INTRODUCTION
Colorectal cancer (CRC) is a major cause of cancer morbidity and mortality worldwide[1], accounting for 8% of all malignant tumors in adults[2]. Historically, chemotherapy has been used for palliation of symptoms and over the last few years, median overall survival (OS) in patients with advanced CRC has been substantially increased from 12 mo to approximately 20-22 mo when all the available chemotherapeutic agents are administered[3]. Oxaliplatin, an integral component of adjuvant and palliative therapy for CRC, is commonly used with infusional 5-fluorouracil/leucovorin (5-FU/LV) to form the FOLFOX4 regimen[4]. Here, we report a rare case of acute coronary syndrome (ACS) believed to have occurred as an adverse effect of FOLFOX4. This case indicates the need for physicians to be aware of the potential for cardiac toxicity to occur in patients being treated with FOLFOX4.
CASE REPORT
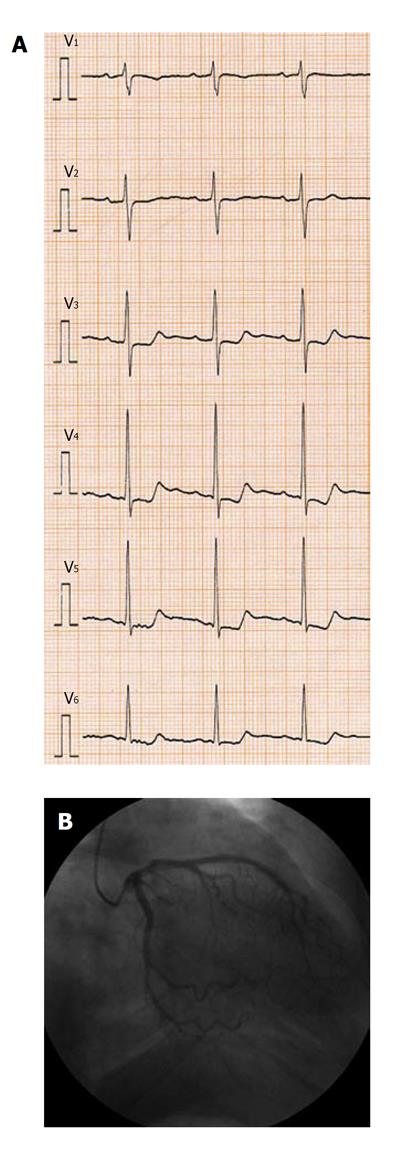
A 71-year-old man underwent a right hemicolectomy and opted not to receive adjuvant chemotherapy. Eight months later, the patient was readmitted to our hospital with chest discomfort. The results of a treadmill exercise test and other cardiological examinations were normal. Further examination revealed the recurrence of colon cancer with multiple liver metastases. The patient, therefore, received FOLFOX4 therapy, up to a total of 5 courses, and showed a partial response. No severe toxicities were observed, apart from grade 4 neutropenia. While receiving the sixth course of the same therapy, the patient complained of chest pain and systemic itching approximately 15 min after the start of the oxaliplatin and leucovorin infusion. The infusion was stopped immediately and an electrocardiogram (ECG) taken while the symptoms were manifest, revealing typical signs of ischemia [ST segment depression (V3-V6) (Figure 1A)].
Figure 1 Cardiological examinations revealed typical signs of variant angina (vasospasm).
A: Electrocardiogram revealed typical signs of ischemia. (ST-segment depression: V3-V6); B: Coronary arteriography showed that the coronary arterial tree vessels were intact.
Nitroglycerin was administered immediately to relieve the symptoms, resulting in the disappearance of the ST segment depression. Coronary arteriography (CAG) conducted on the same day showed that the coronary arterial tree vessels were intact (Figure 1B). Believing the chest pain to have been merely coincidental as the patient had a history of occasional chest discomfort, we continued the same therapy with daily administration of oral calcium channel blocker, benidipine hydrochloride, due to further regression of the metastatic liver tumors. However, during the seventh cycle of chemotherapy, the patient again developed the same chest pain and systemic itching approximately 15 min after the start of the oxaliplatin and leucovorin infusion, necessitating termination of infusion.
