Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.112944
Revised: September 14, 2025
Accepted: November 19, 2025
Published online: January 15, 2026
Processing time: 147 Days and 22.7 Hours
Chemotherapy with an immune checkpoint inhibitor is one of the standard regi
To evaluate the safety and efficacy of nivolumab combined with chemotherapy in patients with AGC and ascites.
We retrospectively collected clinical data from 124 patients with AGC who re
Ascites was detected in 47 patients (38%); 26 (21%) were classified into the HAB group. Patients in the HAB group exhibited a significantly poorer performance status, a higher prevalence of diffuse-type histology, and lower programmed cell death ligand 1 (PD-L1) expression. Combination therapy with FOLFOX and neutropenia was significantly more common in the HAB group. Progression-free survival (PFS) (4.4 months vs 9.3 months, P = 0.0012) and overall survival (OS) (7.3 months vs 21.2 months, P < 0.0001) were significantly poorer in the HAB group. However, an improvement in ascites was observed in 61.5% of patients in the HAB group. PD-L1 ex
Nivolumab plus chemotherapy demonstrated modest efficacy and acceptable toxicity in patients with AGC and HAB.
Core Tip: The combination of chemotherapy and immune checkpoint inhibitors has become standard therapy for advanced gastric cancer (AGC). However, data on efficacy in patients with AGC and high ascites burden (HAB) remain limited. Chemotherapy combined with nivolumab has demonstrated modest efficacy in AGC patients with HAB (progression-free survival: 4.4 months; overall survival: 7.3 months). Chemotherapy with nivolumab improved ascites in 56% of patients. Furthermore, these results showed no correlation with programmed cell death ligand 1 (PD-L1) expression. These results suggest that nivolumab chemotherapy could be a therapeutic option for patients with AGC and HAB, regardless of PD-L1 expression.
- Citation: Matsumoto T, Sugimoto S, Omori R, Makiyama C, Nakasya A, Nagai H, Yasui H, Higashi R, Sasamoto A, Satake H. Efficacy and safety of nivolumab plus chemotherapy in patients with advanced gastric cancer with massive ascites. World J Gastrointest Oncol 2026; 18(1): 112944
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/112944.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.112944
Palliative chemotherapy is the standard of care for unresectable, recurrent, advanced gastric cancer (AGC). In recent years, the addition of immune checkpoint inhibitors to combination therapy with 5-fluorouracil (5-FU) and platinum-based agents has been reported to improve prognosis in several trials. In the CHECKMATE-649 trial of human epidermal growth factor receptor 2 (HER2)-negative AGC, chemotherapy combined with nivolumab significantly prolonged overall survival (OS) compared with chemotherapy alone (13.8 months vs 11.6 months; HR: 0.80; P < 0.001)[1]. In addition, pembrolizumab and tislelizumab show additive effects when combined with chemotherapy, making the combination of chemotherapy and immune checkpoint inhibitors the standard treatment for HER2-negative AGC[2,3].
Peritoneal dissemination is one of the most common forms of metastasis in gastric cancer and can lead to complications such as bowel obstruction and ascites. Massive ascites is observed in approximately 3%-10% of AGC cases, resulting in a poor prognosis[4,5]. Patients with AGC and massive ascites are often excluded from phase III trials. The JCOG1108/WJOG7312G phase II/III trial is the only phase III trial evaluating the efficacy of 5-FU/L-leucovorin (FL) plus paclitaxel (FLTAX) in patients with AGC and massive ascites or impaired oral intake due to peritoneal dissemination. However, the FLTAX regimen did not demonstrate superiority to FL regarding OS (7.3 months vs 6.1 months; P = 0.14)[4]. Similarly, WJOG10517G, a single-arm phase II study evaluating the efficacy of mFOLFOX6 (oxaliplatin plus leucovorin plus 5-FU) in the same AGC patient population, showed that OS was almost identical at 7.4 months[6,7]. However, there are currently no data on the efficacy and safety of combination therapy with chemotherapy and immune checkpoint inhibitors in patients with AGC and massive ascites.
Massive ascites can lead to a poor quality of life due to symptoms such as abdominal pain, abdominal fullness, and vomiting. From a clinical perspective, managing these complications with palliative care is crucial, and new approaches are needed. Therefore, this study aimed to retrospectively evaluate the safety and efficacy of nivolumab combined with chemotherapy in patients with AGC and ascites.
