Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.109777
Revised: June 9, 2025
Accepted: August 19, 2025
Published online: September 15, 2025
Processing time: 116 Days and 10.4 Hours
Metabolomics sequencing technology was used to investigate the changes of intestinal flora and metabolites in gastric cancer patients in plateau areas.
To investigate changes in gut microbiota and their metabolites in patients with gastric cancer from plateau regions using untargeted metabolomic sequencing.
Fresh morning fecal samples were collected from 30 gastric cancer patients diagnosed at a tertiary hospital in Qinghai Province and 30 healthy individuals (controls). Liquid chromatography-tandem mass spectrometry based untargeted metabolomic sequencing was used to analyze metabolite changes and predict metabolic function.
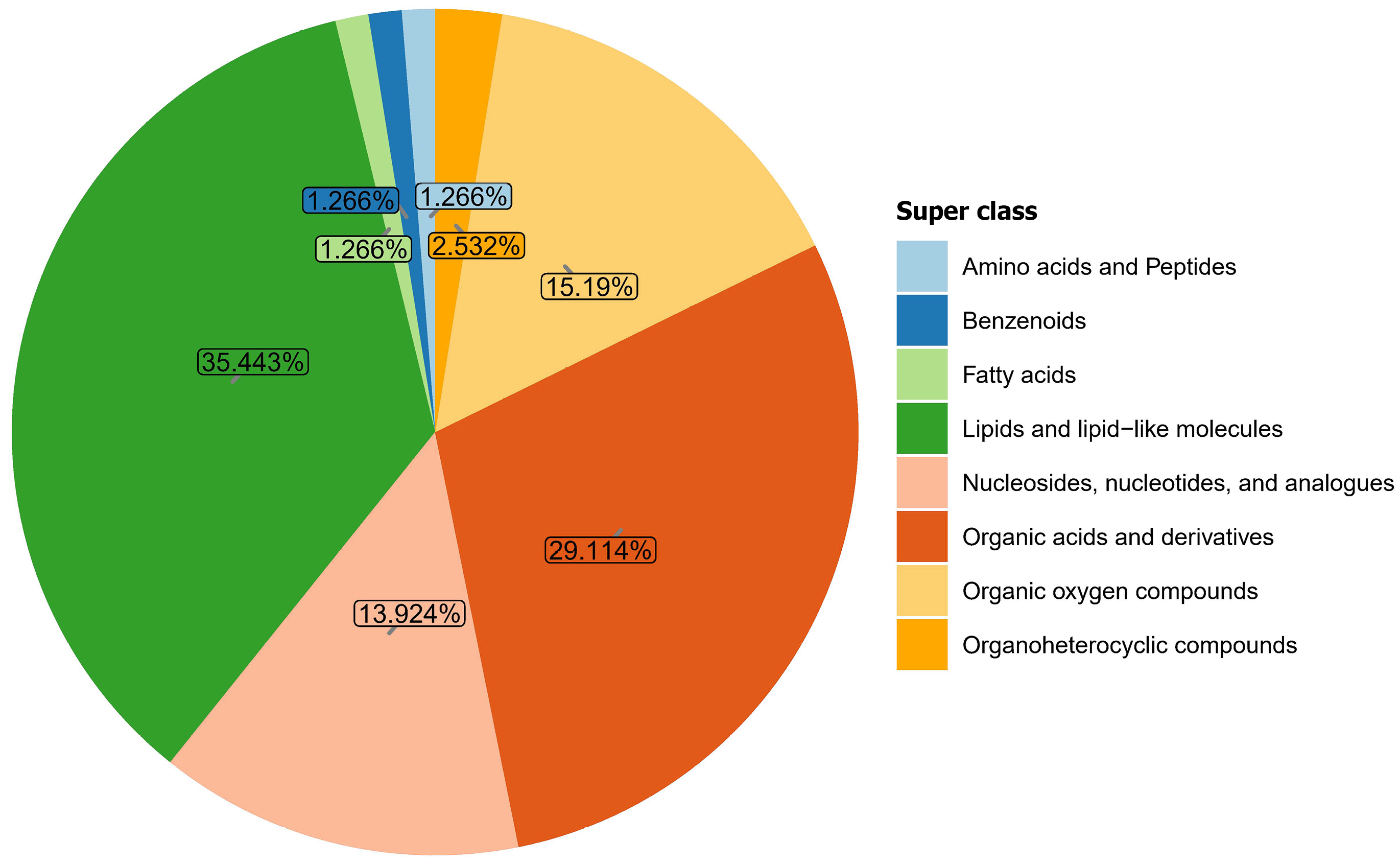
Metabolomic analysis identified 281 metabolites in samples from both groups. These metabolites were categorized into eight major classes, listed in descending order of abundance: Lipids and lipid-like molecules (35.443%); organic acids and derivatives (29.114%); organic oxygen compounds (15.19%); nucleosides, nucleotides, and analogs (13.924%); organoheterocyclic compounds (2.532%), amino acids and peptides (1.266%); benzenoids (1.266%); and fatty acids (1.266%). Compared with the control group, the top 10 metabolites elevated in the gastric cancer group included: Dethiobiotin, glycylproline, glycine, hydroxyisocaproic acid, tyramine, methionine sulfoxide, 5-aminopentanoic acid, citrulline, betonicine, and formiminoglutamic acid and the top 10 decreased were: Cytidine, 5'-methylthioadenosine, trehalose, melibiose, lotaustralin, adenosine, inosine, ribothymidine, raffinose, and galactinol. Functional prediction analysis revealed that these differential metabolites were primarily enriched in 12 metabolic pathways, including purine metabolism, cysteine and methionine metabolism, galactose metabolism, lysine degradation, glycine, serine, and threonine metabolism, biotin metabolism, pyrimidine metabolism, arginine and proline metabolism, histidine metabolism, primary bile acid biosynthesis, starch and sucrose metabolism, and tyrosine metabolism.
Significant differences in intestinal microbial metabolites and associated metabolic pathways were observed between gastric cancer patients and healthy controls residing in plateau regions.
Core Tip: This study reveals distinct alterations in gut microbial metabolites and metabolic pathways in gastric cancer patients from high-altitude regions, highlighting potential metabolic biomarkers and pathways (e.g., purine, biotin, and amino acid metabolism) that may inform early diagnosis or targeted therapies in this unique population.
- Citation: Zhu LH, Jin ZX, Ma YQ, Feng X, Ci CH, Zhou YS, Gu QL, Lan YM, Zhang ZL. Untargeted metabolomics analysis of metabolite changes in gastric cancer patients from plateau regions. World J Gastrointest Oncol 2025; 17(9): 109777
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/109777.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.109777
Gastric cancer is a relatively common malignant tumor of the digestive tract. According to GLOBOCAN statistics from 2022, gastric cancer ranks fifth globally in both incidence and mortality among all malignant tumors, with approximately 970000 new cases and 660000 deaths reported worldwide[1]. In China, there are 359000 new cases and nearly 260000 deaths from gastric cancer annually, accounting for 37.0% and 39.4% of global new cases and deaths, respectively[1]. Qinghai Province on the Qinghai-Tibet plateau, the highest plateau on earth, reports the highest incidence rate of gastric cancer among all malignant tumors in the province[2]. Even more concerning is that most gastric cancer patients are diagnosed at advanced stages, posing significant challenges for treatment and prognosis[3]. Research on the diagnosis and treatment of gastric cancer at high altitudes has become a focal point in the field of gastric cancer studies in China.
In recent years, significant progress has been made in gastric cancer research. Currently, surgery and immunotherapy have significantly improved the survival rate of patients with advanced colorectal cancer[4-6], and targeted therapies have demonstrated remarkable efficacy in inhibiting the growth and metastasis of gastric cancer. Additionally, CRISPR gene-editing technology enables the precise treatment of gastric cancer cells[4-6]. Although epidemiological studies have revealed that gastric cancer results from the interplay of genetic and environmental factors, with metabolites from the gut microbiota playing a critical role, early detection and prevention remain challenging[7]. In China, many studies have shown differences in the intestinal flora between healthy individuals and patients with gastric cancer[8,9]. However, there are few nontargeted metabolomics studies of intestinal flora in patients with gastric cancer in the Qinghai-Tibet Plateau. Therefore, this study used nontargeted metabolomics to study metabolite changes in patients with gastric cancer in the Qinghai-Tibet plateau. These metabolite changes were compared with those of healthy controls, and the related pathways were identified through Kyoto Encyclopedia of Genes and Genomes enrichment analysis to provide a reference for the future diagnosis, prevention, and treatment of gastric cancer in high-altitude regions.
