Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.114021
Revised: November 2, 2025
Accepted: December 1, 2025
Published online: January 15, 2026
Processing time: 124 Days and 0.9 Hours
SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma is a highly aggressive tumor, and spontaneous splenic rupture (SSR) as its presenting manifestation is rarely reported among pancreatic malignancies.
We herein report a rare case of a 59-year-old female who presented with acute left upper quadrant abdominal pain without any history of trauma. Abdominal ima
This study reports a rare case of SMARCB1/INI1-deficient undifferentiated rhab
Core Tip: SMARCB1-deficient undifferentiated rhabdoid carcinoma of the pancreas represents a highly aggressive and rare neoplasm. This study suggests that aggressive multimodal therapy, including surgical resection and chemotherapy, may prolong survival in selected patients; however, the overall prognosis remains poor. Given its distinct molecular characteristics and potential resistance to standard therapies, further research into targeted treatment approaches and prospective clinical studies is urgently warranted.
- Citation: Yao WQ, Ma XY, Wang GH. Clinicopathologic features of SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma: A case report and review of literature. World J Gastrointest Oncol 2026; 18(1): 114021
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/114021.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.114021
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic malignancy. It represents the fourth leading cause of cancer-related mortality worldwide in both men and women, accounting for approximately 90% of all primary pancreatic tumors[1,2]. Pancreatic undifferentiated carcinoma (UDC) is exceedingly rare, representing approximately 2%-7% of all pancreatic tumors[3], with some reports indicating an incidence as low as 0.25%[4]. According to World Health Organization 2019, pancreatic UDC is defined as a carcinoma lacking a definitive line of differentiation. Under this defi
We report the case of a female patient diagnosed with SMARCB1-deficient PURC invading adjacent organs, including the spleen and left kidney. The patient underwent complete surgical resection followed by adjuvant chemotherapy consisting of gemcitabine and nab-paclitaxel and remained in good clinical condition throughout a six-month follow-up period during 6 months of follow-up.
A 59-year-old woman was admitted with a one-day history of persistent, dull pain and discomfort localized to the left upper abdomen.
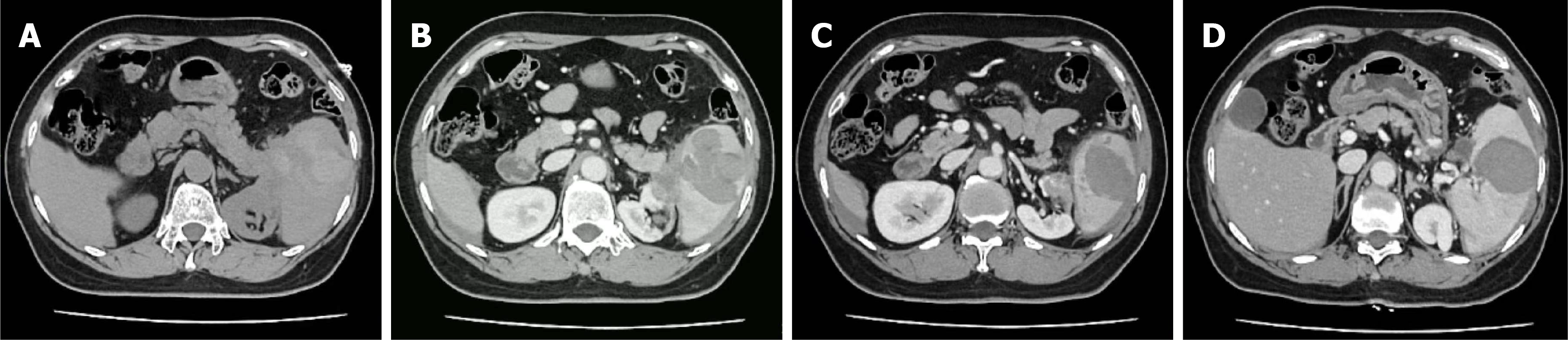
The patient developed left abdominal pain and discomfort for one day without any apparent precipitating factors. She denied trauma, gastrointestinal bleeding, fever, or other systemic symptoms, and the pain persisted without significant relief. Initial abdominal computed tomography (CT) revealed findings suggestive of a splenic hematoma or mass with possible intraperitoneal hemorrhage, as well as multiple pelvic effusions (possible hemoperitoneum). She was admitted with a preliminary diagnosis of non-traumatic splenic rupture for further evaluation and management.
The patient reported a prior history of nephritis, which was not formally treated. She denied hypertension, diabetes mellitus, or other chronic medical conditions.
The patient has no family history of hereditary or neoplastic diseases.
On admission, the patient was alert but fatigued. Pupils were round and equal, with a diameter of 3 mm and reactive to light. The tongue was midline, with no facial asymmetry. Lung auscultation revealed clear breath sounds and a regular cardiac rhythm. Abdominal examination demonstrated mild tenderness in the left upper quadrant without rebound tenderness or guarding. Bilateral Babinski signs were negative. Pelvic compression and thoracic cage compression tests were negative. Percussion tenderness over the thoracic spine was positive, while the lumbar spine showed no tenderness. Neurologic reflexes were normal, and no jaundice or lymphadenopathy was detected.
