Published online Dec 15, 2017. doi: 10.4251/wjgo.v9.i12.492
Peer-review started: August 12, 2017
First decision: August 30, 2017
Revised: August 31, 2017
Accepted: September 14, 2017
Article in press: September 14, 2017
Published online: December 15, 2017
Processing time: 124 Days and 14.8 Hours
A 51-year-old male patient was referred to our hospital because of an incidentally detected cystic mass near the common bile duct (CBD). Imaging studies demonstrated a cystic mass that was suspected to communicate with the CBD. Gastroscopy showed irregular mucosal thickening with hyperemic change in the second portion of the duodenum. A type II choledochal cyst combined with duodenal malignancy was suspected. The patient underwent surgical resection and the histological diagnosis was mucinous adenocarcinoma of the duodenum with cystic metastasis. Although its incidence is extremely rare, care should be taken to check for other sites of malignancy when a pericholedochal cystic mass is detected.
Core tip: Mucinous adenocarcinoma is very rare in the duodenum, and a cystic metastasis from mucinous adenocarcinoma of duodenum has never been reported. This is the first report of primary mucinous adenocarcinoma of duodenum with cystic metastasis. Although rare, careful evaluation with a high suspicion for other sites of malignancy is needed when a pericholedochal cystic mass is detected.
- Citation: Kim YN, Song JS. Cystic metastasis from a mucinous adenocarcinoma of duodenum mimicking type II choledochal cyst: A case report. World J Gastrointest Oncol 2017; 9(12): 492-496
- URL: https://www.wjgnet.com/1948-5204/full/v9/i12/492.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i12.492
The small intestine is the longest gastrointestinal (GI) tract organ, reaching six to seven meters in average length. Despite its length and the large mucosal surface area of the small intestine, only 5% of malignant neoplasms of the GI tract occur in the small intestine[1]. Among them, primary adenocarcinoma of the duodenum represents approximately 25%-52% of malignant neoplasms of the small intestine and 4.6% were mucinous adenocarcinoma[2]. Choledochal cysts are rare, congenital dilatation of the extrahepatic or intrahepatic biliary tree. Among them, type II choledochal cyst, a diverticulum of the common bile duct (CBD), is the rarest type. Here, we present a case of mucinous adenocarcinoma of the duodenum with cystic metastasis, which is extremely rare and was initially misinterpreted as a type II choledochal cyst.
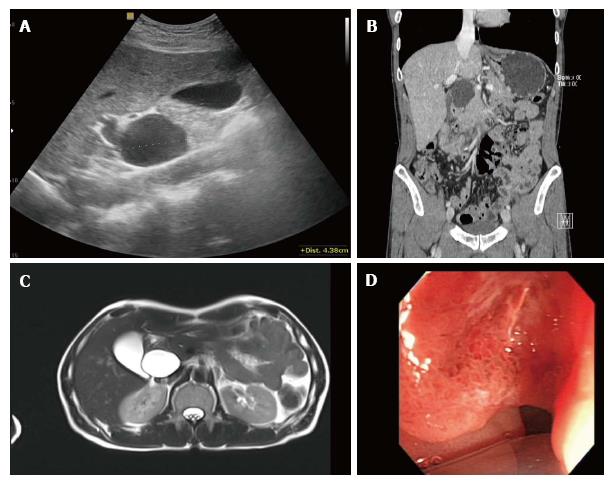
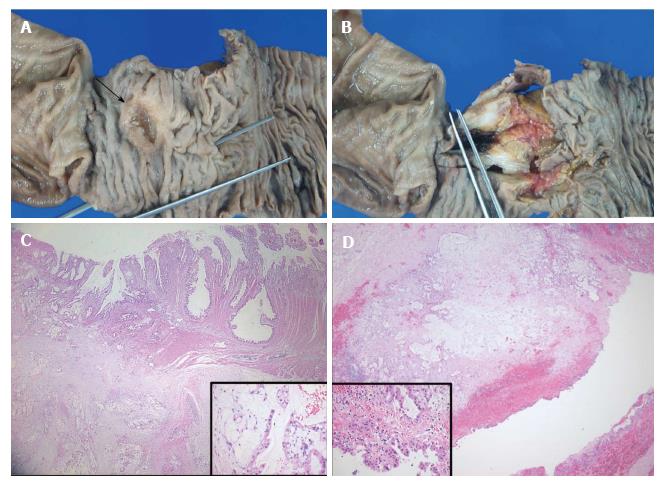
A 51-year-old male patient visited a local hospital because of dyspepsia and epigastric pain. Ultrasonography revealed a 4.5 cm sized cystic mass near the CBD and pancreatic head (Figure 1A). He was transferred to our hospital for further evaluation of the cystic mass. His medical history and laboratory findings were unremarkable. Tumor markers such as alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9 were within normal limits. Contrast-enhanced abdominal computed tomography (CT) showed a homogeneous low-density cystic mass with thin, smooth walls next to the CBD, and there were suspicions of a communication between the two structures (Figure 1B). Under the impression that the lesion was a type II