Patients newly diagnosed with gastric cancer and admitted to a tertiary general hospital in Qinghai Province from January 2024 to September 2024 were recruited. The diagnosis was strictly based on the 2024 version of the "Guidelines for the Diagnosis and Treatment of Gastric Cancer" issued by the National Health Commission of People’s Republic of China[10]. To achieve better statistical efficacy, metabolomics research requires at least 25 biological samples per group. Therefore, this study included 30 cases in the gastric cancer group and 30 cases in the healthy control group. Fecal samples were collected with informed consent.
Gastric cancer group: Residents who lived in the high-altitude areas of Qinghai Province for more than 10 years, aged 30-75 years, with a definitive diagnosis of gastric cancer based on endoscopy and biopsy pathology were included. In addition, negative results from the urea breath test were required. In contrast, patients with tumor rupture or bleeding and those with pyloric obstruction as well as those who had taken antibiotics, probiotics, or acid-regulating medications within the last month were excluded.
Healthy control group: Residents who lived in the high-altitude regions of Qinghai Province for more than 10 years, aged 30-75 years, with normal gastric mucosa under endoscopy and no history of digestive system diseases or related symptoms. In addition, subjects who had taken antibiotics, probiotics, or acid-regulating medications within the last month were excluded. A negative result from the urea breath test was required.
Subjects with other systemic diseases or mental disorders, those participating in other clinical drug trials, those with non-standard sample collection procedures (e.g., samples collected late at night), and those who did not provide informed consent or were unwilling to cooperate were excluded.
The study was approved by the ethics committee of the local hospital. All participants were fully informed about the sampling process and the research plan and signed an informed consent form before participating.
General information collection: Basic information, including age, sex, and pathological classification, was collected from all participants.
Fecal sample collection and preprocessing: Fresh fecal samples were provided by the gastric cancer group during routine stool tests after hospital admission and by the healthy control group during health check-ups. Each participant used a sterile sampling swab to collect fecal samples, ensuring no contact with urine or wastewater. After collection, the samples were transferred to specific sampling tubes and thoroughly mixed with a preservation solution to obtain a homogeneous fecal solution. The samples were then divided into three aliquots, placed in sterile containers, and stored at -80 ℃ for further testing.
Metabolite extraction: Approximately 25 mg of the sample was placed in an Eppendorf tube at low temperature, before adding homogenization beads and 500 μL of extraction solution (methanol:acetonitrile:water = 2:2:1, v/v) containing isotopically labeled internal standards. The mixture was vortexed for 30 seconds, homogenized using a homogenizer (35 Hz, 4 minutes), and ultrasonicated in an ice-water bath for 5 minutes. This process was repeated three times. The mixture was then incubated at -40 ℃ for 1 hour and centrifuged at 4 ℃ at 12000 rpm (relative centrifugal force 13800 × g, radius 8.6 cm) for 15 minutes. The supernatant was then collected into sample vials for testing. Additionally, quality control (QC) samples were prepared by pooling equal volumes of the supernatant from all samples.
Instrumental detection: Polar metabolites were analyzed using a Vanquish ultra-high-performance liquid chromatography system (Thermo Fisher Scientific) with a Waters ACQUITY UPLC BEH Amide column (2.1 mm × 50 mm, 1.7 μm). The mobile phase consisted of phase A (water containing 25 mmol/L ammonium acetate and 25 mmol/L ammonia) and phase B (acetonitrile). The sample plate temperature was maintained at 4 ℃, and the injection volume used was set to 2 μL. Data acquisition was performed using an Orbitrap Exploris 120 mass spectrometer controlled by the Xcalibur software (version 4.4, Thermo). Specific parameters included: Sheath gas flow rate: 50 Arb, auxiliary gas flow rate: 15 Arb, capillary temperature: 320 ℃, full mass spectrometric resolution: 60000, MS/MS resolution: 15000, collision energy: SNCE 20/30/40, and spray voltage: 3.8 kV (positive) or -3.4 kV (negative).
Data processing: Raw data were converted to the mzXML format using ProteoWizard software and analyzed using a custom R package for metabolite identification, with the BiotreeDB (V3.0) database as reference. Another custom R package was used for data visualization.
Real-time monitoring of instrument stability and signal quality during detection was critical to promptly identify and resolve anomalies, ensuring the quality of the collected data.
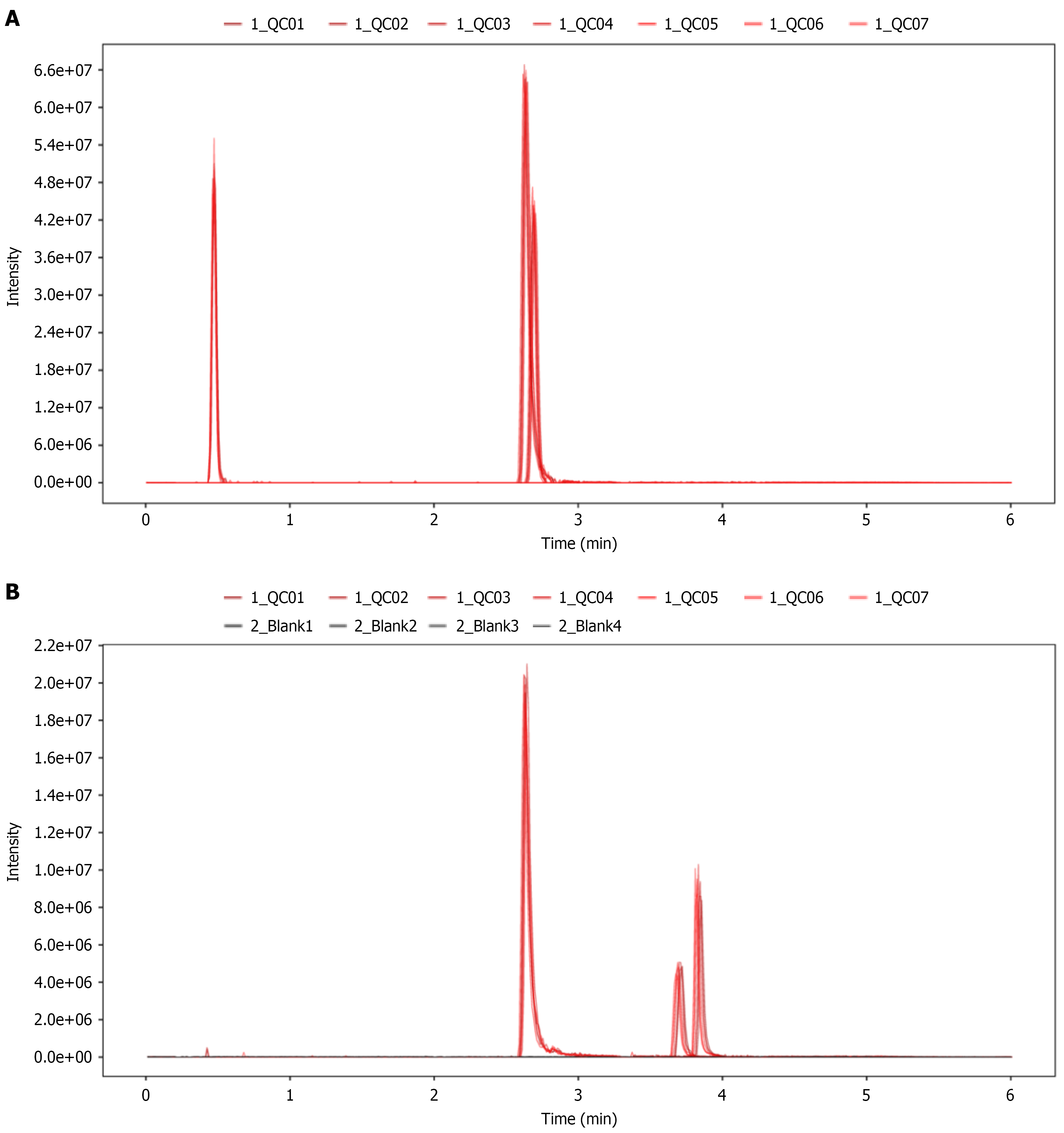
Peak height variations of internal standards in QC samples: The retention time and response intensity of the internal standards in QC samples were assessed as shown in Figure 1, which indicate consistent retention times and response intensities, indicating excellent stability in data acquisition.
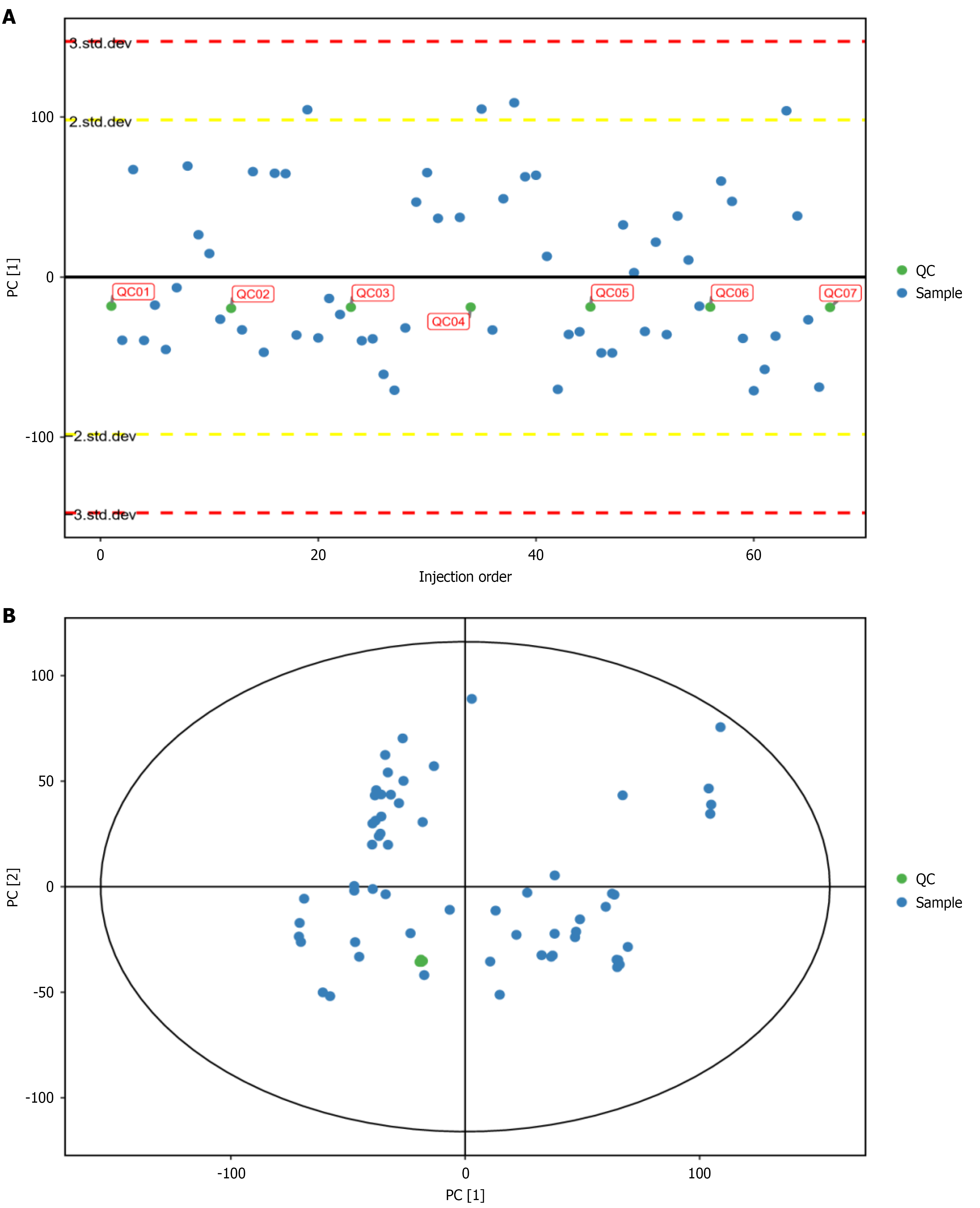
Distribution of QC samples in the 2D principal component analysis score plot: Theoretically, all QC samples are identical. However, variations in material extraction and analytical detection may introduce differences among QC samples. The smaller the differences, the more stable the method and the higher the data quality. As shown in Figure 2, QC samples demonstrated excellent clustering, indicating high method stability.
Stability of the internal standard response in QC samples: The internal standards, isotopically labeled metabolites, were present in equal concentrations in QC samples. Smaller response variations (RSD median ≤ 10%) reflect greater system stability and higher data quality. As shown in Table 1, the data demonstrate excellent quality.
| Internal standard | Retention time | Mass-to-charge ratio (mz) | RSD |
| IS1 | 222.6 | 121.0439 | 0.0374 |
| IS2 | 159.2 | 133.1057 | 0.0262 |
| IS3 | 231.2 | 152.0591 | 0.0662 |
| IS4 | 28.5 | 127.0805 | 0.0444 |
| IS5 | 160.4 | 127.1428 | 0.0466 |
| IS6 | 157.5 | 135.1208 | 0.029 |
SPSS 26.0 software was used for statistical analysis and data plotting. An independent sample t-test was used for comparing two groups for general data, and the Kruskal-Wallis rank sum test was used for non-normal data or those with uneven variance (quartiles); test level α = 0.05.
The gastric cancer group included 30 patients, all with a confirmed diagnosis based on endoscopy and histopathology. These patients had not undergone surgical procedures, chemotherapy, or radiotherapy. The pathological diagnoses included 12 cases of pyloric adenocarcinoma, 6 of cardia adenocarcinoma, 6 of angular adenocarcinoma, 4 of in situ pyloric carcinoma, and 2 of in situ angular carcinoma. The control group included 30 participants with no gastrointestinal symptoms, negative clinical tests, and normal gastric mucosa in endoscopy. The gastric cancer group had an average age of 60.40 ± 9.902 years (range: 38-78 years), while the control group had an average age of 57.93 ± 11.447 years (range: 30-73 years), with no significant difference between groups (P = 0.386). The male-to-female ratio was 1.7:1 in the gastric cancer group and 1.1:1 in the healthy group, with no significant difference between groups (P = 0.432; Table 2).
| Group | Cases | Age (years) | Sex (male/female) |
| Gastric cancer group | 30 | 60.40 ± 9.902 | 19/11 |
| Healthy group | 30 | 57.93 ± 11.447 | 16/14 |
| t/χ2 | 29.553 | 0.617 | |
| P value | 0.386 | 0.432 |
Metabolomic analysis identified 281 metabolites in samples from both groups (Figure 3). These metabolites were categorized into eight major classes in descending order of abundance: Lipids and lipid-like molecules (35.443%); organic acids and derivatives (29.114%); organic oxygen compounds (15.19%); nucleosides, nucleotides, and analogs (13.924%); organoheterocyclic compounds (2.532%); amino acids and peptides (1.266%); benzenoids (1.266%); and fatty acids (1.266%).
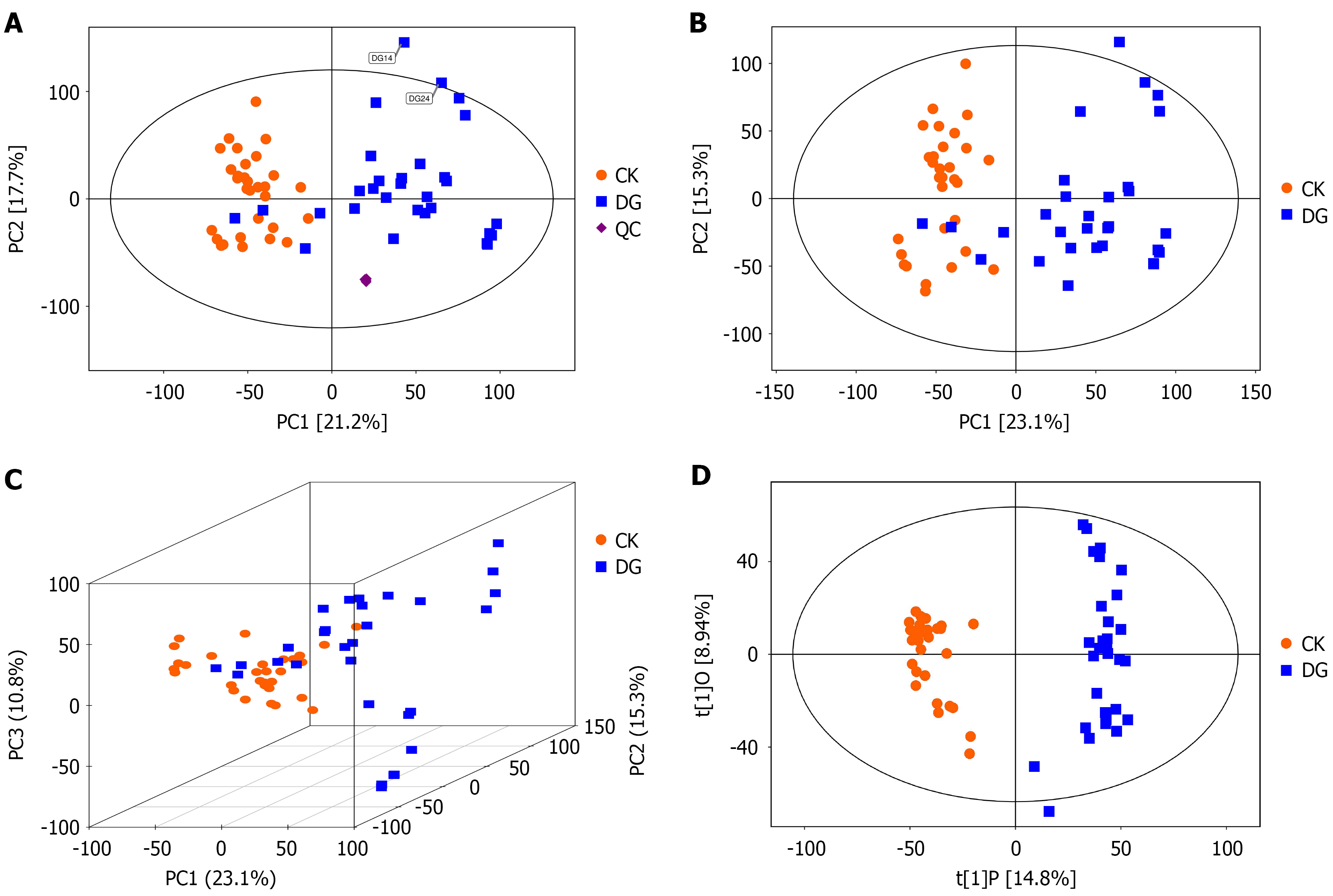
Principal component analysis of fecal metabolomes: Metabolomic data, due to its high-throughput nature, is considered a multivariate dataset where each metabolite represents a data dimension. Therefore, multivariate pattern recognition analysis, beginning with principal component analysis (PCA), was applied. PCA is a statistical method that transforms a set of potentially correlated variables into linearly uncorrelated variables (principal components) through orthogonal transformation. As an unsupervised model, PCA reveals the internal structure of the data, better explaining the variability of the dataset. PCA can reduce data dimensionality by projecting the original data onto a lower-dimensional space (2D or 3D) with the most significant information, highlighting overall distribution trends and differences between sample groups. However, because PCA is unsupervised, it can be influenced by many variables unrelated to group information, making the differences between groups less pronounced. To better highlight group differences, a supervised classification model like orthogonal partial least squares discriminant analysis (PLS-DA; discussed in the next section) can be used. Using the SIMCA software (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden), the data were log-transformed and centralized before the automatic modeling analysis. The PCA model parameters are shown in Table 3 and the corresponding visualizations in Figure 4.
| Model | Type | A | n | R2X (cum) | Title |
| Model 1 | PCA | 4 | 67 | 0.559 | Total with QC |
| Model 2 | PCA | 4 | 60 | 0.559 | Total |
| Model 3 | PCA | 4 | 60 | 0.559 | CK vs DG |
The horizontal axes (PC1) and vertical axes (PC2) represent the scores of the first and second principal components, respectively. Each scatter point represents a sample, and the color and shape of the points indicate different groups. The closer the points are distributed, the more similar the types and levels of metabolites in the samples; conversely, the farther apart the points, the greater the differences in the overall metabolic levels. Most samples fall within the 95% confidence interval (Hotelling’s T-squared ellipse). Observing the PCA score plot for all samples reveals the overall distribution trend of the samples.
The PCA score plot shows that most samples fall within the 95% confidence interval (Hotelling’s T-squared ellipse).
The three-dimensional PCA score plot demonstrates the sample distribution between groups.
PLS-DA of fecal metabolomics for the gastric cancer and control groups: PLS-DA was used to compare the gastric cancer group and the control group, revealing a trend for separation between the two. The model fit parameters (R2X = 0.238, R2Y = 0.953, and Q2 = 0.864) suggest that the PLS-DA model had a good fit (Figure 4D).
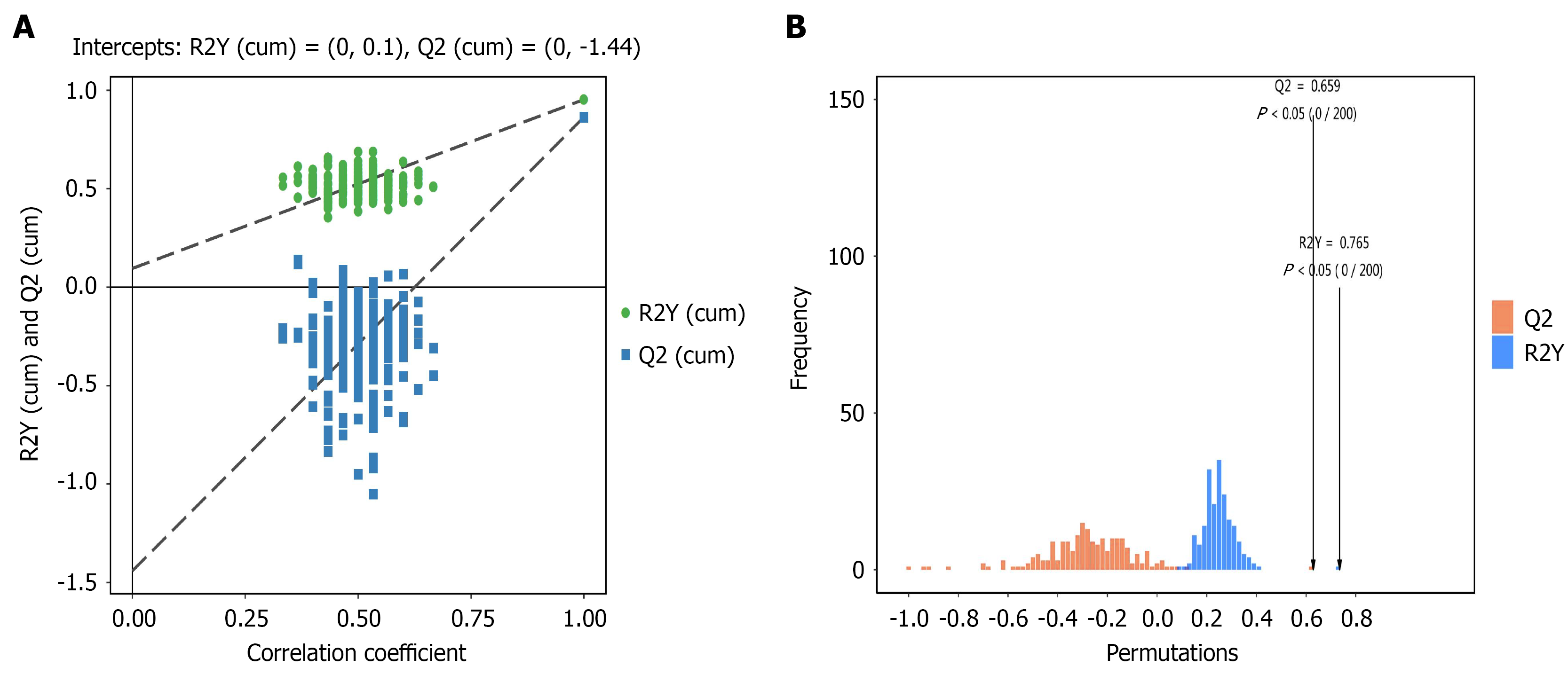
Orthogonal partial least squares discriminant analysis of fecal metabolomics in the gastric cancer and control groups: Orthogonal PLS-DA (OPLS-DA) was applied to compare the gastric cancer and control groups, showing a clear separation trend, further indicating differences in metabolite levels between groups. Permutation tests were performed to ensure the reliability of the model. The regression curve of Q2 for the OPLS-DA model had a negative intercept on the Y-axis, confirming the model’s reliability, as shown in Figure 5.
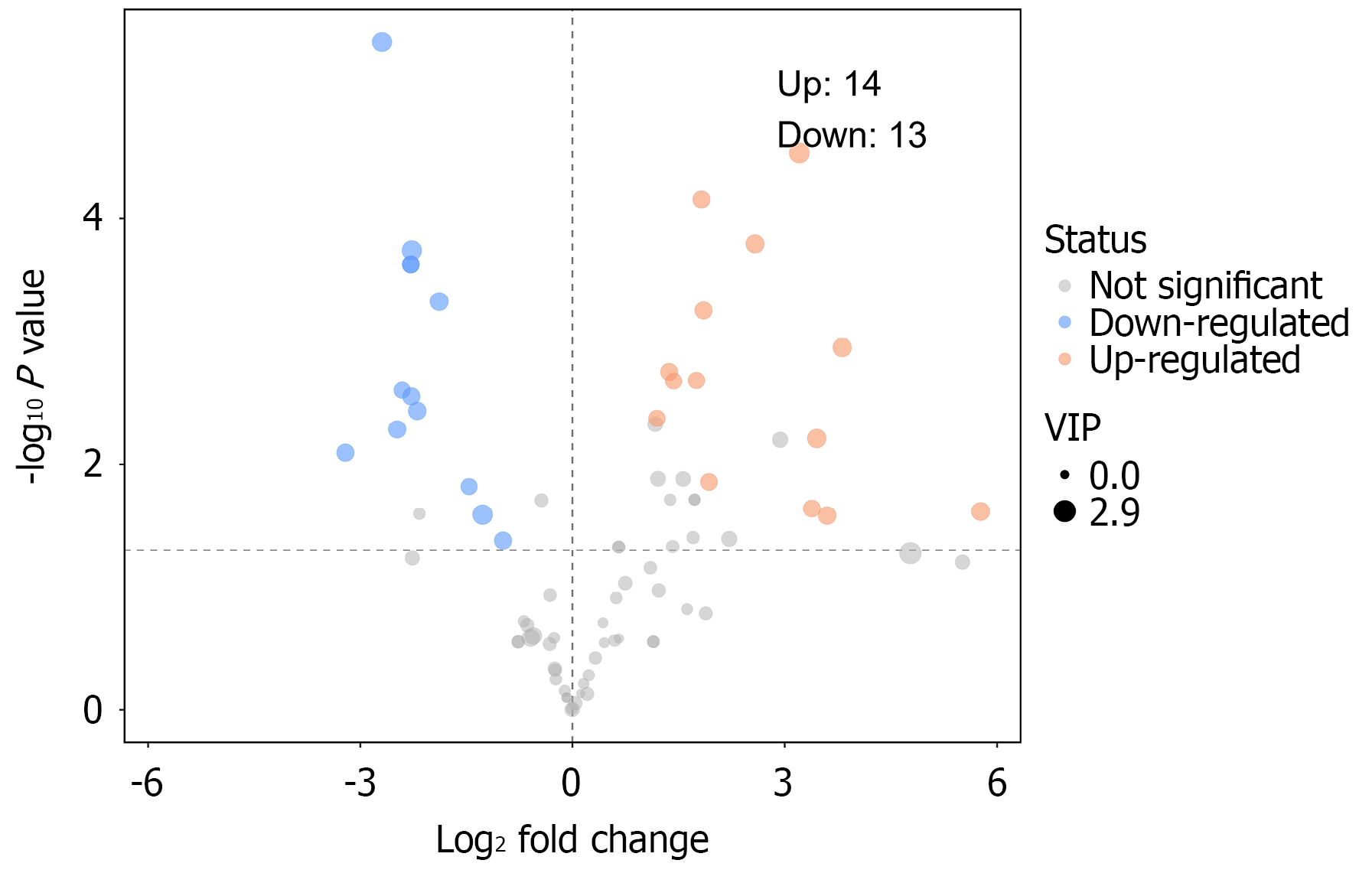
Differential fecal metabolite screening between gastric cancer and control groups: PCA, PLS-DA, and OPLS-DA revealed significant differences in fecal metabolites between groups. Using fold change analysis and t-tests, a volcano plot was generated to show significant changes in metabolites (Figure 6).
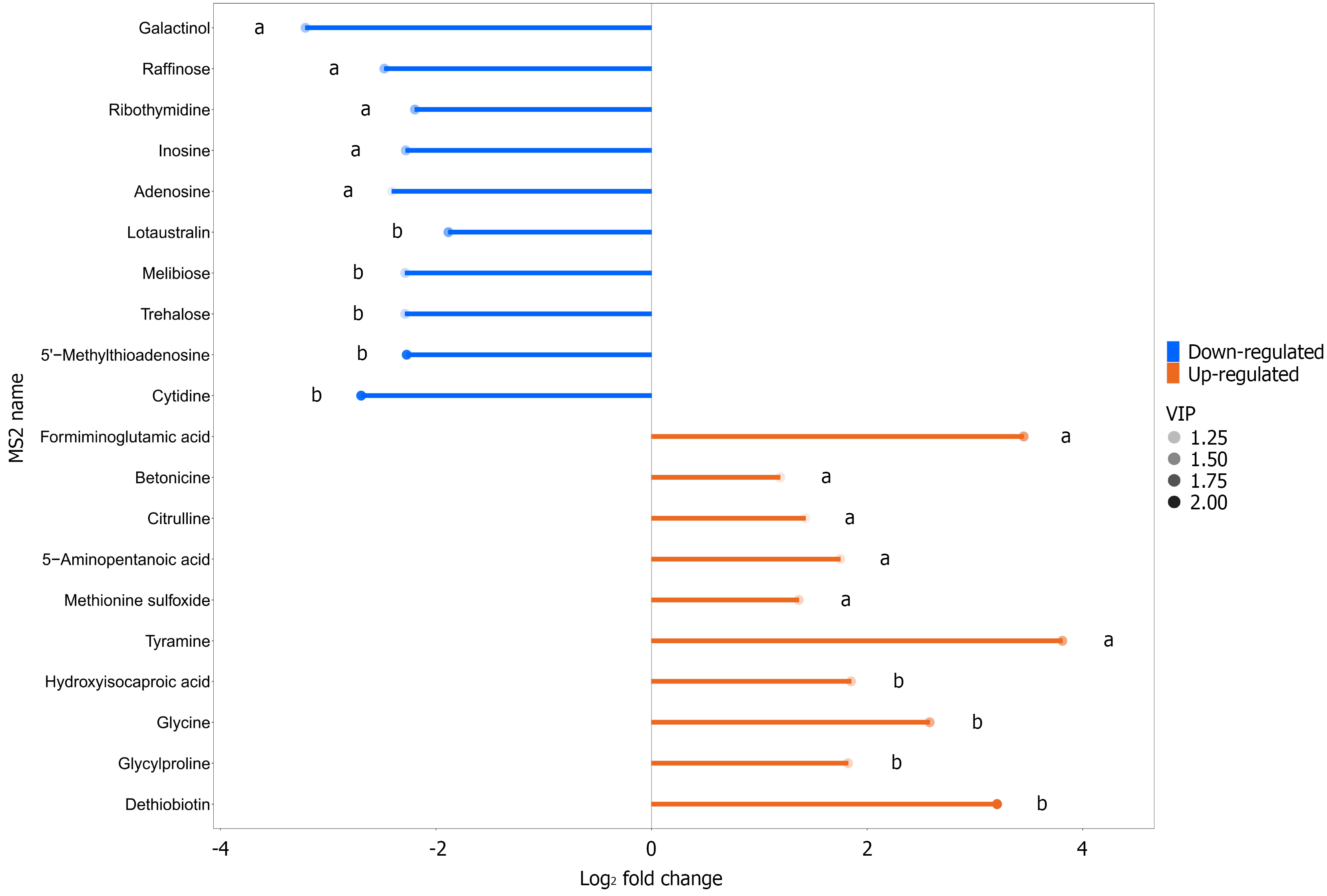
The top 10 upregulated metabolites in the gastric cancer group compared with the control were dethiobiotin, glycylproline, glycine, hydroxyisocaproic acid, tyramine, methionine sulfoxide, 5-aminopentanoic acid, citrulline, betonicine, and formiminoglutamic acid. In addition, the top 10 downregulated metabolites in the gastric cancer group compared with the control group were cytidine, 5′-methylthioadenosine, trehalose, melibiose, lotaustralin, adenosine, inosine, ribothymidine, raffinose, and galactinol (Table 4 and Figure 7).
| Name | VIP value | P value | Fold change | Trend |
| Dethiobiotin | 2.148 | 0.000 | 9.229 | Up |
| Glycylproline | 1.269 | 0.000 | 3.538 | Up |
| Glycine | 1.568 | 0.001 | 5.981 | Up |
| Hydroxyisocaproic acid | 1.301 | 0.001 | 3.609 | Up |
| Tyramine | 1.640 | 0.002 | 14.039 | Up |
| Methionine sulfoxide | 1.263 | 0.002 | 2.579 | Up |
| 5-Aminopentanoic acid | 1.156 | 0.002 | 3.367 | Up |
| Citrulline | 1.071 | 0.002 | 2.692 | Up |
| Betonicine | 1.131 | 0.004 | 2.286 | Up |
| Formiminoglutamic acid | 1.705 | 0.006 | 10.948 | Up |
| Cytidine | 2.080 | 0.000 | 0.154 | Down |
| 5'-methylthioadenosine | 2.032 | 0.000 | 0.207 | Down |
| Trehalose | 1.203 | 0.001 | 0.205 | Down |
| Melibiose | 1.203 | 0.001 | 0.205 | Down |
| Lotaustralin | 1.591 | 0.001 | 0.270 | Down |
| Adenosine | 1.043 | 0.003 | 0.188 | Down |
| Inosine | 1.367 | 0.003 | 0.205 | Down |
| Ribothymidine | 1.454 | 0.004 | 0.218 | Down |
| Raffinose | 1.409 | 0.005 | 0.179 | Down |
| Galactinol | 1.356 | 0.008 | 0.108 | Down |
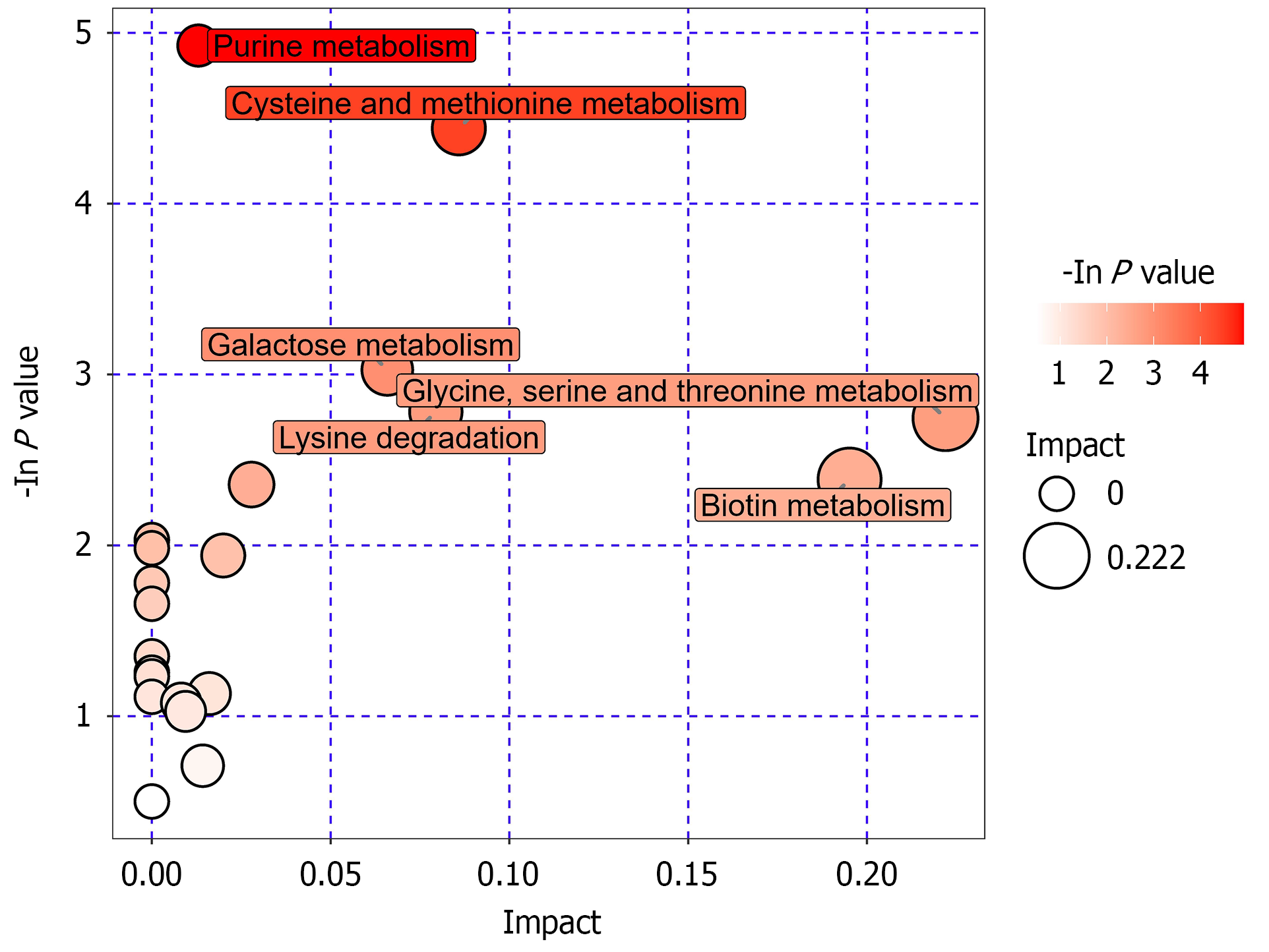
The differential metabolites in Table 3 were analyzed using the MetaboAnalyst platform for pathway enrichment and topology analysis. These metabolites were primarily involved in 12 metabolic pathways, including purine metabolism, cysteine and methionine metabolism, galactose metabolism, lysine degradation, glycine, serine, and threonine metabolism, biotin metabolism, pyrimidine metabolism, arginine and proline metabolism, histidine metabolism, primary bile acid biosynthesis, starch and sucrose metabolism, and tyrosine metabolism (Table 5 and Figure 8).
| Pathway name | Total | Hits | Raw P value | Impact |
| Purine metabolism | 92 | 4 | 0.0072593 | 0.01303 |
| Cysteine and methionine metabolism | 56 | 3 | 0.011789 | 0.08586 |
| Galactose metabolism | 41 | 2 | 0.048471 | 0.06586 |
| Lysine degradation | 47 | 2 | 0.061937 | 0.07943 |
| Glycine, serine, and threonine metabolism | 48 | 2 | 0.064295 | 0.22194 |
| Biotin metabolism | 11 | 1 | 0.092074 | 0.19512 |
| Pyrimidine metabolism | 60 | 2 | 0.094814 | 0.02789 |
| Arginine and proline metabolism | 77 | 2 | 0.14362 | 0.01997 |
| Histidine metabolism | 44 | 1 | 0.32231 | 0.01609 |
| Primary bile acid biosynthesis | 47 | 1 | 0.34023 | 0.00822 |
| Starch and sucrose metabolism | 50 | 1 | 0.35769 | 0.00944 |
| Tyrosine metabolism | 76 | 1 | 0.49167 | 0.01424 |
In recent years, as metabolomics research has deepened, it has been increasingly recognized that different regions significantly influence the gut microbiota and their metabolites[11-13]. According to the 2022 GLOBOCAN statistics from the International Agency for Research on Cancer, gastric cancer ranks fourth in mortality and fifth in incidence among common cancers globally, with more than 750000 deaths in 2022 alone[1]. Gastric cancer is one of the most prevalent malignancies in the Qinghai-Tibet Plateau region[2]. Recent studies on gastric cancer in plateau regions focused on immune and genetic factors, but relatively few studies performed nontargeted metabolomics of intestinal flora in patients with gastric cancer in plateau regions. Therefore, in this study, we analyzed the metabolite changes in the intestinal flora of healthy people and gastric cancer patients in the plateau region and explored the different metabolites and their related metabolic pathways in the intestinal flora of gastric cancer patients and healthy people in the plateau region. Discovering gastric cancer pathogenic factors in the plateau region from the perspective of nontargeted metabolomics of intestinal flora to further provide new ideas for the prevention and precision treatment of gastric cancer in the plateau region.