Emergency biochemical tests revealed mild elevations in γ-glutamyl transferase, alanine aminotransferase (ALT), glucose, and lactate dehydrogenase. The tumor marker panel, including carcinoembryonic antigen, alpha-fetoprotein, carcinomic antigen 125, carcinomic antigen 15-3, carcinomic antigen 19-9, and squamous cell carcinoma antigen, was within normal reference ranges. Postoperatively, serum amyloid A was elevated, while cardiac troponin I remained negative. Inter
The initial CT revealed a heterogeneous splenic lesion with intraperitoneal fluid, raising suspicion of spontaneous splenic rupture (SSR; Figure 1). Follow-up CT obtained one week postoperatively confirmed the absence of the spleen and left kidney, consistent with prior total splenectomy, distal pancreatectomy, and left nephrectomy. No abnormal enhancement was identified in the operative bed.
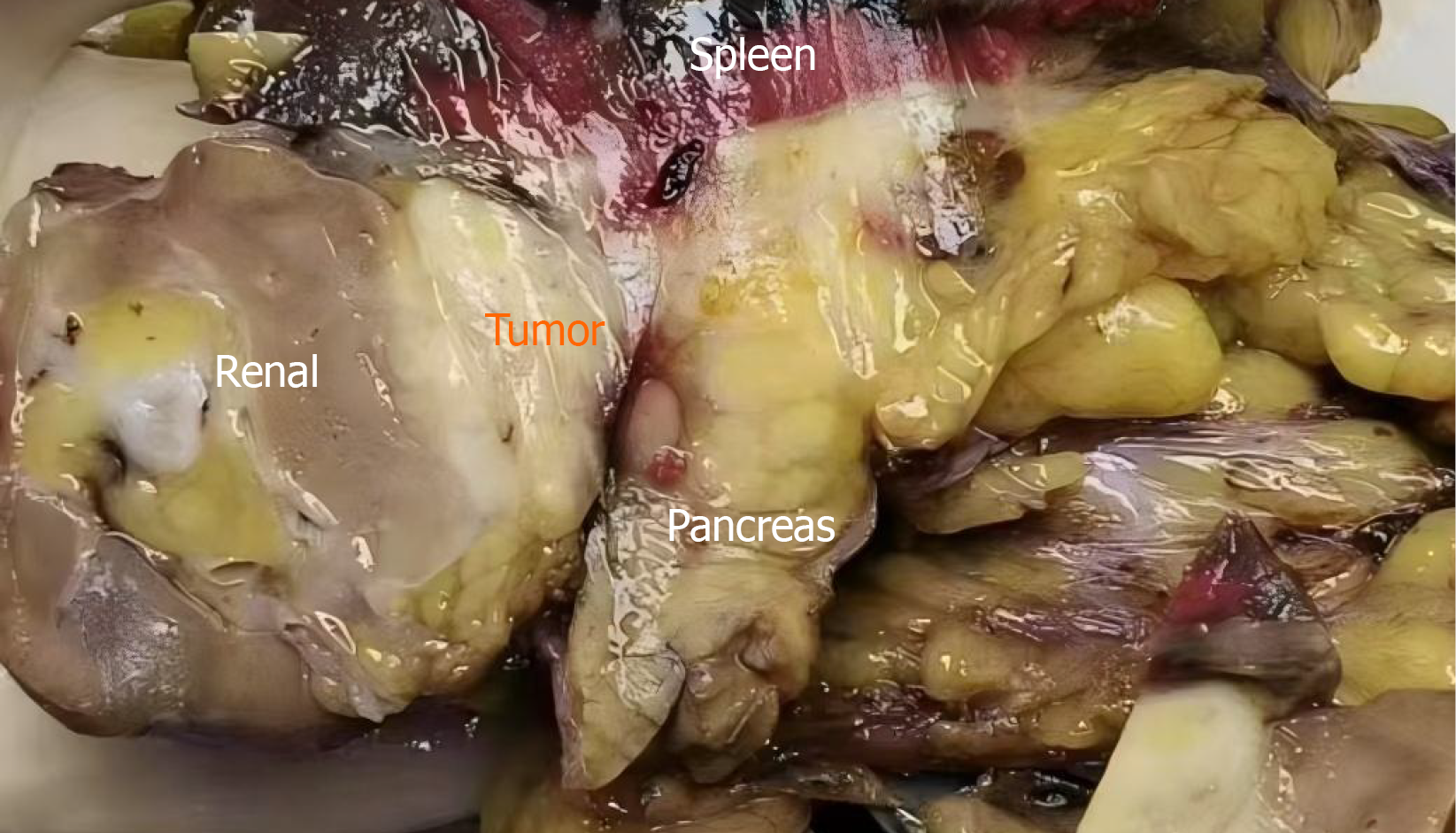
Gross examination: The surgical specimen comprised the distal pancreas, spleen, and left kidney (Figure 2). The pancreas measured appropriately 3 cm × 2 cm × 1.5 cm. The spleen measured 13 cm × 9 cm × 3 cm, and a cystic area measuring 5 cm × 4 cm × 3 cm was identified on the cut surface, with a slightly thickened inner wall. A firm, nodular lesion mea
Microscopic findings: Cytologically, the tumor demonstrated two distinct components - glandular and spindle cell elements. The adenocarcinoma component showed tubular, cribriform, and focal micropapillary architecture. Tumor cells were columnar to cuboidal, possessing eosinophilic cytoplasm and round-to-oval nuclei with mild nuclear pleo
Molecular features: A comprehensive panel of molecular markers was evaluated in both the well-differentiated PDAC and the undifferentiated rhabdoid carcinoma components. The ductal adenocarcinoma exhibited strong immunoreactivity for CK7 and CK-pan, both of which are widely utilized biomarkers to confirm epithelial origin and are particularly valuable in differentiating PDAC from colorectal adenocarcinoma (Figure 4A). In contrast, the PURC component demonstrated complete loss of CK7 expression, while CK-pan staining remained diffusely positive (Figure 4A). Similarly, strong nuclear immunoreactivity for SMARCB1 and SMARCA4 was observed, consistent with intact expression of SWI/SNF chromatin remodeling complex subunits (Figure 4A). Loss of SMARCB1 or SMARCA4 expression is typically observed in atypical teratoid/rhabdoid tumors, with SMARCB1 loss most frequently associated with the anaplastic monomorphic subtype of rhabdoid pancreatic carcinoma[8]. In this case, the PURC component demonstrated complete loss of SMARCB1 expression, while SMARCA4 expression was retained, indicating a selective deficiency within the com
The final diagnosis was T3N1M0 PDAC with SMARCB1-deficient UDC.
As mentioned above, following a multidisciplinary expert consultation, the patient underwent emergency open surgery, including total splenectomy, partial pancreatectomy, and left nephrectomy. Postoperatively, the patient received adjuvant chemotherapy consisting of gemcitabine, nab-paclitaxel, bevacizumab and sintilimab.
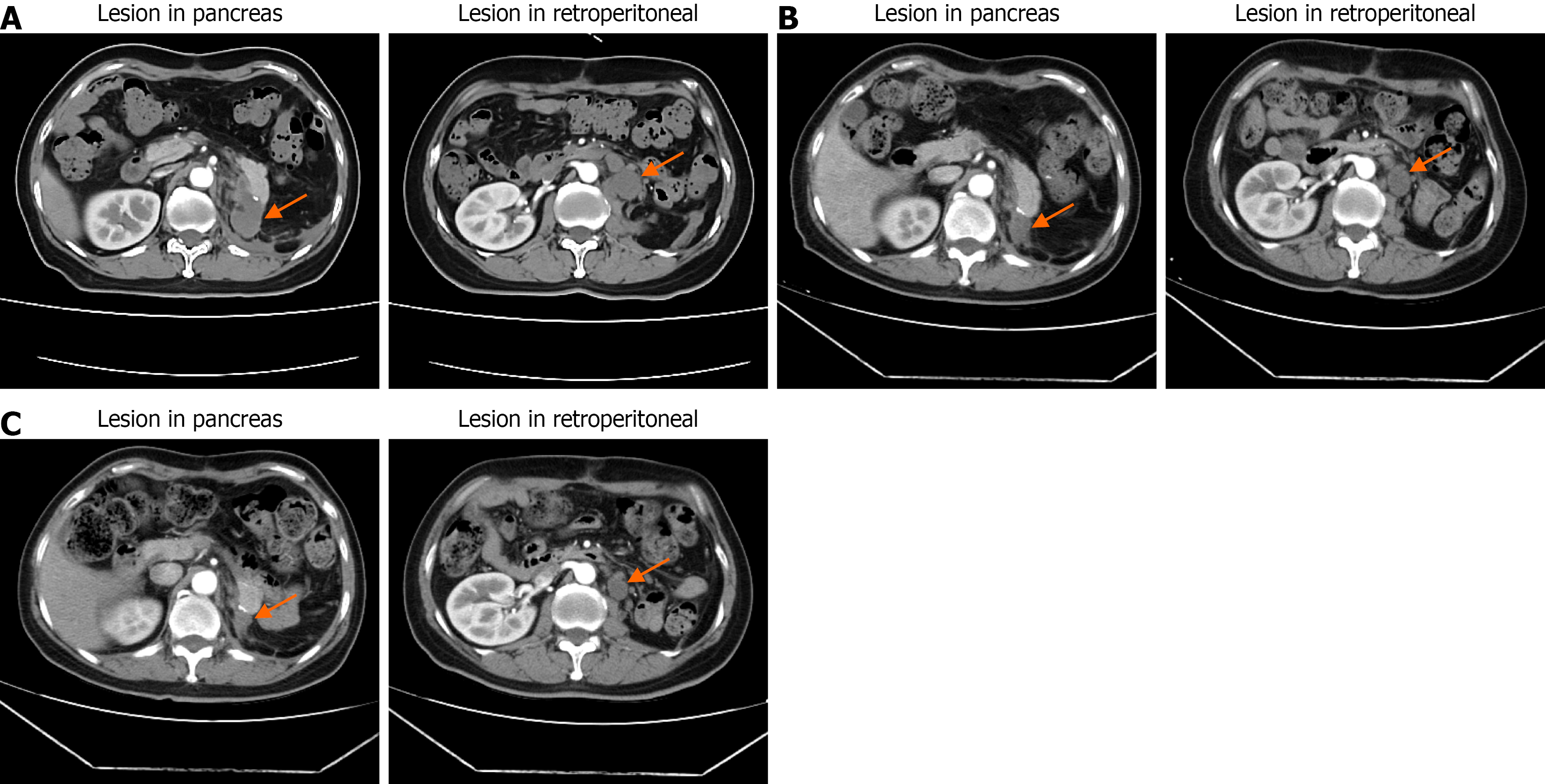
At two months postoperatively, a follow-up contrast-enhanced abdominal CT revealed a soft-tissue mass in the pan
SMARCB1/INI1, a core component of the SWI/SNF chromatin-remodeling complex, regulates gene expression by altering chromatin structure and plays a critical role in diverse biological processes including cell cycle regulation, dif
