Published online Nov 15, 2011. doi: 10.4251/wjgo.v3.i11.153
Revised: September 30, 2011
Accepted: October 7, 2011
Published online: November 15, 2011
Gemcitabine is one of the most used anti-neoplastic drugs with documented activity in almost all major localizations of cancer. In pancreatic cancer treatment, gemcitabine occupies a prominent place as a first line chemotherapy, partly because of the paucity of other efficacious chemotherapy options. In fact, only a minority of pancreatic cancer patients display a response or even stability of disease with the drug. There are currently no clinically applicable means of predicting which patient will derive a clinical benefit from gemcitabine although several proposed markers have been studied. These markers are proteins involved in drug up-take, activation and catabolism or proteins that define the ability of the cell to undergo apoptosis in response to the drug. Several of these markers are reviewed in this paper. We also briefly discuss the possible role of stem cells in drug resistance to gemcitabine.
- Citation: Voutsadakis IA. Molecular predictors of gemcitabine response in pancreatic cancer. World J Gastrointest Oncol 2011; 3(11): 153-164
- URL: https://www.wjgnet.com/1948-5204/full/v3/i11/153.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v3.i11.153
Gemcitabine (2’,2’-difluoro 2’deoxycytidine, dFdC) is a nucleotide analogue used as an anti-neoplastic drug in several types of cancer such as pancreatic, lung, breast, cholangiocarcinomas and sarcomas. The toxicity profile of gemcitabine includes myelotoxicity (with anemia, thrombocytopenia and neutropenia), asthenia, reversible liver function tests elevation, nausea and rare pulmonary toxicity[1]. In view of its efficacy and well-established manageable toxicity profile, gemcitabine is an integral part of many chemotherapy combinations and is used also as monotherapy[2-5].
In pancreatic cancer gemcitabine has been approved as a first line treatment of locally advanced and metastatic disease on the basis of improvements in quality of life measures and moderate prolongation of survival obtained with its use[2]. Most pancreatic cancer patients treated with gemcitabine do not have an objective response to treatment and only a minority obtains stabilization of disease or partial response. In addition, there is currently no clinical means of predicting whether patients will benefit from the drug or only suffer from side effects of treatment. This editorial review will summarize available data on potential molecular predictors.
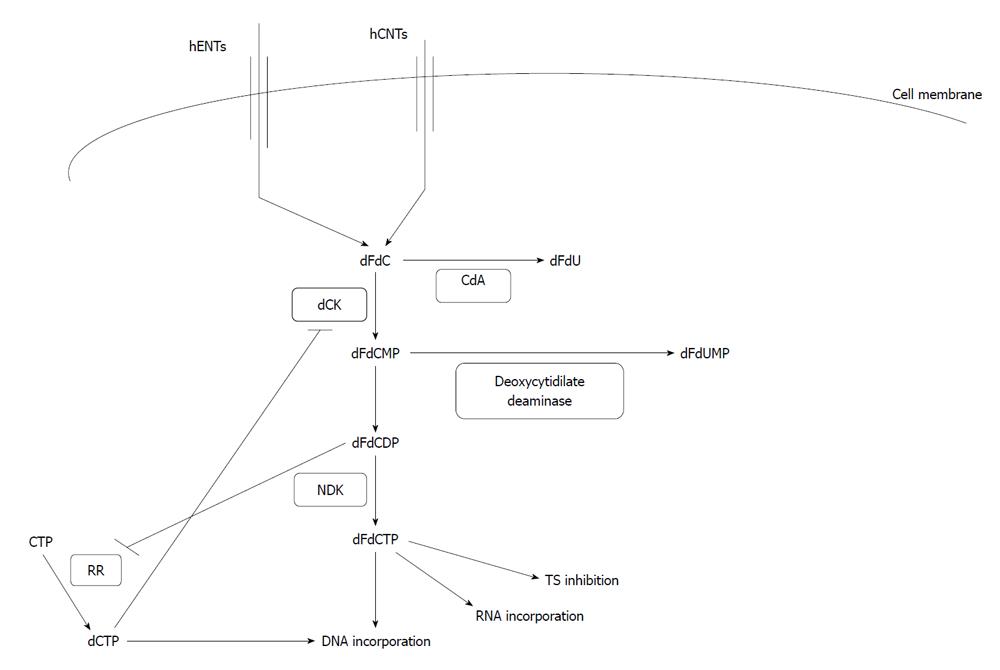
Gemcitabine is taken up by cells with the aid of several transporters including hENT1 and 2 (human equilibrative nucleoside transporter 1 and 2) and hCNT1 and 3 (human concentrative nucleoside transporters 1 and 3) (Figure 1). When intracellular, it is phosphorylated by the enzyme deoxycytidine kinase (dCK) to gemcitabine monophosphate (dFdCMP) and diphosphate (dFdCDP) and subsequently by nucleoside diphosphate kinase to the triphosphate (dFdCTP) form which is the DNA-incorporated metabolite[6,7]. Gemcitabine and gemcitabine monophosphate are catabolized by deoxycytidine deaminase (CdA) and deoxycytidilate deaminase to difluoro-deoxyuridine (dFdU) and difluoro-deoxyuridylate monophosphate (dFdUMP) respectively. The expression of dCK, a rate-limiting enzyme in gemcitabine activation, is enhanced by the binding of its mRNA by the protein Hu antigen R (HuR). Its function is enhanced by gemcitabine diphosphate which inhibits the enzyme ribonucleotide reductase (RR) and prevents the formation of dCTP, an inhibitor of dCK (Figure 1).
After intra-cellular enzymatic conversion to difluoro-deoxycytidine triphosphate (dFdCTP), gemcitabine is incorporated into DNA and causes strand termination, which leads to apoptosis. Gemcitabine-induced DNA lesions are more difficult for DNA repair mechanisms to recognize because after dFdCTP incorporation an additional nucleotide is incorporated into the chain before termination. Nevertheless, the incorporation of a false nucleotide is sensed by the DNA damage machinery which initiates an apoptotic response through binding of a DNA-PK (DNA-dependent protein kinase) complex. This complex includes the regulatory sub-unit heterodimer Ku70/Ku86 and the catalytic sub-unit DNA-PKcs. DNA-PK phosphorylates p53 which is thus protected from mdm2-mediated ubiquitination and subsequent degradation, and is stabilized to perform its apoptotic transcription function[8]. DNA-PK further co-operates with kinases ATM and ATR to phosphorylate histone H2AX[9,10] associated with termination of DNA synthesis[11].
Additional mechanisms of action of gemcitabine include RNA incorporation, RR inhibition and thymidylate synthase (TS) inhibition[6].