We concluded that the patient’s symptoms were due to cardiac toxicity associated with oxaliplatin or leucovorin and chemotherapy was stopped.
DISCUSSION
Treatment of metastatic CRC has changed considerably in recent years. The combination of 5-FU/LV with either bolus (Roswell Park schedule) or infusional (De Gramont schedule) administration combined with a second active drug, either irinotecan[5] or oxaliplatin, is accepted as the mainstay of first line treatment, while the choice of which particular drug to combine with 5-FU/LV does not influence OS[6]. Furthermore, the addition of a molecularly-targeted compound, bevacizumab (BV), to oxaliplatin-based chemotherapy significantly improved progression free survival (PFS) in a first line trial in patients with metastatic CRC[3], although differences in OS did not reach statistical significance[3]. However, many reports from Europe and America have shown that while addition of BV to chemotherapeutic drugs has no correlation with allergic reaction or vasospasm, it does lead to an increase in arterial thromboembolism (ATE)[7-9]. The risk factors for ATE are a history of ATE and an age of 65 years or more[9]. It is interesting to note that the most relevant toxicity of FOLFOX4 is sensory neurotoxicity in general[10], although only a small number of reports to date have described cardiac toxicity due to FOLFOX4[11]. In a large scale phase III trial, the multicenter international study of oxaliplatin/5-FU/LV in the adjuvant treatment of colon cancer, with no addition of BV, FOLFOX4 was superior to 5-FU/LV in patients with resected stage III colon cancer in terms of PFS and OS[12]. In this pivotal trial, André et al[12] reported that the incidence of thromboembolic events among patients who received at least one cycle of the FOLFOX4 was approximately 6% and two patients died suddenly from cardiac causes. Maindrault-Goebel et al[13] reported that increased use of oxaliplatin resulted in the emergence of allergic reactions, including anaphylactic shock. These results suggest that the risk of ACS is rarely increased with repetition of chemotherapy including oxaliplatin, but no additional BV.
We believe that the present case was variant angina as a type of ACS, as the patient’s ECG showed changes that were typical of ischemia and CAG revealed that the coronary arterial tree vessels were intact. Chest pain occurred approximately 15 min after the start of oxaliplatin and leucovorin infusion and BV was not used, indicating that the underlying mechanism was quite different from that of ACS occurring with use of BV. We inferred that the ACS in this case was associated with hypersensitivity to oxaliplatin or leucovorin, with coronary stenosis (vasospasm) occurring as an allergic reaction to either drug.
Paiva et al[14] reported a case of a 55-year-old woman with metastatic colon adenocarcinoma who presented with ACS, probably secondary to arterial vasospasm while receiving continuous intravenous 5-FU infusion (modified FOLFOX6). After the onset of ACS, their patient was treated with raltitrexate plus oxaliplatin and subsequently with irinotecan plus cetuximab, with no other cardiac events.
The difference between their report and the present case was the onset time and drug inferred to be the principal cause. As mentioned above, in our case, ACS occurred during 2 h simultaneous administration of oxaliplatin and leucovorin, strongly suggesting an allergic reaction to either drug. In their case, ACS occurred during continuous injection of 5- FU, suggesting an association with hypersensitivity to 5-FU.
The pathogenesis of 5-FU induced cardiotoxicity is still poorly defined[15]. Mosseri et al[16] studied rings of the aorta from white rabbits and found that 5-FU causes direct, endothelium independent vasoconstriction of vascular smooth cells. This process involved activation of protein kinase C. Other authors addressed the role of endotelin-1, a potent vasoconstrictor[17].
Matsubara et al[18] found a depletion of high energy phosphate compounds of the ventricular myocardium associated with ECG changes in guinea pigs treated with 5-FU at different concentrations. These authors concluded that fluoroacetate was the 5-FU’s metabolite responsible for this myocardial damage. On the other hand, Kuzel et al[19] pointed out a coagulation activation as the cause of ACS associated with 5-FU like ACS occurring with use of BV.
Although FOLFOX4 is an important therapeutic regimen for patients with CRC, few reports have been made to date of ACS due to oxaliplatin or leucovorin. Therefore, the underlying mechanism of the cardiovascular toxicity induced by oxaliplatin or leucovorin seen here remains to be elucidated in further study.