Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.109949
Revised: June 23, 2025
Accepted: August 6, 2025
Published online: September 15, 2025
Processing time: 112 Days and 6.8 Hours
Clinical trial evidence points to chemotherapy’s potential in augmenting the effects of immunotherapy.
To assess the effectiveness of first-line chemoimmunotherapy (CIT) for microsatellite stable (MSS) metastatic colorectal cancer (mCRC) verses standard-of-care (SOC; 5-fluorouracil/leucovorin/oxaliplatin/bevacizumab).
This was a multicenter retrospective cohort study conducted in Peking University First Hospital and Jilin Cancer Hospital. Patients with MSS mCRC who had received first-line treatment were eligible. The Kaplan-Meier method and Cox proportional hazard model were used to evaluate progression-free survival (PFS) and to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). PFS was set as the primary endpoint. Propensity score (PS) was calculated to balance the baseline characteristics of the two cohorts. With PS, we performed three statistical methods, namely inverse probability weighting, PS matching, and additional adjustment for PS with multivariate cox regression.
Between July 2019 and November 2024, 148 eligible patients were enrolled, with 40 and 108 patients assigned to the CIT and SOC cohorts, respectively. At a global median follow-up of 21.4 months, the crude median PFS was 13.5 months (95%CI: 9.77-21.6) in the CIT cohort vs 9.1 months (95%CI: 7.8-10.6) in the SOC cohort, yielding a nonsignificant hazard ratio (HR) of 0.5645 (95%CI: 0.3637-0.8763, P = 0.01; SOC as reference). Multivariate Cox regression analysis, adjusted for sex, age > 60 years, Eastern Cooperative Oncology Group performance status, rat sarcoma mutation, primary tumor location (left vs right) and number of metastatic organs (liver/lung), demonstrated an adjusted HR of 0.55 (95%CI: 0.35-0.87, P = 0.011). PS-based analyses using PS matching (post-matching n = 40 vs 40), PS-adjusted multivariate Cox regression, and inverse probability weighting revealed consistent significant trends favoring CIT, with HRs for CIT of 0.5641 (95%CI: 0.3303-0.9635, P = 0.0361), 0.60 (95%CI: 0.38-0.96, P = 0.034), and 0.57 (95%CI: 0.337-0.973, P = 0.039), respectively.
Efficacy of CIT in MSS mCRC could surpass that of standard first-line chemotherapy. Further research is needed to investigate specific clinical characteristics or biomarkers to identify patients who may derive benefit from CIT.
Core Tip: This study evaluated the effectiveness of chemoimmunotherapy (CIT) as the first-line regimen for microsatellite stable metastatic colorectal cancer vs standard-of-care (5-fluorouracil/leucovorin/oxaliplatin/bevacizumab) in the real world. The efficacy of CIT in microsatellite stable metastatic colorectal cancer could surpass that of standard first-line chemotherapy protocols. Further research is needed to investigate specific clinical characteristics or biomarkers to identify patients who may derive benefit from CIT.
- Citation: Gao Z, Zhou ZF, Wang XY, Song T, Wu SK, Jin X. Chemotherapy plus bevacizumab vs chemoimmunotherapy for metastatic colorectal cancer: Real-world analysis. World J Gastrointest Oncol 2025; 17(9): 109949
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/109949.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.109949
Colorectal cancer (CRC) ranks as the second deadliest malignancy worldwide[1] and carries a growing global socioeconomic burden[2]. Improving outcomes for patients with locally advanced rectal cancer and metastatic CRC (mCRC) remains a significant clinical challenge. mCRC patients face poor prognosis with 5-year survival rates < 15%[3]. Clinically, immune checkpoint inhibitors (ICIs) demonstrate remarkable efficacy in mCRC with high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR). These tumors typically exhibit high tumor mutation burden, creating an inflammatory tumor microenvironment rich in tumor-infiltrating lymphocytes, particularly memory cells and cytotoxic T lymphocytes[4]. However, microsatellite stable (MSS) CRC patients derive minimal benefit from ICI monotherapy. The KEYNOTE-016 study reported objective response rates of 40% (4/10) and progression-free survival (PFS) of 78% (7/9) in dMMR CRC patients, compared to 0% (0/18) and 11% (2/18) respectively in MSS patients[5]. Similarly, KEYNOTE-028 found no clinical benefit in 22 of 23 programmed death-ligand 1 (PD-L1)-positive (PD-L1 ≥ 1%) CRC patients with MSS status[6]. Consequently, MSS/proficient mismatch repair (pMMR) CRC is considered an “immunologically cold” tumor, presenting major challenges for immunotherapy in advanced disease. Transforming these “cold” tumors into “hot” tumors through microenvironment modulation represents a crucial therapeutic goal[7]. Emerging evidence suggests some MSS/pMMR CRC patients may benefit from combination immunotherapy approaches. Various tumor-intrinsic microenvironmental factors contribute to ICI resistance in MSS/pMMR CRC, making immunosuppression modulation a primary target for combination strategies[7]. Current combination approaches include immunotherapy with chemotherapy, radiotherapy, targeted therapy, or dual immunotherapy. Chemoimmunotherapy (CIT) remains the most clinically utilized strategy, as chemotherapy can induce immunogenic cell death and counteract immune evasion, thereby potentiating immunotherapy effects[8]. Specifically, tumor cell destruction releases tumor antigens that activate host immune responses. ICI-chemotherapy combinations have become first-line standard for many solid tumors, with synergistic mechanisms including enhanced immunogenicity, reduced immunosuppression, increased immune cell infiltration, and immunological memory formation.
Several clinical trials are investigating CIT for MSS/pMMR CRC, although current results remain modest. The LEAP-017 trial evaluating lenvatinib plus pembrolizumab in pretreated non-MSI-H/dMMR mCRC failed to meet its overall survival (OS) primary endpoint[9]. The phase 2 CheckMate 9X8 study comparing nivolumab plus modified 5-fluorouracil/Leucovorin/oxaliplatin/bevacizumab with standard care showed potential benefit specifically in RAS mutant/MSS (CMS3 subtype) mCRC patients[10]. The BBCAPX study demonstrated enhanced response rates with sintilimab plus capecitabine and oxaliplatin (CapeOx)/bevacizumab in MSS mCRC patients with acceptable toxicity[11]. The METIMMOX phase 2 trial comparing fludarabine/oxaliplatin plus nivolumab with fludarabine/oxaliplatin alone in MSS mCRC reported median PFS of 6.6 vs 5.6 months[12]. While CIT offers promise for MSS/pMMR CRC patients, further validation of safety/efficacy profiles and optimization of personalized treatment strategies remain imperative.