Sensitivity and resistance to gemcitabine can be attributed to cellular events involved in the drug’s metabolism, which has an impact on its intracellular availability, activation and ability to trigger apoptosis, as well as to cellular changes not affecting drug metabolism but impacting on processes determining the propensity of the cell to respond to gemcitabine toxicity. These cellular changes may be caused by exposure to the drug (secondary resistance) or be independent of such exposure (primary resistance) and associated with the neoplastic process per se[12,13].
Cellular up-take of gemcitabine by nucleotide transporters, a prerequisite for anti-neoplastic action, influences sensitivity to the drug. Both concentrative (sodium-dependent) and equilibrative (sodium-independent) transporters have been implicated in gemcitabine up-take and neoplastic cells in vitro become resistant when transporters are inhibited[14]. In cell lines derived from human pancreatic adenocarcinomas which express endogenous hENT1, exogenous expression of hCNT1 confers additional gemcitabine sensitivity[15]. hENT2 and hCNT3 play also a role in gemcitabine up-take[16].
Levels of transporter expression correlate with sensitivity to gemcitabine in pancreatic cancer patients. In a series of 45 pancreatic cancer patients treated with adjuvant gemcitabine and radiotherapy after tumor resection, expression of hENT1 and hCNT3 was studied by immunohistochemistry[17]. High expression of both transporters was found to be associated with improved overall survival (OS). Disease free survival (DFS) was also better for patients with high hENT1 expression and, in univariate analysis, for high hCNT3 expression, although in multivariate analysis the association displayed a borderline loss of statistical significance. The homogeneity of hENT1 staining in immunohistochemistry may also predict gemcitabine response[18]. Patients with homogeneously positive hENT1 staining in all cancer cells had a better outcome than patients in whom areas of the cancer were negative for hENT1. hENT1 mRNA levels were correlated with OS, DFS and time to disease progression in 83 patients with stage I to IV pancreatic cancer treated with gemcitabine[19]. In this study, hENT1 mRNA was the only mRNA for a gemcitabine metabolism protein showing such a correlation while other mRNAs analyzed (dCK, 5’-nucleotidase, Cytidine deaminase and RRM1 and RRM2) showed no correlation.
In a study of pancreatic cancer using data from a randomized comparison of adjuvant gemcitabine or 5-FU together with radiation in both arms[20], high hENT1 immunostaining correlated with both DFS and OS in the group receiving gemcitabine but not in the group of 5-FU[21]. These data confirm hENT1 as a predictive marker of gemcitabine response in pancreatic cancer but not as prognostic of outcome in general. Interestingly and in contrast, in non-metastatic, lymph node positive gastric cancer patients, high hENT1 expression in lymph nodes has been reported to be prognostic of worse outcomes[22].
Up-regulation of hENT1 is observed after treatment of cells with de novo nucleoside production inhibitors, such as 5-FU or its analogue tegafur[23,24]. This up-regulation sensitizes cells to subsequent treatment with gemcitabine. As a result, use of a 5-FU analogue followed by gemcitabine was more effective in a xenograft tumor model than the reverse sequence or concomitant drug administration[23].
Active export of gemcitabine from pancreatic carcinoma cells is mediated by the multidrug resistance protein 5 (MRP5 also named ABCC5)[25]. Gemcitabine resistant cell lines display up-regulation of MRP5 and small hairpin RNA (shRNA)-silencing of MRP5-reduced gemcitabine resistance.
Higher dCK expression has been associated with a better OS in pancreatic adenocarcinoma patients treated with gemcitabine[26]. When these patients became resistant to the drug, no consistent mutations or change in expression of dCK were observed, implying that the enzyme plays no role in this acquired resistance. Nevertheless, dCK expression down-regulation may be observed in rare cases[26]. Increased patient age is also associated with decreased dCK, possibly attributable to epigenetic silencing of the gene during aging, although this has not been formally tested.
HuR binds to mRNA 3’-untranslated regions with AU or U-rich regions and increases their stability and translation. Transfection of pancreatic cancer cells with HuR sensitized them to gemcitabine but not to other chemotherapy drugs such as cisplatin, etoposide or 5FU[27]. An improved survival was found in gemcitabine-treated pancreatic cancer patients with higher dCK stabilizing HuR protein expression compared with patients with lower expression of this protein. pp32 (also named ANP32A, Acidic Nuclear Protein 32A) is a protein that has been reversibly associated with pancreatic cancer aggressiveness, being suppressed in poorly differentiated pancreatic carcinomas[28]. pp32 is a binding partner of HuR and disrupts its ability to bind mRNAs. As a result, dCK expression is reduced and cells become resistant to gemcitabine when pp32 is up-regulated[29]. Immunohistochemistry data from the same study that demonstrated the predictive value of hENT1 and hCNT3[20] also pointed to a prognostic value of dCK for OS and DFS in patients who received adjuvant gemcitabine, with patients expressing high levels of dCK having significantly better outcomes than their counterparts with low expression levels[30]. In this report, patients in the 5FU receiving arm were not evaluated and consequently the possible predictive value of dCK for gemcitabine response was neither confirmed or refuted.
In a study of mRNA expression of several genes involved in gemcitabine metabolism (hENT1, hENT2, dCK, CdA, 5’-NT, RRM1 and RRM2) in 35 advanced pancreatic cancer patients, only dCK was found to correlate with gemcitabine effectiveness, with patients having a high dCK being more likely to be gemcitabine responders than patients with low dCK mRNA in their tumors[31].
Activity of CdA, the enzyme that catabolizes gemcitabine to difluoro-deoxyuridine has been mainly associated with toxicity outcomes. Patients treated with chemotherapy combinations including gemtitabine who had lower plasma CdA activity, had more severe adverse effects from treatment compared with counterparts with higher plasma CdA activity[32]. In lung cancer, polymorphisms of CdA which confer lower enzymatic activity have further been associated with clinical benefit from gemcitabine/cisplatin chemotherapy, as measured by increased time to progression and OS, in addition to increased toxicity[33]. In an in vitro study, fibroblasts transduced with CdA showed reistance to gemcitabine[34].
RR is the enzyme that converts ribonucleotide 5’-diphosphates to 2’-deoxyribonucleotide 5’-diphosphates. The holoenzyme is made up of two sub-units, RRM1 and RRM2. Production of 2’-deoxyribonucleotide 5’-diphosphates is the rate limiting step for the supply pathway of 2’-deoxyribonucleotide 5’-triphosphates, the building blocks of DNA. There is a reciprocal inhibition between gemcitabine and RR. Gemcitabine, after transformation to dFdCTP, inhibits the activity of RR, while dCTP produced by RR inhibits dCK, increases the activity of dCDA and directly antagonizes dFdCTP for DNA incorporation.
