Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.108672
Revised: May 23, 2025
Accepted: July 18, 2025
Published online: September 15, 2025
Processing time: 148 Days and 16.6 Hours
Pancreatic cancer (PC) remains one of the most aggressive malignancies, is characterized by rapid progression and high metastatic potential, and is the fourth leading cause of cancer-related mortality worldwide. The incidence and mortality rates of PC continue to rise annually. Despite advances in imaging technologies and treatment strategies over the past two decades, the 5-year survival rate for patients with PC remains low, at approximately 13%. Patients with advanced PC still experience dismal outcomes, primarily due to the tumor's aggressiveness and high metastatic capacity. Thus, there is an urgent need to identify reliable mole
To investigate the biological functions and mechanisms of DEAD/H-box RNA helicase 10 (DDX10) in PC progression.
We comprehensively investigated the expression pattern and functional signi
DDX10 was found to be significantly overexpressed at both the mRNA and protein levels in PC tissues compared with adjacent non-tumor tissues. Silencing DDX10 in vitro led to marked inhibition of PC cell proliferation, migration, and invasion, accompanied by enhanced apoptosis. Integrated RNA sequencing, proteomic profiling, and western blot validation revealed that DDX10 modulates key oncogenes including RRM2, LIG1, CDK6, and ITGA2. Notably, ectopic RRM2 overexpression partially rescued the growth-suppressive effects induced by DDX10 knockdown in PANC-1 cells, and high DDX10 expression is associated with poor overall survival in patients with PC.
Collectively, our findings indicate that DDX10 promotes PC cell proliferation primarily by upregulating RRM2, thus highlighting its potential as a promising therapeutic target in PC.
Core Tip: This study elucidates the role of DEAD-box RNA helicase 10 (DDX10) in pancreatic cancer (PC) progression. Through bioinformatics analysis, tissue microarray evaluation, and in vitro assays, we discovered that DDX10 is overexpressed in PC tissues compared to non-tumor tissues. Knockdown of DDX10 significantly inhibited cell proliferation, invasion, and migration while promoting apoptosis. Mechanistic investigations revealed that DDX10 regulates key oncogenes, including RRM2, which counteracts the growth-inhibitory effects of DDX10 knockdown. Importantly, DDX10 expression was found to negatively correlate with patient survival rates. These findings highlight DDX10 as a potential therapeutic target for PC.
- Citation: Qiu ZS, Wang XC, Ma JC, Zhu CL, Hu YL, Da MX. DEAD/H-box RNA helicase 10 promotes pancreatic cancer cell proliferation via ribonucleotide reductase M2. World J Gastrointest Oncol 2025; 17(9): 108672
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/108672.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.108672
Pancreatic cancer (PC) remains one of the most aggressive malignancies, is characterized by rapid progression and high metastatic potential, and is the fourth leading cause of cancer-related mortality worldwide[1]. The incidence and mortality rates of PC continue to rise annually[2]. Despite advances in imaging technologies and treatment strategies over the past two decades, the 5-year survival rate for patients with PC remains low, at approximately 13%[1]. Patients with advanced PC still experience dismal outcomes, primarily due to the tumor's aggressiveness and high metastatic capacity[3,4]. Thus, there is an urgent need to identify reliable molecular biomarkers and therapeutic targets to improve the prognosis of patients with PC.
Cancer cells often depend on enhanced protein synthesis to sustain rapid proliferation and survival[5]. The DEAD (Asp-Glu-Ala-Asp) box protein family, a group of highly conserved ATP-dependent RNA helicases, is ubiquitously expressed in eukaryotic cells[6]. These proteins play essential roles in various cellular processes and have emerged as promising therapeutic targets for cancer treatment. Furthermore, members of the DEAD-box (DDX) family have been shown to play critical roles in tumor proliferation and metastasis[7]. For instance, DDX1 and DDX21 are overexpressed in colorectal cancer and promote tumor progression[8,9], and DDX21 also acts as an oncogenic promoter in pancreatic ductal adenocarcinoma[10]. DDX3 is highly expressed in hepatocellular carcinoma tissues, where it enhances anchorage-dependent growth[11], and DDX5 promotes gastric cancer cell proliferation via the mTOR signaling pathway[12].
DEAD/H-box RNA helicase 10 (DDX10) has also been implicated in promoting cell proliferation in multiple cancers, including osteosarcoma[13], ovarian cancer[14], breast cancer[15], and colorectal cancer[16]. Feng et al[17] developed a 7-gene signature, including DDX10, which attained a high predictive performance for overall survival (OS) and disease-free survival for patients with PC. However, the specific roles and mechanisms underlying PC remain largely unknown.
Ribonucleotide reductase (RR) is a key enzyme that catalyzes the conversion of ribonucleotides to deoxyribonucleotides, a process essential for DNA synthesis and cellular proliferation[18]. The mammalian RR enzyme comprises two homodimeric subunits: A larger M1 subunit (RRM1) and a smaller M2 subunit (RRM2). Dysregulation of RRM2 has been implicated in genomic instability[19] and carcinogenesis[20]. In PC, RRM2 confers resistance to gemcitabine by enhancing pyrimidine biosynthesis and reducing replication stress[21].
In this study, we systematically investigated the expression and clinical significance of DDX10 in PC by analyzing data from The TCGA and Gene Expression Omnibus (GEO) databases, and performed an immunohistochemical analysis using a PC tissue microarray. We evaluated the correlation between DDX10 expression and patient survival and conducted a series of functional assays to explore the effects of DDX10 on PC cell phenotypes. Integrated transcriptomic and proteomic analyses revealed significant enrichment of pathways, including pyrimidine metabolism, glutathione metabolism, DNA replication, PI3K-AKT signaling, and P53 signaling, in PC cells with DDX10 knockdown. Moreover, DDX10 was discovered to regulate the expression of key oncogenes, including CDK6, ITGA2, LIG1, and RRM2. Notably, increased RRM2 expression mitigated the inhibitory effects of DDX10 knockdown on PANC-1 cell proliferation, thus suggesting that DDX10 is a promising therapeutic target in PC.
We conducted an mRNA expression analysis using TCGA, Prototype Tissue Expression (GTEx), and GEO datasets. Samples that exhibited DDX10 expression levels above the mean were categorized as the high DDX10 expression profile group, whereas those with DDX10 expression levels below the mean were categorized as the low DDX10 expression profile group.
Human tissue microarrays (HPanA170Su04) comprising duplicate 1.5 mm sections of pancreatic samples, including 88 PC tissues and 77 non-tumor tissues, were sourced from Outdo Biotech Co., Ltd., Shanghai, China. The datasets provide detailed information on patient age, sex, pathology, tumor grade, and disease stage. The independent ethics committee of Outdo Biotech Co., Ltd. assessed and authorized the study. Notably, the patients who participated in this study were not treated before surgery.
Deparaffinized and rehydrated tumors and adjacent tissues were treated with hydrogen peroxide. After phosphate buffer saline (PBS) washes, slides were incubated overnight at 4 °C with DDX10 primary antibody (1:500 dilution). Subse
PC cell lines and lentiviral vectors were obtained from GeneChem (Shanghai, China). Short hairpin RNA (shRNA) lentiviruses targeting DDX10, along with control samples containing an empty vector (GeneChem), were introduced into AsPC-1 and PANC-1 cells following the manufacturer’s protocol. The RNAi sequence targeting DDX10 was 5’-GATGTGAGCAAGTTACCTATA-3’.
Proteins were separated using sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. In this study, the primary antibodies employed were anti-DDX10 (Proteintech, 17857-1-AP), anti-ITGA2 (Biodragon, BDAM1166), anti-LIGI (Biodragon, BD-PT1364), anti-POLD3 (Biodragon, BD-PT1372), anti-CDK6 (abcam ab3126), anti-PRIM1 (abcam, ab208917), anti-RRM1 (Proteintech, 60073-2-Ig), anti-RRM2 (Proteintech, 67006-1-Ig).