Based on the aforementioned research, the synergistic combination of chemotherapy and anti-programmed death 1 (PD-1) immunotherapy should represent a critical research direction to transform current MSS mCRC treatment paradigms. To investigate the real-world performance of CIT as first-line treatment for MSS mCRC, we have commenced a multicenter retrospective cohort study assessing its safety and efficacy.
This study used a multicenter retrospective cohort research design. Patients with advanced CRC who were treated at Peking University First Hospital and Jilin Cancer Hospital between July 2019 and November 2024 were enrolled. The experimental group consisted of patients who received first-line treatment with chemotherapy combined with anti-PD-1 immunotherapy (CIT cohort), while the control group comprised patients who received conventional treatment (chemotherapy combined with bevacizumab) [standard-of-care therapy (SOC) cohort].
The inclusion criteria were: (1) Histologically or cytologically confirmed unresectable mCRC with measurable lesions based on the Response Evaluation Criteria in Solid Tumors; (2) pMMR or MSS, B-Raf proto-oncogene wild-type; (3) Patients receiving chemotherapy combined with anti-PD-1 immunotherapy, with or without bevacizumab, and those receiving chemotherapy combined with bevacizumab alone; (4) Having not undergone radiotherapy or completed radiotherapy > 4 weeks ago; and (5) Eastern Cooperative Oncology Group score ≤ 2.
The exclusion criteria were: (1) Patients with dMMR or MSI-H, or B-Raf proto-oncogene mutations; (2) Symptomatic brain metastases; (3) Uncontrolled active infection; (4) Dysphagia, intractable vomiting, or known drug absorption disorders; and (5) Patients with symptomatic or high-risk obstruction, bleeding, or perforation, or those who have undergone intestinal stent placement to relieve intestinal obstruction.
In most cases, the chemotherapy regimen comprised an oxaliplatin-based doublet (folinic acid, 5-fluorouracil, oxaliplatin, or CAPEOX) or a topoisomerase inhibitor (folinic acid, 5-fluorouracil, or irinotecan). Anti-PD-1 immunotherapy included penpulimab, pembrolizumab, sintilimab, tislelizumab, and toripalimab. The antiangiogenic agent was bevacizumab.
This study was conducted in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Peking University First Hospital and Jilin Cancer Hospital. The requirement for patient approval or informed consent was waived by the Human Ethics Committee of Peking University First Hospital and Jilin Cancer Hospital, owing to the retrospective nature of the study and the use of anonymous clinical data for analysis.
The study follow-up concluded in May 2025. The primary endpoint, PFS, was measured from enrollment to first disease progression or death. OS was calculated from enrollment to death, with censoring at last follow-up. The neutrophil-to-lymphocyte ratio (NLR) was determined by dividing the absolute neutrophil count by the absolute lymphocyte count (ALC). The lymphocyte-to-monocyte ratio was obtained by taking the quotient of ALC and the absolute monocyte count. Platelet-to-lymphocyte ratio (PLR) was calculated by dividing the absolute platelet count (PLT) by ALC. The systemic immune-inflammation index (SII), was calculated using the formula: PLT × absolute neutrophil count/ALC. Complete blood counts were collected at two time points: Baseline (within 14 days before initial anti-PD-1 therapy) and at 6 weeks post-treatment. The change in NLR (ΔNLR) was defined as the difference between the 6-week post-treatment value and the baseline value.
To minimize confounding bias, propensity score matching (PSM) was performed with a 1:1 ratio via the nearest-neighbor algorithm (MatchIt package[13]). Survival outcomes were estimated by Kaplan-Meier curves, while Cox regression models identified independent prognostic factors with corresponding hazard ratios (HRs) and 95% confidence intervals (CIs). All analyses were conducted using R version 4.4.2.
We conducted three sensitivity analyses to control for bias: Initial Schoenfeld residual testing (survival package[14]) confirmed proportional hazards assumption compliance, followed by appropriate univariate and multivariate regression modeling. Second, PSM analysis with varying matching ratios was applied to PFS and OS. Third, inverse probability of treatment weighting (IPTW) was used to adjust for baseline characteristics and evaluate treatment outcomes.
From July 2019 and November 2024, 148 eligible patients were enrolled. According to the treatment received, 40 and 108 patients were respectively allocated to the CIT cohort and SOC cohort. The study population included 91 males (61.5%) and 57 females (38.5%), with 72 patients (48.6%) aged > 60 years. Right-sided colon cancer accounted for 33.8% (n = 50), 81.6% (n = 120) had undergone primary tumor resection, and 39.9% (n = 59) presented with multiorgan metastases. All cases were pMMR, while RAS mutations were identified in 19% (n = 28) (Table 1).