This retrospective study included consecutive patients with AGC who received chemotherapy plus nivolumab as first-line treatment at Ichinomiyanishi Hospital and Kobe City Medical Center General Hospital between July 2017 and December 2024. All data were retrospectively collected from the patients’ medical records. The inclusion criteria were as follows: (1) Unresectable or metastatic gastric or gastroesophageal junction cancer; (2) Histologically or cytologically confirmed adenocarcinoma; (3) Confirmed HER2-negative cancer; (4) Received nivolumab plus platinum doublet chemotherapy as the first-line treatment; and (5) Evaluable lesions (whether lesions are measurable is irrelevant). This study was approved by the Institutional Review Board of Kobe City Medical Center General Hospital (No. zn250106) and conformed to the guidelines of the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study and the opportunity to opt out. The data cutoff date was April 8, 2025.
The levels of programmed cell death ligand 1 (PD-L1) were measured using an immunohistochemistry PD-L1 antibody assay (Dako 28-8). The combined positive score (CPS) was calculated as the number of PD-L1-staining tumour and immune cells divided by the total number of viable tumour cells, multiplied by 100, with a maximum score of 100. Microsatellite instability was measured by multiplex PCR fragment analysis (MSI Test Kit, FALCO). Claudin (CLDN) 18 expression was assessed using the VENTANA CLDN18 (43-14A) RxDx assay, with positivity defined as 75% of tumour cells showing moderate to strong membranous staining.
Based on studies by Nakajima et al[4] and Masuishi et al[5], we classified ascites using computed tomography (CT) scans as follows: Massive, extending throughout the abdominal cavity; moderate, neither mild nor massive; mild, located only in the upper or lower abdominal cavity; and no ascites, ascites not detected. Cases with massive or moderate ascites were defined as having a high ascites burden (HAB), and cases with mild or no ascites were defined as having a low ascites burden (LAB). An improvement in ascites was defined as a decrease in one or more levels of ascites on the CT scan at the time of evaluation.
The Eastern Cooperative Oncology Group performance status (PS) was defined by clinical oncologists. Tumour response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Toxicity was assessed using the CTCAE version 5.1. Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using two-sample t-tests. In patients with at least one measurable lesion, the tumour response was retrospectively reassessed by the investigator according to the RECIST version 1.1. In cases without evaluable lesions, the treatment efficacy was defined as not being evaluated. OS was assessed from the start of chemotherapy plus nivolumab until death. Patients who were alive or had missing data at the data cutoff point were censored. Progression-free survival (PFS) was assessed from the date of initiation of chemotherapy plus nivolumab until disease progression was confirmed. Patients with no information regarding disease progression were treated as censored cases. OS and PFS were estimated using the Kaplan-Meier method. Statistical analyses were performed using JMP, version 17 (SAS Institute Inc., Cary, NC, United States).
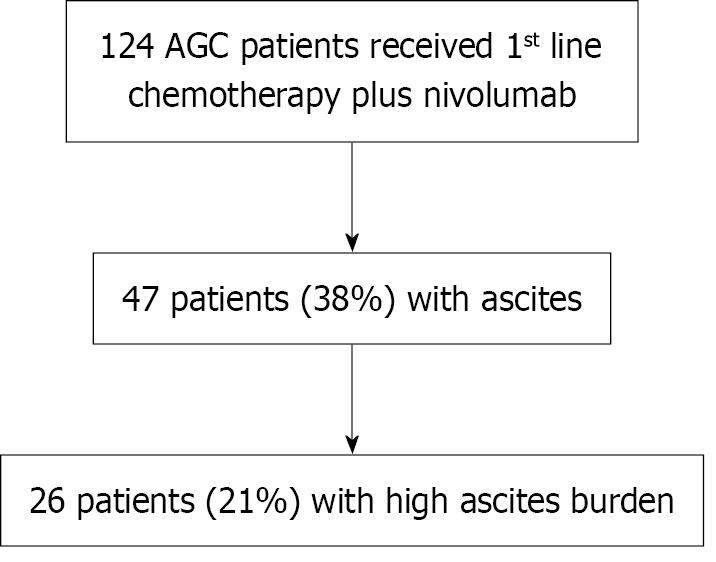
Among the 124 patients with AGC who received chemotherapy plus nivolumab as a first-line regimen, 98 (79%) had LAB and 26 (21%) had HAB (Figure 1). The patient characteristics are presented in Table 1. Poor PS, diffuse histology, and peritoneal dissemination were significantly more common in the HAB group than in the LAB group. PD-L1 expression was predominantly < 1% in the HAB group, whereas expression levels of ≥ 5% were more common in the LAB group. Regarding combination chemotherapy regimens, FOLFOX was administered more frequently in the HAB group, whereas SOX was administered more frequently in the LAB group.
