TO THE EDITOR
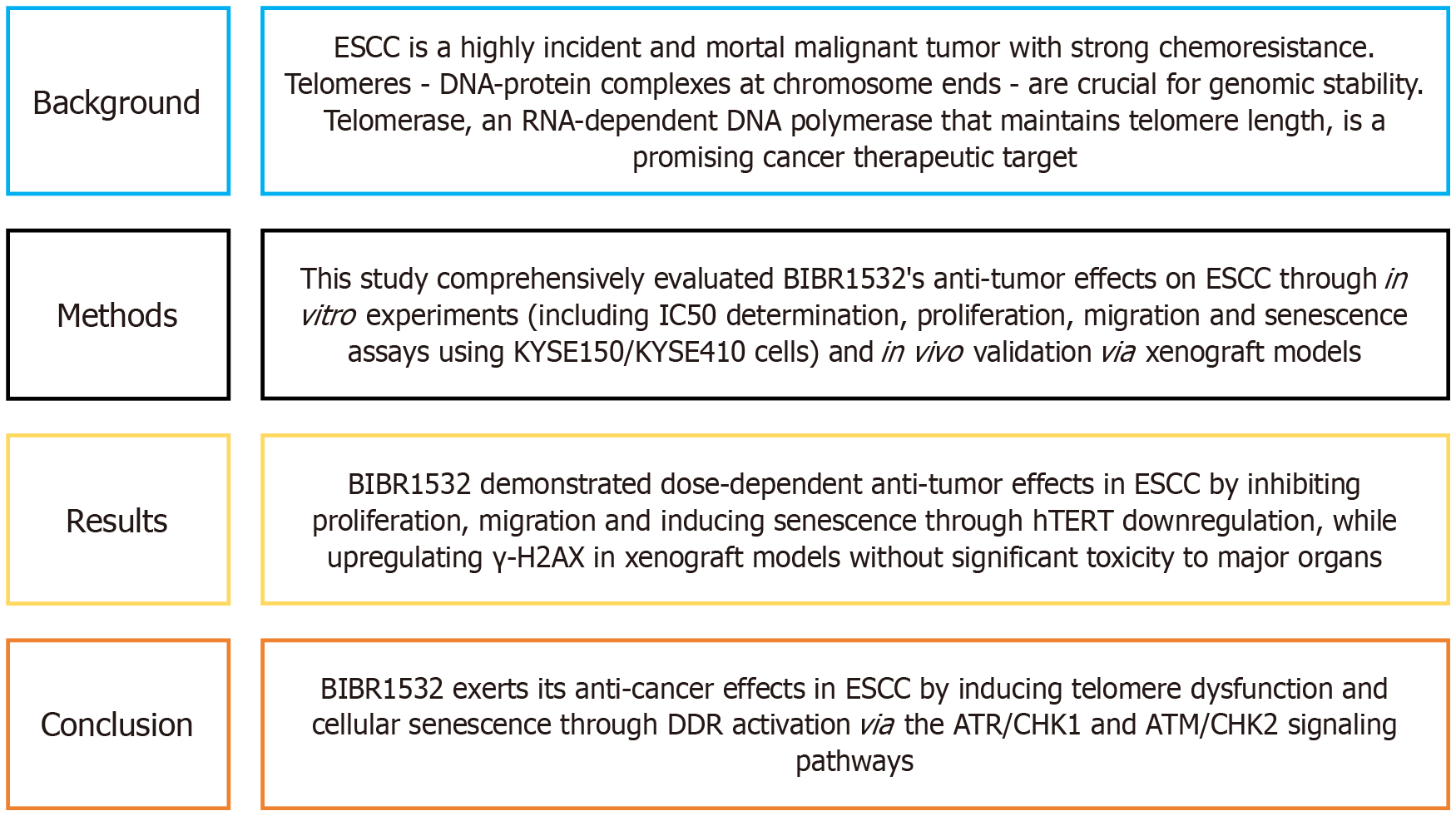
We enjoyed reading the article, which was published in World Journal of Gastrointestinal Oncology[1]. Figure 1 lists the major findings of this article. The main objective was to determine the efficacy and underlying mechanisms of the telomerase inhibitor BIBR1532 in esophageal squamous cell carcinoma (ESCC). The authors determined that BIBR1532 exerts anti-cancer effects on ESCC through the induction of the DNA damage response via the ATR/CHK1 and ATM/CHK2 pathways and the downregulation of telomere-binding protein expression. These findings will advance our understanding of ESCC and the development of targeted therapeutics by providing a rational basis and targeted translational approach for novel telomere regulation-based treatments.
Figure 1 The main information of the published article.