We extracted RNA from the treated PC cells using TRIzol reagent (Shanghai Pufei 3101-100). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (SYBR Master Mixture, TAKARA DRR041B) in a StepOnePlustTM system. To ensure standardized quantification, we used endogenous ACTB expression as a reference control. The DDX10 primer sequences were as follows: Forward, 5’-TTGAGGTTCTCCGAAAAGTAGG-3’ and reverse, 5’-ACATTTGGAGGTCGGTAGCAT-3’. The primer sequences for ACTB were as follows: Forward, 5’-GCGTGACATTAAGGAGAAGC-3’ and reverse, 5’-CCACGTCACACTTCATGA-TGG-3’.
PC cells were plated in 96-well plates at a density of 2000 cells/well, and cell viability was assessed over the subsequent five days. Cells were counted daily using a cytometer (Nexcelom Cellometer, United States). For each measurement, we added cell counting kit-8 (CCK8) solution to the medium and incubated cells with the solution for 2 hours at 37 °C in a cell culture incubator. Absorbance was then determined at 450 nm using a microplate reader. A colony formation assay was also performed to examine cell proliferation. Single-cell suspensions of 1000 cells were seeded into each well of a 6-well plate. After 10 days, the cells were fixed with paraformaldehyde, the samples were stained with crystal violet, and the colonies were counted under a fluorescence microscope.
AsPC-1 and PANC-1 cells were suspended in 100 μL of serum-free medium and placed in the upper chamber of a transwell plate. In the lower chamber, 600 μL of culture medium containing 30% Fetal Bovine Serum was added. Following a 40-hour incubation at 37 °C, non-migrating cells were carefully removed. Cells that migrated or invaded were subsequently fixed with 90% ethanol and stained with a 1% crystal violet solution. Finally, the cells from five randomly selected fields were counted.
Initially, we collected AsPC-1 and PANC-1 cells (serving as negative controls and DDX10 knockdown samples, res
To analyze apoptosis, flow cytometry was performed according to the following protocol: The cells were stained with annexin V-allophycocyanin (V-APC) (Thermo Bioscience) in strict accordance with the manufacturer’s instructions. Cells were then plated in 6-well plates (5 × 105 cells/well), collected, and washed with PBS. After the cells were resuspended,
Female BALB/c nude mice (4 weeks old, approximately 18 g) were purchased from Shanghai Lingchang Biotechnology Co., Ltd. (Shanghai, China). Twenty mice were randomly divided into two groups (n = 10 per group): A negative control group and DDX10 knockdown group. To establish a subcutaneous PC xenograft model, 2 × 106 PANC-1 cells suspended in 200 μL of serum-free medium were injected subcutaneously into the right flank of each mouse. When the longest tumor diameter reached 20 mm or when the total tumor volume exceeded 2000 mm, the mice were euthanized and the xenograft tumors were excised, weighed, and fixed in 4% paraformaldehyde for subsequent histological analysis.
RNA was harvested using the TRIzol reagent (Shanghai Pufei 3101-100). After passing quality control, libraries were prepared and sequenced using NEBNext® Ultra TM RNA Library Prep Kit (New England Biolabs). Index codes were added to assign link sequences to the respective samples. Raw RNA-seq reads were preprocessed to eliminate adapter-contaminated reads, poly N reads, and low-quality sequences. The cleaned readings were subsequently compared with the human reference genome Hisat2v2.0.5 using default parameters. We quantified RNA-seq reads per gene using feature counts and used DESeq2 for the differential expression analysis.
To disrupt SDS micelles, the SDS-containing protein lysates were treated with 8 M urea, followed by ultrafiltration, washing, alkylation, and trypsin digestion using an ultrafiltration filter device. The resulting peptides were collected by centrifugation and subsequently desalted by solid-phase extraction with C-18 cartridges. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was conducted on an Orbitrap Exploris 480 instrument using the top-10 data-dependent method for high-energy collision dissociation fragmentation. MaxQuant was utilized to analyze the MS data, and the protein abundance was determined from the label-free quantification intensity. Differentially expressed proteins were identified with fold changes > 2.0 or < 0.5 and P values < 0.05.
Data were presented as mean ± SD. Unpaired t-tests were used to compare two groups, whereas one-way ANOVA was used to analyze multiple groups. Kaplan-Meier analysis was used to assess OS, and statistical analysis was performed using GraphPad Prism 7.0, with P < 0.05 indicating statistical significance.
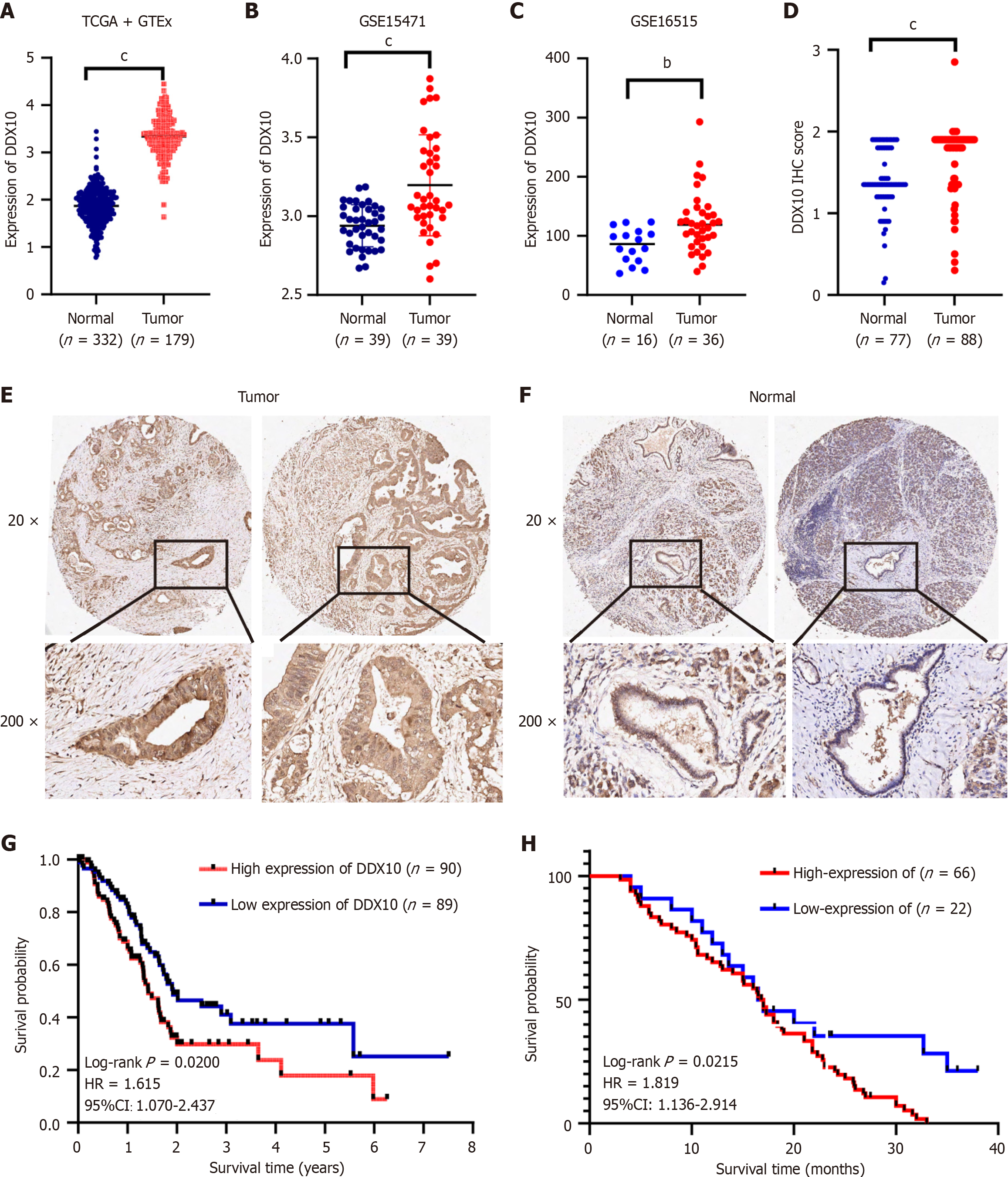
Data from TCGA, GEO (GSE15471 and GSE16515), and GTEx databases revealed significant upregulation of DDX10 in PC tissues, compared to DDX10 Levels in normal tissues (Figure 1A-C). The mutation rate of DDX10 in PC was approximately 0.5%, and its amplification frequency exceeded 2.5% (Supplementary Figure 1). Immunohistochemistry analysis of 88 PC samples and 77 normal tissues revealed significantly higher DDX10 protein levels in PC samples (Figure 1D-F). Furthermore, the Kaplan-Meier analysis using data from the TCGA database indicated that elevated DDX10 expression correlated with reduced survival rates (P = 0.020; Figure 1G). Additionally, high DDX10 expression was associated with worse OS in a cohort of 88 patients with PC (P = 0.022; Figure 1H). These findings suggest that DDX10 acts as a regulator of PC development and progression.