In PDAC, mutations or loss of SWI/SNF complex subunits such as ARID1A, SMARCA4 (BRG1), and SMARCA2 (BRM) are relatively common, whereas loss of SMARCB1 (INI1) remains uncommon[22,23]. However, in pancreatic UDC, the frequency of SMARCB1 loss is significantly higher than in conventional PDAC, suggesting that SMARCB1 deficiency may be closely associated with the tumor dedifferentiation process[8,24]. A previous study demonstrated that SMARCB1 loss significantly correlates with reduced expression of other SWI/SNF subunits, including ARID1A, SMARCC1, and SMARCC2, in PURC[24]. Pancreatic SMARCB-deficient UDC is an exceedingly rare entity. Its clinicopathological characteristics have primarily been described in limited case reports and small series[18]. Clinical presentation is typically non-specific, and diagnosis often occurs at an advanced stage, characterized by local invasion and/or distant metastasis[25,26]. For example, reported symptoms include abdominal pain and weight loss or jaundice[18,27]. Imaging studies usually reveal a pancreatic mass, but its morphology is variable and may mimic other pancreatic neoplasms such as a solid pseudopapillary neoplasm (SPN). This may sometimes be misdiagnosed as other types of pancreatic tumors, such as SPN[18,28].
Common presenting symptoms of pancreatic cancer include persistent upper abdominal or back pain, progressive weight loss, decreased appetite, and jaundice, although some patients are diagnosed incidentally during routine examinations. In this case, the patient’s initial presentation with SSR is exceptionally rare and represents a novel diagnostic challenge in SMARCB1-deficient rhabdoid pancreatic UDC. SSR is most commonly associated with hematologic ma
Studies investigating treatment outcomes in SMARCB1-deficient rhabdoid pancreatic UDC remain scarce, and those available lack sufficient statistical power to inform subtype-specific therapeutic strategies. This underscores an urgent need for robust evidence to inform optimal treatment strategies for this aggressive neoplasm. A systematic literature review of PubMed, EMBASE, MEDLINE, and Cochrane databases identified 55 reported cases of UDC with rhabdoid features (Table 1). Among the reported cases, treatment strategies and associated clinical outcomes were available for 51 patients. Most of the cases underwent surgery or biopsy alone[33-36], four received a combination of surgery and adju
| Ref. | Histology | Age (years)/sex | Site | Size (cm) | Treatment | Metastasis | Prognosis |
| Guillan[34], 1968 | Pleomorphic ADCA | 65/M | Head | 10 | Palliative | Yes | DOD 3 months |
| Pleomorphic ADCA | 59/M | Body/tail | 6 | Palliative | Yes | DOD 3 months | |
| Pleomorphic ADCA | 72/M | Body/tail | 8 | Palliative | Yes | DOD 4 months | |
| Pleomorphic ADCA | 75/M | Body/tail | 9 | Palliative | Yes | DOD 5 months | |
| Pleomorphic ADCA | 62/M | Body/tail | 15 | Palliative | Yes | DOD 3 months | |
| Alguacil-Garcia and Weiland[33], 1977 | Round cell anaplastic carcinoma | 78/M | Body | 30 | Biopsy | Lung, liver | DOD in few days |
| Round cell anaplastic carcinoma | 74/M | Tail | 15 | Surgery + chemotherapy | None | DOD 4 months | |
| Round cell anaplastic carcinoma | 51/F | Head | 5 | Surgery | Lymph node | DOD 1 months | |
| Round cell anaplastic carcinoma | 73/M | Diffuse | 11 | Biopsy | Lymph node, adrenals | DOD in few days | |
| Round cell anaplastic carcinoma | 30/M | Body | 12 | Autopsy | Lymph node, ileum, kidney, thyroid, lung | NS | |
| Tschang et al[39], 1977 | Pleomorphic carcinoma | 67/M | Tail | 3 | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months |