DDR: DNA damage response; ESCC: Esophageal squamous cell carcinoma; hTERT: Human telomerase reverse transcriptase.
After analysing the anti-cancer effects of BIBR1532, it was determined that it could be developed as a chemotherapeutic drug for oesophageal squamous cancer. The discovery of BIBR1532 dates back to 2001[2]; however, it was not translated into a commercial product up to the time of manuscript preparation (March 10, 2025). We believe that the clinical translation of BIBR1532 was significantly impeded by its low druggability, which was the direct result of its poor aqueous solubility[3]. Therefore, improved solubilisation techniques were required to enhance its aqueous solubility[4]. Organic solvents, such as dimethylsulfoxide (DMSO), were used to solubilise BIBR1532, as reported in the literature[5-8]. Notably, the study[1] also utilised DMSO to dissolve BIBR1532, as pure DMSO was the ‘vehicle control’ as described in the materials and methods section. However, it is not practical to develop a DMSO (or other organic solvent)-solubilised BIBR1532 formulation for clinical use. To improve the druggability of BIBR1532, alternative solubilisation technologies must be considered. In this letter, a potent solubilisation technique for BIBR1532 [e.g. solid dispersion (SD)] was proposed following a rational analysis.
CONCEPULISATION
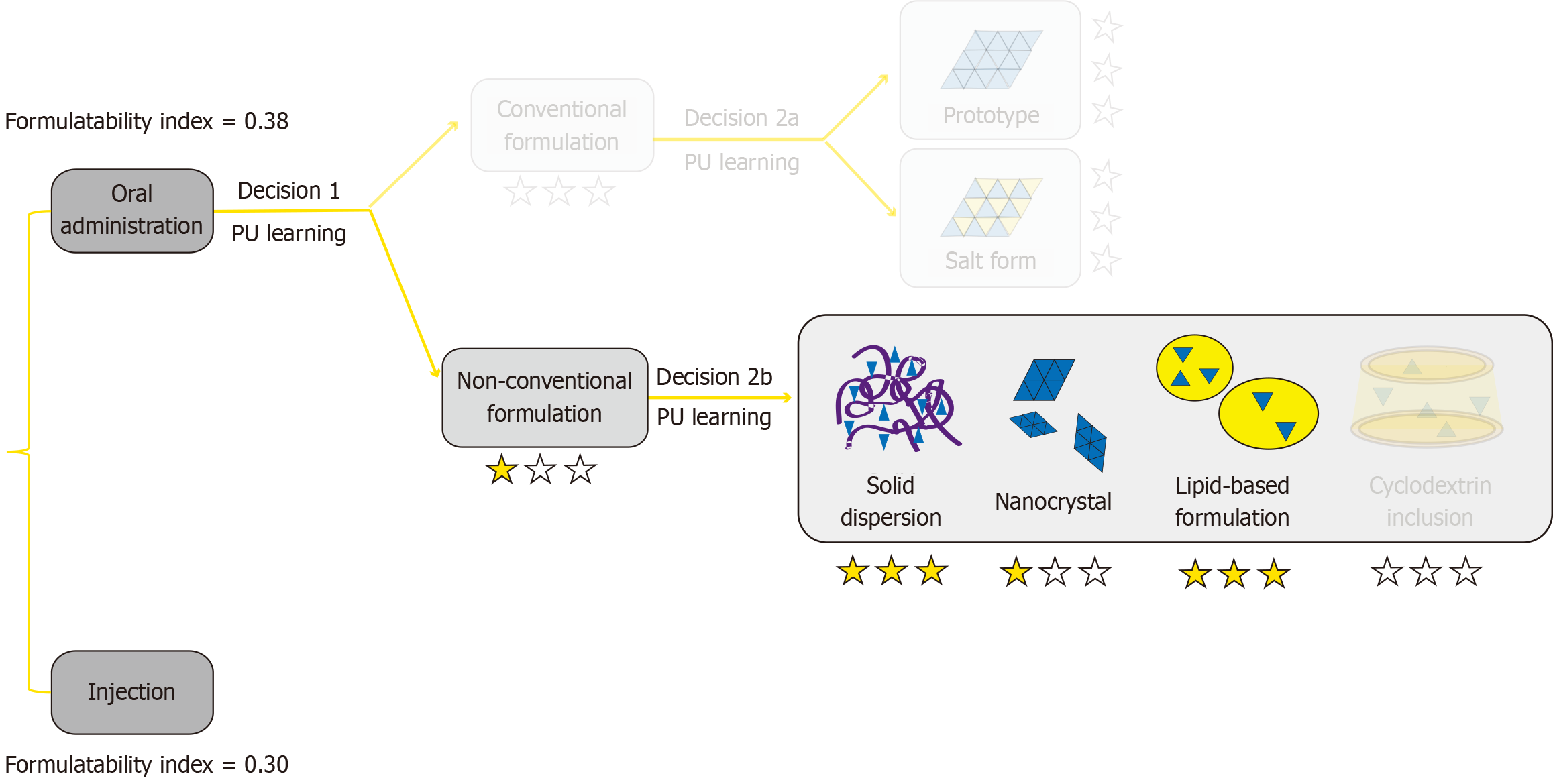
The PubChem database established by the National Library of Medicine was used to obtain the simplified molecular input line entry system (SMILES) identifier for BIBR1532: C/C( = C\C( = O)NC1 = CC = CC = C1C( = O)O)/C2 = CC3 = CC = CC = C3C = C2. The results can be accessed publicly online at https://pubchem.ncbi.nlm.nih.gov/compound/9927531. The SMILES identifier was stored locally on the corresponding author’s personal computer. Next, the SMILES identifier for BIBR1532 was imported into the FormulationDT artificial intelligence platform (http://formulationdt.computpharm.org/) to calculate the most adaptive solubilisation approach for developing a formulation. The results were screen-captured and further analysed. The formulatability index (inversely proportional to the difficulty in formulation development)[9] of the oral drug product (0.38) was higher than that of the injectable drug product (0.30) of BIBR1532, suggesting that it was more feasible to develop an oral drug formulation (Figure 2). Moreover, compared with conventional formulations, non-conventional formulations were more recommended. As the number of stars indicates the recommendation levels, SD and lipid-based formulations were preferred.
Figure 2 Analysis of the FormulationDT artificial intelligence platform.
PU: Positive unlabeled.
A comparison was made between SD and lipid-based formulations based on a literature survey. Lipid-based formulations primarily included lipid-containing self-emulsifying drug delivery systems, self-microemulsifying drug delivery systems and self-nanoemulsifying drug delivery systems. The particle size for the dispersions in these systems was < 250 nm[10]. Thus, lipid-based formulations may be classified as nanoparticulate systems and are considered difficult to scale up for industrial production[11]. SD refers to a system in which therapeutic molecules are dispersed in a solid-state carrier or matrix[12]. They may be further categorised into eutectic systems, glass solutions and suspensions, solid solutions and amorphous SDs[13]. SDs are ‘coarse grain’ systems rather than nanoparticulates, which and may be readily produced by mature manufacturing techniques, such as melt extrusion, spray drying and wet granulation[14]. According to Bhujbal et al[15], approximately 32 SD-based drug products had been approved as of 2021. From the perspective of large-scale manufacturing, the cost and risk of SD are considerably lower compared with lipid-based formulations. Therefore, SD was considered a more promising choice to solubilise BIBR1532.
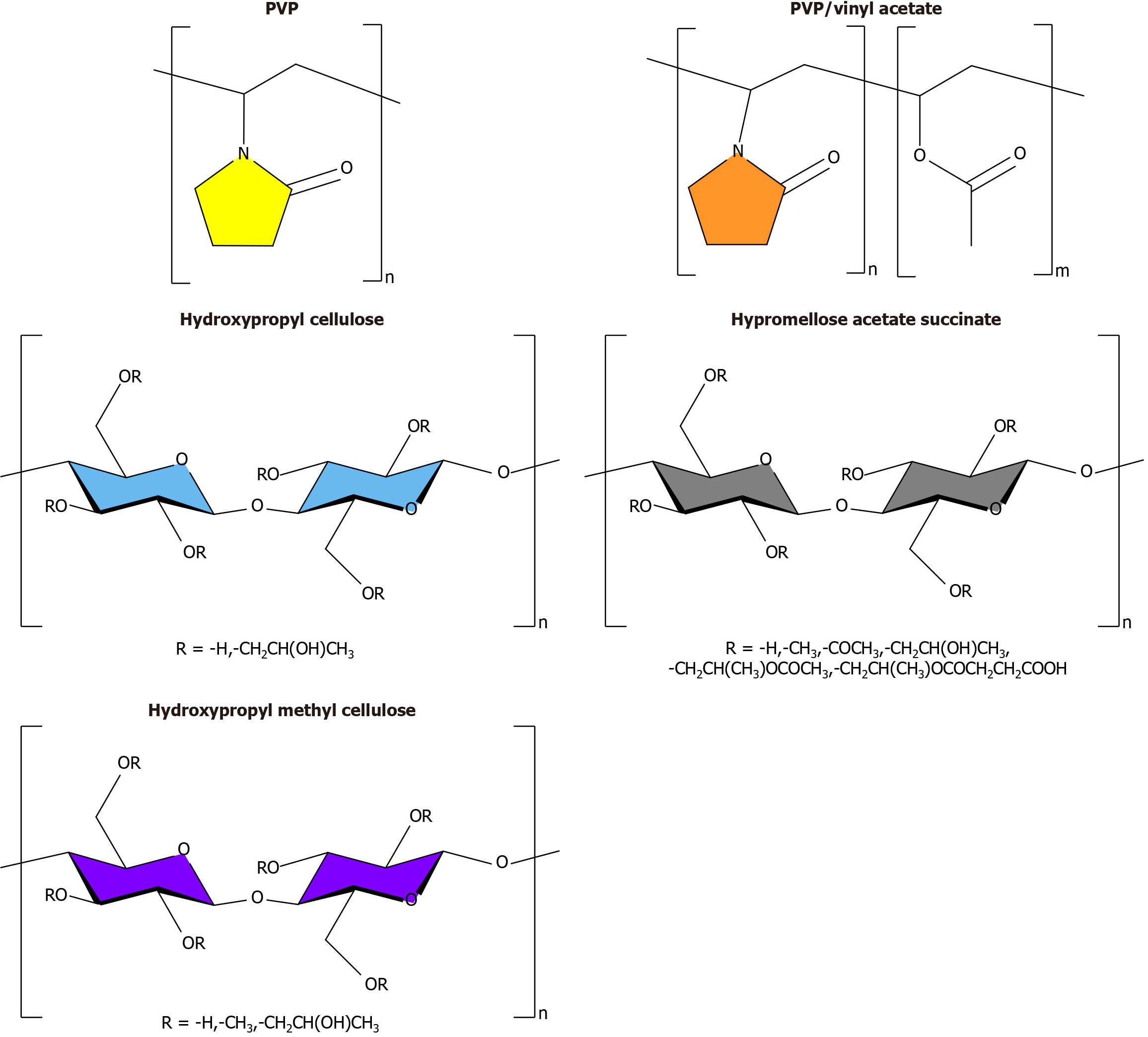
Finally, the appropriate carrier/matrix material to prepare a BIBR1532 SD was assessed. The carrier/matrix materials for the licensed SD products included polyvinylpyrrolidone (PVP), PVP/vinyl acetate (PVP/VA), hydroxypropyl cellulose (HPC), hypromellose acetate succinate (HPMCAS) and hydroxypropyl methylcellulose (HPMC). Their molecular structures are shown in Figure 3[16]. Of these, PVP and PVP/VA exhibited relatively high hygroscopicity, which can absorb moisture during storage and exert detrimental effects on physical stability. HPC exhibited a relatively low glass transition temperature (Tg), which indicated a greater tendency towards crystallisation during storage[17]. HPMCAS and HPMC showed relatively low hygroscopicity and high Tg, and the former substance was generally used for intestinal-targeted delivery because it was insoluble at pH < 5.0[16]. Although intestinal-targeted delivery is not suited for treating oesophageal squamous cancer, selecting HPMC is more reasonable. Therefore, HPMC was considered a potent carrier/matrix material to prepare BIBR1532-loaded SD.
Figure 3 Molecular structures of carrier/matrix materials for the licensed solid dispersion products.
PVP: Polyvinylpyrrolidone.
In summary, we advanced the theory that HPMC-based SDs represent an effective choice to solubilise BIBR1532 to increase its druggability and accelerate its clinical development for the treatment of oesophageal squamous cancer. In future studies, a pilot test on the feasibility of SD fabrication will be conducted. We sincerely acknowledge the authors of the commented article for their valuable contribution, which has inspired our work.
CONCLUSION
BIBR1532 faces significant challenges in drug development because of its extremely poor aqueous solubility, which severely limits its clinical application for the treatment of ESCC. This letter proposes the use of SD technology as a solubilisation strategy. An analysis using an artificial intelligence platform and a literature comparison indicates that SDs offer greater advantages for industrial production than lipid-based nanosystems. Moreover, HPMC with low hygroscopicity and high Tg was identified as an ideal carrier material. This strategy is expected to significantly enhance the dissolution rate and bioavailability of BIBR1532, providing a new pathway for clinical translation. Subsequent studies will require a detailed formulation of BIBR1532-loaded SD along with comprehensive cell-based, animal and clinical trials to verify its efficacy and safety. We anticipate the successful development of this formulation strategy, which offers a novel treatment option for patients with ESCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Masood Z, PhD, PharmD, Professor, Pakistan S-Editor: Luo ML L-Editor: A P-Editor: Zhang L