| All (n = 124) | HAB (n = 26) | LAB (n = 98) | P value | ||
| Age (years) | Median (range) | 70 (23-87) | 71.5 (38-86) | 70 (23-87) | 0.9211 |
| Sex | Male | 82 (66) | 14 (54) | 68 (69) | 0.1641 |
| PS | 0 | 44 (35) | 1 (4) | 43 (44) | < 0.0001 |
| 1 | 60 (48) | 16 (62) | 44 (45) | ||
| 2 or 3 | 20 (16) | 9 (35) | 11 (11) | ||
| Location | Esophago-gastric junction | 20 (16) | 1 (4) | 19 (19) | 0.0716 |
| Gastric | 104 (84) | 25 (96) | 79 (81) | ||
| Gastrectomy | Yes | 34 (27) | 5 (19) | 29 (30) | 0.3345 |
| Pathology | Diffuse type | 75 (60) | 24 (92) | 51 (52) | 0.0009 |
| Number of metastatic organs | 2 or more | 62 (50) | 15 (58) | 47 (48) | 0.5086 |
| Liver metastasis | Yes | 38 (31) | 4 (15) | 34 (35) | 0.0921 |
| Peritoneum dissemination | Yes | 75 (60) | 25 (96) | 50 (51) | < 0.0001 |
| Ascites | Yes | 47 (38) | 26 (100) | 21 (21) | < 0.0001 |
| High ascites burden | Yes | 26 (21) | 26 (100) | 0 | < 0.0001 |
| Regimen | SOX | 67 (54) | 8 (31) | 59 (60) | 0.0037 |
| FOLFOX | 51 (41) | 18 (69) | 33 (34) | ||
| CAPOX | 6 (5) | 0 | 6 (6) | ||
| PD-L1 CPS | Less than 1 | 24 (19) | 10 (38) | 14 (14) | 0.0082 |
| 1 ≤ CPS < 5 | 36 (29) | 6 (23) | 29 (30) | ||
| 5 or more | 45 (36) | 5 (19) | 41 (42) | ||
| Unknown | 19 (15) | 5 (19) | 14 (14) | ||
| MSI | MSI-high | 5 (4) | 0 | 5 (5) | 0.3489 |
| MSS | 74 (60) | 18 (69) | 56 (57) | ||
| Unknown | 45 (36) | 8 (31) | 37 (38) | ||
| CLDN 18.2 | Positive | 14 (11) | 4 (15) | 10 (10) | 0.0767 |
| Negative | 29 (23) | 2 (8) | 27 (28) | ||
| Unknown | 81 (65) | 20 (77) | 61 (62) |
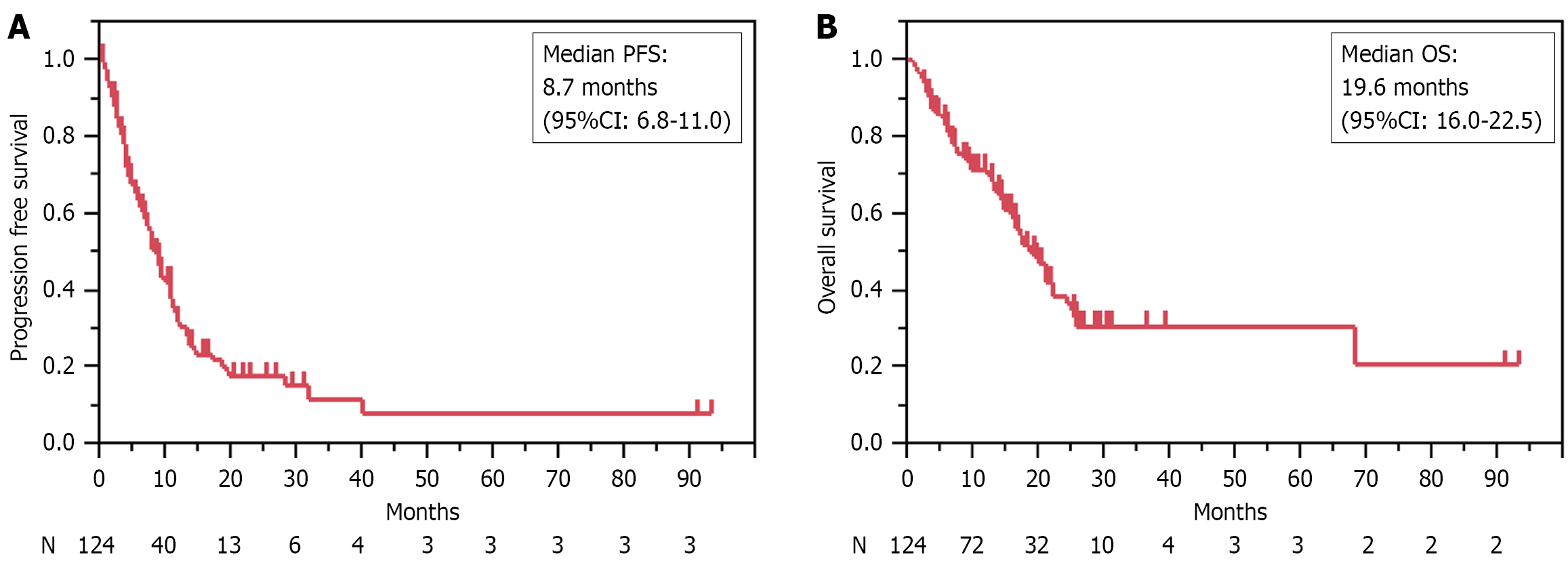
The median follow-up duration was 20.9 months (range: 3.5-94.3 months). The overall response rate (ORR) was 42.3% and the disease control rate (DCR) was 83.9%. The median PFS was 8.7 months (95%CI: 6.8-11.0), and the median OS was 19.6 months (95%CI: 16.0-22.5 months) (Table 2 and Figure 2).