| Pleomorphic carcinoma | 49/M | Tail | NS | Biopsy | Lymph node, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 54/M | Body/tail | NS | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 65/M | Tail | NS | Autopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 72/M | Body | NS | Autopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 61/M | Body | NS | Biopsy | Lymph node, liver, peritoneum | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 72/M | Head/body | 10 | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 65/M | Body/tail | NS | Biopsy + chemotherapy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 69/M | Head | 10 | Biopsy + chemotherapy | Liver, peritoneum | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 65/M | Body/tail | NS | Biopsy + chemotherapy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 72/M | Head | 10 | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 53/M | Diffuse | 10 | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 81/F | Body/tail | 15 | Biopsy | Lymph node, liver, extra-abdominal | DOD median 3 months, mean 6 months | |
| Pleomorphic carcinoma | 79/F | Head | 10 | Biopsy | Lymph node | DOD median 3 months, mean 6 months | |
| Reyes et al[45], 1980 | Pleomorphic giant cell carcinoma | 59/M | Head | Mean 9 | Supportive | Lymph node, widespread | DOD 1 month |
| Pleomorphic giant cell carcinoma | 37/M | Tail | Mean 9 | Chemotherapy | Lymph node, widespread | DOD 13 months | |
| Pleomorphic giant cell carcinoma | 59/M | Head | Mean 9 | Supportive | Lymph node, widespread | DOD 1 month | |
| Pleomorphic giant cell carcinoma | 71/M | Head | Mean 9 | Palliative surgery | Lymph node, widespread | DOD 2 months | |
| Pleomorphic giant cell carcinoma | 70/M | Head | Mean 9 | Supportive | Lymph node, widespread | DOD 3 months | |
| Pleomorphic giant cell carcinoma | 77/M | Head | Mean 9 | Supportive | Lymph node, widespread | DOD 1 month | |
| Pleomorphic giant cell carcinoma | 64/M | Head | Mean 9 | Supportive | Lymph node, widespread | DOD 2 months | |
| Pleomorphic giant cell carcinoma | 62/M | Head | Mean 9 | Palliative surgery | Lymph node, widespread | DOD 1 month | |
| Pleomorphic giant cell carcinoma | 54/M | Body | Mean 9 | Supportive | Lymph node, widespread | DOD 4 months | |
| Nishihara et al[35], 1997 | Carcinoma with rhabdoid features | 52/F | NS | 10 | Surgery | Lymph node, liver | DOD 19 months |
| Al-Nafussi and O'Donnell[46], 1999 | Adeno carcinoma extensive rhabdoid | 77/F | NS | NS | No | Soft tissue, liver, lung, kidney, heart, adrenals | DOD initially |
| Kuroda et al[38], 2007 | Anaplastic carcinoma with rhabdoid features | 68/F | NS | 14 | Palliative | Regional, bronchial | DOD 2 months |
| Anaplastic carcinoma with rhabdoid features | 59/M | NS | 10 | Surgery + chemotherapy | Liver | DOD 2 months | |
| Chadha et al[47], 2004 | Anaplastic pancreatic carcinoma | 74/F | Tail | NS | Palliative | Lymph node, liver, adrenal, peritoneum | DOD 2 weeks |
| Cho et al[37], 2006 | Carcinoma mucinous rhabdoid features | 65/F | Tail | 11 | Surgery + RT | Omentum, mesentery, liver, lung | DOD 12 months |
| Jamali et al[36], 2007 | ADSCA rhabdoid | 75/M | NS | 3 | Surgery | Liver | DOD 6 months |
| Layfield and Bentz[40], 2008 | Pleomorphic giant cell carcinoma | 71/M | Head | NS | Surgery | Liver | Alive 2 months |
| Pleomorphic giant cell carcinoma | 81/F | Head | NS | Palliative | NS | DOD 3 months | |
| Pleomorphic giant cell carcinoma | 59/M | Head | NS | Surgery | Lymph node, liver | Alive 3 months | |
| Pleomorphic giant cell carcinoma | 81/M | Head | NS | Palliative | NS | NS | |
| Pleomorphic giant cell carcinoma | 64/M | Body | NS | Palliative | Lymph node | Alive 4 months | |
| Anaplastic carcinoma | 63/M | NS | 10 | Supportive | Synchronous, liver | DOD 11 days | |