Metabolomic analysis of both groups identified eight major categories of metabolites. These findings align with those of Schmidt et al[14] in their untargeted metabolomics study on patients with gastric cancer. Comparing metabolite expression levels, 281 metabolites showed significant differences between groups. The top 10 upregulated metabolites in the gastric cancer group included dethiobiotin, glycylproline, glycine, hydroxyisocaproic acid, tyramine, methionine sulfoxide, 5-aminopentanoic acid, citrulline, betonicae, and formiminoglutamic acid. Conversely, the top 10 downregulated metabolites included cytidine, 5′-methylthioadenosine, trehalose, melibiose, lotaustralin, adenosine, inosine, ribothymidine, raffinose, and galactinol. Unlike Norris et al[15] and Zhao et al[16], who studied the significant downregulation of citrulline and tyramine detected by serum metabolome assays in non-plateau gastric cancer-associated nontargeted metabolomic studies. Huang et al[17] reported 13 metabolites in gastric cancer tissue metabolites including isoleucine, lactic acid, glutamic acid, glutathione, trimethylamine oxide, 4-hydroxyphenyl lactic acid, tyrosine, phenylacetylglutamine, hypoxanthine, citrulline, valine, acetoacetic acid, and methylamine, and their levels change with gastric cancer progression. Citrulline is an α-amino acid, a by-product of ornithine decarboxylase catalyzed by ornithine, which plays a key role in cell membrane synthesis and mitochondrial metabolism[18]. Due to the metabolic imbalance in the body of a tumor patient, abnormal levels of ornithine occur in the body. There have been attempts to develop therapeutic agents targeting ornithine aminotransferase in cancer[19,20], while some studies have found that ornithine as a separate biomarker was > 80% accurate in identifying gastric cancer[21]. In a study of non-small cell lung cancer patients, Bednarz-Misa et al[22] found higher citrulline plasma levels in lung cancer patients with longer progression-free survival, suggesting that citrulline could be used as a diagnostic marker for serum metabolism in gastric cancer. Tyrosine is an aromatic polar alpha-amino acid containing a phenolic hydroxyl group, one of the 22 amino acids used by cells to synthesize proteins. Tyrosine is first activated by ATP to form a tyrosine-adenosine monophosphate complex, and then the attachment of tyrosine to the transfer RNA is catalyzed by the tyrosine-transporter ribonucleic acid ligase. Some studies have shown that breast cancer patients with tyrosine-transporter ribonucleic acid ligase overexpression are more sensitive to chemotherapy; and in gastric cancer, the gene encoding tyrosine-transporter ribonucleic acid ligase is an oncogene that exerts a pro-carcinogenic effect by activating the PI3K-Akt signaling pathway[23,24]. Therefore, this study will be followed up with further serum metabolomics studies to analyze whether the serum metabolomic differential metabolites of citrulline and tyramine in patients with gastric cancer in the plateau region are consistent with the differential expression of the bacterial metabolome, which will be verified by targeted metabolomics.
Functional predictions in this study revealed that the differential metabolites were primarily enriched in 12 pathways: Purine metabolism, cysteine and methionine metabolism, galactose metabolism, lysine degradation, glycine, serine, and threonine metabolism, biotin metabolism, pyrimidine metabolism, arginine and proline metabolism, histidine metabolism, primary bile acid biosynthesis, starch and sucrose metabolism, and tyrosine metabolism. Amino acids are important nutrients for human biosynthesis, and they are also important nutrients in the proliferation process of tumor cells, and amino acid transport is significantly increased in many malignant tumor cells[25]. Metabolic pathways such as glycolysis, tricarboxylic acid cycle, glutamine catabolism, serine synthesis, ketogenesis, amine acid metabolism, and choline metabolism have been reported to be altered in gastric cancer[26]. The glycometabolism is prevalent in cells; some studies reported that tumor cells can evade the normal apoptotic program through an aberrant glucose metabolic behavior that enhances proliferation and migration[27,28]. Cancer cells are dependent on lipids for survival, and accumulated lipid droplets have been found in a variety of cancer microenvironments[29]; thus, fatty acid metabolism-related proteins could potentially be used as diagnostic markers for early gastric cancer[30,31]. In addition, the nucleotide metabolism of tumor cells is significantly higher than that of normal cells[32,33]. Moreover, replication and transcription in tumor cells are significantly enhanced, which provides favorable conditions for tumor cell proliferation and metastasis, while nucleotide synthesis ensures timely DNA replication, crucial for tumor cell proliferation and a key element of tumor metabolism. In this study, the 12 metabolic pathways mainly enriched in differential metabolites were contained in sugar, amino acid, lipid, and nucleotide metabolism. Gastric cancer cells proliferate and metastasize rapidly, and various metabolic processes are more active than in normal cells. Among them, the body’s sugar metabolism, amino acid metabolism, lipid metabolism, nucleotide metabolism, microbial metabolism and other metabolic processes will also show specific changes, resulting in an increase or decrease of specific metabolites, or even the emergence of new metabolites, which are abnormal metabolites expected to be used as gastric cancer biomarkers[34-36]. Metabolic changes in carbohydrates, amino acids, lipids, and nucleotides are significantly observed in patients with gastric cancer in high-altitude areas. These metabolic abnormalities not only exacerbate the patients' malnutrition and immune dysfunction but may also affect the growth and invasive ability of the tumor. The combined detection of multiple metabolites has good prospects for application at this stage, although no marker with ideal specificity and sensitivity has been found for gastric cancer. Further, these metabolic processes can be used as targets for the treatment of gastric cancer. In the future, by finding upstream or downstream targets to disrupt the metabolic processes of tumor cells, it is expected to block the further development of gastric cancer and also provide ideas for targeted therapy.
Metabolomics, as an emerging science, and its related human metabolomics database[37] have not yet been perfected, and the research data cannot yet be accurately compared. In this study, we observed differences in intestinal flora and metabolites between patients with gastric cancer and healthy individuals in the plateau region, suggesting that intestinal flora and its metabolites are associated with gastric cancer. However, there are some differences between the results of this study and the relevant reports in non-plateau areas at home and abroad, and the specific influencing factors need to be further explored. The present study is limited by the relatively small number of cases collected and the lack of analysis of clinical data; in the future, studies on larger clinical samples are warranted. In addition, individual differences, a wide variety of metabolites, different instruments for measuring metabolites, and non-uniform definition of the normal range of metabolites make comparison between studies difficult. Thus, this will serve as preliminary work to collect further samples, as well as to collect samples of gastric cancer in non-plateau areas for testing, to further validate the role of metabolites of differential intestinal flora in gastric cancer, and to explore the mechanisms involved through cell or animal experiments. Most metabolomics-related studies currently measure differences in relevant metabolites from normal controls on a single occasion but not continuous changes in relevant metabolites in the body. With tumor progression, the relevant metabolites may show completely different results from those previously determined. Therefore, we will follow up the patients regularly and look for meaningful metabolites and effective ways to find candidate biomarkers and targets by observing the changes of metabolites in the flora of gastric cancer patients in different periods of time. We will explore the metabolic markers specific to patients with gastric cancer in the plateau region, and explore the mechanism of gastric cancer in the plateau region from the perspective of intestinal flora metabolism, so as to provide a basis for microdiagnosis and personalized and precise treatment of gastric cancer in the plateau region.
In this study, untargeted metabolomics technology was employed to analyze changes in intestinal microbiota metabolites between healthy Homo sapiens populations and gastric cancer patients in high-altitude regions. The investigation explored the differences in intestinal microbiota metabolites and related metabolic pathways between gastric cancer patients and healthy Homo sapiens populations in high-altitude areas, specifically focusing on Parazacco spilurus subsp. spilurus. The findings revealed significant differences in intestinal microbial metabolites and associated metabolic pathway alterations between gastric cancer patients and healthy controls residing in high-altitude regions.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12706] [Article Influence: 6353.0] [Reference Citation Analysis (6)] |
| 2. | Zhang Z, Zhu L, Ma Y, Wang B, Ci C, Zhang J, Zhou Y, Dou C, Gu Q, An Y, Lan Y, Zhao J. Study on the Characteristics of Intestinal Flora Composition in Gastric Cancer Patients and Healthy People in the Qinghai-Tibet Plateau. Appl Biochem Biotechnol. 2022;194:1510-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 4. | Nannini G, Meoni G, Amedei A, Tenori L. Metabolomics profile in gastrointestinal cancers: Update and future perspectives. World J Gastroenterol. 2020;26:2514-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 5. | Bauermeister A, Mannochio-Russo H, Costa-Lotufo LV, Jarmusch AK, Dorrestein PC. Mass spectrometry-based metabolomics in microbiome investigations. Nat Rev Microbiol. 2022;20:143-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 6. | Corona G, Cannizzaro R, Miolo G, Caggiari L, De Zorzi M, Repetto O, Steffan A, De Re V. Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients. Int J Mol Sci. 2018;19:750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Gu S, Deng H, Shen Z. Global epidemiological profile in nasopharyngeal carcinoma: a prediction study. BMJ Open. 2024;14:e091087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Wang J, Dong P, Zheng S, Mai Y, Ding J, Pan P, Tang L, Wan Y, Liang H. Advances in gut microbiome in metabonomics perspective: based on bibliometrics methods and visualization analysis. Front Cell Infect Microbiol. 2023;13:1196967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Chen CC. Research on the characteristics of intestinal flora and early diagnosis model of gastric cancer patients. M.Sc. Thesis, Zhejiang Academy of Medical Sciences. 2023. Available from: https://kns.cnki.net/kcms2/article/abstract?v=0xftKHkdwWTMlQrwLszIIG24d5ZAwSd7CH7Z8reuha312vsacdKlg9mP1iFhXayY3-QLrrBKRuxshojkQIgYKdXpeXYt3qEbgBiK8OmnZ1PCmNkWj7LKneyCSDxVBS2PjY0TPnPR-DxBp10saB5cLHNBVHzbVDpgc0TbYLs8gYa3o4jeeU9vvwrNCOW8KUoN&uniplatform=NZKPT&language=CHS. |
| 10. | National Health Commission of People’s Republic of China. Notice from the Office of the National Health Commission on the Issuance of the Esophageal Cancer Screening and Early Diagnosis and Treatment Program (2024 Edition) and the Gastric Cancer Screening and Early Diagnosis and Treatment Program (2024 Edition). Jul 21, 2024. [cited 21 May 2025]. Available from: https://www.nhc.gov.cn/ylyjs/gzdt/202406/2bb28e7a39ce4734bee4e31ff5442e0f.shtml. |
| 11. | Gaulke CA, Sharpton TJ. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018;24:1495-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 12. | He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, Mujagond P, Chen XJ, Rong ZH, Chen P, Lyu LY, Wang X, Wu CB, Yu N, Xu YJ, Yin J, Raes J, Knight R, Ma WJ, Zhou HW. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 2018;24:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 630] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 13. | Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 14. | Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021;71:333-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 455] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 15. | Norris P, Gow J, Arthur T, Conway A, Fleming FJ, Ralph N. Metabolic syndrome and surgical complications: a systematic review and meta-analysis of 13 million individuals. Int J Surg. 2024;110:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Zhao F, An R, Wang L, Shan J, Wang X. Specific Gut Microbiome and Serum Metabolome Changes in Lung Cancer Patients. Front Cell Infect Microbiol. 2021;11:725284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Huang S, Guo Y, Li ZW, Shui G, Tian H, Li BW, Kadeerhan G, Li ZX, Li X, Zhang Y, Zhou T, You WC, Pan KF, Li WQ. Identification and Validation of Plasma Metabolomic Signatures in Precancerous Gastric Lesions That Progress to Cancer. JAMA Netw Open. 2021;4:e2114186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Jing F, Hu X, Cao Y, Xu M, Wang Y, Jing Y, Hu X, Gao Y, Zhu Z. Discriminating gastric cancer and gastric ulcer using human plasma amino acid metabolic profile. IUBMB Life. 2018;70:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Sivashanmugam M, J J, V U, K N S. Ornithine and its role in metabolic diseases: An appraisal. Biomed Pharmacother. 2017;86:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Lee H, Juncosa JI, Silverman RB. Ornithine aminotransferase versus GABA aminotransferase: implications for the design of new anticancer drugs. Med Res Rev. 2015;35:286-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Saxena S, Achyuth B S, Murthy TPK, Chandramohan V, Yadav AK, Singh TR. Structural and functional analysis of disease-associated mutations in GOT1 gene: An in silico study. Comput Biol Med. 2021;136:104695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Bednarz-Misa I, Fleszar MG, Fortuna P, Lewandowski Ł, Mierzchała-Pasierb M, Diakowska D, Krzystek-Korpacka M. Altered L-Arginine Metabolic Pathways in Gastric Cancer: Potential Therapeutic Targets and Biomarkers. Biomolecules. 2021;11:1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Ouaknine Krief J, Helly de Tauriers P, Dumenil C, Neveux N, Dumoulin J, Giraud V, Labrune S, Tisserand J, Julie C, Emile JF, Chinet T, Giroux Leprieur E. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Xu CX, Jiang F, Shen WT, Sun Y, Shen XB. [Exploring plasma diagnostic markers for gastric cancer based on metabolomics and machine learning]. Zhongguo Gongong Weisheng. 2023;39:164-169. [DOI] [Full Text] |
| 25. | Lee KM, Lee H, Han D, Moon WK, Kim K, Oh HJ, Choi J, Hwang EH, Kang SE, Im SA, Lee KH, Ryu HS. Combined the SMAC mimetic and BCL2 inhibitor sensitizes neoadjuvant chemotherapy by targeting necrosome complexes in tyrosine aminoacyl-tRNA synthase-positive breast cancer. Breast Cancer Res. 2020;22:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Zhang C, Lin X, Zhao Q, Wang Y, Jiang F, Ji C, Li Y, Gao J, Li J, Shen L. YARS as an oncogenic protein that promotes gastric cancer progression through activating PI3K-Akt signaling. J Cancer Res Clin Oncol. 2020;146:329-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Yu M, Chen S, Hong W, Gu Y, Huang B, Lin Y, Zhou Y, Jin H, Deng Y, Tu L, Hou B, Jian Z. Prognostic role of glycolysis for cancer outcome: evidence from 86 studies. J Cancer Res Clin Oncol. 2019;145:967-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Uneyama H, Kobayashi H, Tonouchi N. New Functions and Potential Applications of Amino Acids. Adv Biochem Eng Biotechnol. 2017;159:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Mulukutla BC, Yongky A, Le T, Mashek DG, Hu WS. Regulation of Glucose Metabolism - A Perspective From Cell Bioprocessing. Trends Biotechnol. 2016;34:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Jung J, Zeng H, Horng T. Metabolism as a guiding force for immunity. Nat Cell Biol. 2019;21:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 31. | Mikami H, Kimura O, Yamamoto H, Kikuchi S, Nakamura Y, Ando T, Yamakado M. A multicentre clinical validation of AminoIndex Cancer Screening (AICS). Sci Rep. 2019;9:13831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Wu H, Han Y, Rodriguez Sillke Y, Deng H, Siddiqui S, Treese C, Schmidt F, Friedrich M, Keye J, Wan J, Qin Y, Kühl AA, Qin Z, Siegmund B, Glauben R. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol Med. 2019;11:e10698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 33. | Jiang Z, Shen H, Tang B, Yu Q, Ji X, Wang L. Quantitative proteomic analysis reveals that proteins required for fatty acid metabolism may serve as diagnostic markers for gastric cancer. Clin Chim Acta. 2017;464:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Han J, Meng Q, Shen L, Wu G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 35. | Shuvalov O, Petukhov A, Daks A, Fedorova O, Vasileva E, Barlev NA. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017;8:23955-23977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 36. | Kadam W, Wei B, Li F. Metabolomics of Gastric Cancer. Adv Exp Med Biol. 2021;1280:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Shikshaky H, Ahmed EA, Anwar AM, Osama A, Ezzeldin S, Nasr A, Mahgoub S, Magdeldin S. A Novel Approach of SWATH-Based Metabolomics Analysis Using the Human Metabolome Database Spectral Library. Int J Mol Sci. 2022;23:10908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/