| Characteristics | Levels | n (%) |
| Age (year) | ≤ 60 | 76 (51.4) |
| > 60 | 72 (48.6) | |
| Gender | Male | 91 (61.5) |
| Female | 57 (38.5) | |
| ECOG score | 0-1 | 136 (91.9) |
| 2 | 12 (8.1) | |
| Location | Right colon | 50 (33.8) |
| Left colon and rectum | 98 (66.2) | |
| Surgery | No | 27 (18.4) |
| Yes | 120 (81.6) | |
| No. of metastases | 1 | 89 (60.1) |
| ≥ 2 | 59 (39.9) | |
| Liver metastasis | No | 64 (43.2) |
| Yes | 84 (56.8) | |
| Lung metastasis | No | 106 (71.6) |
| Yes | 42 (28.4) | |
| RAS | Wild type | 22 (14.9) |
| KRAS mutation | 26 (17.6) | |
| NRAS mutation | 2 (1.4) | |
| Unknown | 98 (66.2) | |
| Group | SOC | 108 (73.0) |
| Chemoimmunotherapy | 40 (27.0) |
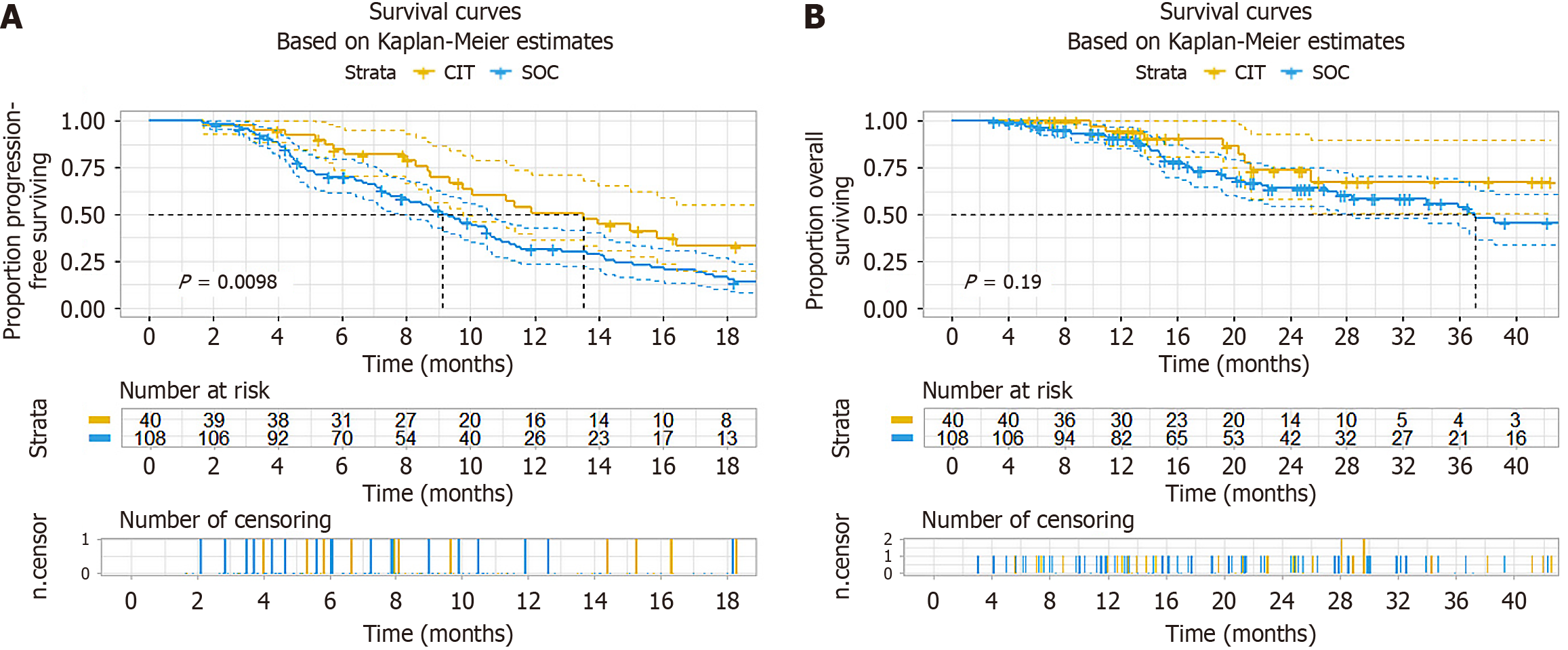
With a median follow-up of 21.4 months (as of May 2025), the overall cohort demonstrated a median PFS of 10 months. The crude PFS was 13.5 (95%CI: 9.77-21.6) months in CI cohort vs 9.13 (7.8-10.6) months in SOC cohort. The PFS improvement achieved significance (HR = 0.56, 95%CI: 0.36-0.88, P = 0.011) (Figure 1A). However, no significant OS benefit was seen (HR = 0.61, 95%CI: 0.28-1.30, P = 0.197) (Figure 1B). Multivariate Cox regression analysis, adjusted for sex, age > 60 years, Eastern Cooperative Oncology Group performance status, RAS mutation, primary tumor location (left vs right) and number of metastatic organs (liver/Lung), demonstrated an adjusted HR of 0.55 (95%CI: 0.35-0.87, P = 0.011) in PFS (Table 2) and HR of 0.7 (95%CI: 0.32-1.52, P = 0.367) in OS (Table 3).
| Dependent: Survival (PFS/30, status PFS) | All | HR (univariable) | HR (multivariable) | |
| Gender | Male | 90 (61.2) | - | - |
| Female | 57 (38.8) | 0.83 (0.57-1.21, P = 0.335) | - | |