| Agaimy et al[8], 2015 | Rhabdoid | 76/M | Head | 5 | Surgery | NS | DOD 1 month |
| Rhabdoid, angiosarcoma-like | 44/F | Head | 6 | Surgery | NS | NS | |
| Rhabdoid, pseudopapillary acantholytic gland-like spaces | 72/M | Head | 4 | Surgery | Liver, lymph node nodes | DOD postoperatively | |
| Rhabdoid, prominent neutrophils and focal glandular formation | 61/M | Tail | 5 | Surgery | Intra-abdominal, stomach | NS | |
| Sano et al[41], 2014 | Rhabdoid | 68/F | Body/tail | 10 | Palliative | Liver, kidneys, lungs, right adrenal gland, omentum, peritoneum | DOD in 2 weeks |
| Ohike et al[42], 2007 | Rhabdoid, solid/diffuse | 35/F | Head | 6 | Chemotherapy | Liver | DOD in 7 months |
| Tahara et al[43], 2018 | Rhabdoid, solid/diffuse | 67/F | Body | 1.9 | Chemotherapy | Liver, lung, bone, tongue, right thigh | DOD in 6 months |
| King et al[44], 2021 | Rhabdoid; monomorphic epithelioid | 59/F | Tail | 1.6 | Neoadjuvant FOLFIRINOX; distal pancreatectomy; adjuvant gemcitabine/paclitaxel | Liver | Disease-free 20 months |
| Our case | Rhabdoid | 59/F | Tail | 4.5 | Gemcitabine/nab-paclitaxel; bevacizumab/toripalimab | Spleen, kidney, retroperitoneum | Alive 6 months |
In this case, despite locally advanced disease at presentation, upfront resection combined with adjuvant gemcitabine and nab-paclitaxel has resulted in six months of disease-free survival to date, suggesting that aggressive multimodal management may confer benefit in selected patients. Given the absence of standardized treatment protocols, current management is largely extrapolated from PDAC guidelines. According to the National Comprehensive Cancer Network guidelines, R0 surgical resection remains the cornerstone for localized disease, while adjuvant chemotherapy - typically gemcitabine-based - is indicated to reduce the risk of recurrence[49]. For unresectable or metastatic disease, FOLFIRINOX or gemcitabine/nab-paclitaxel are preferred systemic therapies[49]. However, the distinct molecular biology of SMA
SMARCB1-deficient rhabdoid pancreatic UDC represents a rare and highly aggressive tumor subtype with limited published evidence to guide optimal management. Our case, together with previously reported cases, suggests that ag
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68558] [Article Influence: 13711.6] [Reference Citation Analysis (201)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (11)] |
| 3. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6048] [Article Influence: 3024.0] [Reference Citation Analysis (4)] |
| 4. | Ishihara S, Miyakawa S, Takada T, Takasaki K, Nimura Y, Tanaka M, Miyazaki M, Nagakawa T, Kayahara M, Horiguchi A. Status of surgical treatment of biliary tract cancer. Dig Surg. 2007;24:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2742] [Article Influence: 457.0] [Reference Citation Analysis (3)] |
| 6. | Çağlayan D, Karakurt Eryilmaz M, Korkmaz M, Karaağaç M, Hendem E, Artaç M. Is pancreatic giant cell tumor resistant to standard chemotherapy? Anticancer Drugs. 2022;33:758-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Gkountakos A, Mafficini A, Lou E, Malleo G, Salvia R, Calicchia M, Silvestris N, Racila E, Amin K, Veronese N, Brunetti O, Antonini P, Ingravallo G, Mattiolo P, Saponaro C, Nappo F, Simbolo M, Bariani E, Lonardi S, Fassan M, Milella M, Lawlor RT, Scarpa A, Luchini C. Genomic characterization of undifferentiated sarcomatoid carcinoma of the pancreas. Hum Pathol. 2022;128:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Agaimy A, Haller F, Frohnauer J, Schaefer IM, Ströbel P, Hartmann A, Stoehr R, Klöppel G. Pancreatic undifferentiated rhabdoid carcinoma: KRAS alterations and SMARCB1 expression status define two subtypes. Mod Pathol. 