Over-expression of the RRM2 sub-unit of RR in pancreatic cancer cell lines decreases their sensitivity to gemcitabine while RRM2 knock-down with siRNA enhances gemcitabine sensitivity in vitro in cell lines and in vivo in human pancreatic cancer xenografts in mice[35]. Interestingly RRM2 knock-down also decreased transcription factor nuclear factor-κB (NF-κB) activity. An investigation of RRM2 levels in patient samples showed that pancreatic cancer patients with low levels of RRM2 mRNA in their diagnostic biopsy showed significantly better survival than patients with high RRM2 mRNA levels[36]. This study comprised 31 patients who were all treated with gemcitabine.
The RRM1 sub-unit of RR has also been found to be associated with gemcitabine resistance in vitro as it was the most up-regulated gene in a screen comparing gene expression between a pancreatic cancer cell line and its gemcitabine-resistant derivative[37]. Knocking down RRM1 by RNA interference restored gemcitabine sensitivity to the level of the parental line. In 18 recurrent pancreatic cancer patients treated with gemcitabine, those patients who had low RRM1 mRNA levels in their biopsies had a significant better survival than their high-expressing counterparts[37].
In an effort to combine the most important gemcitabine metabolizing genes in a model of gemcitabine sensitivity prediction, Nakano et al[38] proposed the ratio of mRNA expression of hENT1 x dCK/RRM1 x RRM2 as predicting sensitivity to gemcitabine of cell lines in vitro. This ratio decreased as cell lines acquired resistance to the drug whereas individual gene mRNA levels were not correlated with gemcitabine sensitivity in this in vitro model. In a therapeutic context, simultaneous transfection of gemcitabine resistant pancreatic cancer cells with dCK and uridine monophosphate kinase and knock-down of RRM2 and TS with siRNAs sensitized these cells to gemcitabine[39].
Finally, several single nucleotide polymorphisms of various gemcitabine metabolism-associated genes which affect activity of the respective proteins have been associated with gemcitabine activity and toxicity[40].
Intracellular pathways with a role in the pathogenesis of malignancy are associated with resistance to gemcitabine-induced cell death without directly affecting intracellular drug retention and activation but instead blocking drug-induced death signaling. These pathways start from surface molecules such as receptor tyrosine kinases and adhesion mediators and also include down-stream kinases,other modulators and transcription factors.
Phosphatidylinositol-3 kinase/AKT/NF-κB axis
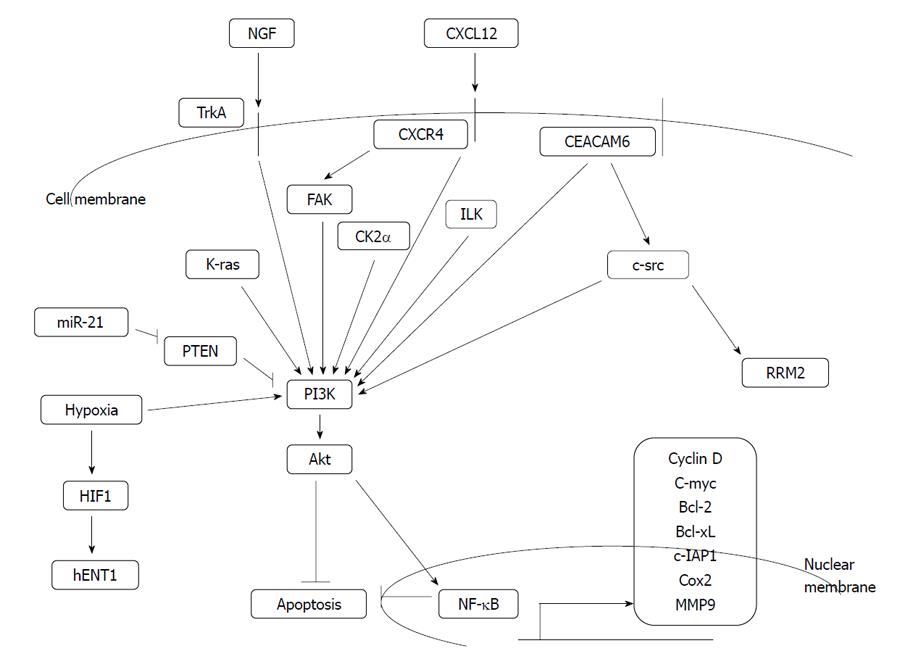
This important pathway involves activation of anti-apoptotic kinase akt (alternatively named PKB, protein kinase B) down-stream of k-ras and phosphatidylinositol-3 kinase (PI3K) kinase (Figure 2). K-ras carries activating mutations in the majority of pancreatic cancer patients and thus, gemcitabine resistance due to hyper-activated akt may be clinically relevant. Akt activates anti-apoptotic transcription factor NF-κB, other transcription factors and anti-apoptotic modulators.
Both in vitro studies using pancreatic cancer cell lines[41] and in vivo human pancreatic cell line xenograft studies in mice[42] have shown sensitization to gemcitabine following pharmacologic inhibition of PI3K by wortmannin or LY294002. Nevertheless, another study of xenografts of a variety of human pancreatic cell lines in SCID mice showed that akt inhibition by wortmannin did not always sensitize xenografted tumors to gemcitabine treatment, but some xenografts had a tumor resistance promoting effect in contrast to a sensitizing effect in the same cell lines in culture[43]. These results argue for the importance of host factors in modulating tumor response to treatment. In a cell line study, inhibition of PI3K/akt with LY294002 failed to sensitize cells to gemcitabine[44]. In contrast, inhibition of NF-κB pharmacologically or through the use of an inhibitor IκBα super-repressor sensitized resistant cell lines to gemcitabine.Notably, although LY294002 inhibited akt kinase, it had no effect on NF-κB down-stream, possibly due to concomitant activation of NF-κB by other up-stream signals which may explain its failure to have a gemcitabine-sensitizing effect.
Both canonical and non-canonical NF-κB pathways are activated in many cases of pancreatic cancer[45,46]. Apart from constitutive activation, this transcription factor, which confers drug resistance, is further induced by hormones such as pro-gastrin[47] and chemotherapeutic agents. In pancreatic cancer, knock-down of NF-κB sub-unit p65 (RelA) had a synergistic effect with gemcitabine in inhibiting both a gemcitabine-sensitive and a gemcitabine-resistant cell line in vitro and in mice xenografts in vivo[48]. Gemcitabine alone led to NF-κB up-regulation. In contrast, another group[49], using various pancreatic cancer cell lines, both gemcitabine-sensitive and resistant, showed that p65 knock-down had a synergistic effect with gemcitabine only in sensitive lines in vitro and in animal xenografts. In this instance no up-regulation of NF-κB by gemcitabine was noted. It is unclear whether these somewhat conflicting results are due to different experimental conditions, the different gemcitabine-resistant cell lines used for the in vivo experiments or other factors. Down-regulation of NF-κB by isoflavone genistein also showed synergy with gemcitabine in inhibiting the growth of pancreatic cancer lines[50]. Curcumin, another natural product, and the vitamin E analogue γ-tocotrienol acted synergistically with gemcitabine in inhibiting pancreatic cancer cells in vitro and in vivo and inhibited NF-κB and its target genes cyclin D1, c-myc, bcl-2, bcl-xL, c-IAP1, cox-2 and MMP9[51,52]. Glycogen synthase kinase 3 (GSK3) inhibition, either pharmacologically or by siRNA, was found to down-regulate NF-κB activity but did not confer synergistic activity to gemcitabine in all pancreatic cancer cell lines tested[53]. This is expected because GSK3 has several other cellular targets and effects. For example its inhibition stabilizes and activates β-catenin signaling[54].
Hypoxic conditions activate the PI3K/akt/NF-κB pathway and decrease the sensitivity of pancreatic cancer cells to gemcitabine in vitro[55]. ERK phosphorylation and activation was also observed in these conditions. Pharmacological inhibition of epidermal growth factor receptor (EGFR), up-stream of PI3K and of akt, by small molecule inhibitors PKI166 and LY294002, reversed gemcitabine resistance in this model. Pharmacological inhibition of ERK had only a partial effect. NF-κB DNA binding activity was inhibited by EGFR and akt inhibition but not by ERK inhibition, an observation that may explain re-sensitization to gemcitabine. Hypoxia also activates transcription factor HIF-1, which suppresses hENT-1[56]. This effect would be expected to contribute to gemcitabine resistance.
TrkA is the receptor tyrosine kinase for nerve growth factor, the expression of which has been shown to correlate with gemcitabine resistance in vitro[57]. Pancreatic cancer cell lines with higher TrkA expression and kinase activity were more resistant to gemcitabine than a cell line with lower TrkA expression and activity. siRNA for TrkA decreased IC50 (the concentration resulting in proliferation inhibition by 50%) of gemcitabine in resistant cell lines from 40-50 nmol/L to less than 10 nmol/L and increased the fraction of apoptotic cells from 15% to 30%. Knocking down TrkA resulted in inhibition of down-stream akt kinase, as measured by inhibition of phosphorylation of a fusion GSK3 protein in an in vitro assay. It remains plausible that proliferation inhibition and gemcitabine sensitization by TrkA inhibition passes through additional pathways down-stream of TrkA.
High expression of chemokine receptor CXCR4, the receptor for CXCL12 (also named SDF-1α, stromal-derived factor 1α), correlates with poorer survival in pancreatic cancer patients after resection[58]. The CXCR4/CXCL12 axis produces activation of focal adhesion kinase (FAK), akt and ERK in vitro and produces also gemcitabine resistance in pancreatic cancer cells[59] through activation of the expression of NF-κB, β-catenin and target proteins bcl-2, bcl-xL, survivin and Notch-1. A CXCR4 inhibitor, AMD3100, was able to reverse gemcitabine resistance.
Plasma concentrations of large variants of the extra-cellular matrix protein tenascin C were higher in pancreatic cancer patients than controls and were even higher in gemcitabine-resistant patients[60]. The interaction of an alternatively spliced form of tenascin C with membrane protein Annexin A2 activates the PI3K/Akt/NF-κB axis and confers gemcitabine resistance in pancreatic cells. In this model, gemcitabine resistance was reversed by NF-κB inhibition using pyrolidine dithiocarbamate or siRNA.
Notch-3, one of the Notch cell surface receptors which undergo proteolytic cleavage after ligand binding and are translocated to the nucleus to act as co-activators of transcription, has been shown to be involved in gemcitabine resistance[61]. siRNA for notch-3 sensitizes pancreatic cancer cells to gemcitabine in vitro. Akt activity as measured by GSK3 phosphorylation was inhibited after Notch-3 knock-down.
Expression of carcinoembryonic antigen cell adhesion molecule 6 (CEACAM6), a CEA family member of glycosylphosphatidyl inositol-linked surface molecules, confers resistance to gemcitabine in pancreatic cancer cells[62]. Gemcitabine-resistant cells in which CEACAM6 is permanently knocked-down by shRNA expression become sensitized to the drug. Reciprocally, sensitive cells that did not express CEACAM6 at baseline become resistant to gemcitabine when transfected with CEACAM6. CEACAM6 signals through c-src and akt kinases, as witnessed by the induction of activity of these kinases in transfected cells.
Integrin-linked kinase (ILK) is a serine threonine kinase that links adhesion signaling to down-stream cell signal transduction. It is involved in the activation of the PI3K/akt pathway. Pharmacological inhibition of ILK by the small molecule inhibitor QLT0254 decreased akt Ser473 phosphorylation in pancreatic cancer xenografts. QLT0254 also inhibited phosphorylation of targets of mTOR, kinase S6K and S6 ribosomal protein as well as phosphorylation of signal transducer and activator of transcription 3 but not of GSK3 and ERK[63]. Mice treated with the ILK inhibitor displayed a smaller volume of xenografts after 3 wk of treatment than controls. Nevertheless QLT0254 did not produce a significant reduction in the proliferation or apoptosis of the xenografts after single agent exposure. In combination with gemcitabine, the ILK inhibitor produced a small additional growth inhibition; xenografts used in these experiments were also sensitive to gemcitabine monotherapy.
Phosphorylation of FAK, another important adhesion and integrin-associated kinase, has been associated with resistance of pancreatic cell lines to gemcitabine[64]. FAK phosphorylation resulted in akt phosphorylation and activation as well as poor phosphorylation of anti-apoptotic bcl-2 family members. Inhibition of laminin-induced FAK phosphorylation with siRNA or with over-expression of FAK-related non-kinase, an endogenous inhibitor, enhanced gemcitabine sensitivity in vitro and in a nude mouse xenograft model[64,65].
Non-receptor tyrosine kinase src has increased activity in gemcitabine-resistant pancreatic cancer cell lines and its pharmacological inhibition diminished this resistance[66]. Similarly, c-src suppression by siRNA sensitized cells to gemcitabine and increased their apoptosis[67]. Akt activity, as measured by GSK3 phosphorylation, was inhibited by c-src RNA interference. Pharmacological inhibition of c-src decreased the expression of RR sub-unit RRM2, which was over-expressed in gemcitabine resistant cell lines[66].