choledochal cyst, which is a discrete diverticulum of the extrahepatic bile duct, magnetic resonance (MR) imaging and endoscopic ultrasound (EUS) were done. The cystic mass showed low signal intensity on the T1-weighted MR image and high SI on the T2-weighted MR image with nearly imperceptible walls and there was no evidence of an enhancing solid portion in the cyst (Figure 1C). EUS also revealed a 4.5 cm sized cystic mass which seemed to be connected with the CBD, and gastroscopy showed irregular mucosal thickening with hyperemic change in the second portion of the duodenum (Figure 1D). Based on these findings, the patient underwent Whipple’s operation under the impression the lesion was a type II choledochal cyst with extrinsic compression of the duodenum, and the possibility of combined duodenal malignancy due to the mucosal lesion in the duodenum. An examination of the resected specimen revealed a duodenal cancer in the second portion of the duodenum 2.5 cm proximal to the ampulla of Vater, and the cystic mass did not show communication with the CBD (Figure 2A and B). The histological diagnosis was mucinous adenocarcinoma of the duodenum with cystic metastasis and subpyloric lymph node metastasis (Figure 2C and D). The postoperative course of the patient was uneventful. The patient was disease-free 12 mo after the initial diagnosis. However, the patient died 18 mo after the recurrence.
We initially suspected a type II choledochal cyst combined with duodenal malignancy due to the mucosal lesion in the duodenum and the gastroscopic biopsy revealed a moderate degree of dysplasia. All of the imaging studies showed a well-marginated, homogeneously thin-walled cyst adjacent to the CBD which is regarded as a diverticulum of the extrahepatic bile duct, and the duodenal lesion was invisible. Since surgical resection is generally considered for the treatment of choledochal cysts, the patient underwent Whipple’s operation, and the patient was confirmed to have mucinous adenocarcinoma of the duodenum with cystic metastasis and subpyloric lymph node metastasis.
Mucinous adenocarcinoma is one of the histologic subtypes of carcinoma and is very rare in the duodenum. A recent study from South Korea by Chang et al[3]. revealed that 54.8% of small intestinal carcinomas were located in the duodenum and 4.6% were mucinous adenocarcinoma. Due to its rarity, to the best of our knowledge, this is the first case report of primary mucinous adenocarcinoma of duodenum with cystic metastasis. Although there are several studies in the literature describing the imaging findings of small bowel carcinoma including duodenal carcinoma[3-6], there are no previous reports reporting the imaging findings of mucinous adenocarcinoma of the duodenum. According to previous studies, duodenal cancer typically appears as an irregular thickening of the duodenal wall with regional lymph node enlargement on CT. Since the duodenal lesion of our patient was flat and small (2.0 cm), the primary lesion in the second portion of the duodenum and metastatic lymph node in the subpyloric area were missed on initial imaging studies including CT and magnetic resonance imaging (MRI). In a retrospective review of CT and MRI, the metastatic lymph node in the subpyloric area was identified. However, the primary lesion was invisible.