2015;28:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 9. | McIntyre CA, Grimont A, Park J, Meng Y, Sisso WJ, Seier K, Jang GH, Walch H, Aveson VG, Falvo DJ, Fall WB, Chan CW, Wenger A, Ecker BL, Pulvirenti A, Gelfer R, Zafra MP, Schultz N, Park W, O'Reilly EM, Houlihan SL, Alonso A, Hissong E, Church GM, Mason CE, Siolas D, Notta F, Gonen M, Dow LE, Jarnagin WR, Chandwani R. Distinct clinical outcomes and biological features of specific KRAS mutants in human pancreatic cancer. Cancer Cell. 2024;42:1614-1629.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Zhu Z, Chen X, Guo A, Manzano T, Walsh PJ, Wills KM, Halliburton R, Radko-Juettner S, Carter RD, Partridge JF, Green DR, Zhang J, Roberts CWM. Mitotic bookmarking by SWI/SNF subunits. Nature. 2023;618:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Versteege I, Sévenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1178] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 12. | Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol. 2020;17:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 496] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 13. | Yang H, Zhou L, Zhong G, Li X, Wang Y. SMARCB1 (INI-1)-Deficient Sinonasal Carcinoma: A Case Report and Literature Review. Ear Nose Throat J. 2025;104:NP154-NP158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Agaimy A, Weichert W. SMARCA4-deficient Sinonasal Carcinoma. Head Neck Pathol. 2017;11:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Tischkowitz M, Huang S, Banerjee S, Hague J, Hendricks WPD, Huntsman DG, Lang JD, Orlando KA, Oza AM, Pautier P, Ray-Coquard I, Trent JM, Witcher M, Witkowski L, McCluggage WG, Levine DA, Foulkes WD, Weissman BE. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clin Cancer Res. 2020;26:3908-3917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Zhou P, Fu Y, Tang Y, Jiang L, Wang W. Thoracic SMARCA4-deficient tumors: a clinicopathological analysis of 52 cases with SMARCA4-deficient non-small cell lung cancer and 20 cases with thoracic SMARCA4-deficient undifferentiated tumor. PeerJ. 2024;12:e16923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Zhong Z, Zhong Y, Li C, Li Y, Xu L, Fu S. SMARCB1-deficient poorly differentiated testicular carcinoma: a case report. Front Oncol. 2025;15:1554352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Hua Y, Soni P, Larsen D, Zreik R, Leng B, Rampisela D. SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma mimicking solid pseudopapillary neoplasm: A case report and review of the literature. World J Gastroenterol. 2020;26:5520-5526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Radko-Juettner S, Yue H, Myers JA, Carter RD, Robertson AN, Mittal P, Zhu Z, Hansen BS, Donovan KA, Hunkeler M, Rosikiewicz W, Wu Z, McReynolds MG, Roy Burman SS, Schmoker AM, Mageed N, Brown SA, Mobley RJ, Partridge JF, Stewart EA, Pruett-Miller SM, Nabet B, Peng J, Gray NS, Fischer ES, Roberts CWM. Targeting DCAF5 suppresses SMARCB1-mutant cancer by stabilizing SWI/SNF. Nature. 2024;628:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108:547-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Agaimy A, Daum O, Märkl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF Complex-deficient Undifferentiated/Rhabdoid Carcinomas of the Gastrointestinal Tract: A Series of 13 Cases Highlighting Mutually Exclusive Loss of SMARCA4 and SMARCA2 and Frequent Co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016;40:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Chen IY, Ettel MG, Bell PD, Huber AR, Findeis-Hosey JJ, Wang W, Hezel AF, Dunne RF, Drage MG, Agostini-Vulaj D. SWI/SNF chromatin remodeling complex in pancreatic ductal adenocarcinoma: Clinicopathologic and immunohistochemical study. Hum Pathol. 