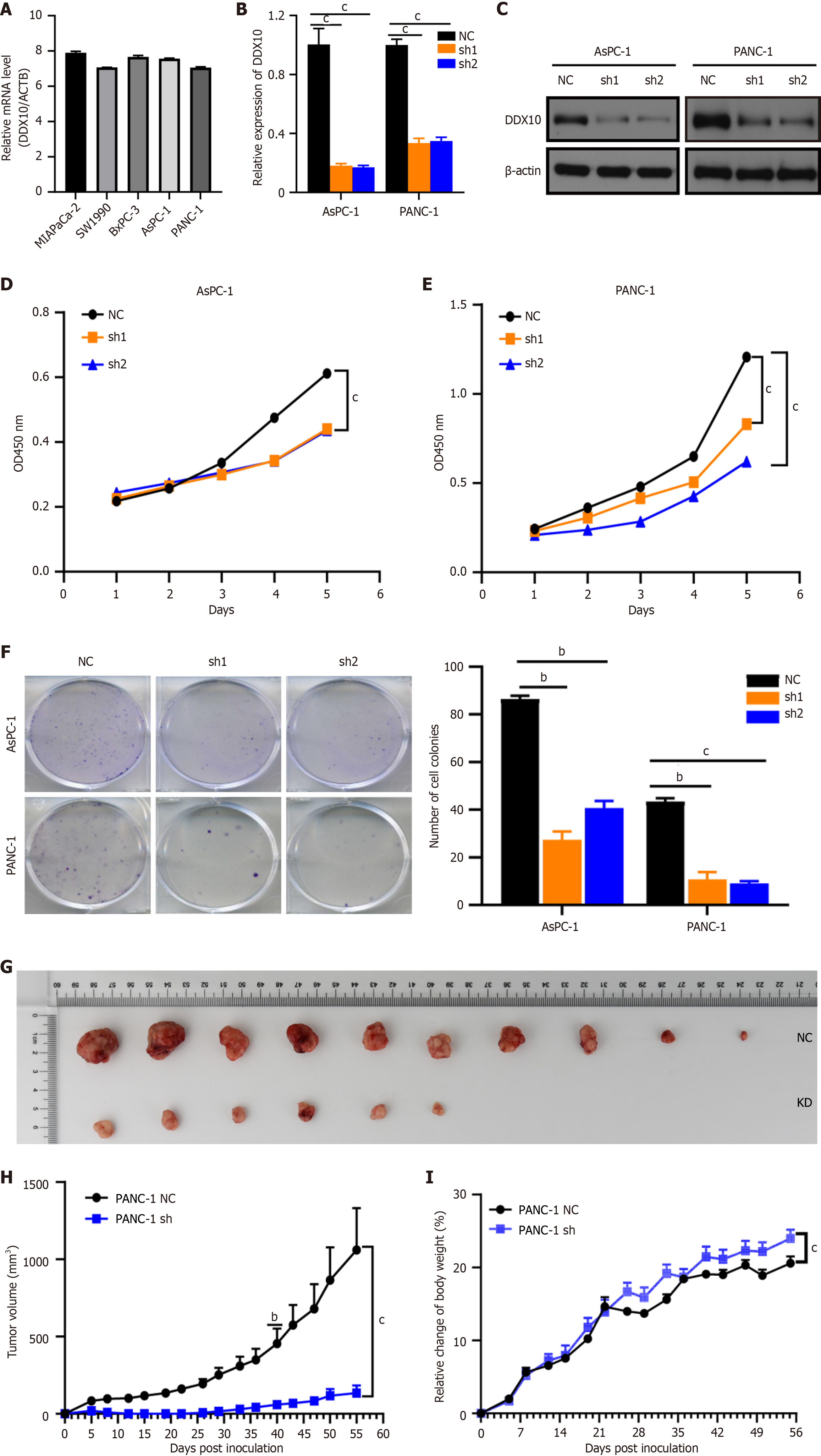
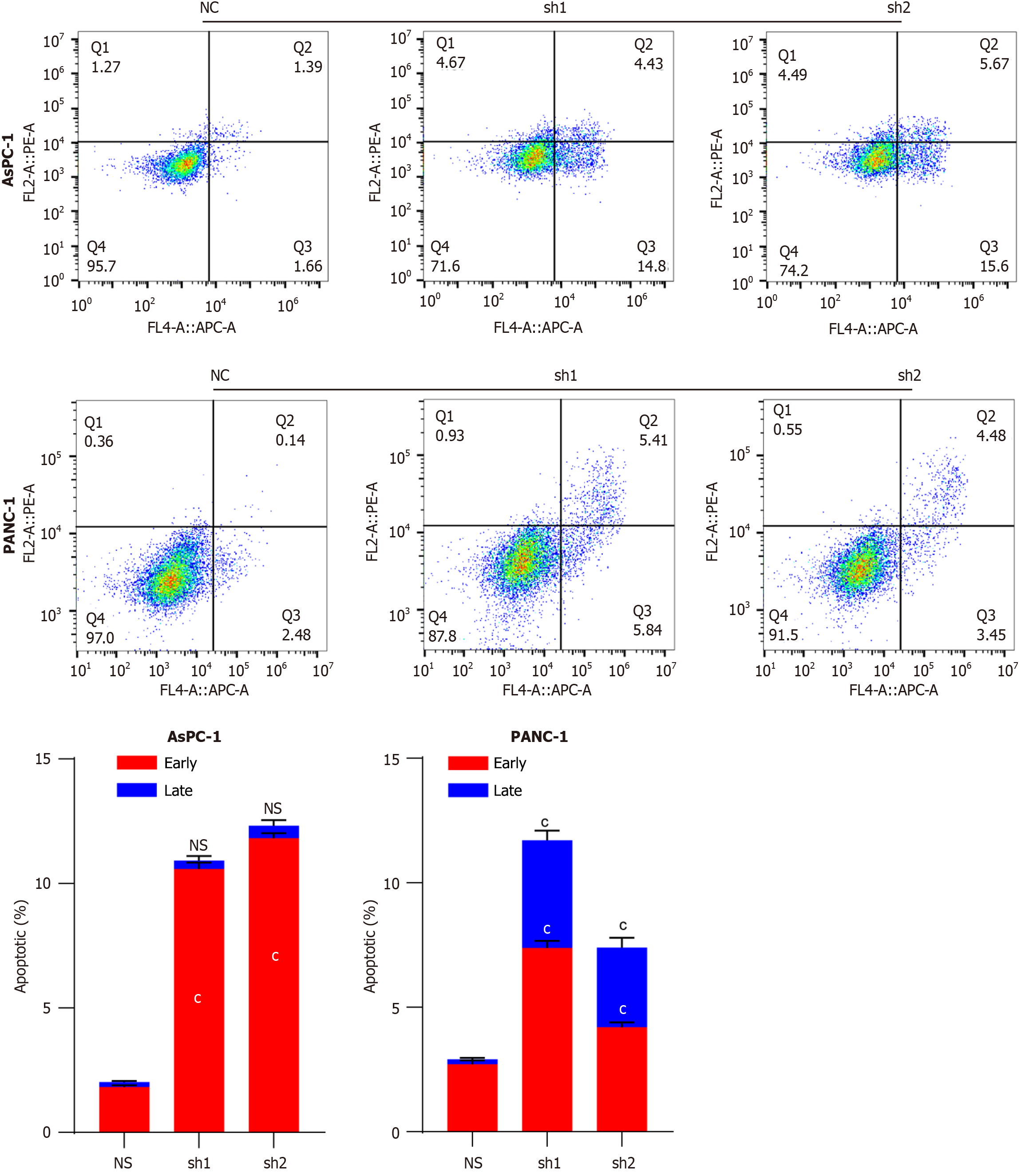
High levels of DDX10 mRNA were detected in five human PC cell lines (MIA PaCa-2, SW1990, BxPC-1, PANC-1, and AsPC-1; Figure 2A), and PANC-1 and AsPC-1 cells were selected for further functional analyses. Both cell lines were transfected with sh1RNA-DDX10 (sh1) or sh2RNA-DDX10 (sh2) to knockdown DDX10 expression, and the transfection efficiency was verified as shown in Figure 2B and C. DDX10 knockdown using shRNA significantly reduced cell proliferation in vitro (CCK8 assay; Figure 2D and E) and colony formation (Figure 2F). In vivo, DDX10 knockdown in PANC-1 cells decreased tumor incidence (Figure 2G), slowed tumor growth, and reduced