| Age (year) | ≤ 60 | 75 (51.0) | - | - |
| > 60 | 72 (49.0) | 1.02 (0.71-1.48, P = 0.915) | - | |
| ECOG score | 0-1 | 135 (91.8) | - | - |
| 2 | 12 (8.2) | 1.68 (0.88-3.23, P = 0.117) | - | |
| Primary tumor location | Right colon | 49 (33.3) | - | - |
| Left colon and rectum | 98 (66.7) | 1.11 (0.76-1.64, P = 0.587) | - | |
| No. of metastatic organs | 1 | 88 (59.9) | - | - |
| ≥ 2 | 59 (40.1) | 1.39 (0.96-2.02, P = 0.082) | 1.13 (0.76-1.67, P = 0.550) | |
| Liver metastasis | No | 64 (43.5) | - | - |
| Yes | 83 (56.5) | 1.33 (0.92-1.94, P = 0.133) | - | |
| Lung metastasis | No | 105 (71.4) | - | - |
| Yes | 42 (28.6) | 1.03 (0.68-1.56, P = 0.891) | - | |
| RAS | Wild type | 22 (15.0) | - | - |
| Mutation | 28 (19.0) | 1.86 (0.98-3.53, P = 0.057) | 1.95 (1.01-3.75, P = 0.045) | |
| Unknown | 97 (66.0) | 1.48 (0.87-2.53, P = 0.152) | 1.65 (0.95-2.86, P = 0.076) | |
| Group | Control group | 107 (72.8) | - | - |
| Experimental group | 40 (27.2) | 0.56 (0.36-0.88, P = 0.011) | 0.55 (0.35-0.87, P = 0.011) | |
| Dependent: Survival (OS/30, status OS) | All, n (%) | HR (univariable) | HR (multivariable) | |
| Gender | Male | 90 (61.2) | - | - |
| Female | 57 (38.8) | 0.74 (0.41-1.34, P = 0.316) | 0.94 (0.51-1.74, P = 0.848) | |
| Age (year) | ≤ 60 | 75 (51.0) | - | - |
| > 60 | 72 (49.0) | 1.25 (0.71-2.19, P = 0.434) | 0.98 (0.55-1.74, P = 0.942) | |
| ECOG score | 0-1 | 135 (91.8) | - | - |
| 2 | 12 (8.2) | 1.03 (0.37-2.87, P = 0.957) | - | |
| Primary tumor location | Right colon | 49 (33.3) | - | - |
| Left colon and rectum | 98 (66.7) | 1.00 (0.56-1.78, P = 0.992) | - | |
| No. of metastatic organs | 1 | 88 (59.9) | - | - |
| ≥ 2 | 59 (40.1) | 1.49 (0.85-2.60, P = 0.164) | - | |
| Liver metastasis | No | 64 (43.5) | - | - |
| Yes | 83 (56.5) | 3.55 (1.88-6.69, P < 0.001) | 3.48 (1.80-6.72, P < 0.001) | |
| Lung metastasis | No | 105 (71.4) | - | - |
| Yes | 42 (28.6) | 0.73 (0.38-1.39, P = 0.335) | - | |
| RAS | Wild type | 22 (15.0) | - | - |
| Mutation | 28 (19.0) | 0.92 (0.41-2.07, P = 0.842) | - | |
| Unknown | 97 (66.0) | 0.63 (0.31-1.27, P = 0.195) | - | |
| Group | Control group | 107 (72.8) | - | - |
| Experimental group | 40 (27.2) | 0.61 (0.28-1.30, P = 0.197) | 0.70 (0.32-1.52, P = 0.367) | |
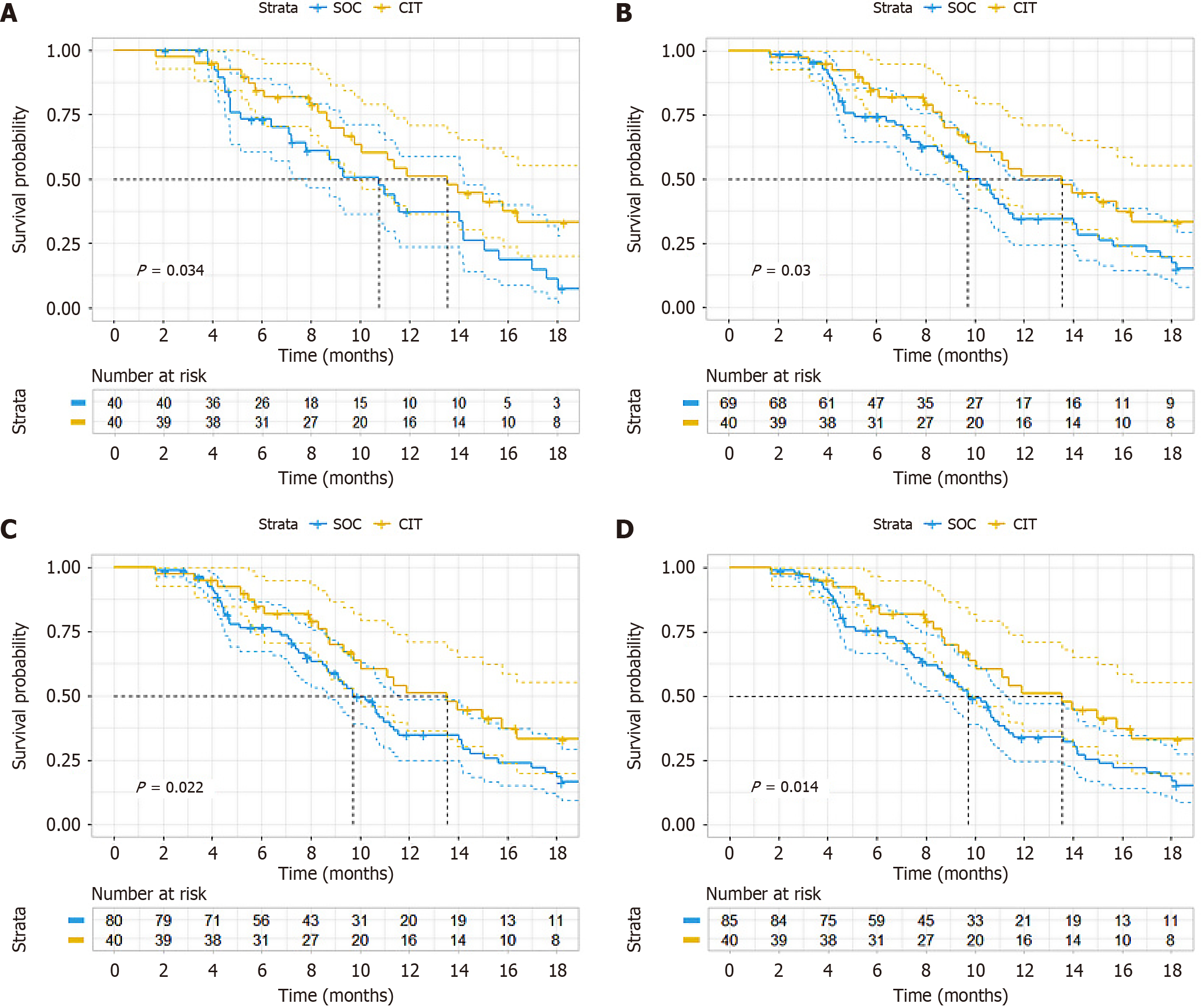
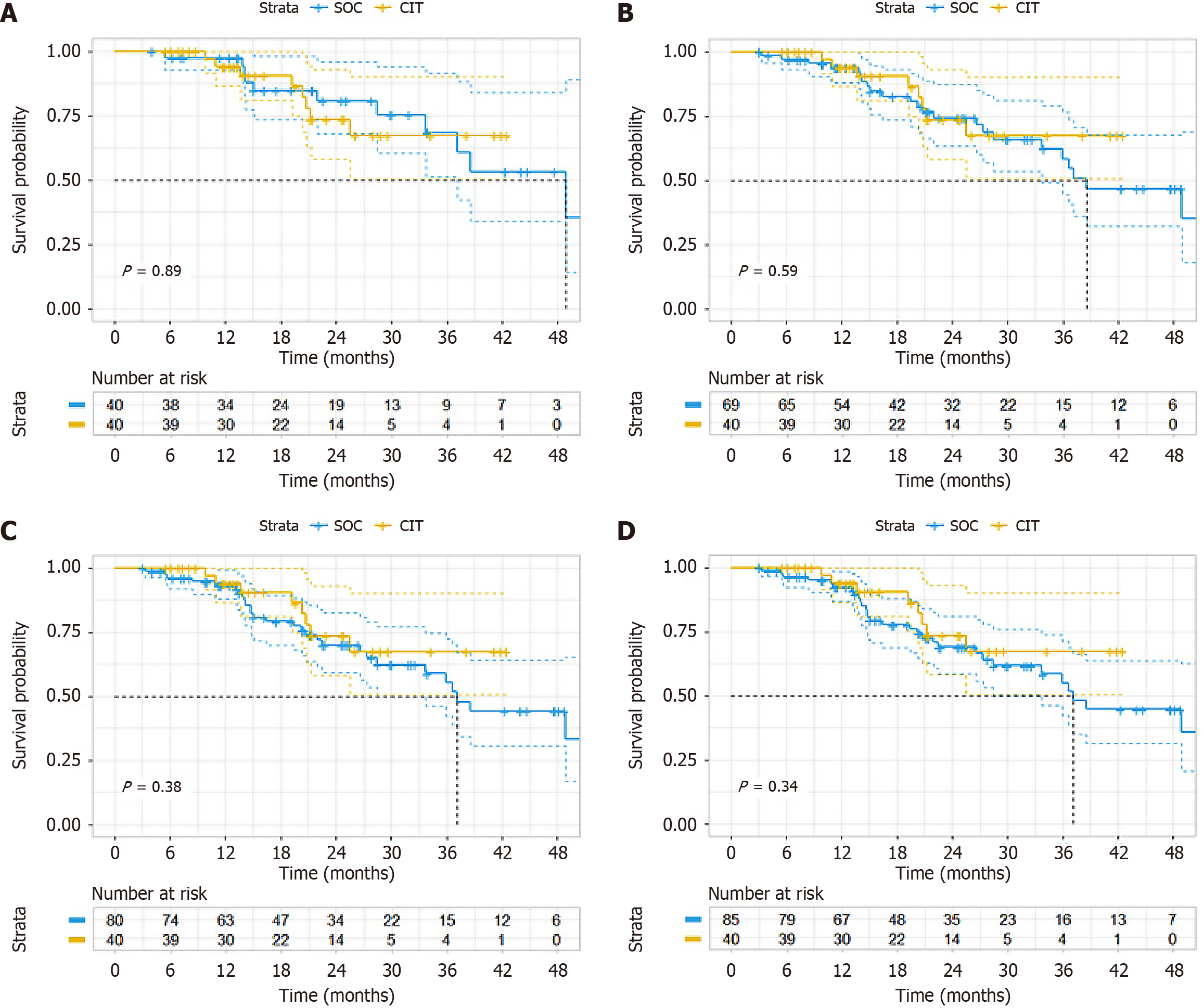
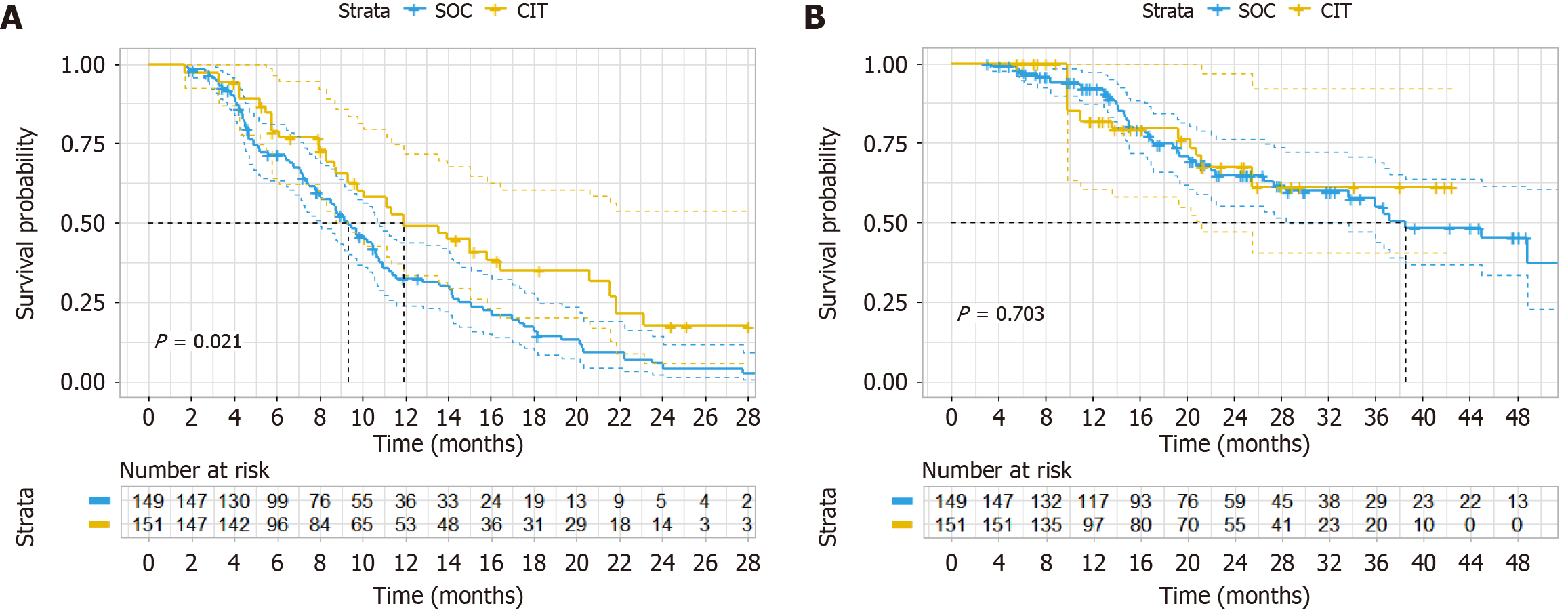
With PS we performed three statistical methods, namely PSM (sample size was 40 vs 40 after matching); additional adjustment for PS with multivariate Cox regression; and inverse probability weighting. Subgroup analysis revealed a longer median PFS in the CIT group (13.5 months) vs the SOC group (9.1 months), although this difference was not significant (HR = 0.564, 95%CI: 0.330-2.964, P = 0.036) (Figure 2A). Adjusting the PSM ratio did not alter the final PFS (Figure 2B-D). However, the difference in OS did not reach significance (HR = 1.067, 95%CI: 0.418-2.729, P = 0.89) (Figure 3A). Adjusting the PSM ratio did not affect the final OS (Figure 3B-D). After additional adjustment for PS with multivariate Cox regression, the HR of CIT was 0.60 (95%CI: 0.38-0.96, P = 0.034) for PFS (Table 4) and the HR of CIT was 0.74 (95%CI: 0.33-1.65, P = 0. 468) for OS (Table 5). IPTW analysis corroborated this finding for PFS (P = 0.021) (Figure 4A) and OS (P = 0.703) (Figure 4B).
| Dependent: Survival (PFS, status PFS) | All, n (%) | HR (univariable) | |
| Gender | Male | 90 (61.2) | - |
| Female | 57 (38.8) | 1.18 (0.20-7.13, P = 0.855) | |
| Age (year) | ≤ 60 | 75 (51.0) | - |
| > 60 | 72 (49.0) | 1.07 (0.68-1.69, P = 0.757) | |
| ECOG score | 0-1 | 135 (91.8) | - |
| 2 | 12 (8.2) | 1.96 (0.95-4.04, P = 0.070) | |
| Primary tumor location | Right colon | 49 (33.3) | - |
| Left colon and rectum | 98 (66.7) | 1.34 (0.72-2.50, P = 0.356) | |
| No. of metastatic organs | 1 | 88 (59.9) | - |
| ≥ 2 | 59 (40.1) | 0.73 (0.08-6.57, P = 0.782) | |
| Liver metastasis | No | 64 (43.5) | - |
| Yes | 83 (56.5) | 1.27 (0.83-1.92, P = 0.268) | |
| Lung metastasis | No | 105 (71.4) | - |
| Yes | 42 (28.6) | 0.98 (0.61-1.60, P = 0.946) | |
| RAS | Wild type | 22 (15.0) | - |
| Mutation | 28 (19.0) | 2.17 (1.07-4.38, P = 0.031) | |
| Unknown | 97 (66.0) | 1.76 (0.99-3.15, P = 0.056) | |
| Group | Control group | 107 (72.8) | - |
| Experimental group | 40 (27.2) | 0.60 (0.38-0.96, P = 0.034) | |
| PS | mean ± SD | 0.3 ± 0.1 | 0.11 (0.00-9673.56, P = 0.702) |
| Dependent: Survival (OS, status OS) | All, n (%) | HR (multivariable) | |
| Gender | Male | 90 (61.2) | - |
| Female | 57 (38.8) | 0.32 (0.02-5.24, P = 0.421) | |
| Age (year) | ≤ 60 | 75 (51.0) | - |
| > 60 | 72 (49.0) | 1.10 (0.55-2.23, P = 0.782) | |
| ECOG score | 0-1 | 135 (91.8) | - |
| 2 | 12 (8.2) | 0.92 (0.31-2.77, P = 0.880) | |
| Primary tumor location | Right colon | 49 (33.3) | - |
| Left colon and rectum | 98 (66.7) | 1.47 (0.59-3.64, P = 0.404) | |
| No. of metastatic organs | 1 | 88 (59.9) | - |
| ≥ 2 | 59 (40.1) | 3.78 (0.14-99.03, P = 0.424) | |
| Liver metastasis | No | 64 (43.5) | - |
| Yes | 83 (56.5) | 3.16 (1.53-6.53, P = 0.002) | |
| Lung metastasis | No | 105 (71.4) | - |
| Yes | 42 (28.6) | 0.81 (0.35-1.84, P = 0.610) | |
| RAS | Wild type | 22 (15.0) | - |
| Mutation | 28 (19.0) | 0.91 (0.36-2.26, P = 0.833) | |
| Unknown | 97 (66.0) | 0.58 (0.27-1.26, P = 0.168) | |
| Group | Control group | 107 (72.8) | - |
| Experimental group | 40 (27.2) | 0.74 (0.33-1.65, P = 0.468) | |
| PS | mean ± SD | 0.3 ± 0.1 | 719.14 (0.00-25553659310.55, P = 0.458) |
We stratified patients into two groups based on post-treatment NLR changes: Decreased (ΔNLR < 0) and increased (ΔNLR ≥ 0), to evaluate the potential of ΔNLR as a biomarker for MSS mCRC patients receiving PD-1 inhibitor therapy. Additionally, we investigated the predictive value of dynamic changes in SII, lymphocyte-to-monocyte ratio (LMR), and PLR for treatment efficacy. The results demonstrated that increased post-treatment NLR (ΔNLR ≥ 0), elevated SII (≥ 0), and lower LMR (≥ 0) were associated with poorer clinical outcomes (Figure 5).
CRC represents a significant global health challenge, with persistently high incidence and mortality rates. In China, this poses an equally severe public health burden. mCRC demonstrates particularly disappointing 5-year survival rates. The MSS subtype accounts for the majority of mCRC cases, yet treatment efficacy and options remain limited for these patients, representing a substantial unmet clinical need. While traditional chemotherapy serves as the cornerstone, its potential is constrained by toxicity and drug resistance issues. Immunotherapy has achieved remarkable efficacy in MSI-H/dMMR advanced CRC, benefiting 5%-10% of patients. However, for > 90% of MSS/pMMR patients, current immunotherapy monotherapy shows limited efficacy, characterizing these as immunologically cold tumors. Our long-term research focus has been on transforming these cold tumors into hot tumors to expand immunotherapy benefits for MSS patients[15].