| Total (n = 124) | HAB (n = 26) | LAB (n = 98) | P value | |
| Best overall response | ||||
| Complete response | 2 | 0 | 2 | |
| Partial response | 48 | 8 | 40 | |
| Stable disease | 49 | 10 | 39 | |
| Progressive disease | 19 | 6 | 13 | |
| Not evaluated | 6 | 2 | 4 | |
| Objective response rate (%) | 42.3 | 33.3 | 44.9 | 0.3615 |
| Disease control rate (%) | 83.9 | 75.0 | 86.2 | 0.2152 |
| Improvement of ascites (%) | 59.6 | 61.5 | 57.1 | 0.7745 |
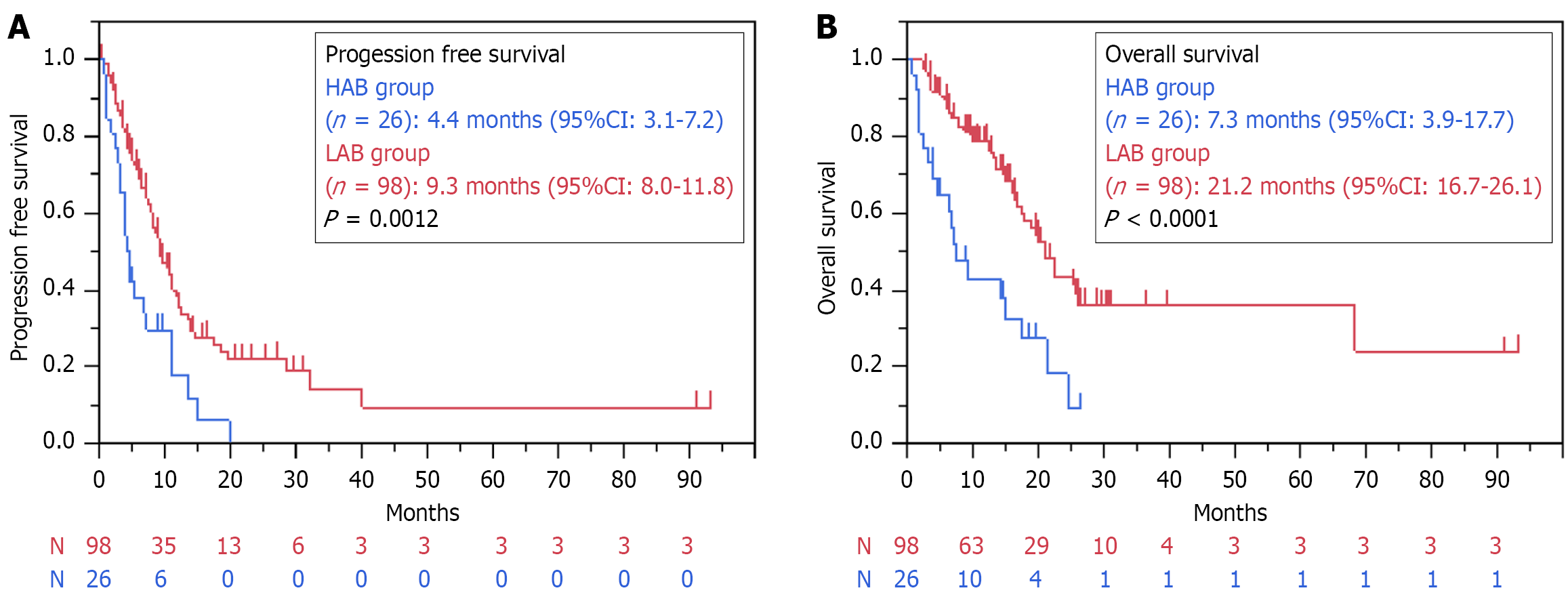
When comparing the HAB and LAB groups, the median PFS (4.4 months vs 9.3 months, P = 0.0012) and OS (7.3 months vs 21.2 months, P < 0.0001) were significantly lower in the HAB group (Figure 3). There were no significant differences in the ORR (33.3% vs 44.9 %, P = 0.3615) and DCR (75% vs 86.2%, P = 0.2152) between the groups. Ascites showed an overall improvement rate of 59.6%, with rates of 61.5% and 57.1% in the HAB and LAB groups, respectively (P = 0.7745).
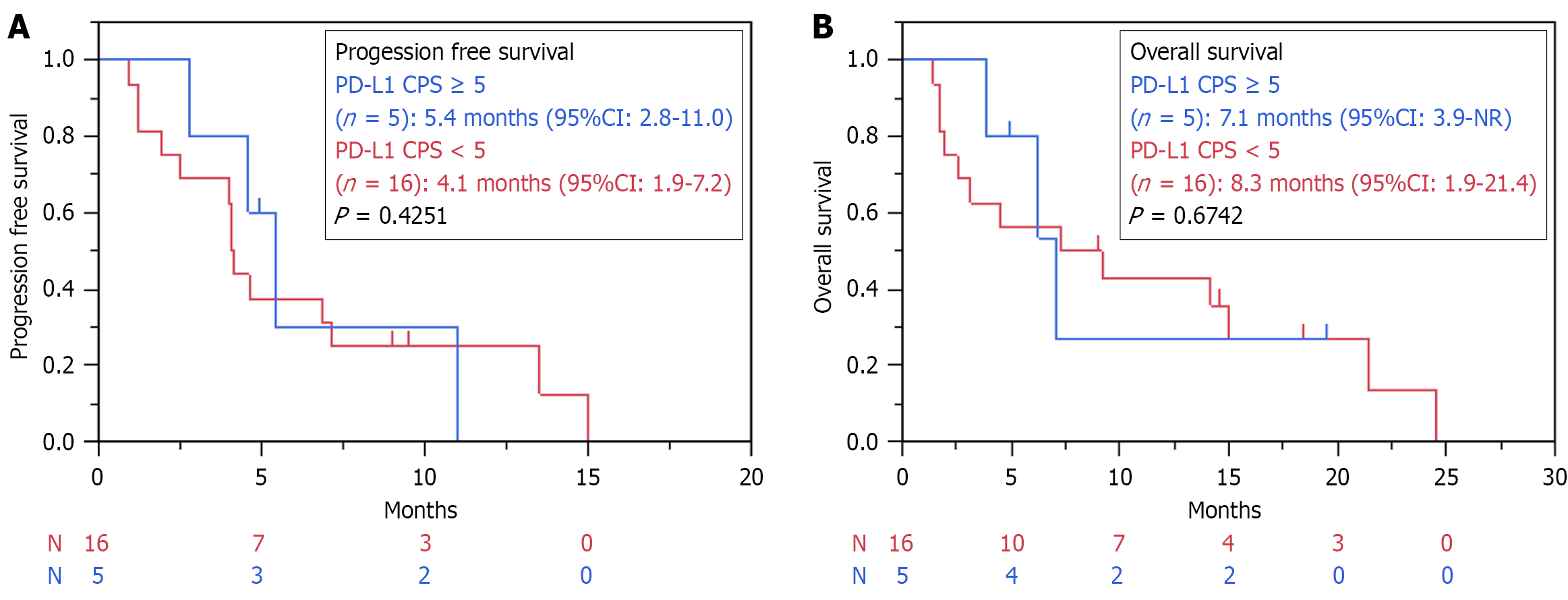
We performed an exploratory analysis to investigate the relationship between PD-L1 expression and the treatment response. The CPS for PD-L1 was measured in 105 patients. When comparing CPS ≥ 5 and CPS < 5, no statistically significant differences were observed in PFS (9.2 months vs 7.5 months, P = 0.3557) and OS (17.4 months vs 19.6 months, P = 0.9501) in the overall population (Supplementary Figure 1). Similarly, in the HAB group, no statistically significant differences in PFS (5.4 months vs 4.1 months, P = 0.4251) and OS (7.1 months vs 8.3 months, P = 0.6742) were observed between CPS ≥ 5 and CPS < 5 (Figure 4). The ascites improvement rate was 56% in patients with a PD-L1 CPS of < 5, and 60% in patients with a CPS of ≥ 5 (P = 1.000).
We performed univariate and multivariate analyses of PFS and OS, using the following variables: Age, PS, prior gastrec
The adverse events are listed in Tables 3 and 4. When comparing adverse events of all grades, peripheral neuropathy was significantly more common in the LAB group (23% vs 44%, P = 0.014). When comparing grade 3 or higher adverse events, neutropenia was significantly more common in the HAB group (46% vs 18%, P = 0.01). When comparing the immune-related adverse events between the two groups, skin reactions were significantly more common in the LAB group (0% vs 16%, P = 0.023), whereas no significant difference was observed in the incidence of grade 3 or higher immune-related adverse events. The overall transition rate to second-line treatment was 69%, with rates of 54% in the HAB group and 74% in the LAB group (P = 0.1255).
| All (n = 124) | HAB (n = 26) | LAB (n = 98) | P value | |||||
| All | Grade 3 or more | All | Grade 3 or more | All | Grade 3 or more | All | Grade 3 or more | |
| 111 (90) | 51 (41) | 23 (88) | 14 (54) | 88 (90) | 37 (38) | 1.000 | 0.179 | |
| Neutropenia | 44 (35) | 30 (24) | 12 (46) | 12 (46) | 32 (33) | 18 (18) | 0.258 | 0.010 |
| Anaemia | 18 (15) | 7 (6) | 4 (15) | 3 (12) | 14 (14) | 4 (4) | 0.546 | 0.159 |
| Low platelet | 17 (14) | 6 (5) | 3 (12) | 2 (8) | 14 (14) | 4 (4) | 1.000 | 0.281 |
| Nausea | 30 (24) | 2 (2) | 5 (19) | 0 | 25 (26) | 2 (2) | 0.794 | 1.000 |
| Fatigue | 42 (34) | 1 (1) | 6 (23) | 0 | 36 (37) | 1 (1) | 0.246 | 1.000 |
| Anorexia | 34 (27) | 5 (4) | 6 (23) | 1 (4) | 28 (29) | 4 (4) | 0.631 | 1.000 |
| Peripheral sensory neuropathy | 49 (40) | 1 (1) | 6 (23) | 0 | 43 (44) | 1 (1) | 0.014 | 1.000 |
| Stomatitis | 6 (5) | 0 | 0 | 0 | 6 (6) | 0 | 0.342 | - |
| Diarrhoea | 20 (16) | 3 (2) | 2 (8) | 1 (4) | 18 (18) | 2 (2) | 0.240 | 1.000 |
| Constipation | 11 (9) | 0 | 0 | 0 | 11 (11) | 0 | 0.118 | - |
| Oedema | 6 (5) | 0 | 0 | 0 | 6 (6) | 0 | 0.583 | - |
| Allergy | 9 (7) | 7 (6) | 1 (4) | 1 (4) | 8 (8) | 6 (6) | 0.201 | 0.342 |
| Dysgeusia | 10 (8) | 0 | 2 (8) | 0 | 8 (8) | 0 | 0.714 | - |
| Infection | 10 (8) | 4 (3) | 3 (12) | 2 (8) | 7 (7) | 2 (2) | 0.395 | 0.281 |
| Hand-foot syndrome | 5 (4) | 0 | 0 | 0 | 5 (5) | 0 | 0.583 | - |
| All (n = 124) | HAB (n = 26) | LAB (n = 98) | P value | |||||
| All | Grade 3 or more | All | Grade 3 or more | All | Grade 3 or more | All | Grade 3 or more | |
| Any adverse event | 45 (36) | 12 (10) | 6 (23) | 2 (8) | 39 (40) | 10 (10) | 0.168 | 1.000 |
| Skin reaction | 16 (13) | 0 | 0 | 0 | 16 (16) | 0 | 0.023 | - |
| Hypothyroidism | 7 (6) | 0 | 1 (4) | 0 | 6 (6) | 0 | 1.000 | - |
| Adrenal insufficiency | 5 (4) | 2 (2) | 0 | 0 | 5 (5) | 2 (2) | 0.583 | 1.000 |
| Hypopituitary function | 2 (2) | 0 | 0 | 0 | 2 (2) | 0 | 1.000 | - |
| Pneumonitis | 9 (7) | 3 (2) | 4 (15) | 1 (4) | 5 (5) | 2 (2) | 0.091 | 0.510 |
| Colitis | 6 (5) | 2 (2) | 0 | 0 | 6 (6) | 2 (2) | 0.342 | 0.579 |
| Hepatitis | 2 (2) | 2 (2) | 0 | 0 | 2 (2) | 2 (2) | 1.000 | 1.000 |
| Nephritis | 2 (2) | 1 (1) | 1 (4) | 0 | 1 (1) | 1 (1) | 0.377 | 1.000 |
| Myositis | 1 (1) | 1 (1) | 0 | 0 | 1 (1) | 1 (1) | 1.000 | 1.000 |
| Polymyalgia | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 | 1.000 | - |
| Arthritis | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 | 1.000 | - |
| Cholangitis | 1 (1) | 1 (1) | 1 (4) | 1 (4) | 0 | 0 | 1.000 | 1.000 |
To our knowledge, this is the first retrospective observational study to evaluate the efficacy and safety of chemotherapy combined with nivolumab in patients with AGC and massive ascites. A higher proportion of patients in the HAB group received FOLFOX than in the LAB group (69% vs 34%). This is probably because the efficacy and safety of FOLFOX have been reported more extensively in patients with AGC accompanied by ascites[4,5]. Although chemotherapy plus nivolu
PD-L1 expression is known to be a predictive biomarker for the efficacy of immune checkpoint inhibitors[1,2], and previous reports have suggested that chemotherapy in combination with nivolumab further prolonged OS in patients with PD-L1 CPS ≥ 5[1]. In our study, most patients in the HAB group had a PD-L1 CPS of < 5; however, no correlation between PD-L1 expression and OS was observed. In contrast, the addition of nivolumab to chemotherapy has been re
In the HAB group, diffuse-type histology was significantly more common than in the LAB group. Furthermore, an analysis of other biomarkers showed a trend towards a higher prevalence of increased CLDN18.2 expression in the HAB group. Several studies have reported that CLDN18.2 expression is higher in diffuse AGC than in intestinal AGC[8-14]. An integrated analysis of the SPOTLIGHT and GLOW trials in CLDN18.2-positive AGC suggested that zolubetuximab may show promising efficacy, even in the diffuse type[15]. These results suggest that treatment selection based on biomarkers other than PD-L1, such as CLDN18.2, may become increasingly important for improving OS in AGC with HAB.
Recently, several trials have investigated the efficacy of intraperitoneal chemotherapy for AGC with peritoneal dissemination. In the phase III PHOENIX-GC trial that compared intraperitoneal and intravenous paclitaxel plus S-1 with S-1 plus cisplatin, the benefit of intraperitoneal administration was not observed at the primary endpoint of OS. However, when adjusted for ascites, the HR for OS was 0.59 (95%CI: 0.39-0.87, P = 0.008), indicating significantly better outcomes in the intraperitoneal group[16]. In the phase III DRAGON-01 trial, which compared intraperitoneal and intravenous paclitaxel plus S-1 with S-1 plus paclitaxel alone, patients with AGC without distant metastases other than peritoneal dissemination were enrolled, with over 60% having ascites of 300 mL or more. At the primary OS endpoint, intraperitoneal administration significantly improved the OS (19.4 months vs 13.9 months, HR: 0.66, P = 0.0056)[17]. These results suggest that the combination of systemic chemotherapy and intraperitoneal administration may be beneficial in AGC with HAB, warranting further investigation.
This study has some limitations. First, it was a retrospective study with a relatively small sample size. The efficacy of chemotherapy combined with nivolumab for managing ascites is controversial, and prospective interventional studies are warranted. A further limitation is that, given the retrospective study design, we were unable to assess changes in quality of life. A multicentre phase II study of mFOLFOX6 plus nivolumab in gastric cancer with severe peritoneal metastases, WJOG16322G (Japan Registry of Clinical Trials 041220164.), is ongoing[18], and the results are eagerly awaited. Second, biomarker exploration in this trial was insufficient. Currently, treatment development targeting not only PD-L1 and microsatellite instability, but also CLDN18.2 and FGFR2, is progressing for gastric cancer. Future studies on AGC with HAB should include further biomarker analyses.
In conclusion, chemotherapy plus nivolumab showed a modest improvement in OS, tolerable safety, and improvement in ascites in patients with AGC with HAB. As this was a retrospective, small-scale study, future prospective studies are warranted to establish more effective treatments for AGC with HAB.
We gratefully thank the participants of this study and their families, as well as the study investigators, teams, and data managers (Mizuho Takahashi, Kie Takeda, and Ryo Ogura) for their contributions to the publication of this study.
| 1. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 2222] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 2. | Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, Rivera F, Alves GV, Garrido M, Shiu KK, Fernández MG, Li J, Lowery MA, Çil T, Cruz FM, Qin S, Luo S, Pan H, Wainberg ZA, Yin L, Bordia S, Bhagia P, Wyrwicz LS; KEYNOTE-859 investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 416] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 3. | Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, Zimina AV, Bai Y, Shi J, Lee KW, Wang J, Poddubskaya E, Pan H, Rha SY, Zhang R, Hirano H, Spigel D, Yamaguchi K, Chao Y, Wyrwicz L, Disel U, Cid RP, Fornaro L, Evesque L, Wang H, Xu Y, Li J, Sheng T, Yang S, Li L, Moehler M, Xu RH; RATIONALE-305 Investigators. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. 2024;385:e078876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 4. | Nakajima TE, Yamaguchi K, Boku N, Hyodo I, Mizusawa J, Hara H, Nishina T, Sakamoto T, Shitara K, Shinozaki K, Katayama H, Nakamura S, Muro K, Terashima M. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer. 2020;23:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Masuishi T, Kadowaki S, Kondo M, Komori A, Sugiyama K, Mitani S, Honda K, Narita Y, Taniguchi H, Ura T, Ando M, Mishima H, Muro K. FOLFOX as First-line Therapy for Gastric Cancer with Severe Peritoneal Metastasis. Anticancer Res. 2017;37:7037-7042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Masuishi T, Nakajima TE, Yamazaki K, Hironaka S, Kudo C, Yoshimura K, Muro K. WJOG10517G: a multicenter Phase II study of mFOLFOX6 in gastric cancer patients with severe peritoneal metastases. Future Oncol. 2020;16:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hara H, Masuishi T, Ando T, Kawakami T, Yamamoto Y, Sugimoto N, Shiraishi K, Esaki T, Negoro Y, Tsuzuki T, Sawai H, Nakamura M, Inagaki T, Shinohara Y, Kawakami H, Kawakami K, Katsuya H, Maeda O, Fujita Y, Yoshimura K, Nakajima T, Muro K. P-99 A multicenter phase II study of mFOLFOX6 in advanced gastric cancer patients with severe peritoneal metastases: WJOG10517G. Ann Oncol. 2022;33:S284-S285. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Wu LW, Jang SJ, Shapiro C, Fazlollahi L, Wang TC, Ryeom SW, Moy RH. Diffuse Gastric Cancer: A Comprehensive Review of Molecular Features and Emerging Therapeutics. Target Oncol. 2024;19:845-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Baek JH, Park DJ, Kim GY, Cheon J, Kang BW, Cha HJ, Kim JG. Clinical Implications of Claudin18.2 Expression in Patients With Gastric Cancer. Anticancer Res. 2019;39:6973-6979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49:870-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Coati I, Lotz G, Fanelli GN, Brignola S, Lanza C, Cappellesso R, Pellino A, Pucciarelli S, Spolverato G, Guzzardo V, Munari G, Zaninotto G, Scarpa M, Mastracci L, Farinati F, Realdon S, Pilati P, Lonardi S, Valeri N, Rugge M, Kiss A, Loupakis F, Fassan M. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer. 2019;121:257-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Shitara K, Van Cutsem E, Lordick F, Enzinger PC, Ilson DH, Shah MA, Xu R, Lonardi S, Yamaguchi K, Hung Y, Kukielka-Budny B, Bhattacharya PP, Matsangou M, Li R, Moran DM, Ranganath R, Pophale R, Ajani JA. Final overall survival results from phase 3 SPOTLIGHT study evaluating zolbetuximab + mFOLFOX6 as first-line (1L) treatment for patients (pts) with claudin 18 isoform 2 (CLDN18.2)+, HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma. J Clin Oncol. 2024;42:4036-4036. [DOI] [Full Text] |
| 13. | Nakayama I, Qi C, Chen Y, Nakamura Y, Shen L, Shitara K. Claudin 18.2 as a novel therapeutic target. Nat Rev Clin Oncol. 2024;21:354-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 14. | Shitara K, Xu RH, Ajani JA, Moran D, Guerrero A, Li R, Pavese J, Matsangou M, Bhattacharya P, Ueno Y, Wang X, Shah MA. Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer. 2024;27:1058-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 15. | Shitara K, Shah MA, Lordick F, Van Cutsem E, Ilson DH, Klempner SJ, Kang YK, Lonardi S, Hung YP, Yamaguchi K, Enzinger P, Nakajima T, Matsangou M, Cao Y, Li R, Moran D, Pophale R, Oh M, Ranganath R, Ajani JA, Xu RH. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N Engl J Med. 2024;391:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 16. | Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, Imamoto H, Kodera Y, Uenosono Y, Amagai K, Kadowaki S, Miwa H, Yamaguchi H, Yamaguchi T, Miyaji T, Kitayama J. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol. 2018;36:1922-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 17. | Yan C, Yang Z, Shi Z, Lu S, Shi M, Nie M, Chen J, Wu D, Mou Y, Xu Y, Wang Y, Liu X, Cao H, Gu J, Yu J, Liu K, Liu X, Zhang J, Yin K, Zhu Z. Intraperitoneal and intravenous paclitaxel plus S-1 versus intravenous paclitaxel plus S-1 in gastric cancer patients with peritoneal metastasis: Results from the multicenter, randomized, phase 3 DRAGON-01 trial. J Clin Oncol. 2025;43:327-327. [DOI] [Full Text] |
| 18. | Wakabayashi M, Masuishi T, Ogata T, Hanamura F, Furuta M, Yamamoto Y, Kawakami K, Hirano H, Kito Y, Izawa N, Takahashi N, Matsumoto T, Kawakami H, Ando T, Minashi K, Kudo C, Yoshimura K, Muro K. A multicenter Phase II study of mFOLFOX6 plus nivolumab for gastric cancer with severe peritoneal metastases: WJOG16322G. Future Oncol. 2025;21:2135-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/