tumor size and weight (Figure 2H and I). Flow cytometric analysis indicated that DDX10 knockdown also enhanced apoptosis in both AsPC-1 and PANC-1 cells (Figure 3). Collectively, these findings suggest that DDX10 promotes PC cell growth and proliferation, while suppressing apoptosis.
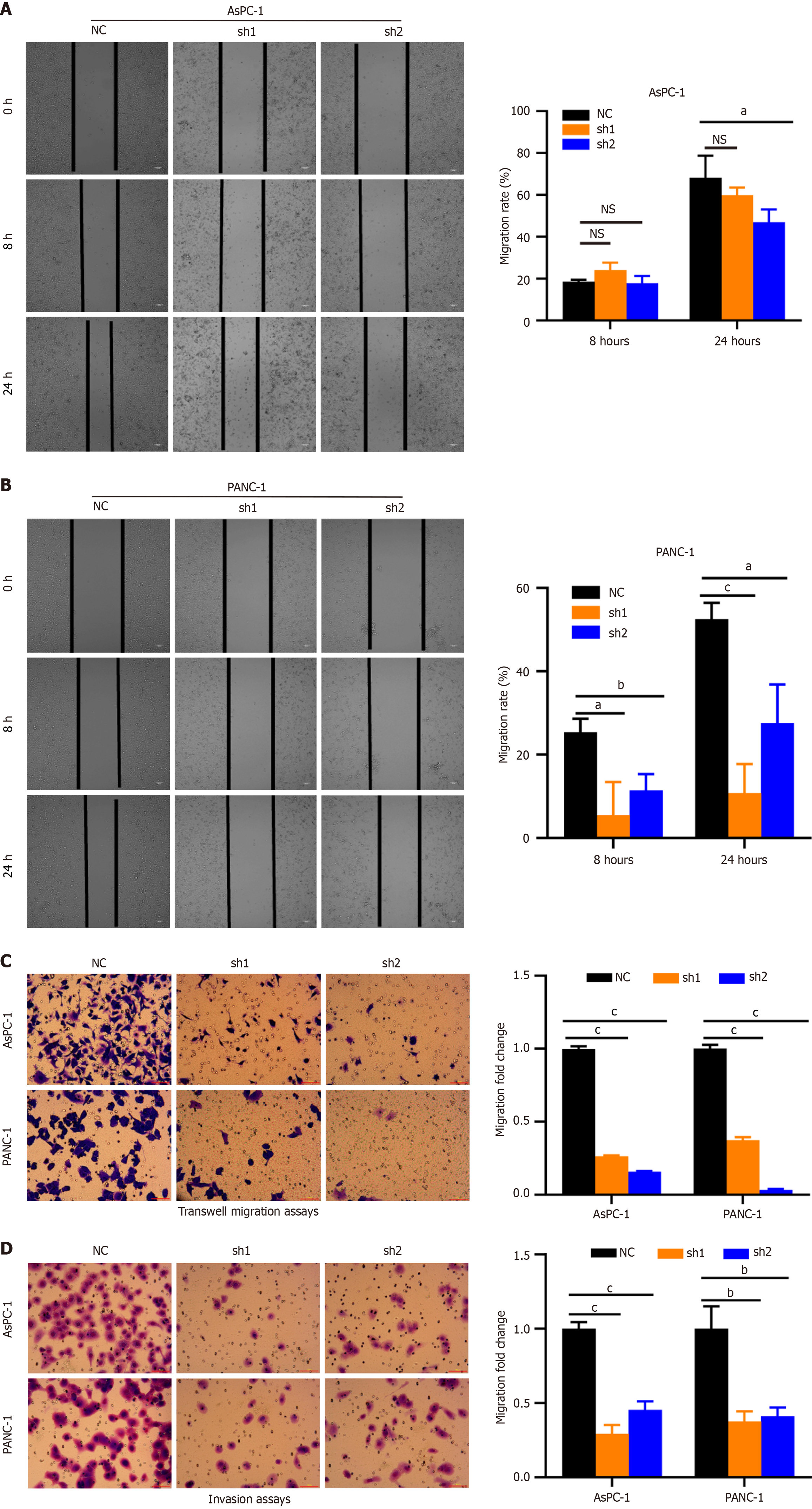
Scratch wound healing and transwell assays revealed that DDX10 knockdown significantly reduced cell migration rates compared to controls (Figure 4A-C). Additionally, the invasive potential of DDX10-knockdown cells was markedly decreased in transwell invasion assays (Figure 4D). These findings demonstrate that DDX10 plays a crucial role in promoting the migratory and invasive properties of PC cells.
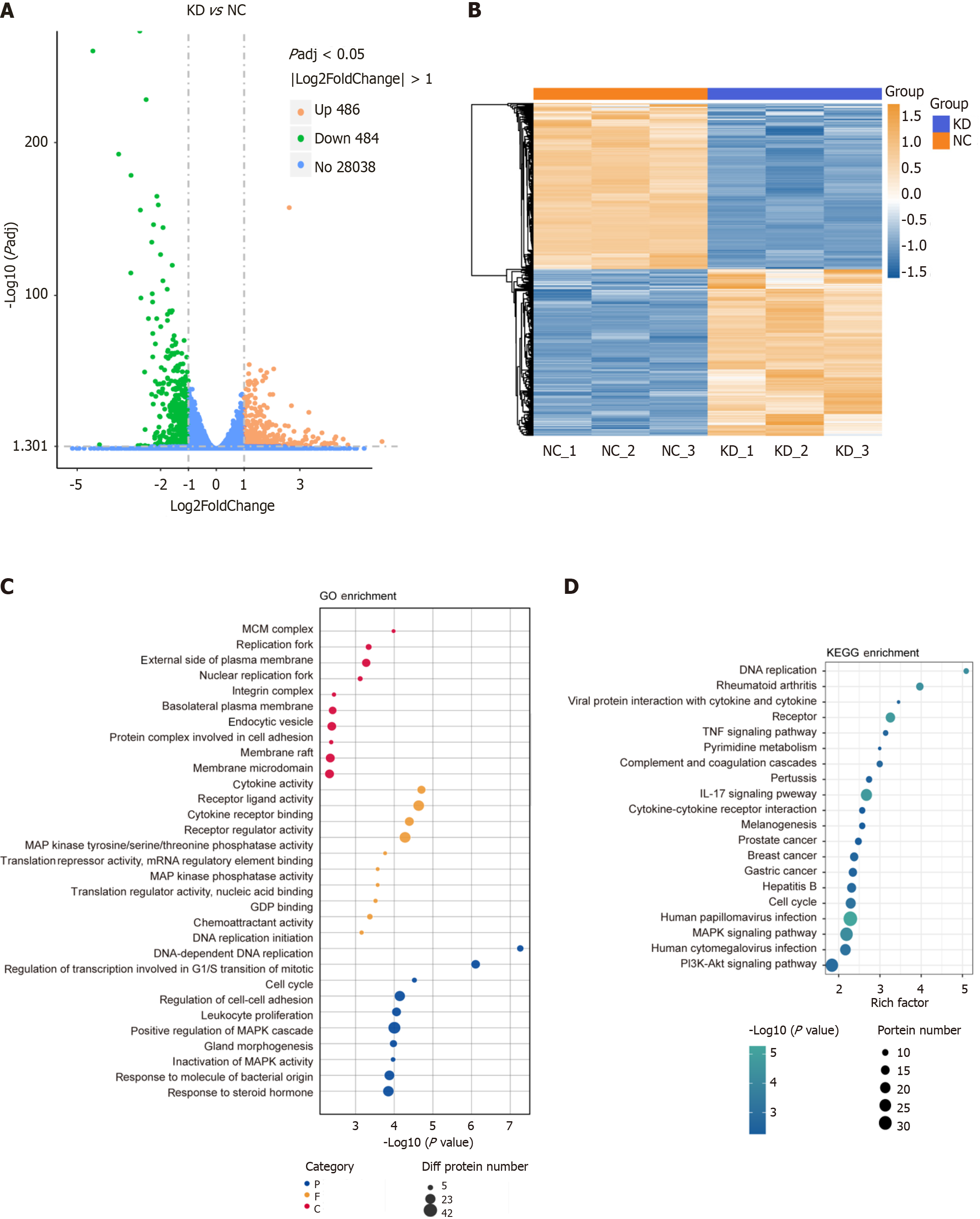
DDX10 knockdown affects cellular signaling pathways in PC cells. RNA-seq and label-free quantitative proteomics were performed on PANC-1 cells following DDX10 knockdown. RNA-seq identified 970 differentially expressed genes (486 upregulated and 484 downregulated; Figure 5A) using hierarchical clustering, which revealed distinct expression profiles (Figure 5B). The functional enrichment analysis highlighted terms related to DNA replication, cell adhesion, transcription/translation regulation (Figure 5C), and KEGG pathways including DNA replication, cell cycle, and PI3K/AKT signaling (Figure 5D).
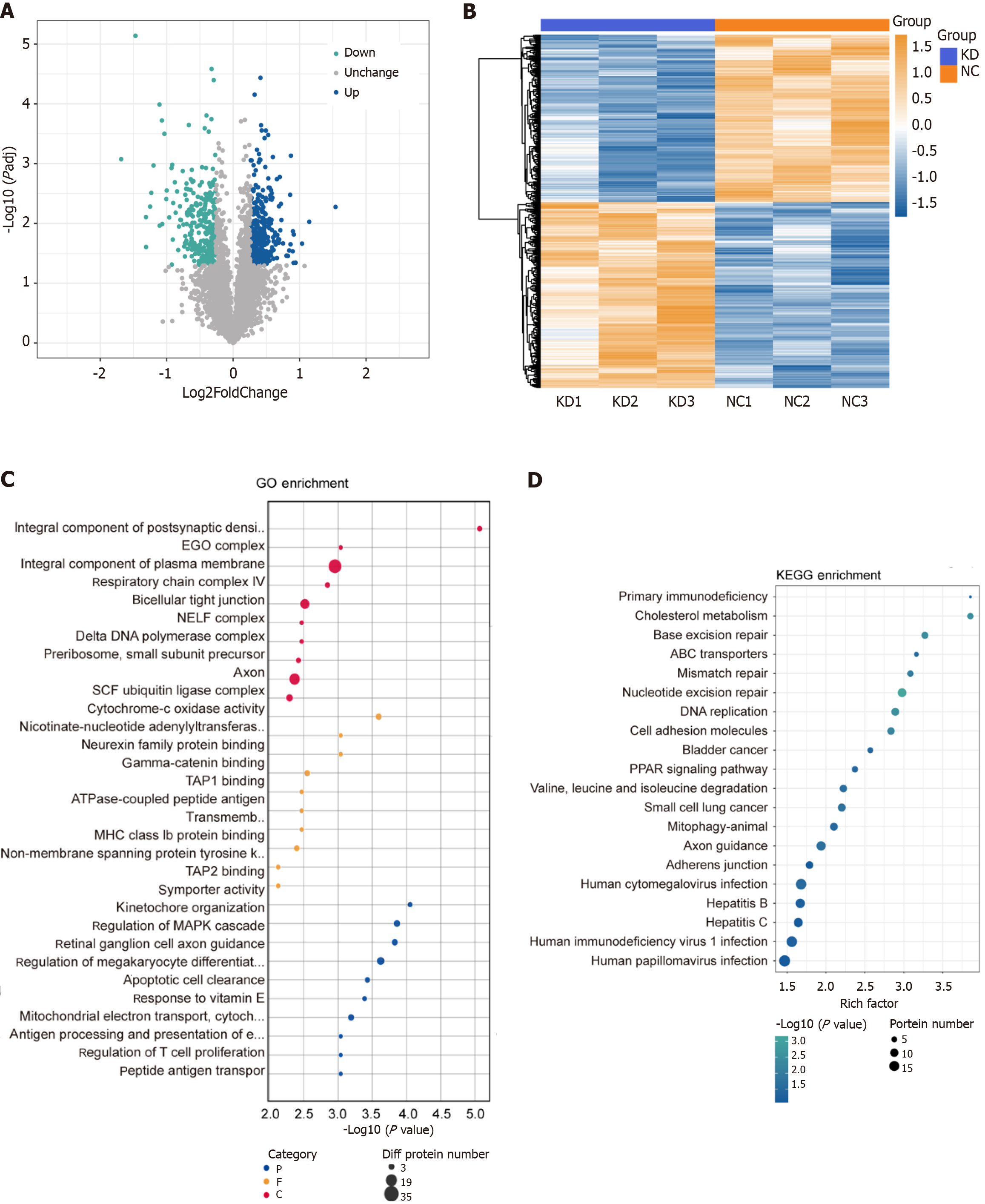
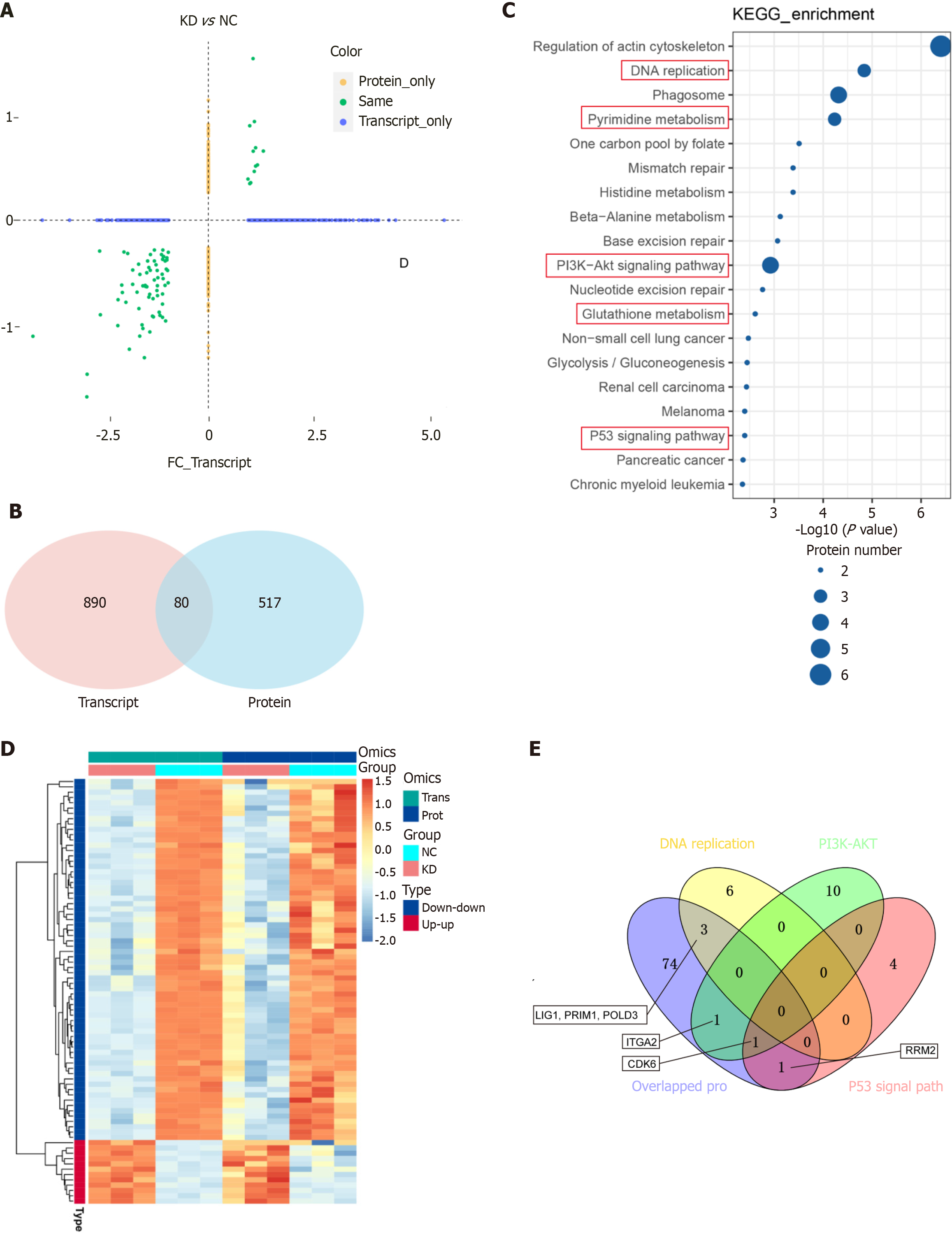
The proteomic analysis detected 597 differentially expressed proteins (314 upregulated and 283 downregulated; Figure 6A), and clustering and enrichment analyses identified key pathways, such as cholesterol metabolism and DNA replication (Figure 6B-D). The integration of RNA-seq and proteomic data revealed 80 overlapping genes with consistent expression trends (Figure 7A and B), which were primarily enriched in pathways that included pyrimidine metabolism and PI3K/AKT signaling (Figure 7C and D). Notably, ITGA2, CDK6, LIG1, PRIM1, POLD3, and RRM2 were identified as potential key mediators of DDX10 function in PC (Figure 7E).
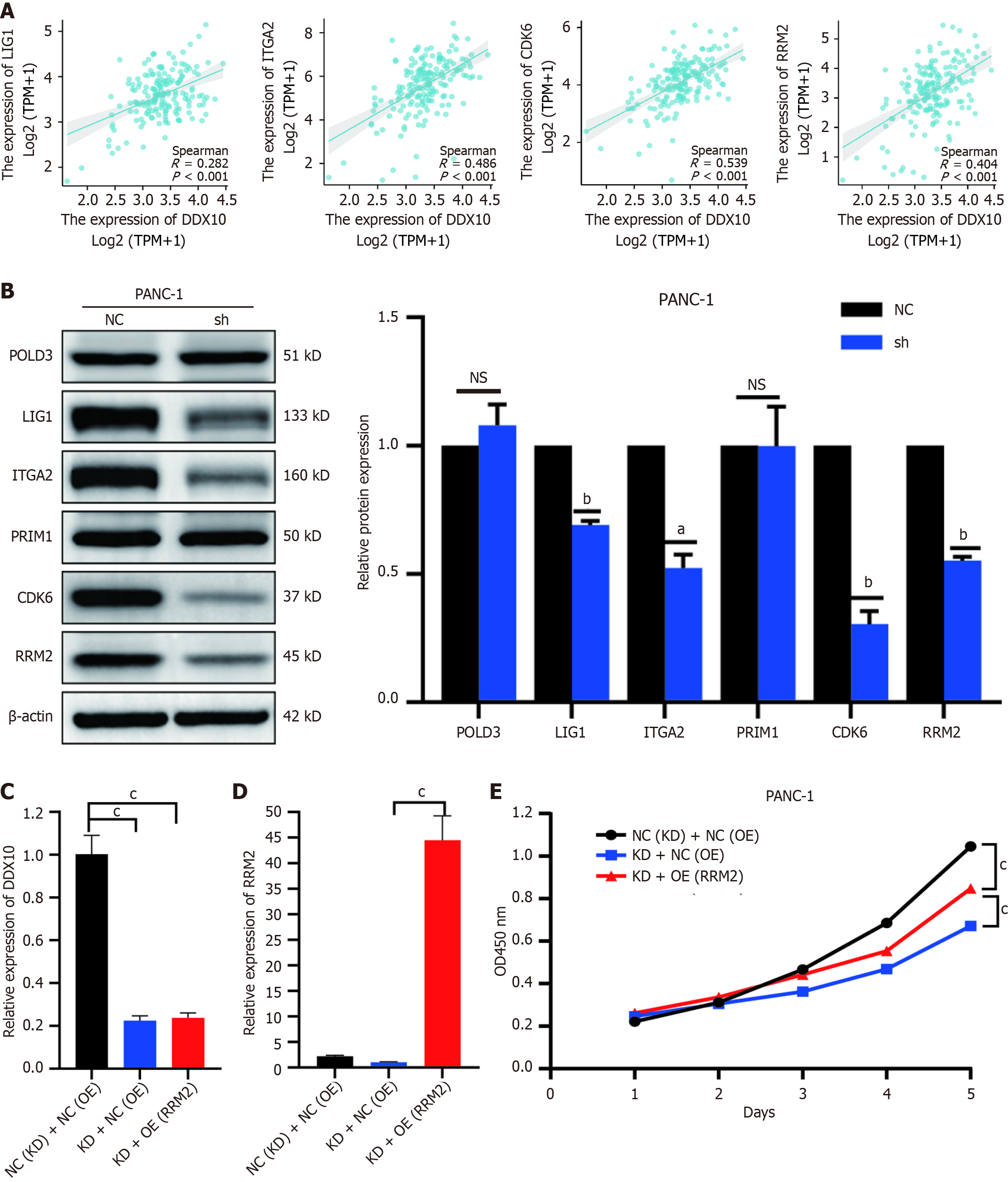
To investigate the correlation between the six identified genes (ITGA2, CDK6, LIG1, PRIM1, POLD3, and RRM2) and DDX10, we analyzed PC data from TCGA using the Xiantao academic platform (https://www.xiantaozi.com). The results showed that all six proteins were positively correlated with the clinical significance of DDX10 (Figure 8A and Supplementary Figure 2). The analysis also showed that ITGA2, RRM2, LIG1, and CDK6 expression levels were significantly reduced in DDX10-knockdown (shDDX10) cells (Figure 8B). The KEGG enrichment analysis highlighted the significant involvement of the P53 signaling pathway, with CDK6 and RRM2 as key players. Given the limited efficacy of CDK6 inhibitors in the clinic, we focused on RRM2 as a potential therapeutic target. Functional rescue experiments in PANC-1 cells revealed that RRM2 overexpression (OE) restored cell proliferation in DDX10-knockdown cells (Figure 8C-E). These findings suggest that RRM2 is a critical mediator in the modulation of PC cell growth in response to DDX10.
The findings of the present study provide new insights into the role and underlying mechanisms of the RNA helicase DDX10 in driving PC cell proliferation. Immunohistochemical analysis revealed significantly higher DDX10 Levels in PC tissues than in non-tumorous pancreatic tissues. Furthermore, data from microarray analyses sourced from the TCGA and GEO repositories further confirmed that DDX10 expression was markedly elevated in cancerous tissues compared to that in normal pancreatic tissues. The functional role of DDX10 was examined in two PC cell lines, AsPC-1 and PANC-1. Silencing of DDX10 using shRNA resulted in significant reductions in cell proliferation, migration, and invasion, whereas apoptosis was notably increased compared to that in cells infected with control shRNA. These findings suggest that DDX10 plays a key role in pancreatic tumor progression. Additionally, our integrative omics analysis revealed that DDX10 enhanced PC cell proliferation by regulating RRM2. To the best of our knowledge, this is the first study to exa
DDX10, a member of the DEAD-box family of RNA helicases, is implicated in the progression of various cancers. Previous studies have linked DDX10 OE to a poor prognosis in osteosarcoma, whereas its deficiency inhibits tumor progression by suppressing MAPK signaling[13]. Our transcriptome pathway enrichment analysis revealed significant enrichment of the MAPK signaling pathway, which suggests a similar potential role for DDX10 in PC. Notably, the DDX family of proteins is a key regulator of RNA splicing and is involved in spliceosome assembly. For example, DDX10 promotes colorectal cancer cell proliferation and metastasis by modulating RPL35 splicing[16], while DDX39B enhances FUT3 mRNA levels by influencing FUT3 precursor mRNA splicing[22]. In ovarian cancer, DDX10 promotes cell proliferation via the Akt/NF-κB signaling pathway, even at low expression levels[14]. Despite limited research, these studies underscore the need for further exploration of DDX10 mechanisms in various cancers.
In the present study, a combination of transcriptome and proteome analyses identified 80 differentially expressed genes with consistent trends. GO functional enrichment analysis revealed that these genes were primarily involved in biological processes such as transcriptional regulation of the G1/S transition in the mitotic cell cycle and DNA synthesis. At the molecular level, these genes were associated with protein complex binding, enzymatic activity, and ubiquitin-protein ligase activity. The KEGG pathway analysis highlighted significant enrichment in pathways related to DNA replication, pyrimidine metabolism, drug metabolism (other enzymes), P53 signaling, and PI3K-AKT signaling. These findings suggest that DDX10 may influence PC cell behavior by interacting with protein complexes and enzymes, thus potentially regulating the cell cycle and DNA synthesis via the P53, PI3K-AKT, and pyrimidine metabolism pathways. Further studies indicated that RRM2, a key enzyme in pyrimidine metabolism[21,23], plays a crucial role in gemcitabine resistance by enhancing pyrimidine biosynthesis and reducing DNA replication stress[21]. OE of RRM2 in gemcitabine-resistant KBGem cells increases the dCTP pool, which competes with gemcitabine[24]. Gambogic acid down-regulates RR, inhibits PC cell invasion, and regulates gemcitabine activity[25]. Given the poor clinical response to gemcitabine (< 20%)[26], exploring the relationship between DDX10 and RRM2 is essential for improving chemotherapeutic strategies for PC.
RRM1 and RRM2 are rate-limiting enzymes in pyrimidine metabolism that are essential for maintaining the deoxyribonucleoside triphosphate (dNTP) pool and for the efficient DNA replication processes that are integral to cancer progression[27-29]. We observed a positive correlation between DDX10 and RRM2 expression in PC tissues. RRM2 OE reversed the inhibitory effects of DDX10 knockdown on PC cell proliferation, supporting the hypothesis that DDX10 promotes cancer progression by regulating RRM2 expression. RRM2 is also a prognostic biomarker in several cancers, including colon[30], breast[31], and pancreatic[32] cancers, and its OE enhances cell migration, invasion, and proliferation. RRM2 is directly regulated by the p53 transcription factor, which is activated by cellular stress signals such as oxidative stress, DNA damage, nutrient deficiency, and hypoxia[33]. Prior research has indicated that SETDB1 suppresses p53 expression, whereas its absence upregulates p53 expression and promotes apoptosis, thus inhibiting pancreatic ductal adenocarcinoma development[34]. In addition, UBE2T mediates p53 degradation, thereby promoting gemcitabine resistance in PC cells[21]. Given the involvement of RRM2 in DNA replication and gemcitabine resistance, and p53 the regulatory role, DDX10 may accelerate PC progression via the p53/RRM2 axis. Further experimental validation is required to better understand these hypotheses.
In conclusion, our study revealed that DDX10 OE in PC tissues is correlates with poor patient survival. Knockdown of DDX10 using RNA interference significantly inhibited PC cell proliferation, migration, and invasion and promoted apoptosis. DDX10 also affects the expression of key oncogenes such as RRM2, ITGA2, CDK6, and LIG1. Furthermore, DDX10 mediates oncogenic effects in PC by regulating RRM2 expression Further investigation of DDX10 as a potential therapeutic target may facilitate the development of personalized treatment strategies for patients with PC.