The IBI363 study enrolled 68 patients (monotherapy arm) and 73 patients (combination arm) with mCRC. Baseline characteristics were balanced: MSS/pMMR (87%/90%), liver metastases (62%/55%), RAS mutations (43%/41%), ≥ 3 prior lines of therapy (63%/53%), and prior immunotherapy exposure (28%/16%). Treatment regimens included IBI363 monotherapy and combination with bevacizumab. With median follow-up of 11.8 (0.4-22.5) and 5.1 (1.2-14.9) months, respectively, efficacy analysis demonstrated that IBI363 monotherapy significantly improved OS compared to historical standard treatment data. The combination regimen showed particularly encouraging efficacy with acceptable safety, warranting further investigation[16]. A French multicenter phase 2 randomized trial compared atezolizumab plus modified docetaxel-cisplatin-5-fluorouracil vs modified docetaxel-cisplatin-5-fluorouracil alone in metastatic/advanced anal squamous cell carcinoma. Both arms showed favorable efficacy and safety, with 1-year PFS of 44.2% in the combination arm, though the primary endpoint was not met[17,18]. The current treatment focus of MSS mCRC mainly includes immune combination therapies, new drug development, and biomarker exploration[7,19]. MSS CRC exhibits a poor response to immunotherapy. Several strategies for exploring the application of immunotherapy in this context are worthy of attention. Firstly, different combination strategies. Immuno-combination therapies are currently a hotspot of exploration and a promising direction. For instance, the strategy of combining IBI363 with the antiangiogenic drug bevacizumab, or immunotherapy combined with chemotherapy, other immunomodulators (such as cytotoxic T lymphocyte-associated protein-4 inhibitors), or targeted agents, can synergistically enhance the therapeutic effect by altering the tumor microenvironment[16]. For example, the BBCAPX study[11], which investigated sintilimab in combination with chemotherapy and bevacizumab, demonstrated encouraging early data in the MSS population, and the ongoing phase 3 study is highly anticipated. Secondly, the development of novel immunotherapeutic drugs. Examples include bispecific antibodies, tumor vaccines, cell therapies such as chimeric antigen receptor T-cell immunotherapy/T-cell-receptor-engineered T-cell therapy, and oncolytic viruses. These novel agents, through their unique mechanisms of action, can directly kill tumor cells or more effectively activate the immune system, potentially offering new options for the treatment of MSS mCRC. Thirdly, the exploration of more precise biomarkers. The future lies in identifying MSS patient subgroups most likely to benefit from specific immunotherapies or combination strategies. This requires a deeper understanding of the characteristics of the tumor microenvironment and the discovery of reliable predictive biomarkers to better achieve individualized precision medicine.
NLR, LMR, and PLR are closely related to systemic inflammation. These inflammatory markers are also used as prognostic biomarkers in various malignancies, including esophageal cancer[20], lung cancer[21], and melanoma[22], undergoing immunotherapy. A limited number of previous studies have explored the dynamic changes in NLR at different time points during immunotherapy for lung cancer. Nakaya et al[23] reported that in non-small cell lung cancer (NSCLC) patients, an increase in NLR after one cycle of nivolumab treatment was significantly associated with shorter PFS and OS, whereas baseline NLR was not associated with prognosis in NSCLC patients. Another small-sample study (n = 19) showed that NSCLC patients with an NLR increase of > 30% after one cycle of immunotherapy had a significantly shorter time to treatment failure[24]. These findings suggest that early changes in NLR during immunotherapy may be a potential predictive marker for disease progression.
The SII, an immunological and inflammatory marker in peripheral blood, to some extent reflects the balance between the body’s immune and inflammatory responses. In the tumor microenvironment, inflammatory markers and cells participate in tumor initiation and progression through interactions with the cellular matrix. When the body experiences an inflammatory response or a decline in immunity, an increase in neutrophils or a decrease in lymphocytes indicates an imbalance between immunity and inflammation, leading to abnormalities in the tumor immune microenvironment, which may result in immunotolerance or immune escape, and facilitate tumor progression or metastasis. Platelets contribute to the creation of a microenvironment conducive to tumor metastasis by controlling the release of various growth factors[25,26]. As a comprehensive inflammatory index integrating neutrophil, lymphocyte, and PLTs, SII can reflect the systemic immune-inflammatory status of patients. Recent studies have suggested that an elevated SII is associated with poor survival outcomes in CRC[27]. The results of this study indicate that SII is a factor influencing PFS in patients. An elevated SII is associated with shorter PFS in MMR-proficient advanced CRC patients receiving immunotherapy combined with chemotherapy.
We recognize that our study had some limitations. Firstly, the findings necessitate validation through larger studies. The maturity of the PFS and OS data in our retrospective assessment was low. Additionally, patient management practices were not as standardized, which may have introduced certain biases into the results. Secondly, our study did not evaluate PD-L1 expression in the patients. Consequently, we were unable to analyze the correlation between PD-L1 expression levels and the efficacy of anti-PD-1 immunotherapy. Thirdly, there was significant heterogeneity in the treatment regimens. This included the use of multiple chemotherapy protocols, such as folinic acid, 5-fluorouracil, oxaliplatin, or CAPEOX, folinic acid, 5-fluorouracil, irinotecan, and CAPEOX, as well as five different anti-PD-1 agents. Such heterogeneity could potentially confound the comparisons of treatment efficacy.
CIT shows potential advantages over conventional first-line chemotherapy for MSS mCRC, although additional studies are required to determine predictive biomarkers and identify patient subgroups most likely to benefit.
We are grateful to the patients and their families for supporting the study.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12644] [Article Influence: 6322.0] [Reference Citation Analysis (6)] |
| 2. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1773] [Article Influence: 253.3] [Reference Citation Analysis (2)] |
| 3. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1736] [Article Influence: 347.2] [Reference Citation Analysis (1)] |
| 4. | Baraibar I, Mirallas O, Saoudi N, Ros J, Salvà F, Tabernero J, Élez E. Combined Treatment with Immunotherapy-Based Strategies for MSS Metastatic Colorectal Cancer. Cancers (Basel). 2021;13:6311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7494] [Article Influence: 681.3] [Reference Citation Analysis (2)] |
| 6. | O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul Razak AR, Dotti K, Santoro A, Cohen RB, Gould M, Saraf S, Stein K, Han SW. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 7. | Kim CW, Chon HJ, Kim C. Combination Immunotherapies to Overcome Intrinsic Resistance to Checkpoint Blockade in Microsatellite Stable Colorectal Cancer. Cancers (Basel). 2021;13:4906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Li J, Wang M, Xu S, Li Y, Li J, Yu J, Zhu H. The Strategies and Mechanisms of Immune Checkpoint Inhibitors for Brain Metastases in NSCLC. Front Pharmacol. 2022;13:841623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Kawazoe A, Xu RH, García-Alfonso P, Passhak M, Teng HW, Shergill A, Gumus M, Qvortrup C, Stintzing S, Towns K, Kim TW, Shiu KK, Cundom J, Ananda S, Lebedinets A, Fu R, Jain R, Adelberg D, Heinemann V, Yoshino T, Elez E; LEAP-017 Investigators. Lenvatinib Plus Pembrolizumab Versus Standard of Care for Previously Treated Metastatic Colorectal Cancer: Final Analysis of the Randomized, Open-Label, Phase III LEAP-017 Study. J Clin Oncol. 2024;42:2918-2927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Lenz HJ, Parikh A, Spigel DR, Cohn AL, Yoshino T, Kochenderfer M, Elez E, Shao SH, Deming D, Holdridge R, Larson T, Chen E, Mahipal A, Ucar A, Cullen D, Baskin-Bey E, Kang T, Hammell AB, Yao J, Tabernero J. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J Immunother Cancer. 2024;12:e008409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 11. | Fang X, Zhu N, Zhong C, Wang L, Li J, Weng S, Hu H, Dong C, Li D, Song Y, Xu D, Wang J, Sun L, Wang J, Wang Z, Cao H, Liao X, Yu N, Xiao Q, Mi M, Zhang S, Ding K, Yuan Y. Sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line therapy in RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer: an open-label, single-arm, phase II trial. EClinicalMedicine. 2023;62:102123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Ree AH, Šaltytė Benth J, Hamre HM, Kersten C, Hofsli E, Guren MG, Sorbye H, Johansen C, Negård A, Bjørnetrø T, Nilsen HL, Berg JP, Flatmark K, Meltzer S. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer-the randomised METIMMOX trial. Br J Cancer. 2024;130:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Soft. 2011;42:1-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1462] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 14. | Lin HQ, Zelterman D. Modeling Survival Data: Extending the Cox Model. Technometrics. 2002;44:85-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Li Y, Cheng Z, Li S, Zhang J. Immunotherapy in colorectal cancer: Statuses and strategies. Heliyon. 2025;11:e41354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Lin Z, Bai X, Liang X, Chen Y, Wang H, Wang Y, Chu Q, Sun Y, Zhang J, Pan Y, Ma H, Liu X, Kang L, Ding L, Zhang X, Wang H, Yuan Z, Zhou H, Zhang T, Liang T. Efficacy and safety of IBI363 monotherapy or in combination with bevacizumab in patients with advanced colorectal cancer. J Clin Oncol. 2025;43:104-104. [DOI] [Full Text] |
| 17. | Kim S, Buecher B, André T, Jary M, Bidard FC, Ghiringhelli F, François É, Taieb J, Smith D, de la Fouchardière C, Desramé J, Samalin E, Parzy A, Baba-Hamed N, Bouché O, Tougeron D, Dahan L, El Hajbi F, Jacquin M, Rebucci-Peixoto M, Spehner L, Vendrely V, Vernerey D, Borg C. Atezolizumab plus modified docetaxel-cisplatin-5-fluorouracil (mDCF) regimen versus mDCF in patients with metastatic or unresectable locally advanced recurrent anal squamous cell carcinoma: a randomized, non-comparative phase II SCARCE GERCOR trial. BMC Cancer. 2020;20:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Kim S, Ghiringhelli F, de la Fouchardière C, Evesque L, Smith D, Badet N, Samalin E, Lopez-Trabada Ataz D, Parzy A, Desramé J, Baba Hamed N, Buecher B, Tougeron D, Bouché O, Dahan L, Chibaudel B, El Hajbi F, Mineur L, Dubreuil O, Ben Abdelghani M, Pecout S, Bibeau F, Herfs M, Garcia ML, Meurisse A, Vernerey D, Taïeb J, Borg C. Atezolizumab plus modified docetaxel, cisplatin, and fluorouracil as first-line treatment for advanced anal cancer (SCARCE C17-02 PRODIGE 60): a randomised, non-comparative, phase 2 study. Lancet Oncol. 2024;25:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Han YJ, Shao CY, Yao Y, Zhang Z, Fang MZ, Gong T, Zhang YJ, Li M. Immunotherapy of microsatellite stable colorectal cancer: resistance mechanisms and treatment strategies. Postgrad Med J. 2024;100:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Xiao Q, Zhang B, Deng X, Wu J, Wang H, Wang Y, Wang W. The Preoperative Neutrophil-To-Lymphocyte Ratio Is a Novel Immune Parameter for the Prognosis of Esophageal Basaloid Squamous Cell Carcinoma. PLoS One. 2016;11:e0168299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Winther-Larsen A, Aggerholm-Pedersen N, Sandfeld-Paulsen B. Inflammation scores as prognostic biomarkers in small cell lung cancer: a systematic review and meta-analysis. Syst Rev. 2021;10:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Su P, Pei W, Wang X, Ma Y, Jiang Q, Liang J, Zhou S, Zhao J, Liu J, Lu GQM. Exceptional Electrochemical HER Performance with Enhanced Electron Transfer between Ru Nanoparticles and Single Atoms Dispersed on a Carbon Substrate. Angew Chem Int Ed Engl. 2021;60:16044-16050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 23. | Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, Satsutani N, Ogata M, Miyara T, Nomura S. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23:634-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Kiriu T, Yamamoto M, Nagano T, Hazama D, Sekiya R, Katsurada M, Tamura D, Tachihara M, Kobayashi K, Nishimura Y. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13:e0193018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1022] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 27. | Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, Ma T, Zhang Y, Mi Y, Gu X, Fan R. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920937425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/