatment solution for high-risk group of SPN. Further, another report concluded that SPN are radiosensitive, and can be successfully treated by using radiation therapy[18]. Future clinical and molecular studies are required to provide more precise tools to predict the biological behavior of SPN.
Abdominal distension.
Abdominal mass.
Pancreatic mucinous neoplasms.
All labs were within normal limits.
Mesenchymal neoplasm.
Solid pseudopapillary neoplasm (SPN).
Complete cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy.
Grading and staging play an important role in treatment and prognosis.
SPN: Solid pseudopapillary neoplasm.
Future clinical and molecular studies are required to provide more precise tools to predict the biological behavior of SPN.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ikura Y, Lin J, Ramia JM, Şendur MAN S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world’s literature. Surgery. 1995;118:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumors of the Digestive System. 4th edn. Lyon: International Agency for Research on Cancer 2010; . |
| 4. | Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A, Nakache R. Solid pseudopapillary neoplasm of the pancreas: Management and long-term outcome. Eur J Surg Oncol. 2017;43:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Marchegiani G, Andrianello S, Massignani M, Malleo G, Maggino L, Paiella S, Ferrone CR, Luchini C, Scarpa A, Capelli P. Solid pseudopapillary tumors of the pancreas: Specific pathological features predict the likelihood of postoperative recurrence. J Surg Oncol. 2016;114:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Guo M, Luo G, Jin K, Long J, Cheng H, Lu Y, Wang Z, Yang C, Xu J, Ni Q. Somatic Genetic Variation in Solid Pseudopapillary Tumor of the Pancreas by Whole Exome Sequencing. Int J Mol Sci. 2017;18:pii: E81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 9. | Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 327] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4521] [Article Influence: 226.1] [Reference Citation Analysis (0)] |
| 11. | Haque S, Dietz R, Perez MC. Recognizing the distinct cytomorphologic features of solid pseudopapillary neoplasm of the pancreas. J Gastrointest Oncol. 2016;7:E13-E16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | O’Rahilly R. The timing and sequence of events in the development of the human digestive system and associated structures during the embryonic period proper. Anat Embryol (Berl). 1978;153:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Jutric Z, Rozenfeld Y, Grendar J, Hammill CW, Cassera MA, Newell PH, Hansen PD, Wolf RF. Analysis of 340 Patients with Solid Pseudopapillary Tumors of the Pancreas: A Closer Look at Patients with Metastatic Disease. Ann Surg Oncol. 2017;24:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW, Kim SW; Korean Pancreatic Surgery Club. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 2014;260:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Yang F, Yu X, Bao Y, Du Z, Jin C, Fu D. Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature. Surgery. 2016;159:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 16. | Tajima Y, Kohara N, Maeda J, Inoue K, Kitasato A, Natsuda K, Irie J, Adachi T, Kuroki T, Eguchi S. Peritoneal and nodal recurrence 7 years after the excision of a ruptured solid pseudopapillary neoplasm of the pancreas: report of a case. Surg Today. 2012;42:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Honore C, Goere D, Dartigues P, Burtin P, Dumont F, Elias D. Peritoneal carcinomatosis from solid pseudopapillary neoplasm (Frantz’s tumour) of the pancreas treated with HIPEC. Anticancer Res. 2012;32:1069-1073. [PubMed] |
| 18. | Fried P, Cooper J, Balthazar E, Fazzini E, Newall J. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985;56:2783-2785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Miyazaki Y, Miyajima A, Maeda T, Yuge K, Hasegawa M, Kosaka T, Kikuchi E, Kameyama K, Jinzaki M, Nakagawa K. Extrapancreatic solid pseudopapillary tumor: case report and review of the literature. Int J Clin Oncol. 2012;17:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Hibi T, Ojima H, Sakamoto Y, Kosuge T, Shimada K, Sano T, Sakamoto M, Kitajima M, Yamasaki S. A solid pseudopapillary tumor arising from the greater omentum followed by multiple metastases with increasing malignant potential. J Gastroenterol. 2006;41:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Deshpande V, Oliva E, Young RH. Solid pseudopapillary neoplasm of the ovary: a report of 3 primary ovarian tumors resembling those of the pancreas. Am J Surg Pathol. 2010;34:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | He S, Yang X, Zhou P, Cheng Y, Sun Q. Solid pseudopapillary tumor: an invasive case report of primary ovarian origin and review of the literature. Int J Clin Exp Pathol. 2015;8:8645-8649. [PubMed] |
| 23. | Fukunaga M. Pseudopapillary solid cystic tumor arising from an extrapancreatic site. Arch Pathol Lab Med. 2001;125:1368-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, Iwanaga T, Nakaizumi A, Fujita M, Wada A. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990;85:597-601. [PubMed] |
| 25. | Guo X, Li N, Ren K, Wu L, Ma LI, Wu S, Xie F, Feng Z. Extrapancreatic solid pseudopapillary tumors: A clinicopathological analysis of two cases. Mol Clin Oncol. 2016;4:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Junzu G, Yanbin S, Suxia W, Janjun D. A case of extrapancreatic solid pseudopapillary tumor in the retroperitoneum. Jpn J Radiol. 2012;30:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Zhu H, Xia D, Wang B, Meng H. Extrapancreatic solid pseudopapillary neoplasm: Report of a case of primary retroperitoneal origin and review of the literature. Oncol Lett. 2013;5:1501-1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Chen Q, Lu W, Lv W. Overlap of microcystic stromal tumor and primary solid pseudopapillary neoplasm of the ovary. Int J Clin Exp Pathol. 2015;8:11792-11797. [PubMed] |
| 29. | Cheuk W, Beavon I, Chui DT, Chan JK. Extrapancreatic solid pseudopapillary neoplasm: report of a case of primary ovarian origin and review of the literature. Int J Gynecol Pathol. 2011;30:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Walter T, Hommell-Fontaine J, Hervieu V, Adham M, Poncet G, Dumortier J, Lombard-Bohas C, Scoazec JY. Primary malignant solid pseudopapillary tumors of the gastroduodenal area. Clin Res Hepatol Gastroenterol. 2011;35:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Stoll LM, Parvataneni R, Johnson MW, Gui D, Dorigo O, Sullivan P. Solid pseudopapillary neoplasm, pancreas type, presenting as a primary ovarian neoplasm. Hum Pathol. 2012;43:1339-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |