Published online Feb 15, 2026. doi: 10.4251/wjgo.v18.i2.113922
Revised: November 21, 2025
Accepted: December 18, 2025
Published online: February 15, 2026
Processing time: 111 Days and 21.8 Hours
The management of advanced colorectal cancer (CRC) is challenging due to limi
To validate the efficacy and safety of FZJDXYG and determine its effects on immune function in patients with advanced CRC.
In this multi-center, double-blind, randomized, placebo-controlled trial, 78 eligible patients were randomly assigned at a ratio of 1:1 to the treatment group (receiving FZJDXYG plus standard care) or the control group (receiving placebo plus standard care) for 12 weeks.
The treatment group experienced significant reductions in carcinoembryonic antigen and carbohydrate antigen 19-9 levels (both P < 0.05), with significantly greater reductions in these two markers compared to the control group (both P < 0.05). Immunologically, the treatment group exhibited significant increases in CD3+ and CD4+ cell counts and CD4+/CD8+ ratio, and decreased CD8+ levels, with all changes superior to those in the control group (all P < 0.05). The Karnofsky Performance Status score was also significantly higher in the treatment group (P < 0.05). Crucially, the incidence of adverse events was significantly lower in the treatment group than in the control group (P < 0.05).
FZJDXYG effectively ameliorates clinical symptoms, enhances immune function, and reduces chemotherapy-induced toxicities in patients with advanced CRC with spleen deficiency and stasis toxin syndrome.
Core Tip: The combination of Fuzheng Jiedu Xiaoyong granules (FZJDXYG) with first-line therapy for advanced colorectal cancer (CRC) demonstrates significant clinical efficacy with minimal adverse effects. It can effectively modulate immune dysfunction and improve patients’ quality of life. A parallel, randomized, controlled trial confirmed the significant efficacy and high safety of FZJDXYG in treating advanced CRC patients. Moreover, FZJDXYG significantly increased CD4+ T cell abundance, reduced CD8+ T cell abundance, and increased the CD4+/CD8+ ratio in patients, with significant differences before and after treatment, as well as between the treatment and control group.
- Citation: Qin HY, Li Z, Cong PW, Mi D, Li FZ, Hu X, Li GX. Clinical efficacy of Fuzheng Jiedu Xiaoyong granules in advanced colorectal cancer (spleen deficiency and stasis toxin syndrome). World J Gastrointest Oncol 2026; 18(2): 113922
- URL: https://www.wjgnet.com/1948-5204/full/v18/i2/113922.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i2.113922
Colorectal cancer (CRC) is a leading malignancy of the digestive system. In 2020, it ranked third globally for both incidence and mortality, with a persistently high burden of new cases that constitutes a major public health challenge[1]. Treatment options for advanced CRC are limited and primarily rely on chemotherapy, targeted therapy, and immunotherapy. While these modalities offer clinical benefits, their utility is often constrained by the significant adverse effects of chemotherapy and the development of drug resistance to targeted agents, resulting in suboptimal patient quality of life (QoL) and therapeutic outcomes[2]. Consequently, exploring novel therapeutic strategies is a critical priority. Recent research indicates that natural compounds from Chinese herbal medicine can inhibit cancer cell proliferation and modu
The “Tumors as Sore and Ulcer” theory is a modern therapeutic framework for oncology rooted in classical TCM literature, where tumors were classified as a type of yong (carbuncle) or ju (deep-rooted abscess). The canonical text Wei Ji Bao Shu: Yong Ju Wu Fa (The Precious Book of Medical Aid: Chapter on the Five Manifestations of Abscesses) explicitly links ai (cancer) to conditions including ju and yong, stating: “Among the five manifestations of yong and ju, the first is ‘ai’... the third is ‘ju’... and the fifth is ‘yong’“. It posits that both internal and external pathogenic factors can disrupt qi and blood circulation, leading to the local accumulation of phlegm, dampness, qi, and blood stasis, which coalesce to form “ai du” (cancer-toxin)[4]. This mechanism is analogous to the pathogenesis of yong and ju, which also arise from qi-blood stagnation and pathogenic retention. This shared etiology supports the therapeutic principle: “‘ai and ‘yang’ (abscesses and ulcers) are analogous, and their pattern differentiation and treatment can be interrelated” (Ai Yang Xiang Lei, Bian Zhi Xiang Can)[5]. Notably, internal treatment strategies for sores and ulcers—tuo (promoting), bu (tonifying), and xiao (eliminating)—as well as external therapies, have shown significant efficacy and unique advantages in cancer management[6].
Fuzheng Jiedu Xiaoyong granules (FZJDXYG), a botanical formula for treating advanced CRC, has demonstrated marked clinical efficacy in alleviating symptoms and enhancing patients’ QoL. Our present multi-center randomized controlled trial (RCT) enrolled eligible patients with advanced CRC, who were allocated to a treatment group or a control group using a random number table. By comparing changes in outcome measures between these two groups, this study aims to further evaluate the safety and efficacy of FZJDXYG and to define its potential role in managing advanced CRC.
This multi-center, double-blind RCT was conducted from August 2023 to February 2025. A total of 78 patients with unresectable stage IIIC or IV CRC were recruited from the Second Affiliated Hospital of Liaoning University of Traditional Chinese Medicine and the Liaoning Cancer Hospital & Institute. In addition to standard care, participants were randomly assigned at a 1:1 ratio to receive either FZJDXYG or a placebo for the 12-week intervention period. The intervention was terminated prematurely in cases of severe adverse events (AEs) or disease progression.
Written informed consent was obtained from all patients prior to their inclusion in the study.
The sample size was calculated based on data from previous relevant studies. The primary efficacy endpoint was change in the tumor marker carcinoembryonic antigen (CEA). A superiority test was used with a two-sided significance level (α) of 0.05 and a power (1-β) of 0.90 (β = 0.10). The following formula for comparing the means of two samples in a superiority trial was used, where μT and μC represent the mean values for the treatment and control groups, respectively. Based on prior research, μC was 13.02, and we hypothesized μT to be 8.75, with a standard deviation (σ) of 5.26. The non-inferiority margin (Δ) was set to 0, and the allocation ratio (K) between the treatment and control groups was 1:1. Substituting these values into the formula yielded a required sample size (nC) of 31.38, which was rounded up to 32 patients per group. Based on an estimated 20% dropout rate, the final sample size was increased to 39 patients per group, for a total of 78 patients.
Randomization was performed using a random number table. The allocation sequence was computer-generated using SAS statistical software and stratified by study center. The randomization codes were distributed in sequentially numbered, sealed blocks to each participating center, along with the corresponding medication kits. Investigators assigned medication kits to participants in the order of their enrollment based on matching sequential numbers. The patients, clinical investigators, data collectors, and statisticians were all unaware of the group assignments.
The two groups were matched at baseline, with no statistically significant differences in gender, age, primary tumor location, metastasis site, time since initial diagnosis, or prior treatment modalities (all P > 0.05; Table 1).
| Item | Treatment group | Control group | χ2/t | P value | |
| Sex | Male | 27 | 25 | ||
| Female | 12 | 14 | 0.058 | 0.81 | |
| Age (years) | 60.53 ± 7.88 | 61.20 ± 10.00 | -0.3271 | 0.745 | |
| Tumor location | Colon | 23 | 22 | ||
| Rectum | 16 | 17 | 0.053 | 0.81 | |
| Metastatic site | Liver | 26 | 24 | ||
| Lungs | 16 | 14 | 0.013 | 0.908 | |
| Time since initial diagnosis (month) | > 6 | 11 | 15 | ||
| < 6 | 28 | 24 | 0.923 | 0.337 | |
| Prior treatment modalities | Surgery | 16 | 16 | ||
| Chemotherapy | 12 | 16 | 0.086 | 0.769 | |
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Liaoning University of Traditional Chinese Medicine [Approval No. 2023(XS)-003-02(FS)].
Diagnosis based on Western medicine: CRC diagnosis was established according to the Chinese Guidelines for the Diagnosis and Treatment of Colorectal Cancer (2023 edition)[7], based on a combination of clinical manifestations, endoscopic findings, imaging studies (computed tomography and magnetic resonance imaging), and pathological findings. Clinical staging was determined using the 8th edition of the American Joint Committee on Cancer staging manual[8]. Eligible patients were diagnosed with unresectable stage IIIC or IV CRC.
Diagnosis based on TCM: The TCM diagnosis of “spleen deficiency and stasis toxin syndrome” (Pi Xu Yu Du Zheng) was based on the National Standard of the People’s Republic of China: Clinical Terminology of Traditional Chinese Medicine - Syndromes[9]. The diagnostic criteria were as follows: (1) Primary symptoms: Fatigue and lack of strength, poor appetite, abdominal distention, and abdominal pain; (2) Secondary symptoms: Palpable abdominal mass, loose stools or constipation, nausea and vomiting, and borborygmus; and (3) Tongue and pulse: A pale-purple tongue with ecchymosis or petechia and a wiry-thready or thin-choppy pulse. The diagnosis was confirmed if a patient presented with three or more primary symptoms, or two primary symptoms plus three or more secondary symptoms, in conjunction with the tongue and pulse findings.
Inclusion criteria: (1) Conform to both the Western medicine and TCM diagnostic criteria outlined above; (2) Have an estimated life expectancy of > 6 months and a Karnofsky Performance Status (KPS) score of ≥ 60; (3) Aged 18-75 years; (4) Have baseline hematological, hepatic, and renal function parameters meeting the standards required for chemotherapy; and (5) Be deemed suitable for treatment with the capecitabine and oxaliplatin (CAPOX) regimen plus bevacizumab.
Exclusion criteria: (1) Had tumors that were surgically resectable according to TNM staging but were not operated on for other reasons; (2) Had other severe concomitant diseases, including significant cardiovascular, cerebrovascular, hepatic, renal, and/or hematological disorders; (3) Had a known history of allergies or were allergic to the drugs investigated; (4) Had cognitive impairment or psychiatric disorders that would preclude compliance with the study protocol; (5) Were pregnant, lactating, or planning a pregnancy during the trial period; or (6) Had participated in another clinical trial within the preceding 3 months.
Withdrawal criteria: (1) The patient voluntarily withdrew from research; (2) Loss of follow-up due to non-compliance with medication and/or scheduled visits; (3) Occurrence of an allergic reaction or a serious AE; (4) Development of other complications or special physiological changes during the trial that made continued participation inadvisable; or (5) Poor compliance with the treatment protocol. Data from participants who withdrew from the study were not included in the final statistical analysis.
Removal criteria: (1) The patients were found to have met the exclusion criteria or not met the inclusion criteria after randomization (i.e., erroneously enrolled); (2) They did not receive any study medication after enrollment or had no follow-up visits; or (3) They used any medications prohibited by the study protocol, making it impossible to evaluate efficacy.
Standard care (for both groups): All patients received the CAPOX regimen plus bevacizumab. (1) Oxaliplatin injection [10 mL: 50 mg/vial, Qilu Pharmaceutical (Hainan Province) Co., Ltd., NMPA Approval No. H20203216]: 130 mg/m2, intravenous infusion on day 1; (2) Capecitabine tablets (0.5 g/tablet, Qilu Pharmaceutical Co., Ltd., NMPA Approval No. H20133361): 1000 mg/m2, administered orally twice daily from day 1 to day 14, followed by a 7-day rest period; and (3) Bevacizumab injection (4 mL: 100 mg/vial, Qilu Pharmaceutical Co., Ltd., NMPA Approval No. S20190040): 7.5 mg/kg, intravenous infusion on day 1. This regimen was administered in 21-day cycles for a total of 4 cycles.
Treatment group: In addition to standard care, patients received FZJDXYG (Sichuan Neo-Green Pharmaceutical Technology Development Co., Ltd.), which contain ginseng, Astragali Radix, Chinese angelica, Poria, Hairyvein Agrimonia herb, figwort root, Flos Lonicerae, licorice root, sargentgloryvine stem, and hedyotis. The dosage was one bag taken orally twice daily, which was administered in 21-day courses for 4 consecutive courses.
Control group: In addition to standard care, patients received a placebo (Sichuan Neo-Green Pharmaceutical Technology Development Co., Ltd.), formulated with 5% of the crude drug amount plus starch, excipients, and flavoring agents to mimic the appearance, smell, and taste of FZJDXYG. The dosage was one bag taken orally twice daily for 4 consecutive 21-day courses.
Primary efficacy endpoints: (1) Serum tumor markers: Serum CEA and carbohydrate antigen 19-9 (CA19-9) levels were measured before and after treatment using an enzyme-linked immunosorbent assay; and (2) T-cell subsets: The levels of CD3+, CD4+, and CD8+ T cells, as well as the CD4+/CD8+ ratio, were measured in peripheral blood before and after treatment using flow cytometry.
Secondary efficacy endpoints: (1) QoL score: The KPS scale[10] was used to assess QoL. A professional physician performed the assessment once before treatment and once after treatment completion; (2) Objective tumor response: Radiological examinations were performed before and after treatment to measure the largest diameter and volume of tumors. A maximum of two lesions per organ and up to five lesions in total were selected as target lesions. Measurable lymph nodes were defined as those with a short axis ≥ 15 mm. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1[11]; (3) TCM symptom score: TCM symptoms were scored before and after treatment. Primary symptoms (fatigue, poor appetite, abdominal distention, and abdominal pain) were graded as none (0), mild (2), moderate (4), or severe (6), whereas secondary symptoms (palpable abdominal mass, loose stools, constipation, nausea/vomiting, and/or borborygmus) were graded as none (0), mild (1), moderate (2), or severe (3); and (4) TCM efficacy evaluation: The therapeutic efficacy was assessed according to previous literature and the Guiding Principles for Clinical Research of New TCM Drugs (Trial)[12,13]. The efficacy index was calculated using the Nimo
AEs, including myelosuppression, gastrointestinal reactions, and hepatic or renal dysfunction, were recorded and graded according to NCI-CTCAE version 5.0[14]. AEs were classified into five grades, with grade 0 indicating no AEs and grade 4 indicating the most severe toxicity.
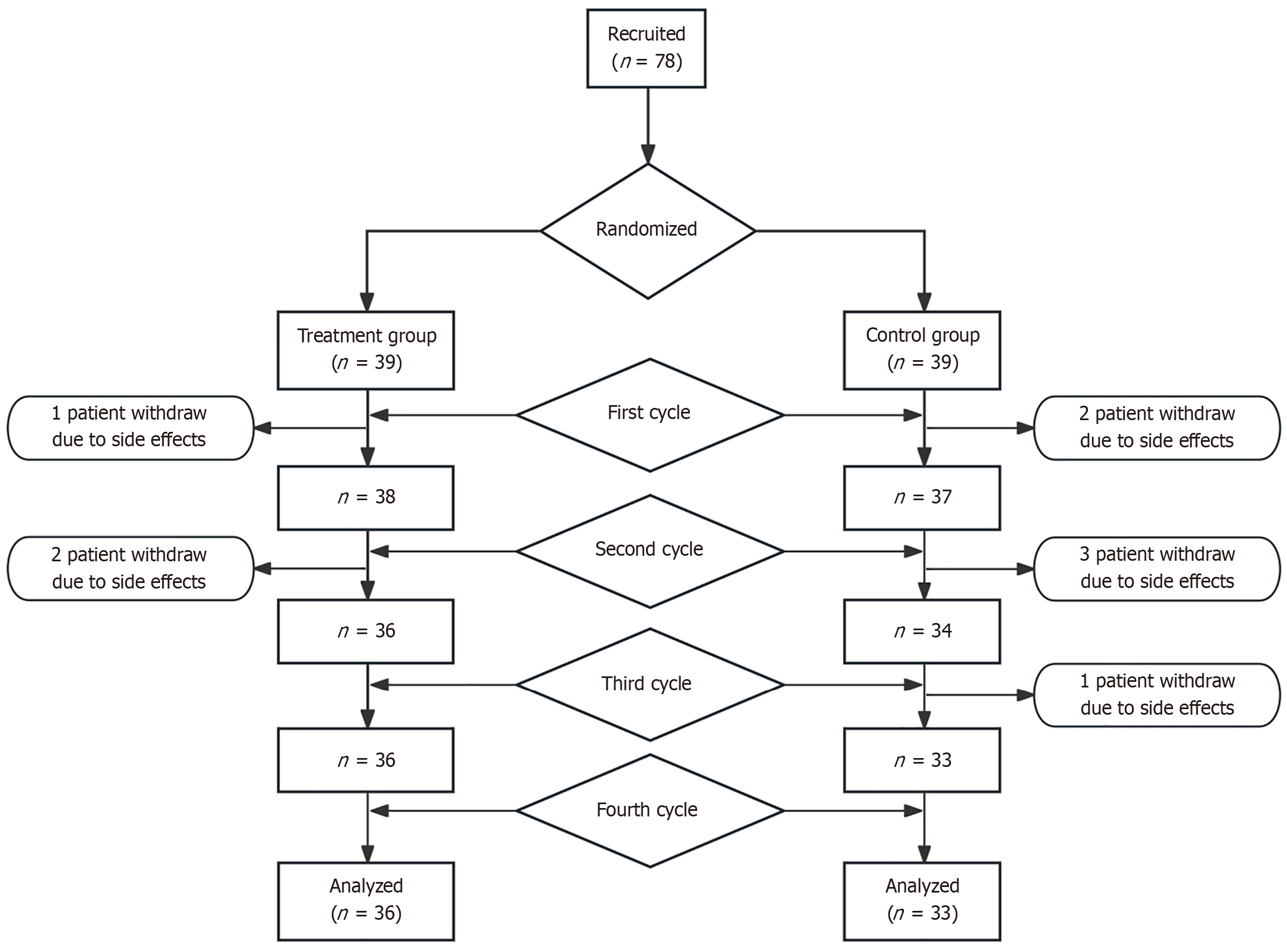
Investigators completed Case Report Forms (CRFs) in a timely manner based on the original source documents, ensuring completeness, accuracy, and verification. After review by a data manager, the data from the original CRFs were double-entered into a password-protected online database. Following data import, the data manager and the principal investigator performed a second verification to correct any discrepancies before finalizing the dataset. Upon completion of data collection, the designated statistician performed data analysis. In the present study, three participants withdrew from the treatment group, leaving 36 for the final analysis. In the control group, six participants withdrew, leaving 33 for the final analysis (Figure 1).
Statistical analysis was performed using SPSS version 23.0. Quantitative data are expressed as mean ± SD. For normally distributed data, a two-sample t-test was used for between-group comparisons. For data that were not normally distributed, the non-parametric Mann-Whitney U test was used. Categorical data are presented as n (%) and were compared using the χ2 test. A two-sided P value of < 0.05 was considered statistically significant.
Prior to treatment, there were no significant differences in serum CEA and CA19-9 levels between the two groups (both P > 0.05). After treatment, both groups showed a significant reduction in CEA and CA19-9 levels compared to their baseline values (both P < 0.05). Notably, the reductions in both CEA and CA19-9 were significantly greater in the treatment group than in the control group (both P < 0.05; Table 2).
| Group | n | CEA (ng/mL) | P value | CA199 (U/mL) | P value | ||
| Before treatment | After treatment | Before treatment | After treatment | ||||
| Treatment group | 36 | 157.80 ± 220.10 | 54.70 ± 132.15a,b | 0.023 | 224.09 ± 315.70 | 45.60 ± 77.96a,b | 0.009 |
| Control group | 33 | 185.06 ± 307.65 | 82.32 ± 137.10b | 0.049 | 213.36 ± 405.23 | 145.47 ± 294.89b | 0.045 |
| P value | 0.343 | 0.041 | 0.866 | 0.046 | |||
At baseline, there were no significant differences in the proportions of T-cell subsets between the two groups (all P > 0.05). Following intervention, the treatment group exhibited significant increases in the percentages of CD3+ and CD4+ T cells and the CD4+/CD8+ ratio compared to baseline (all P < 0.05). Their post-treatment levels were also significantly higher than those in the control group (all P < 0.05). In contrast, the control group did not show any significant changes in these parameters from baseline (all P > 0.05). Following treatment, the proportion of CD8+ cells in peripheral serum of the treatment group significantly decreased (P < 0.05); in contrast, the proportion of CD8+ cells in the control group increased after intervention, but this increase was not statistically significant (P > 0.05). Furthermore, inter-group comparison revealed that the post-treatment CD8+ proportion in the treatment group was significantly lower than that in the control group (P < 0.05; Table 3).
| Group | n | CD3+ (%) | P value | CD4+ (%) | P value | CD8+ (%) | P value | CD4+/CD8+ | P value | ||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||||||
| Treatment group | 36 | 68.90 ± 11.01 | 76.66 ± 10.20a,b | 0.003 | 32.47 ± 7.80 | 37.44 ± 7.27a,b | 0.04 | 31.17 ± 6.9 | 28.38 ± 4.51a,b | 0.046 | 1.10 ± 3.88 | 1.35 ± | 0.014 |
| Control group | 33 | 69.05 ± 12.85 | 71.94 ± 8.90 | 0.291 | 31.63 ± 6.66 | 33.29 ± 7.27 | 0.347 | 31.97 ± 9.75 | 32.35 ± 7.96 | 0.862 | 1.10 ± 0.48 | 1.17 ± 0.44 | 0.791 |
| P value | 0.956 | 0.045 | 0.62 | 0.02 | 0.659 | 0.012 | 0.99 | 0.032 | |||||
The baseline KPS scores were comparable between the two groups (P > 0.05). After treatment, the KPS score was si
In the treatment group, 36 patients completed the study (three withdrew), yielding a disease control rate (DCR) of 91.67%. In the control group, 33 patients completed the study (six withdrew), with a DCR of 87.88%. There was no statistically significant difference in the DCR between the two groups (P > 0.05; Table 5).
| Group | n | Complete response | Partial response | Stable disease | Progressive disease | Disease control rate, n (%) |
| Treatment group | 36 | 0 | 7 | 26 | 3 | 33 (91.67) |
| Control group | 33 | 0 | 6 | 23 | 4 | 29 (87.88) |
| χ2 | 0.015 | |||||
| P value | 0.903 |
Before treatment, the TCM symptom scores were similar between the two groups (P > 0.05). After four treatment cycles, both groups demonstrated a significant reduction in symptom scores compared to baseline (both P < 0.05). However, the reduction was significantly more pronounced in the treatment group than in the control group (P < 0.05). The total effective rate based on TCM response assessment was 86.11% in the treatment group, which was significantly higher than that (66.67%) in the control group (P < 0.05; Tables 6 and 7).
| Group | n | Clinical cure | Marked improvement | Moderate improvement | Stable/deterioration | Total effective rate, n (%) |
| Treatment group | 36 | 0 | 13 | 18 | 5 | 31 (86.11)a |
| Control group | 33 | 0 | 8 | 13 | 12 | 22 (66.67) |
| χ2 | 4.357 | |||||
| P value | 0.037 |
Both groups experienced AEs of varying severity. The incidence of myelosuppression (including reductions in white blood cells, neutrophils, hemoglobin, and platelets), as well as nausea, vomiting, and diarrhea, was significantly lower in the treatment group compared to the control group (all P < 0.05). There were no significant differences in the incidence of other AEs between the two groups, such as elevated alanine aminotransferase/aspartate aminotransferase, hypertension, palmar-plantar erythrodysesthesia syndrome, or paresthesia (P > 0.05; Table 8).
| Adverse events | Grade | 0 | 1 | 2 | 3 | 4 | Incidence (%) | χ2 | P value |
| WBC reduction | Treatment group | 29 | 5 | 2 | 0 | 0 | 19a | ||
| Control group | 19 | 10 | 2 | 1 | 1 | 42 | 4.29 | 0.038 | |
| NE reduction | Treatment group | 31 | 3 | 1 | 1 | 0 | 14a | ||
| Control group | 21 | 5 | 4 | 1 | 2 | 36 | 4.68 | 0.03 | |
| HGB reduction | Treatment group | 21 | 15 | 0 | 0 | 0 | 41a | ||
| Control group | 11 | 19 | 3 | 0 | 0 | 67 | 3.90 | 0.048 | |
| PLT reduction | Treatment group | 26 | 10 | 1 | 0 | 0 | 31a | ||
| Control group | 15 | 15 | 3 | 0 | 0 | 55 | 4.06 | 0.04 | |
| ALT/AST elevation | Treatment group | 29 | 7 | 0 | 0 | 0 | 19 | ||
| Control group | 25 | 8 | 0 | 0 | 0 | 24 | 0.23 | 0.63 | |
| Hypertension | Treatment group | 11 | 15 | 7 | 3 | 0 | 69 | ||
| Control group | 7 | 12 | 9 | 5 | 0 | 79 | 0.78 | 0.38 | |
| Nausea/vomiting | Treatment group | 31 | 4 | 1 | 0 | 0 | 13a | ||
| Control group | 21 | 8 | 3 | 1 | 0 | 36 | 4.68 | 0.030 | |
| Diarrhea | Treatment group | 28 | 8 | 0 | 0 | 0 | 22a | ||
| Control group | 17 | 12 | 2 | 2 | 0 | 48 | 5.23 | 0.022 | |
| Palmar-plantar erythrodysesthesia syndrome | Treatment group | 25 | 10 | 1 | 0 | 0 | 30 | ||
| Control group | 19 | 12 | 2 | 0 | 0 | 42 | 1.05 | 0.306 | |
| Paresthesia | Treatment group | 27 | 9 | 0 | 0 | 0 | 25 | ||
| Control group | 23 | 9 | 1 | 0 | 0 | 30 | 0.243 | 0.62 |
Despite improved 5-year survival rates for advanced CRC, significant challenges remain, with currently available treatment paradigms primarily centered on chemotherapy, targeted therapy, and immunotherapy. The CAPOX regimen combined with bevacizumab is a standard first-line treatment that effectively targets cancer cells and prolongs patient survival[15]. However, therapeutic outcomes are inconsistent due to significant inter-individual variability. Furthermore, treatment efficacy is often limited by drug-related toxicities or a poor performance status in patients with long-standing disease, resulting in suboptimal clinical outcomes.
FZJDXYG is a modified formula derived from “Simiao Yong’an decoction”, a renowned classical prescription for treating Yang-type sores and ulcers, which has also been reported for use in treating malignant tumors[16]. The formula is composed of ginseng, Astragali Radix, Chinese angelica, Poria, Hairyvein Agrimonia herb, figwort root, Flos Lonicerae, licorice root, sargentgloryvine stem, and hedyotis, with therapeutic effects including tonifying the spleen, replenishing qi, resolving toxins, and eliminating sores. Modern pharmacological research indicates that the active compounds in ginseng, Astragali Radix, and Chinese angelica can inhibit inflammatory factors, enhance immune responses, alleviate immunosuppression, and regulate immune balance, thereby contributing to the clearance of cancer cells[17-19]. Poria has been shown to inhibit colon cancer cell proliferation, migration, and invasion by inducing cell cycle arrest, apoptosis, and necrosis[20]. Studies have also confirmed that hedyotis contains 58 active anti-tumor components[21] that suppress colon cancer cell proliferation through various mechanisms and pathways including apoptosis, autophagy, and ferroptosis[22]. By using ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry and network pharmacology, Yu et al[23] demonstrated that four major active components in figwort root were enriched in signaling pathways closely related to cancer. This body of pharmacological evidence provides a strong rationale for the anti-tumor properties of FZJDXYG.
The toxicities associated with chemotherapy for advanced CRC cause significant patient suffering. Consequently, the primary goals of therapy have expanded beyond simply extending survival to include alleviating physical and psychological distress and improving QoL. The present study is the first multi-center RCT to confirm the potential value of FZJDXYG in ameliorating clinical symptoms and enhancing patients’ QoL. Such advantages were particularly evident in reducing specific side effects such as nausea, diarrhea, and myelosuppression (all P < 0.05). CEA and CA19-9, the most commonly used tumor markers for CRC, are specific indicators for monitoring postoperative recurrence, metastasis, and prognosis[24]. By comparing CEA and CA19-9 serum levels between the two groups, our study objectively validated the therapeutic efficacy of FZJDXYG.
Tumor development and progression are closely linked to immune dysfunction. As a critical component of immune cells within the tumor microenvironment, functionally distinct subsets of T-lymphocytes participate in tumorigenesis and represent a key cellular mechanism in tumor progression and metastasis. CD4+ T cells are helper T-lymphocytes that induce the proliferation of other immune cells to exert anti-tumor effects, whereas CD8+ T cells are suppressor T-lympho
However, the present study has several limitations. First, the relatively small sample size may affect the generalizability of the results. Second, the follow-up period was short, which precluded a thorough assessment of the long-term safety and efficacy of FZJDXYG. Furthermore, the 5% crude drug amount in the placebo and the lack of withdrawals’ data imputation in this study may introduce bias into the results. Although our research has revealed the potential of FZJDXYG in improving biomarkers, immune function, and QoL and reducing AEs in patients with advanced CRC, future studies should involve larger sample sizes, extended follow-up periods, and more rigorous designs to comprehensively validate its clinical application value.
FZJDXYG demonstrates significant therapeutic efficacy in the treatment of patients with advanced CRC by improving tumor marker levels, enhancing immune function, elevating QoL, and reducing the incidence of AEs.
We would like to thank the patients for their participation and commitment to the clinical trial.
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2496] [Article Influence: 624.0] [Reference Citation Analysis (2)] |
| 2. | Chiorean EG, Nandakumar G, Fadelu T, Temin S, Alarcon-Rozas AE, Bejarano S, Croitoru AE, Grover S, Lohar PV, Odhiambo A, Park SH, Garcia ER, Teh C, Rose A, Zaki B, Chamberlin MD. Treatment of Patients With Late-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. JCO Glob Oncol. 2020;6:414-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Sun T, He YF, Li S, Zhu L. [Value of modified yiqi qingdu huayu decoction combined with XELOX regimen in treatment of elderly colon cancer]. Liaoning Zhongyi Zazhi. 2024;51:94-98. [DOI] [Full Text] |
| 4. | Liu R, Hua BJ. [Current Status and Analysis of Multidisciplinary Treatment Modalities with Traditional Chinese Medicine for Patients with Cancer]. Zhongguo Zhongliu. 2014;23:311-315. [DOI] [Full Text] |
| 5. | Wang GB, Jiang XC, Liu FD, Pang B, Hua BJ, Piao BK. [Application of sores and ulcers theory in tumor treatment]. Zhongyi Zazhi. 2021;62:1294-1298. [DOI] [Full Text] |
| 6. | Bai WX, Rong Z. [Exploration of theoretical basis for differentiation and treatment of colorectal cancer from the perspective of carbuncle and gangrene and its clinical application]. Zhongyi Zhongliuxue Zazhi. 2023;5:12-17. [DOI] [Full Text] |
| 7. | Hospital Authority of National Health Commission of the People's Republic of China; Chinese Society of Oncology Chinese Medical Association. [Chinese protocol of diagnosis and treatment of colorectal cancer of the National Health Commission (2023 edition)]. Xiaohua Zhongliu Zazhi (Dianziban). 2023;43:602-630. [DOI] [Full Text] |
| 8. | Brierley J, Gospodarowicz M, O'Sullivan B. The principles of cancer staging. Ecancermedicalscience. 2016;10:ed61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | National Health Commission, National Administration of Traditional Chinese Medicine. [Clinic terminology of traditional Chinese medical diagnosis and treatment —Part 2: syndromes/patterns]. 16 Nov 2020. Available from: https://www.gov.cn/zhengce/zhengceku/2020-11/24/content_5563703.htm. |
| 10. | McNair KM, Zeitlin D, Slivka AM, Lequerica AH, Stubblefield MD. Translation of Karnofsky Performance Status (KPS) for use in inpatient cancer rehabilitation. PM R. 2023;15:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22774] [Article Influence: 1339.6] [Reference Citation Analysis (1)] |
| 12. | Zheng XY. Guiding Principles for Clinical Research of New TCM Drugs (Trial). Beijing: China Medical Science and Technology Press, 2002. |
| 13. | Liu X, Yuan C, Lu X, Dong T, He G, Su D, Wang R, Jing L, Cai G, Ren J. Efficacy and influencing factors of Insect Compound Particle combined with chemotherapy for mismatch repair-related locally advanced stage III CRC who had undergone surgery and achieved R0 resection: a multicenter, double-blind, randomized, placebo-controlled clinical trial protocol. Ann Transl Med. 2023;11:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 565] [Article Influence: 94.2] [Reference Citation Analysis (2)] |
| 15. | Tao H, Wu FD, Zhao XL, Fu JP, Zhang XJ. [Effect of bevacizumab combined with oxaliplatin and capecitabine on efficacy, immune function and tumor markers in patients with advanced colon cancer]. Guoji Jianyan Yixue Zazhi. 2022;43:1500-1503. [DOI] [Full Text] |
| 16. | Wu Y, Peng HY. [Research Progress of Simiao Yong' an Decoction in the Treatment of Malignant Cancer Related Complications]. Guangming Zhongyi. 2023;38:1200-1203. [DOI] [Full Text] |
| 17. | Du Y, Di Q, Zhao XX. [Research progress on chemical composition and pharmacological effects of ginseng]. Yaoxue Qianyan. 2025;29:1576-1592. [DOI] [Full Text] |
| 18. | Zeng W, Zhou SQ, Huang J, Tan LJ, Li ZX, Liu F. [Research progress on active components and pharmacological mechanisms of astragali radix in immunoregulation]. Shanghai Zhongyiyao Zazhi. 2025;59:80-89. [DOI] [Full Text] |
| 19. | Sabeel Z, Liang Y, Hao M, Ying L, Guo R, Chen R, Li X, Yu C, Yang Z. A comprehensive review of antitumor properties of Angelica species and their antitumor-responsible constituents and the underlying molecular mechanisms involved in tumor inhibition. Phytother Res. 2023;37:2187-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | He PF, Gao M, Wen JH, Shen YM, Wang YX, Liang ZQ. [Research progress on the pharmacological effects of Poria]. Yunnan Zhongyi Zhongyao Zazhi. 2024;45:83-87. [DOI] [Full Text] |
| 21. | Han X, Zhang X, Wang Q, Wang L, Yu S. Antitumor potential of Hedyotis diffusa Willd: A systematic review of bioactive constituents and underlying molecular mechanisms. Biomed Pharmacother. 2020;130:110735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Li BC, Wang BX, Qiu ZT, Dong YS, Liu SJ. [The systematic overview of the anti-intestinal cancer pharmacological activity and mechanism of action of hedyotis diffusa willd]. Xiandai Shengwu Yixue Jinzhan. 2024;24:4777, 4798-4800. [DOI] [Full Text] |
| 23. | Yu SJ, Kong XB, Jin X, Shan MY, Cheng G, Wang PL, Li WL, Zhao PY, Sheng YJ, He BQ, Shi Q, Li HQ, Zhao QM, Qin LP, Meng XY. Systematic elucidation of the effective constituents and potential mechanisms of Scrophulariae Radix against neoplasm based on LC-MS, network pharmacology, and molecular docking approaches. Front Plant Sci. 2025;16:1615076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Zhao GC, Chen QQ, Pang L, Cheng Z, Mai FQ. [Serum levels of miR-24-3p, CEA and CA199 in patients with colon cancer and their diagnostic value]. Xiandai Zhongliu Yixue. 2021;29:3981-3984. [DOI] [Full Text] |
| 25. | Yu XJ, Qiu MS. [Clinical application of CD4+ and CD8+ T lymphocytes in the diagnosis and treatment of colorectal cancer]. Jianyan Yixue Yu Linchuang. 2021;18:2758-2762. [DOI] [Full Text] |
| 26. | Jin J, Wu SL, Guo KJ. [Expression of peripheral blood CD4+ and CD8+ cells and PD-1 levels of patients with colon cancer and its clinical significance]. Zhejiang Chuangshang Waike. 2024;29:7-9, 13. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/