2024;144:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Tsuda M, Fukuda A, Kawai M, Araki O, Seno H. The role of the SWI/SNF chromatin remodeling complex in pancreatic ductal adenocarcinoma. Cancer Sci. 2021;112:490-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Yamamoto T, Kohashi K, Yamada Y, Kawata J, Sakihama K, Matsuda R, Koga Y, Aishima S, Nakamura M, Oda Y. Relationship between cellular morphology and abnormality of SWI/SNF complex subunits in pancreatic undifferentiated carcinoma. J Cancer Res Clin Oncol. 2022;148:2945-2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Mugaanyi J, Lu C, Huang J, Lu C. Undifferentiated Pancreatic Carcinomas, Clinical Features and Therapeutic Options: What We Know. Cancers (Basel). 2022;14:6102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Imaoka H, Ikeda M, Umemoto K, Sunakawa Y, Ueno M, Ueno H, Ozaka M, Kuwahara T, Okano N, Kanai M, Hisano T, Suzuki Y, Asagi A, Shioji K, Todaka A, Tsuji K, Ikezawa K, Miki I, Komatsu Y, Akutsu N, Yamashita T, Okuyama H, Furuse J, Nagano H. Comprehensive review of undifferentiated carcinoma of the pancreas: from epidemiology to treatment. Jpn J Clin Oncol. 2023;53:764-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Mugaanyi J, Lu C, Lu C, Wang C. Extended pancreato-duodenectomy coupled with adjuvant chemotherapy for SMARCB1/INI1 deficient pancreatic carcinoma: A case report and literature review. Int J Surg Case Rep. 2021;82:105938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | La Rosa S, Bongiovanni M. Pancreatic Solid Pseudopapillary Neoplasm: Key Pathologic and Genetic Features. Arch Pathol Lab Med. 2020;144:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Hajri A, Yaqine K, El Massi S, Errguibi D, Boufettal R, El Jai SR, Chehab F. Spontaneous splenic rupture: A rare complication of acute myeloid leukemia. Report of a case. Ann Med Surg (Lond). 2021;65:102286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Moya Sánchez E, Medina Benítez A. Atraumatic splenic rupture as a complication of acute exacerbation of chronic pancreatitis, an unusual disease. Rev Esp Enferm Dig. 2017;109:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Ando K, Ohkuni Y, Kaneko N, Narita M. Spontaneous rupture of the splenic metastasis in acinar cell carcinoma. Pancreas. 2009;38:836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Ekanayake LS, Jenkins D, Swade S, Patel HP, Bruce D. Locally Invasive Pancreatic Adenocarcinoma Presenting in the Splenic Flexure: A Rare Case Report. Cureus. 2020;12:e8085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Alguacil-Garcia A, Weiland LH. The histologic spectrum, prognosis, and histogenesis of the sarcomatoid carcinoma of the pancreas. Cancer. 1977;39:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Guillan RA. Pleomorphic adenocarcinoma of the pancreas. An analysis of five cases. Cancer. 1968;21:1072-1079. [PubMed] [DOI] [Full Text] |
| 35. | Nishihara K, Katsumoto F, Kurokawa Y, Toyoshima S, Takeda S, Abe R. Anaplastic carcinoma showing rhabdoid features combined with mucinous cystadenocarcinoma of the pancreas. Arch Pathol Lab Med. 1997;121:1104-1107. [PubMed] |
| 36. | Jamali M, Serra S, Chetty R. Adenosquamous carcinoma of the pancreas with clear cell and rhabdoid components. A case report. JOP. 2007;8:330-334. [PubMed] |