Choledochal cysts are a rare congenital anomaly of the intrahepatic or extrahepatic biliary tree and is known to occur in 1 in 100000 to 1 in 150000 live births[7]. Choledochal cysts occur more frequently in Asian populations, with more than two-thirds of all reported cases originating in Asia. Traditionally, choledochal cysts presented predominantly in young age with the triad of abdominal pain, palpable right upper quadrant mass, and intermittent jaundice. Nonetheless, recent analyses show increasing numbers of adults presenting with choledochal cysts[8]. According to Todani’s classification, choledochal cyst can be divided into 5 types: Type I, a cystic or fusiform dilatation of the CBD, which is subdivided into saccular, segmental, and diffuse types; type II, a diverticulum arising from the CBD; type III, choledochocele or a bulbous dilation of the terminal CBD within the ampulla of Vater; type IV, multiple intrahepatic and extrahepatic cysts; and type V, intrahepatic bile duct cysts or Caroli disease[9]. Among them, type II cysts are the most rare form of choledochal cysts, usually making up less than 2%-5% of cases[10]. They usually manifest as a pericholedochal cystic mass, of various shapes, some being gallbladder-like, and others being diverticulum-like. Choledochal cysts have been associated with an approximately 20 to 50-fold increase in biliary malignancies when compared with the general population[11]. The risk of malignancy in type II choledochal cysts has been estimated to range from 7%-9%, which is a slightly lower than the risk for other types of choledochal cysts (14.3% in the third decade)[12]. Current recommendations for management of choledochal cysts is surgical resection regardless of cyst type, including hepaticojejunostomy, Whipple procedure, partial liver resection, or liver transplantation[13].
In conclusion, we have described the first case of a mucinous adenocarcinoma of the duodenum with cystic metastasis. Even though the incidence of this particular type of cancer is extremely low, careful evaluation with a high suspicion for other sites of malignancy must be done when a pericholedochal cystic mass is detected incidentally.
A 51-year-old male patient was admitted because of incidentally detected cystic mass near the common bile duct (CBD).
About 4.5 cm sized cystic mass near the CBD, with irregular mucosal thickening in the second portion of the duodenum.
Type II choledochal cyst combined with duodenal malignancy.
Laboratory findings were unremarkable, including tumor markers such as alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9.
Findings from gastroscopy, ultrasonography, computed tomograph, and magnetic resonance imaging led to a diagnosis of type II choledochal cyst with extrinsic compression of the duodenum, and the possibility of combined duodenal malignancy.
Mucinous adenocarcinoma of the duodenum with cystic metastasis and subpyloric lymph node metastasis.
Whipple’s operation.
Mucinous adenocarcinoma of the duodenum is very rare, and this is the first case report of primary mucinous adenocarcinoma of duodenum with cystic metastasis.
Although rare, careful evaluation with a high suspicion for other sites of malignancy is needed when a pericholedochal cystic mass is detected.
Manuscript source: Unsolicited Manuscript
Specialty type: Oncology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Agrawal S, Espinel J, Sitarz R S- Editor: Chen K L- Editor: A E- Editor: Lu YJ
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8235] [Article Influence: 457.5] [Reference Citation Analysis (11)] |
| 2. | Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135:1070-1074; discussion 1074-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Chang HK, Yu E, Kim J, Bae YK, Jang KT, Jung ES, Yoon GS, Kim JM, Oh YH, Bae HI. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol. 2010;41:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 4. | Angelelli G, Macarini L, Giordano G, Di Giulio G. [The role of computerized tomography in the study of duodenal carcinoma]. Radiol Med. 1991;82:450-454. [PubMed] |
| 5. | Blandino A, Scribano E, Gaeta M, Loria G, Pandolfo I. [Computerized tomography in gaseous hypotonic duodenography in the study of the pancreatico-duodenal area]. Radiol Med. 1994;88:784-788. [PubMed] |
| 6. | Sailer J, Zacherl J, Schima W. MDCT of small bowel tumours. Cancer Imaging. 2007;7:224-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Dhupar R, Gulack B, Geller DA, Marsh JW, Gamblin TC. The changing presentation of choledochal cyst disease: an incidental diagnosis. HPB Surg. 2009;2009:103739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 848] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol. 1987;82:20-24. [PubMed] |
| 11. | Søreide K, Søreide JA. Bile duct cyst as precursor to biliary tract cancer. Ann Surg Oncol. 2007;14:1200-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Voyles CR, Smadja C, Shands WC, Blumgart LH. Carcinoma in choledochal cysts. Age-related incidence. Arch Surg. 1983;118:986-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 144] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Metcalfe MS, Wemyss-Holden SA, Maddern GJ. Management dilemmas with choledochal cysts. Arch Surg. 2003;138:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |