Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.112630
Revised: August 30, 2025
Accepted: November 18, 2025
Published online: January 15, 2026
Processing time: 163 Days and 16.4 Hours
Experimental therapies targeting immune and stromal cells, such as mast cells, cancer-associated fibroblasts, dendritic cells, and tumor endothelial cells, in the treatment of gastrointestinal solid tumors pose new and complex surgical and medico-legal challenges. These innovative treatments require that informed consent not be limited to simple acceptance of the medical procedure, but instead reflect a true relational and cognitive process grounded in understanding, free choice, and the ability to revoke consent at any time. In particular, it is essential that the patient understands the experimental nature of the therapy, its deve
Core Tip: Experimental therapies targeting immune stromal cells in the treatment of gastrointestinal tumors pose surgical and medico-legal challenges. Because these treatments are innovative, the patient's informed consent must reflect a genuinely relational and cognitive process grounded in understanding and free choice. It is essential that the patient understand the experimental nature of the therapy, the potential benefits and risks, as well as the implications for their health and personal dignity. Furthermore, when a patient participates in a non-standardized treatment, they must be informed that the outcomes, whether positive or negative, are not yet known with certainty.
- Citation: Vescio F, Curcio S, Aquila I, Ammendola M, Tarallo AP. Right patient approach to experimental stromal cell therapies for gastrointestinal tumors. World J Gastrointest Oncol 2026; 18(1): 112630
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/112630.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.112630
The introduction of experimental therapies involving stromal immune cells such as mast cells, cancer-associated fi
In cases where stromal cell-based treatments are proposed, therapies that may induce complex immunomodulatory effects or trigger angiogenic pathways that are not yet fully understood, it is essential that patients are fully aware that they are undergoing a non-standardized, investigational treatment[7]. The possible outcomes, whether beneficial or harmful, remain uncertain. This reality demands a particularly careful approach to patient communication[8,9]. Explanations must be clear, scientifically accurate, and accessible, with particular attention to ensuring that the patient has truly understood the information presented[10].
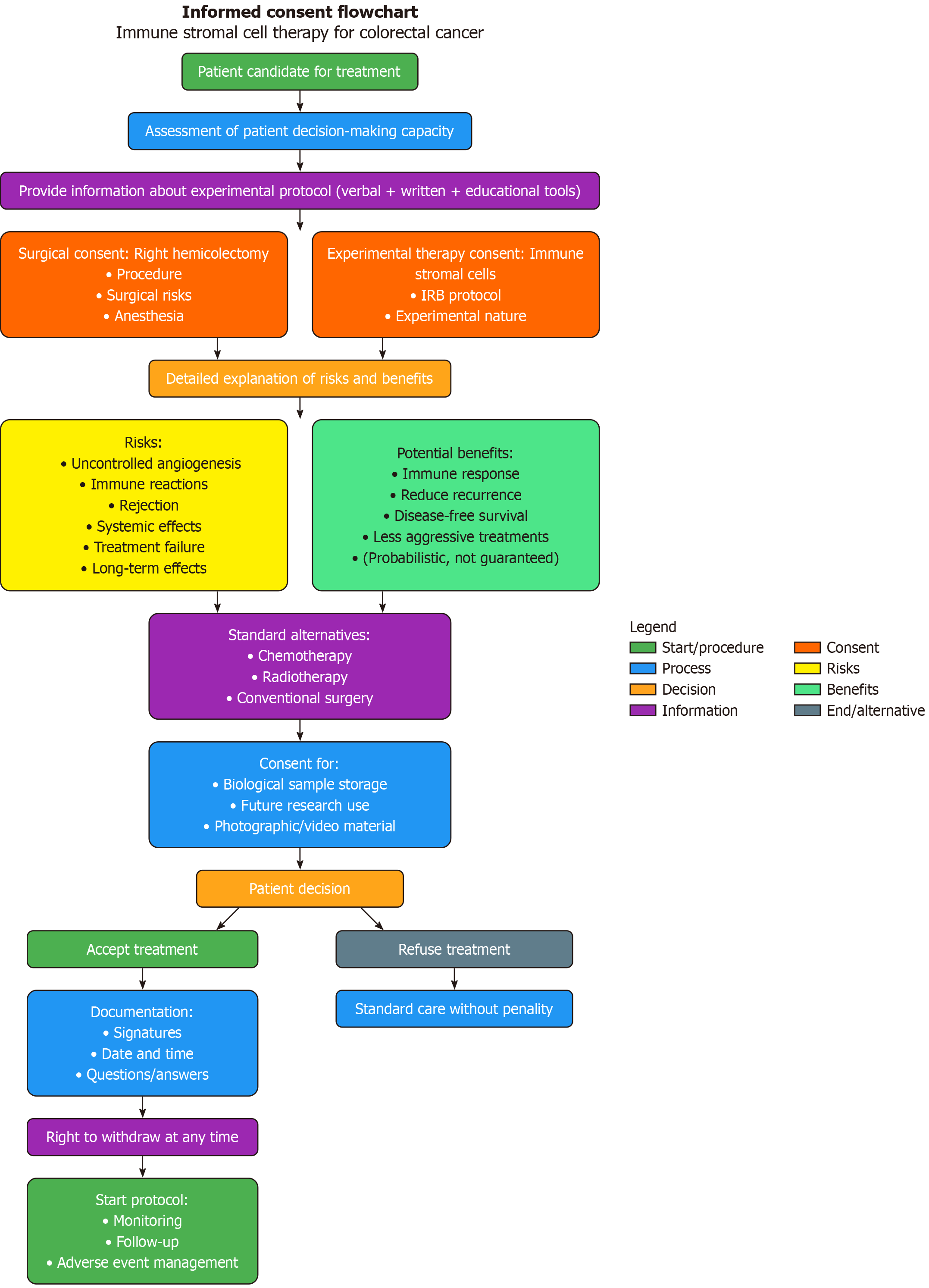
The informed consent form must contain clear and comprehensive information, including the following elements[11] (Figure 1).
Purpose of the experimental treatment: A clear explanation of the biological rationale for the protocol, such as the use of stromal cells to inhibit or modulate tumor growth through controlled immunological or pro-angiogenic mechanisms[12].
Procedure description: A detailed outline that distinguishes among the surgical phase, adjuvant therapies, and the experimental treatment itself, specifying what the patient will undergo at each stage[13].
Foreseeable risks: Including, but not limited to, unintended stimulation of tumor angiogenesis, abnormal inflammatory responses, immunologic rejection, unpredictable systemic effects, potential therapeutic failure, and unknown long-term consequences[14].
Potential benefits: Presented only in probabilistic terms, never as guaranteed outcomes. It is important to emphasize that the treatment is still under evaluation and not yet validated[15].
Available standard therapeutic alternatives: A clear description of conventional treatment options (e.g., chemotherapy, radiation therapy, conventional surgery), including their respective efficacy rates and associated risks[16].
Biological sample storage: Information on the possible future use of collected biological samples for diagnostic or research purposes, in accordance with privacy laws and bioethical standards[17].
Insurance coverage and patient protection: Details about compensation or coverage for any harm potentially caused by the experimental treatment, as provided by applicable clinical trial regulations[18].
Right to withdraw at any time: A clear statement that the patient may withdraw from the study at any moment, without affecting access to standard care and without any form of penalty or disadvantage[19].
In the setting of operable colorectal cancer, experimental therapies involving stromal cells may be integrated as adjuvant treatment (administered after surgical resection to prevent recurrence or metastasis) or as neoadjuvant treatment (ad
The consent form should include: (1) A modular structure with separate, clearly labeled sections for standard surgical procedures (e.g., right hemicolectomy), conventional oncologic treatments, and stromal cell-based experimental protocols[8,9,11,13]; (2) Explicit choice fields, such as “I consent / I do not consent” for each section, allowing the patient to provide selective agreement[19]; (3) A separate signature field for the authorization of biological sample use, specifying the purpose (diagnostic, therapeutic, or research)[4]; (4) A dedicated section documenting the explanations provided, as well as an invitation to ask further questions at any time, both before and during study participation[18]; (5) A statement clearly affirming that withdrawal of consent is an absolute right, which may be exercised at any moment without any negative impact on the continuity or quality of standard medical care[3]; and (6) An explicit mention of the approving IRB, including contact information for questions, concerns, or complaints regarding the study protocol[17].
In conclusion, the introduction of experimental stromal cell therapies demands renewed attention to the quality of the informed consent process[21]. It is essential that patients receive personalized, thorough, and up-to-date information not limited to paper-based documentation, but supplemented by an individualized clinical discussion. This in
Informed consent must be seen as a dynamic process, developed over time, and not merely a formal or bureaucratic act[18,19]. From a medico-legal perspective, the adequacy of informed consent is not only a core requirement for the le
The integration of experimental cell-based therapies into the treatment of radically operated colorectal cancer must be grounded in a fully informed, properly documented, and ethically sound consent, in strict adherence to the principles of patient autonomy, proportionality, prudence, and justice. Such an approach bridges clinical care, scientific research, and the protection of individual dignity.
| 1. | Liu X, Li X, Wei H, Liu Y, Li N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front Immunol. 2023;14:1209056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Li C, Lu Y, Liu C, Yang W. Tumor microenvironment-mediated immune tolerance in development and treatment of gastric cancer. Front Immunol. 2022;13:1016817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 3. | European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) (Text with EEA relevance). 2016: 1-88. Available from: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng. |
| 4. | Presidenza del Consiglio dei Ministri. Decreto Legislativo 30 giugno 2003, n. 196. Codice in materia di protezione dei dati personali. 2003. Available from: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:decreto.legislativo:2003-06-30;196. |
| 5. | Monaco GP, Smith G. Informed consent in complementary and alternative medicine: current status and future needs. Semin Oncol. 2002;29:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | PharmaState Academy. EudraLex Volume 4 - Good Manufacturing Practice Guidelines. Part IV: GMP requirements for Advanced Therapy Medicinal Products. 2017. Available from: https://member.pharmastate.academy/good-manufacturing-practice-guidelines/. |
| 7. | Qiao T, Ding C, Yu S, Li W, Zhao Y, Wang G. Diversity of mast cell subpopulations in the tumor microenvironment of colorectal cancer and their prognostic implications. Cancer Immunol Immunother. 2025;74:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1074] [Article Influence: 214.8] [Reference Citation Analysis (16)] |
| 9. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 931] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 10. | Moll FH, Krischel M. [The genesis of informed consent in the context of medical research ethics 1900-1931]. Urologie. 2023;62:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | European Medicines Agency. Guideline on human cell-based medicinal products. EMEA/CHMP/410869/2006. 2008. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-human-cell-based-medicinal-products_en.pdf#:~:text=This%20guideline%20replaces%20the%20Points%20to%20Consider%20on,heterogeneity%20of%20human%20cell-based%20products%2C%20including%20combination%20products. |
| 12. | Zhang F, Ma Y, Li D, Wei J, Chen K, Zhang E, Liu G, Chu X, Liu X, Liu W, Tian X, Yang Y. Cancer associated fibroblasts and metabolic reprogramming: unraveling the intricate crosstalk in tumor evolution. J Hematol Oncol. 2024;17:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 13. | Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Komi DEA, Redegeld FA. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin Rev Allergy Immunol. 2020;58:313-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 15. | Tang J, Chen Y, Wang C, Xia Y, Yu T, Tang M, Meng K, Yin L, Yang Y, Shen L, Xing H, Mao X. The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics. MedComm (2020). 2024;5:e663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, Boutros M, McClane J, Feldman LS, Steele SR. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017;60:761-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | European Union. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC Text with EEA relevance. 2014: 1-76. Available from: https://eur-lex.europa.eu/eli/reg/2014/536/oj/eng. |
| 18. | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH harmonised guideline integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2). 2016. Available from: https://ichgcp.net/. |
| 19. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 20185] [Article Influence: 1552.7] [Reference Citation Analysis (9)] |
| 20. | Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 628] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 21. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1601] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/