| 37. | Cho YM, Choi J, Lee OJ, Lee HI, Han DJ, Ro JY. SMARCB1/INI1 missense mutation in mucinous carcinoma with rhabdoid features. Pathol Int. 2006;56:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Kuroda N, Iwamura S, Fujishima N, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, Lee GH. Anaplastic carcinoma of the pancreas with rhabdoid features and hyaline globule-like structures. Med Mol Morphol. 2007;40:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Tschang TP, Garza-Garza R, Kissane JM. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer. 1977;39:2114-2126. [PubMed] [DOI] [Full Text] |
| 40. | Layfield LJ, Bentz J. Giant-cell containing neoplasms of the pancreas: an aspiration cytology study. Diagn Cytopathol. 2008;36:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Sano M, Homma T, Hayashi E, Noda H, Amano Y, Tsujimura R, Yamada T, Quattrochi B, Nemoto N. Clinicopathological characteristics of anaplastic carcinoma of the pancreas with rhabdoid features. Virchows Arch. 2014;465:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Ohike N, Sato M, Hisayuki T, Imataka H, Sato S, Wada Y, Saito K, Takahashi M, Tajiri T, Kunimura T, Morohoshi T. Immunohistochemical analysis of nestin and c-kit and their significance in pancreatic tumors. Pathol Int. 2007;57:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Tahara S, Takeyari M, Morii E. Usefulness of cytological analysis in the diagnosis of pancreatic undifferentiated rhabdoid carcinoma. Pathol Int. 2018;68:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | King DA, Rahalkar S, Bingham DB, Fisher GA. Pancreatic INI1-deficient undifferentiated rhabdoid carcinoma achieves complete clinical response on gemcitabine and nab-paclitaxel following immediate progression on FOLFIRINOX: a case report. J Gastrointest Oncol. 2021;12:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Reyes CV, Crain S, Wang T. Pleomorphic giant cell carcinoma of the pancreas: a review of nine cases. J Surg Oncol. 1980;15:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Al-Nafussi A, O'Donnell M. Poorly differentiated adenocarcinoma with extensive rhabdoid differentiation: clinicopathological features of two cases arising in the gastrointestinal tract. Pathol Int. 1999;49:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Chadha MK, LeVea C, Javle M, Kuvshinoff B, Vijaykumar R, Iyer R. Anaplastic pancreatic carcinoma. A case report and review of literature. JOP. 2004;5:512-515. [PubMed] |
| 48. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5897] [Article Influence: 393.1] [Reference Citation Analysis (23)] |
| 49. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 821] [Article Influence: 164.2] [Reference Citation Analysis (0)] |
| 50. | Sasaki M, Kato D, Murakami K, Yoshida H, Takase S, Otsubo T, Ogiwara H. Targeting dependency on a paralog pair of CBP/p300 against de-repression of KREMEN2 in SMARCB1-deficient cancers. Nat Commun. 2024;15:4770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 51. | Shen W, Pan Y, Zou S. Response to PD-1 inhibitor in SMARCB1‑deficient undifferentiated rectal carcinoma with low TMB, proficient MMR and BRAF V600E mutation: a case report and literature review. Diagn Pathol. 2024;19:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 52. | Xue Y, Zhu X, Meehan B, Venneti S, Martinez D, Morin G, Maïga RI, Chen H, Papadakis AI, Johnson RM, O'Sullivan MJ, Erdreich-Epstein A, Gotlieb WH, Park M, Judkins AR, Pelletier J, Foulkes WD, Rak J, Huang S. SMARCB1 loss induces druggable cyclin D1 deficiency via upregulation of MIR17HG in atypical teratoid rhabdoid tumors. J Pathol. 2020;252:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/