The authors thank TCGA and GEO for providing the public data. We thank Genechem of Shanghai for their technical guidance on this experiment.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6068] [Article Influence: 3034.0] [Reference Citation Analysis (4)] |
| 2. | Wang Q, Liu J, Yang Z. Global, regional, and national burden of pancreatic cancer from 1990 to 2021, with projections for 25 years: a systematic analysis for the Global Burden of Disease Study 2021. Eur J Cancer Prev. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Li YJ, Wu JY, Wang JM, Xiang DX. Emerging nanomedicine-based strategies for preventing metastasis of pancreatic cancer. J Control Release. 2020;320:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Mosalem OM, Abdelhakeem A, Abdel-Razeq NH, Babiker H. Pancreatic ductal adenocarcinoma (PDAC): clinical progress in the last five years. Expert Opin Investig Drugs. 2025;34:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Kovalski JR, Kuzuoglu-Ozturk D, Ruggero D. Protein synthesis control in cancer: selectivity and therapeutic targeting. EMBO J. 2022;41:e109823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 6. | Andrisani O, Liu Q, Kehn P, Leitner WW, Moon K, Vazquez-Maldonado N, Fingerman I, Gale M Jr. Biological functions of DEAD/DEAH-box RNA helicases in health and disease. Nat Immunol. 2022;23:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Ali MAM. The DEAD-box protein family of RNA helicases: sentinels for a myriad of cellular functions with emerging roles in tumorigenesis. Int J Clin Oncol. 2021;26:795-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Tanaka K, Ikeda N, Miyashita K, Nuriya H, Hara T. DEAD box protein DDX1 promotes colorectal tumorigenesis through transcriptional activation of the LGR5 gene. Cancer Sci. 2018;109:2479-2489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Song A, Liu B, Li W, Chen B, Gui P, Zhang H, Zhu C, Xu Y, Jiang T, Song J. Competitive binding between DDX21 and SIRT7 enhances NAT10-mediated ac(4)C modification to promote colorectal cancer metastasis and angiogenesis- DDX21 promotes colorectal cancer metastasis. Cell Death Dis. 2025;16:353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Wu S, Sun X, Hua R, Hu C, Qin L. DDX21 functions as a potential novel oncopromoter in pancreatic ductal adenocarcinoma: a comprehensive analysis of the DExD box family. Discov Oncol. 2024;15:333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Yu H, Liu Y, Niu C, Cheng Y. Diosgenin increased DDX3 expression in hepatocellular carcinoma. Am J Transl Res. 2018;10:3590-3599. [PubMed] |
| 12. | Du C, Li DQ, Li N, Chen L, Li SS, Yang Y, Hou MX, Xie MJ, Zheng ZD. DDX5 promotes gastric cancer cell proliferation in vitro and in vivo through mTOR signaling pathway. Sci Rep. 2017;7:42876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Shi JH, Hao YJ. DDX10 overexpression predicts worse prognosis in osteosarcoma and its deletion prohibits cell activities modulated by MAPK pathway. Biochem Biophys Res Commun. 2019;510:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Gai M, Bo Q, Qi L. Epigenetic down-regulated DDX10 promotes cell proliferation through Akt/NF-κB pathway in ovarian cancer. Biochem Biophys Res Commun. 2016;469:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Jiao X, Hooper SD, Djureinovic T, Larsson C, Wärnberg F, Tellgren-Roth C, Botling J, Sjöblom T. Gene rearrangements in hormone receptor negative breast cancers revealed by mate pair sequencing. BMC Genomics. 2013;14:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Zhou X, Liu Z, He T, Zhang C, Jiang M, Jin Y, Wu Z, Gu C, Zhang W, Yang X. DDX10 promotes the proliferation and metastasis of colorectal cancer cells via splicing RPL35. Cancer Cell Int. 2022;22:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 17. | Feng Z, Qian H, Li K, Lou J, Wu Y, Peng C. Development and Validation of a 7-Gene Prognostic Signature to Improve Survival Prediction in Pancreatic Ductal Adenocarcinoma. Front Mol Biosci. 2021;8:676291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Knappenberger AJ, Ahmad MF, Viswanathan R, Dealwis CG, Harris ME. Nucleoside Analogue Triphosphates Allosterically Regulate Human Ribonucleotide Reductase and Identify Chemical Determinants That Drive Substrate Specificity. Biochemistry. 2016;55:5884-5896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Krawic C, Luczak MW, Valiente S, Zhitkovich A. Atypical genotoxicity of carcinogenic nickel(II): Linkage to dNTP biosynthesis, DNA-incorporated rNMPs, and impaired repair of TOP1-DNA crosslinks. J Biol Chem. 2023;299:105385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Chen G, Luo Y, Warncke K, Sun Y, Yu DS, Fu H, Behera M, Ramalingam SS, Doetsch PW, Duong DM, Lammers M, Curran WJ, Deng X. Acetylation regulates ribonucleotide reductase activity and cancer cell growth. Nat Commun. 2019;10:3213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Jiang X, Ma Y, Wang T, Zhou H, Wang K, Shi W, Qin L, Guan J, Li L, Long B, Wang J, Guan X, Ye H, Yang J, Yu Z, Jiao Z. Targeting UBE2T Potentiates Gemcitabine Efficacy in Pancreatic Cancer by Regulating Pyrimidine Metabolism and Replication Stress. Gastroenterology. 2023;164:1232-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 22. | He C, Li A, Lai Q, Ding J, Yan Q, Liu S, Li Q. The DDX39B/FUT3/TGFβR-I axis promotes tumor metastasis and EMT in colorectal cancer. Cell Death Dis. 2021;12:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Giang LH, Wu KS, Lee WC, Chu SS, Do AD, Changou CA, Tran HM, Hsieh TH, Chen HH, Hsieh CL, Sung SY, Yu AL, Yen Y, Wong TT, Chang CC. Targeting of RRM2 suppresses DNA damage response and activates apoptosis in atypical teratoid rhabdoid tumor. J Exp Clin Cancer Res. 2023;42:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | Zhan Y, Jiang L, Jin X, Ying S, Wu Z, Wang L, Yu W, Tong J, Zhang L, Lou Y, Qiu Y. Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed Pharmacother. 2021;133:110996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Xia G, Wang H, Song Z, Meng Q, Huang X, Huang X. Gambogic acid sensitizes gemcitabine efficacy in pancreatic cancer by reducing the expression of ribonucleotide reductase subunit-M2 (RRM2). J Exp Clin Cancer Res. 2017;36:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Dorjee P, Long ZW. A mixed treatment comparison of toxicity of gemcitabine combined with different targeted drugs in the treatment of advanced or metastatic pancreatic cancer. Cancer Biol Ther. 2018;19:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 795] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 28. | Shu Z, Li Z, Huang H, Chen Y, Fan J, Yu L, Wu Z, Tian L, Qi Q, Peng S, Wei C, Xie Z, Li X, Feng Q, Sheng H, Li G, Wei D, Shan C, Chen G. Cell-cycle-dependent phosphorylation of RRM1 ensures efficient DNA replication and regulates cancer vulnerability to ATR inhibition. Oncogene. 2020;39:5721-5733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Tian L, Chen C, Guo Y, Zhang F, Mi J, Feng Q, Lin S, Xi N, Tian J, Yu L, Chen Y, Cao M, Lai C, Fan J, Zhang Y, Chen G. mTORC2 regulates ribonucleotide reductase to promote DNA replication and gemcitabine resistance in non-small cell lung cancer. Neoplasia. 2021;23:643-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Moon SU, Lee JH, Shah M, Yoon S, Woo HG. RRM2 Is a CTNNB1 Transport Regulator Promoting Colon Cancer Progression. Anticancer Res. 2024;44:2471-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Shi SC, Zhang Y, Wang T. High RRM2 expression has poor prognosis in specific types of breast cancer. PLoS One. 2022;17:e0265195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Du Z, Zhang Q, Xiang X, Li W, Yang Q, Yu H, Liu T. RRM2 promotes liver metastasis of pancreatic cancer by stabilizing YBX1 and activating the TGF-beta pathway. iScience. 2024;27:110864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Ou A, Zhao X, Lu Z. The potential roles of p53 signaling reactivation in pancreatic cancer therapy. Biochim Biophys Acta Rev Cancer. 2022;1877:188662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Ogawa S, Fukuda A, Matsumoto Y, Hanyu Y, Sono M, Fukunaga Y, Masuda T, Araki O, Nagao M, Yoshikawa T, Goto N, Hiramatsu Y, Tsuda M, Maruno T, Nakanishi Y, Hussein MS, Tsuruyama T, Takaori K, Uemoto S, Seno H. SETDB1 Inhibits p53-Mediated Apoptosis and Is Required for Formation of Pancreatic Ductal Adenocarcinomas in Mice. Gastroenterology. 2020;159:682-696.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/