In another in vitro study using RNA interference to knock-down the catalytic sub-units of casein kinase 2 (CK2), CK2α and CK2α’, decrease of either of these sub-units increased gemcitabine sensitivity in a resistant pancreatic cancer cell line. CK2α interference favored apoptotic cell death with activation of kinases MKK4 and JNK, while CK2α’ interference produced signs of necrotic cell death with concomitant activation of the PI3K/akt pathway[68]. Other investigators, though experimenting with a different pancreatic cell line, found that gemcitabine produced apoptosis and phosphorylated and activated MKK3/6 and p38MAPK but not JNK[69]. Moreover, pharmacological inhibition of p38MAPK, but not JNK, inhibited gemcitabine-induced apoptosis.
miR-21 is an oncogenic microRNA which has been found to be over-expressed in several types of cancer including pancreatic cancer[70]. Seventy nine per cent of pancreatic cancer patient samples showed miR-21 expression by in situ hybridization while miR-21 was weakly expressed in only 27% of chronic pancreatitis specimens and 8% of normal pancreas. Node negative pancreatic cancer patients with a strong miR-21 expression had a statistically significant poorer OS than their counterparts with a weak or negative staining[70]. Among the targets miR-21 suppresses are protein programmed cell death 4 and phosphatase PTEN. In contrast matrix metalloproteinases MMP2 and MMP5 and vascular endothelial growth factor are up-regulated when primary pancreatic cancer cells are transfected with miR-21. Patients with pancreatic cancer and a high expression of miR-21 have been found to have a poorer prognosis and be resistant to gemcitabine treatment compared with patients with low miR-21[71]. Inhibitors of activated akt reversed gemcitabine resistance in cells expressing high levels of miR-21 in vitro, supporting the role of PTEN suppression in miR-21 resistance. Treatment of gemcitabine resistant pancreatic cancer cell lines with the curcumin analogue CDF down-regulated miR-21, induced PTEN and reversed gemcitabine resistance[72]. The same sensitization effect was observed with miR-21 knocking-down by antisense oligonucleotides[73].
Signals communicated from pancreatic cancer cells to neighboring stroma fibroblasts, through the Hedgehog pathway, are important in inducing proliferation of fibroblasts and creating a desmoplastic stroma around the tumor mass[74]. Desmoplastic stroma is often a characteristic of human pancreatic adenocarcinoma and may act as a barrier to drug delivery in vivo. A Hedgehog inhibitor decreased fibroblast levels in vivo in a mouse model and synergized with gemcitabine in increasing the survival of mice bearing pancreatic tumors[74].
Interferon stimulated gene 15 (ISG15) is a ubiquitin-like protein which, similar to ubiquitin, is linked to target proteins through enzymatic reactions involving an activating enzyme, conjugating enzymes and ligases[75]. Dozens of cellular proteins can be ISGylated and this covalent link leads to modulation of sub-cellular localization and activity of target proteins. ISG15 expression is increased in gemcitabine resistant pancreatic cancer cell lines and knocking-down the protein with siRNA reverses this resistance[76].
TMS1 (target of methylation-induced silencing 1, also called ASC, apoptosis-associated Speck-like protein containing a CARD domain) is a tumor suppressor that is itself suppressed in several types of cancer. TMS1 structure includes an amino-terminal PYD domain (pyrin domain) and a carboxy-terminal CARD domain (caspase recruitment domain). Based on this structure, TMS1 is capable of promoting apoptosis but also of regulating NF-κB signaling[77]. TMS1 is repressed by methylation in pancreatic cells and its up-regulation through transfection or pre-treatment with the demethylating agent 5-azacytidine increases sensitivity to gemcitabine[78].
Bcl-2 family proteins include both pro-apoptotic and anti-apoptotic members[79] and regulate mitochondrial release of pro-apoptotic factors. In the pancreatic cancer cell line Capan-1, prolonged exposure to gemcitabine produced resistance to the drug and led to up-regulation of anti-apoptotic bcl-2 family members bcl-xL and mcl1[80]. In contrast, another pancreatic cell line, Mia-PaCa-2 was sensitized by prolonged gemcitabine exposure and neither expression of bcl-xL nor of mcl-1 was changed significantly. In another study, suppression of mcl-1 by shRNA led to growth inhibition of pancreatic cancer cells in vitro and in xenografts and produced gemcitabine sensitization[81].
Phosphorylation of heat shock protein 27 (Hsp27), a chaperone protein functioning in the unfolded protein response, is mediated by several kinases, such as MAPK activated protein kinase 2 and 5, protein kinase D and akt and results in apoptosis inhibition and proliferation inhibition[82]. Phosphorylation of Hsp27 is associated with resistance to various chemotherapy agents in a number of cancers and has been shown to be increased in a pancreatic cancer cell line resistant to gemcitabine compared with a sensitive clone[83]. Knocking-down Hsp27 with siRNA or suppressing it with a benzylidene lactam compound or with interferon γ reversed this resistance[84-86].
In addition to NF-κB, the expression and function of other transcription factors influences pancreatic cancer cell sensitivity to gemcitabine.
Transcription factor activating protein 2α (AP-2α), one of five members of the human AP-2 family of transcription factors, has tumor suppressing properties in breast and colon cancer[87,88]. In pancreatic cancer cells, AP2α over-expression reduces proliferation in vitro, in a xenograft model[89], and reduces cell migration in vitro. Clones that over-express AP2α are also more sensitive to gemcitabine treatment. Interestingly, clones where there is a moderate over-expression of AP2α show a greater effect, while higher expression results in a lower proliferation and gemcitabine sensitization advantage compared with control cells.
Transcription factor E26 transformation specific sequence 1 (Ets1), belonging to a family of transcription factors with homology to avium erythroblastosis virus E26 gene v-ets, is involved in cell proliferation and differentiation[90]. Members of the family are involved in recurrent translocations associated with prostate cancer[91,92]. Pancreatic cancer cell lines, made resistant to gemcitabine with gradual increasing exposure to the drug, display up-regulation of Ets1 and siRNA-mediated Ets1 silencing restores gemcitabine sensitivity[93]. Ets1 target genes MMP1 and uPA are down-regulated secondary to Ets1 suppression but it remains unknown if they are directly involved in resistance reversal or if other targets are included.
Cancer stem cells constitute a minority of tumor bulk in most cancers but are important because they are able to repopulate tumors after therapeutic attack due to their inherent drug resistance. They are characterized by surface antigens that are preferentially expressed compared to the rest of the tumor cells. Among these antigens, CD44, the hyaluronan surface receptor, is expressed in stem cells from various cancers. It contributes to cell survival and metastasis potential by facilitating communication of cancer cells with their environment but also participating in cell extravasation and motility[94]. CD44+ pancreatic cancer cells were enriched during the acquisition of resistance to gemcitabine by pancreatic cancer cell lines in vitro[95]. CD44+ cells display increased expression of ABCB1 (MDR1) transporter that confers multi-drug resistance. Moreover, pancreatic cancer patients whose tumors stained positive for CD44 had a poorer outcome than CD44 negative patients.
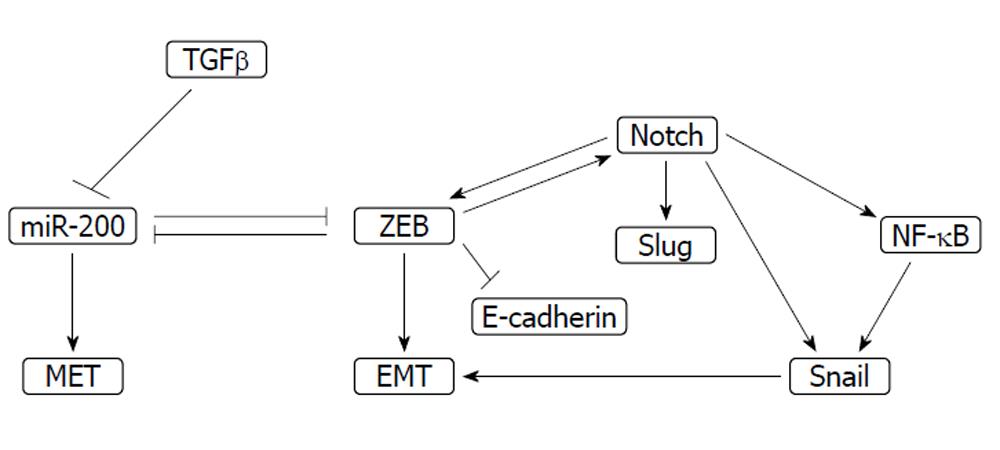
Tumor suppressing microRNAs of the miR-200 family are down-regulated in cells resistant to gemcitabine[72]. The miR-200 family consists of five members belonging to two sub-groups based on their target sequences. Sub-group I is comprised of miR-200a and miR-141 and sub-group II of miR-200b, miR-200c and miR-429. These miRs play a significant role in epithelial mesenchymal transition (EMT) through inhibition of transcription repressors Zinc finger E-box binding homeobox 1 (ZEB1) and ZEB2 (Figure 3). ZEB repressors inhibit reciprocally miR-200s[96]. Thus a feedback loop is established which works physiologically during development and in cancer during metastasis. ZEB activity promotes EMT and miR-200 family activity promotes the reverse process, mesenchymal to epithelial transition. miR-200 down-regulation has been observed in different cancers. In pancreatic cancer miR-200 family expression is variable in different cell lines[97] but it correlates inversely with gemcitabine resistance[98]. miR-200b and c are suppressed in gemcitabine-resistant cell lines which display EMT morphology with fibroblastoid features. In contrast ZEB1 is up-regulated and its target E-cadherin is suppressed[99]. Treatment of these cells with the natural flavinoid, isoflavone or with 3’,3’-diindolyl-methane (DIM) reverses this phenotype, down-regulates ZEB1 and up-regulates E-cadherin. The EMT phenotype is associated with Notch signaling and inhibition of Notch results in reversal of the phenotype as well as ZEB1, slug, snail and NF-κB down-regulation[100]. Reciprocally, ZEB1 induces Notch signaling constituting a feed-forward loop that promotes stemness and drug resistance[101]. miR-200b and c induction is observed after treatment with the curcumin analogue CDF which, as mentioned previously, concomitantly down-regulates miR-21 and induces gemcitabine sensitivity in initially resistant cell lines[72].
DIM and an isoflavone mixture additionally induce miR-146a in pancreatic cancer cells and down-regulate its targets interleukin 1 receptor-associated kinase 1, NF-κB and EGFR[102], which could also contribute to gemcitabine resistance reversal.
The let-7 family of miRs: let-7b, let-7c, let-7d and let-7e, are down-regulated in gemcitabine-resistant pancreatic cancer cells[98]. This family of miRs is associated with suppression of the stem cell phenotype[103].
Stable transfection of pancreatic cells with transforming growth factor β (TGFβ) increases their invasion potential in vitro, although it decreases their proliferation. Knocking-down of TGFβ receptor TβRII by siRNA increased pancreatic cell sensitivity to gemcitabine compared with clones in which siRNA was ineffective and TβRII continued to be expressed[104]. Up-regulation of protein kinase Cα was observed after cell treatment with TβRII and may mediate resistance to gemcitabine but also to cisplatin in this model. TGFβ signaling plays a role in EMT and as a result constitutes a link between this transition and drug resistance[105]. TGFβ suppresses miR-200 microRNAs favoring ZEB transcription modifiers, thereby promoting EMT[106]. In addition, it co-operates with both Notch signaling[107] and transcription repressor Snail[108] in EMT promotion. Snail is induced by NF-κB[109], indicating the co-operation of the two drug resistance-inducing pathways in EMT.
Thus, it becomes evident from the discussion above that stem cells possess specific intra-cellular circuits involving, among others, miRs, ZEBs and CD44 that promote both EMT and drug resistance.
Response to gemcitabine is dependent on both pharmacokinetic and pharmacodynamic factors that define intra-cellular drug availability, activation and metabolism as well as the way the malignant cell responds to the toxic lesion incited by the agent. Membrane transporters and enzymes that are involved in gemcitabine activation and catabolism have been implicated in gemcitabine response. Multiple pro-survival signal pathways culminating in the PI3K/Akt/NF-κB axis activation are important in gemcitabine resistance. There is cross-talk with signals favoring the EMT and the stem cell phenotype. As the cellular events effecting gemcitabine resistance are revealed and the causative lesions, as opposed to resistance-secondary events better clarified, new clinical opportunities will arise. Selecting patients that will most probably benefit from the drug and rationally combining it with sensitizing agents should lead to improved clinical outcomes.
| 1. | Ryan DP, Garcia-Carbonero R, Chabner BA. Cytidine analogues. Cancer chemotherapy and biotherapy. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins 2006; 183-211. |
| 2. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 3. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3338] [Article Influence: 208.6] [Reference Citation Analysis (15)] |
| 4. | Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, Lluch A, Llombart A, du Bois A, Kreienberg R. Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol. 2009;27:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2484] [Cited by in RCA: 2511] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 6. | Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17 Suppl 5:v7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 583] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Wong A, Soo RA, Yong WP, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev. 2009;41:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Achanta G, Pelicano H, Feng L, Plunkett W, Huang P. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res. 2001;61:8723-8729. [PubMed] |
| 9. | Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462-42467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1482] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 10. | Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst). 2004;3:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 787] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 11. | Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer. 2007;97:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, Crawford CR, Cass CE. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998;58:4349-4357. [PubMed] |
| 15. | García-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2',2'-difluorodeoxycytidine- induced cytotoxicity. Clin Cancer Res. 2003;9:5000-5008. [PubMed] |
| 16. | Damaraju VL, Damaraju S, Young JD, Baldwin SA, Mackey J, Sawyer MB, Cass CE. Nucleoside anticancer drugs: the role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene. 2003;22:7524-7536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 242] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Maréchal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, Cass CE, Young J, Salmon I, Devière J. Human equilibrative nucleoside transporter 1 and human concentrative nucleoside transporter 3 predict survival after adjuvant gemcitabine therapy in resected pancreatic adenocarcinoma. Clin Cancer Res. 2009;15:2913-2919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, Cass C, Lai R, Mackey JR. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10:6956-6961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 316] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Giovannetti E, Del Tacca M, Mey V, Funel N, Nannizzi S, Ricci S, Orlandini C, Boggi U, Campani D, Del Chiaro M. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 550] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 21. | Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 22. | Santini D, Vincenzi B, Fratto ME, Perrone G, Lai R, Catalano V, Cass C, Ruffini PA, Spoto C, Muretto P. Prognostic role of human equilibrative transporter 1 (hENT1) in patients with resected gastric cancer. J Cell Physiol. 2010;223:384-388. [PubMed] |
| 23. | Nakahira S, Nakamori S, Tsujie M, Takeda S, Sugimoto K, Takahashi Y, Okami J, Marubashi S, Miyamoto A, Takeda Y. Pretreatment with S-1, an oral derivative of 5-fluorouracil, enhances gemcitabine effects in pancreatic cancer xenografts. Anticancer Res. 2008;28:179-186. [PubMed] |
| 24. | Pressacco J, Mitrovski B, Erlichman C, Hedley DW. Effects of thymidylate synthase inhibition on thymidine kinase activity and nucleoside transporter expression. Cancer Res. 1995;55:1505-1508. [PubMed] |
| 25. | Hagmann W, Jesnowski R, Löhr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 2010;12:740-747. [PubMed] |
| 26. | Sebastiani V, Ricci F, Rubio-Viqueira B, Kulesza P, Yeo CJ, Hidalgo M, Klein A, Laheru D, Iacobuzio-Donahue CA. Immunohistochemical and genetic evaluation of deoxycytidine kinase in pancreatic cancer: relationship to molecular mechanisms of gemcitabine resistance and survival. Clin Cancer Res. 2006;12:2492-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Costantino CL, Witkiewicz AK, Kuwano Y, Cozzitorto JA, Kennedy EP, Dasgupta A, Keen JC, Yeo CJ, Gorospe M, Brody JR. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69:4567-4572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Brody JR, Witkiewicz A, Williams TK, Kadkol SS, Cozzitorto J, Durkan B, Pasternack GR, Yeo CJ. Reduction of pp32 expression in poorly differentiated pancreatic ductal adenocarcinomas and intraductal papillary mucinous neoplasms with moderate dysplasia. Mod Pathol. 2007;20:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Williams TK, Costantino CL, Bildzukewicz NA, Richards NG, Rittenhouse DW, Einstein L, Cozzitorto JA, Keen JC, Dasgupta A, Gorospe M. pp32 (ANP32A) expression inhibits pancreatic cancer cell growth and induces gemcitabine resistance by disrupting HuR binding to mRNAs. PLoS One. 2010;5:e15455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Maréchal R, Mackey JR, Lai R, Demetter P, Peeters M, Polus M, Cass CE, Salmon I, Devière J, Van Laethem JL. Deoxycitidine kinase is associated with prolonged survival after adjuvant gemcitabine for resected pancreatic adenocarcinoma. Cancer. 2010;116:5200-5206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Ashida R, Nakata B, Shigekawa M, Mizuno N, Sawaki A, Hirakawa K, Arakawa T, Yamao K. Gemcitabine sensitivity-related mRNA expression in endoscopic ultrasound-guided fine-needle aspiration biopsy of unresectable pancreatic cancer. J Exp Clin Cancer Res. 2009;28:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Ciccolini J, Dahan L, André N, Evrard A, Duluc M, Blesius A, Yang C, Giacometti S, Brunet C, Raynal C. Cytidine deaminase residual activity in serum is a predictive marker of early severe toxicities in adults after gemcitabine-based chemotherapies. J Clin Oncol. 2010;28:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Tibaldi C, Giovannetti E, Vasile E, Mey V, Laan AC, Nannizzi S, Di Marsico R, Antonuzzo A, Orlandini C, Ricciardi S. Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2008;14:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Eliopoulos N, Cournoyer D, Momparler RL. Drug resistance to 5-aza-2'-deoxycytidine, 2',2'-difluorodeoxycytidine, and cytosine arabinoside conferred by retroviral-mediated transfer of human cytidine deaminase cDNA into murine cells. Cancer Chemother Pharmacol. 1998;42:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Itoi T, Sofuni A, Fukushima N, Itokawa F, Tsuchiya T, Kurihara T, Moriyasu F, Tsuchida A, Kasuya K. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Nakahira S, Nakamori S, Tsujie M, Takahashi Y, Okami J, Yoshioka S, Yamasaki M, Marubashi S, Takemasa I, Miyamoto A. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer. 2007;120:1355-1363. [PubMed] |
| 38. | Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Réjiba S, Bigand C, Parmentier C, Hajri A. Gemcitabine-based chemogene therapy for pancreatic cancer using Ad-dCK: : UMK GDEPT and TS/RR siRNA strategies. Neoplasia. 2009;11:637-650. [PubMed] |
| 40. | Okazaki T, Javle M, Tanaka M, Abbruzzese JL, Li D. Single nucleotide polymorphisms of gemcitabine metabolic genes and pancreatic cancer survival and drug toxicity. Clin Cancer Res. 2010;16:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Ng SSW MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60:5451-5455. [PubMed] |
| 42. | Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7:3269-3275. [PubMed] |
| 43. | Pham NA, Tsao MS, Cao P, Hedley DW. Dissociation of gemcitabine sensitivity and protein kinase B signaling in pancreatic ductal adenocarcinoma models. Pancreas. 2007;35:e16-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, Schäfer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 399] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 45. | Sclabas GM, Fujioka S, Schmidt C, Evans DB, Chiao PJ. NF-kappaB in pancreatic cancer. Int J Gastrointest Cancer. 2003;33:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Wharry CE, Haines KM, Carroll RG, May MJ. Constitutive non-canonical NFkappaB signaling in pancreatic cancer cells. Cancer Biol Ther. 2009;8:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Rengifo-Cam W, Umar S, Sarkar S, Singh P. Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-{kappa}B. Cancer Res. 2007;67:7266-7274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Kong R, Sun B, Jiang H, Pan S, Chen H, Wang S, Krissansen GW, Sun X. Downregulation of nuclear factor-kappaB p65 subunit by small interfering RNA synergizes with gemcitabine to inhibit the growth of pancreatic cancer. Cancer Lett. 2010;291:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 49. | Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, Lopez-Berestein G, Vivas-Mejia PE, Sood AK, McConkey DJ. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143-8151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064-9072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 458] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70:8695-8705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Mamaghani S, Patel S, Hedley DW. Glycogen synthase kinase-3 inhibition disrupts nuclear factor-kappaB activity in pancreatic cancer, but fails to sensitize to gemcitabine chemotherapy. BMC Cancer. 2009;9:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 55. | Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 290] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Liu D, Zhang Y, Dang C, Ma Q, Lee W, Chen W. siRNA directed against TrkA sensitizes human pancreatic cancer cells to apoptosis induced by gemcitabine through an inactivation of PI3K/Akt-dependent pathway. Oncol Rep. 2007;18:673-677. [PubMed] |
| 58. | Maréchal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, Closset J, Devière J, Salmon I, Van Laethem JL. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100:1444-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 60. | Gong XG, Lv YF, Li XQ, Xu FG, Ma QY. Gemcitabine resistance induced by interaction between alternatively spliced segment of tenascin-C and annexin A2 in pancreatic cancer cells. Biol Pharm Bull. 2010;33:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Yao J, Qian C. Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol. 2010;27:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. A novel role for carcinoembryonic antigen-related cell adhesion molecule 6 as a determinant of gemcitabine chemoresistance in pancreatic adenocarcinoma cells. Cancer Res. 2004;64:3987-3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | Yau CY, Wheeler JJ, Sutton KL, Hedley DW. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT0254, inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth, and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 2005;65:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Huanwen W, Zhiyong L, Xiaohua S, Xinyu R, Kai W, Tonghua L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol Cancer. 2009;8:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Duxbury MS, Ito H, Benoit E, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting focal adhesion kinase enhances pancreatic adenocarcinoma gemcitabine chemosensitivity. Biochem Biophys Res Commun. 2003;311:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J Am Coll Surg. 2004;198:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Kreutzer JN, Ruzzene M, Guerra B. Enhancing chemosensitivity to gemcitabine via RNA interference targeting the catalytic subunits of protein kinase CK2 in human pancreatic cancer cells. BMC Cancer. 2010;10:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Habiro A, Tanno S, Koizumi K, Izawa T, Nakano Y, Osanai M, Mizukami Y, Okumura T, Kohgo Y. Involvement of p38 mitogen-activated protein kinase in gemcitabine-induced apoptosis in human pancreatic cancer cells. Biochem Biophys Res Commun. 2004;316:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 71. | Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, Leon LG, Pollina LE, Groen A, Falcone A. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70:4528-4538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 72. | Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 73. | Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 74. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2602] [Article Influence: 153.1] [Reference Citation Analysis (22)] |
| 75. | Pitha-Rowe IF, Pitha PM. Viral defense, carcinogenesis and ISG15: novel roles for an old ISG. Cytokine Growth Factor Rev. 2007;18:409-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Ina S, Hirono S, Noda T, Yamaue H. Identifying molecular markers for chemosensitivity to gemcitabine in pancreatic cancer: increased expression of interferon-stimulated gene 15 kd is associated with intrinsic chemoresistance. Pancreas. 2010;39:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | McConnell BB, Vertino PM. TMS1/ASC: the cancer connection. Apoptosis. 2004;9:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Ramachandran K, Miller H, Gordian E, Rocha-Lima C, Singal R. Methylation-mediated silencing of TMS1 in pancreatic cancer and its potential contribution to chemosensitivity. Anticancer Res. 2010;30:3919-3925. [PubMed] |
| 79. | Voutsadakis IA. Apoptosis and the pathogenesis of lymphoma. Acta Oncol. 2000;39:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Wei SH, Dong K, Lin F, Wang X, Li B, Shen JJ, Zhang Q, Wang R, Zhang HZ. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother Pharmacol. 2008;62:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 83. | Taba K, Kuramitsu Y, Ryozawa S, Yoshida K, Tanaka T, Maehara S, Maehara Y, Sakaida I, Nakamura K. Heat-shock protein 27 is phosphorylated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2010;30:2539-2543. [PubMed] |
| 84. | Taba K, Kuramitsu Y, Ryozawa S, Yoshida K, Tanaka T, Mori-Iwamoto S, Maehara S, Maehara Y, Sakaida I, Nakamura K. KNK437 downregulates heat shock protein 27 of pancreatic cancer cells and enhances the cytotoxic effect of gemcitabine. Chemotherapy. 2011;57:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, Mikuria K, Fujimoto M, Maehara S, Maehara Y, Okita K, Nakamura K, Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345-1350. [PubMed] |
| 86. | Mori-Iwamoto S, Taba K, Kuramitsu Y, Ryozawa S, Tanaka T, Maehara S, Maehara Y, Okita K, Nakamura K, Sakaida I. Interferon-gamma down-regulates heat shock protein 27 of pancreatic cancer cells and helps in the cytotoxic effect of gemcitabine. Pancreas. 2009;38:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Eckert D, Buhl S, Weber S, Jäger R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 88. | Orso F, Penna E, Cimino D, Astanina E, Maione F, Valdembri D, Giraudo E, Serini G, Sismondi P, De Bortoli M. AP-2alpha and AP-2gamma regulate tumor progression via specific genetic programs. FASEB J. 2008;22:2702-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Jonckheere N, Fauquette V, Stechly L, Saint-Laurent N, Aubert S, Susini C, Huet G, Porchet N, Van Seuningen I, Pigny P. Tumour growth and resistance to gemcitabine of pancreatic cancer cells are decreased by AP-2alpha overexpression. Br J Cancer. 2009;101:637-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 91. | Voutsadakis IA, Papandreou CN. The ubiquitin-proteasome system in prostate cancer and its transition to castration resistance. Urol Oncol. 2010;Epub ahead of print. [PubMed] |
| 92. | Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2923] [Cited by in RCA: 2984] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 93. | Khanna A, Mahalingam K, Chakrabarti D, Periyasamy G. Ets-1 expression and gemcitabine chemoresistance in pancreatic cancer cells. Cell Mol Biol Lett. 2011;16:101-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 888] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 95. | Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 96. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1365] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 97. | Kent OA, Mullendore M, Wentzel EA, López-Romero P, Tan AC, Alvarez H, West K, Ochs MF, Hidalgo M, Arking DE. A resource for analysis of microRNA expression and function in pancreatic ductal adenocarcinoma cells. Cancer Biol Ther. 2009;8:2013-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704-6712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 99. | Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820-5828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 722] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 100. | Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400-2407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 499] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 101. | Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 102. | Li Y, Vandenboom TG, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 103. | Mallanna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol. 2010;344:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 104. | Chen Y, Yu G, Yu D, Zhu M. PKCalpha-induced drug resistance in pancreatic cancer cells is associated with transforming growth factor-beta1. J Exp Clin Cancer Res. 2010;29:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 105. | Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 106. | Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686-1698. [PubMed] |
| 107. | Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 601] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 108. | Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 109. | Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
Peer reviewer: Masayuki Ohtsuka, MD, PhD, Department of General Surgery, Graduate School of Medicine, Chiba University, 1-8-1, Inohana, Chuoh-ku, Chiba 260-8670, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM