Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.106910
Revised: June 3, 2025
Accepted: July 23, 2025
Published online: September 15, 2025
Processing time: 138 Days and 0.3 Hours
Pancreatic cancer is a highly aggressive malignancy with a dismal prognosis, primarily due to its late diagnosis. Current gold-standard diagnostic methods, such as tissue histopathological examination, are invasive and carry risks of complications (e.g., bleeding, infection, pancreatic fistula), limiting their routine use. Serum tumor markers [carbohydrate antigen 19-9 (CA-19-9), carcinoembryonic antigen (CEA)] have been widely studied for non-invasive screening, but their single use lacks sufficient sensitivity and specificity for early detection. Emerging research suggests that inflammatory and stromal-related molecules, such as soluble intercellular adhesion molecule-1 (sICAM-1) (involved in tumor cell adhesion and metastasis) and chitinase-3-like protein 1 (CHI3 L1) (a marker of tissue remodeling and inflammation), may complement traditional markers in improving diagnostic accuracy. However, their combined utility in pancreatic cancer diagnosis, particularly for differentiating early-stage tumors, remains unclear.
To explore the application value of serum tumor markers (CA-19-9, CEA) and serum sICAM-1 and CHI3 L1 in the early diagnosis of pancreatic cancer.
From October 2021 to October 2024, 51 patients with pancreatic cancer were selected for the pancreatic cancer group, and 51 healthy examinees were selected for the healthy group during the same period. The value of serum tumor markers in combination with serum sICAM-1 and CHI3 L1 for the early diagnosis of pancreatic cancer was assessed.
Comparison of age, gender, body mass index, and drinking and smoking histories between the two groups was not statistically significant (P > 0.05); serum tumor marker (CA-19-9, CEA), serum sICAM-1 and CHI3 L1 levels were higher in the pancreatic cancer group (P < 0.05); pancreatic cancer patients of stage II had higher serum tumor marker (CA-19-9, CEA), serum sICAM-1, and CHI3 L1 values (P < 0.05); serum tumor markers, serum sICAM-1, and CHI3 L1 were positively correlated with pancreatic cancer (P < 0.05) and showed a positive correlation with stage I and stage II. Pancreatic cancer showed a positive correlation (P < 0.05); multifactor logistic regression analysis showed that serum tumor markers (CA-19-9, CEA), serum sICAM-1 and CHI3 L1 were independent risk factors for pancreatic cancer (P < 0.05); serum tumor markers (CA-19-9, CEA), serum sICAM-1, serum tumor marker (CA-19-9, CEA) and serum sICAM-1 and CHI3 L1 had area under the curves (AUCs) of 0.750, 0.724, 0.585, and 0.562 for pancreatic cancer diagnosis, respectively; AUCs for stage I diagnosis were 0.766, 0.752, 0.622, and 0.572 and for stage II diagnosis were 0.783, 0.758, 0.626, and 0.671, respectively, and the AUCs for the combined diagnosis of pancreatic cancer, stage I pancreatic cancer, and stage II pancreatic cancer were 0.782, 0.824, and 0.862, respectively (P < 0.05).
The combined detection of serum tumor markers with serum sICAM-1 and serum CHI3 L1 can significantly improve the accuracy and sensitivity of the diagnosis of pancreatic cancer and its different stages.
Core Tip: Serum levels of soluble intercellular adhesion molecule-1 and chitinase-3-like protein 1 are markedly elevated in pancreatic cancer patients, with these biomarkers showing strong correlations with tumor invasiveness, metastatic potential, and patient prognosis. This study is to investigate the value of serum tumour markers in combination with serum soluble intercellular adhesion molecule-1 and serum chitinase-3-like protein 1 in the early diagnosis of pancreatic cancer. The combined detection can significantly improve the accuracy and sensitivity of the diagnosis of pancreatic cancer and its different stages, providing a new effective concept for the early diagnosis of pancreatic cancer.
- Citation: Deng QX, Tang XL, Yuan GJ, Jian Q, Chen XH, Wu LJ. Study of serum tumor markers in the early diagnosis of pancreatic cancer. World J Gastrointest Oncol 2025; 17(9): 106910
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/106910.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.106910
Pancreatic cancer is a highly malignant tumor with a complex etiology and pathogenesis involving multiple factors such as smoking, alcohol consumption, high-fat diet, obesity, and genetic predisposition[1]. Genetic mutations and abnormal expression play pivotal roles in the development and progression of pancreatic cancer, where genetic alterations lead to uncontrolled cell proliferation, inhibition of apoptosis, and enhanced invasive and metastatic capabilities, ultimately resulting in tumor formation[1]. Early symptoms of pancreatic cancer are often nonspecific. However, as the disease progresses, clinical manifestations such as abdominal pain, jaundice, and weight loss may emerge. Due to the deep anatomical location of the pancreas, symptoms typically appear only when the tumor has reached a considerable size, leading to late-stage diagnosis in most patients[2-5]. The global incidence of pancreatic cancer continues to rise annually, with an extremely poor prognosis. The 5-year survival rate remains below 10%, dropping to approximately 3% for patients with advanced metastatic disease[6]. In the diagnosis of pancreatic cancer, numerous challenges are faced. Among them, histopathological examination serves as the gold standard for pancreatic cancer diagnosis, but its invasive nature and potential risks, such as bleeding, infection, pancreatic fistula, etc., limit its widespread application[7]. Additionally, puncture biopsy is prone to false-negative results due to insufficient sampling or inaccurate sampling sites. For patients with poor physical condition or unfavorable tumor location, puncture biopsy is generally difficult to perform[8].
Histopathological examination remains the gold standard for pancreatic cancer diagnosis. However, its invasive nature and associated risks such as bleeding, infection, and pancreatic fistula limit widespread clinical application[7]. Furthermore, needle biopsy often faces challenges including insufficient sampling, false-negative results due to inaccurate targeting, and technical difficulties in patients with poor physical condition or unfavorable tumor locations[8]. Serum tumor markers [carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA)] play significant roles in pancreatic cancer diagnosis. CA19-9 demonstrates high positivity rates in pancreatic cancer patients, while the combined detection of CEA and CA19-9 significantly enhances diagnostic accuracy despite CEA’s nonspecific elevation in various malignancies[9,10]. Additionally, serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and chitinase-3-like protein 1 (CHI3 L1) are markedly elevated in pancreatic cancer patients, with these biomarkers showing strong correlations with tumor invasiveness, metastatic potential, and patient prognosis[11,12]. In the field of tumor diagnosis, serum tumor marker testing aims to reflect the presence and characteristics of tumors through blood analysis. Therefore, an ideal tumor marker should exhibit high sensitivity and specificity, thereby allowing for the early detection of tumors while assisting in clinical diagnosis, treatment monitoring, and prognostic evaluation. However, no single serum tumor marker has currently achieved full satisfaction of these requirements. Consequently, the combined detection of multiple markers to enhance diagnostic accuracy has emerged as a prominent research focus. This study aims to investigate the clinical value of combined detection of Serum tumor markers (CA-19-9, CEA) with sICAM-1 and CHI3 L1 in the early diagnosis of pancreatic cancer. The detailed findings are reported below.
Study population: This retrospective study enrolled 51 pancreatic cancer patients (Pancreatic Cancer Group) and 51 healthy individuals undergoing routine physical examinations (Healthy Group) from October 2021 to October 2024 at our hospital.
Inclusion criteria: (1) Pancreatic Cancer Group patients met the diagnostic criteria outlined in the Expert Consensus on Integrated Traditional Chinese and Western Medicine Diagnosis and Treatment of Pancreatic Cancer; (2) Availability of complete clinical and pathological data; and (3) All participants provided written informed consent.
Exclusion criteria: (1) Presence of other malignancies; Recent history of tumor resection surgery; (2) Concurrent severe organ failure (e.g., hepatic or renal failure); and (3) Prior chemotherapy or other antitumor therapies before enrollment.
Demographic and clinical data were collected from participants, including age, sex, body mass index, history of alcohol consumption, smoking status, serum tumor marker levels (CA19-9, CEA), and serum levels of sICAM-1 and CHI3 L1.
Reference ranges in healthy populations: The normal range of CA-19-9 is 0-37 U/mL (values > 37 U/mL suggest a potential malignant tumor). The normal range of CEA is < 5 ng/mL (values > 5 ng/mL suggest a potential malignant tumor). The normal range of sICAM-1 is 100-200 μg/L (values > 200 μg/L suggest a potential malignant tumor). The normal range of CHI3 L1 is < 79 ng/mL (values > 79 ng/mL suggest a potential malignant tumor).
Detection methods: (1) Sample preparation: Fasting venous blood (5 mL) was collected from participants and centrifuged at 3000 rpm for 10 minutes to separate the supernatant for subsequent analyses; and (2) Serum tumor markers (CA-19-9, CEA) were measured using the Roche cobas 6000 automated analyzer via electrochemiluminescence immunoassay. sICAM-1 and CHI3 L1 were quantified using enzyme-linked immunosorbent assay with an Agilent Cary 3500 microplate reader. The enzyme-linked immunosorbent assaykits for CA19-9 and CEA were obtained from Thermo Fisher Scientific and AmyJet Scientific Inc., respectively.
Data were analyzed using SPSS version 26.0. Continuous variables conforming to a normal distribution were expressed as mean ± SD and compared between groups via independent samples t-test. Categorical variables were summarized as frequency and percentage (%), with intergroup differences assessed by χ2 test. Multivariate logistic regression analysis was employed to identify correlations between serum tumor markers (CA-19-9, CEA), serum sICAM-1, CHI3 L1 Levels, and pancreatic cancer risk. Receiver operating characteristic (ROC) curves were generated using the rms package in R 4.3.2 to evaluate diagnostic performance. A two-tailed P < 0.05 was considered statistically significant.
No statistically significant differences were observed between the two groups in age, sex, body mass index, alcohol consumption history, or smoking history (P > 0.05) (Table 1).
| Parameter | Pancreatic cancer group (n = 51) | Healthy group (n = 51) | χ2/t | P value | |
| Age (years), mean ± SD | 56.41 ± 2.79 | 56.38 ± 2.40 | 0.0558 | 0.954 | |
| Sex, male | 22 (43.14) | 24 (47.06) | 0.158 | 0.691 | |
| BMI (kg/m2) | 22.37 ± 1.41 | 21.56 ± 1.44 | - | - | |
| Smoking history | Yes | 31 (60.78) | 29 (56.86) | 0.162 | 0.687 |
| No | 20 (39.22) | 22 (43.14) | - | - | |
| Alcohol history | Yes | 40 (78.43) | 38 (74.51) | 3.539 | 0.060 |
| No | 11 (21.57) | 13 (25.49) | - | - | |
| Heart rate (beats/minute), mean ± SD | 79.27 ± 4.38 | 78.53 ± 3.15 | 0.980 | 0.330 | |
| Systolic BP (mmHg), mean ± SD | 124.76 ± 5.81 | 125.17 ± 5.32 | 0.372 | 0.711 | |
| Diastolic BP (mmHg), mean ± SD | 74.33 ± 2.78 | 75.29 ± 2.38 | 1.873 | 0.064 | |
Patients in the Pancreatic Cancer Group exhibited significantly higher serum levels of tumor markers (CA19-9, CEA), sICAM-1, and CHI3 L1 compared to the Healthy Group (P < 0.05), as detailed in Table 2.
| Parameter | Pancreatic cancer group (n = 51) | Healthy group (n = 51) | χ2/t | P value |
| CA-19-9 (U/mL) | 128.41 ± 10.79 | 28.38 ± 3.40 | 63.145 | < 0.001 |
| CEA (ng/mL) | 11.96 ± 2.23 | 4.64 ± 0.87 | 21.839 | < 0.001 |
| sICAM-1 (μg/L) | 242.57 ± 21.41 | 153.56 ± 12.44 | 25.671 | < 0.001 |
| CHI3 L1 (ng/mL) | 153.13 ± 27.97 | 68.68 ± 7.32 | 20.860 | < 0.001 |
Patients with stage II pancreatic cancer exhibited significantly higher serum levels of tumor markers (CA19-9, CEA), sICAM-1, and CHI3 L1 compared to those with stage I disease (P < 0.05), as shown in Table 3.
| Group | n | CA-19-9 (U/mL) | CEA (U/mL) | sICAM-1 (μg/L) | CHI3 L1 (ng/mL) |
| Stage I pancreatic cancer | 24 | 98.43 ± 6.21 | 8.62 ± 1.12 | 213.51 ± 26.31 | 167.92 ± 5.47 |
| Stage II pancreatic cancer | 27 | 131.37 ± 22.58 | 12.39 ± 2.22 | 254.82 ± 25.79 | 192.46 ± 11.39 |
| t | - | 10.045 | 10.828 | 8.007 | 13.870 |
| P value | - | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The Pearson correlation analysis revealed that Serum tumor markers (CA-19-9, CEA), serum sICAM-1, and CHI3 L1 Levels were positively correlated with pancreatic cancer (P < 0.05). Specifically, these markers showed positive correlations with stage I and stage II pancreatic cancer (P < 0.05), as shown in Table 4.
| Parameter | Pancreatic cancer | Stage I pancreatic cancer | Stage II pancreatic cancer | |||
| r | P value | r | P value | r | P value | |
| CA-19-9 | 0.639 | < 0.001 | 0.641 | < 0.001 | 0.678 | < 0.001 |
| CEA | 0.625 | < 0.001 | 0.632 | < 0.001 | 0.662 | < 0.001 |
| sICAM-1 | 0.529 | < 0.001 | 0.552 | < 0.001 | 0.583 | < 0.001 |
| CHI3 L1 | 0.517 | < 0.001 | 0.534 | < 0.001 | 0.571 | < 0.001 |
Multivariate logistic regression analysis showed that Serum tumor markers (CA-19-9, CEA), serum sICAM-1, and CHI3 L1 were independent risk factors for pancreatic cancer (P < 0.05) (Table 5).
| Factor | β-value | SE | χ2 | P value | OR | 95%CI | |
| Lower | Upper | ||||||
| CA-19-9 (U/mL) | 0.059 | 0.028 | 4.440 | 0.035 | 1.061 | 1.004 | 1.121 |
| CEA (ng/mL) | 0.035 | 0.013 | 7.249 | 0.007 | 1.036 | 1.010 | 1.062 |
| sICAM-1 (μg/L) | 0.071 | 0.016 | 19.691 | 0.000 | 1.074 | 1.040 | 1.108 |
| CHI3 L1 (ng/mL) | 0.076 | 0.028 | 7.367 | 0.007 | 1.079 | 1.021 | 1.140 |
| Constant | -3.143 | 0.834 | 14.202 | 0.000 | - | - | - |
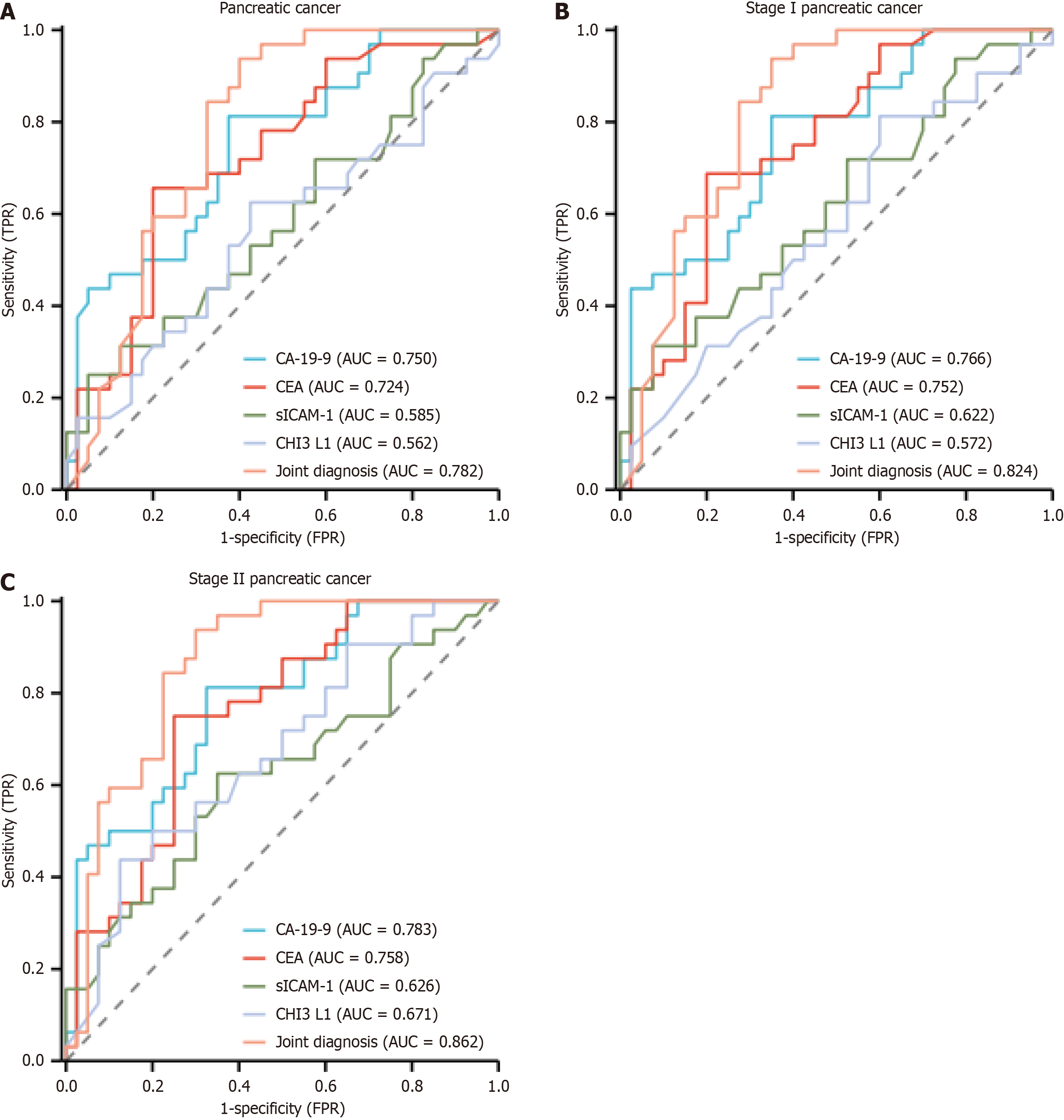
The AUCs for diagnosing pancreatic cancer using serum tumor markers (CA-19-9, CEA) (CA-19-9, CEA), serum sICAM-1, and CHI3 L1 were 0.750, 0.724, 0.585, and 0.562, respectively. For stage I, the AUCs were 0.766, 0.752, 0.622, and 0.572; for stage II, the AUCs were 0.783, 0.758, 0.626, and 0.671. The combined diagnostic AUCs for pancreatic cancer, stage I, and stage II pancreatic cancer were 0.782, 0.824, and 0.862, respectively (P < 0.05) (Figure 1).
Pancreatic cancer is associated with common risk factors such as smoking, unhealthy diet, excessive alcohol consumption, environmental pollution, genetic predisposition, diabetes, and chronic pancreatitis. Its pathogenesis is complex, involving mutations in genes like K-ras and P53, as well as dysregulation of molecular pathways related to inflammation, immune evasion, and angiogenesis[13-15]. Early symptoms of pancreatic cancer are often nonspecific and insidious, including abdominal pain, weight loss, and jaundice, making early diagnosis challenging. Most patients are diagnosed at advanced stages, leading to poor treatment outcomes. Histopathological examination remains the gold standard for diagnosis, but its invasiveness and potential complications (e.g., bleeding, infection) limit its clinical utility[16,17]. Additionally, tumor heterogeneity in pancreatic cancer may introduce significant variability in pathological findings. Serum tumor markers (CA-19-9, CEA), serum sICAM-1, and CHI3 L1 Levels play critical roles in early diagnosis and prognosis assessment. While CA-19-9 exhibits high sensitivity for pancreatic cancer, its levels can also be elevated in benign conditions. CEA, though less specific, is associated with various gastrointestinal malignancies. Both sICAM-1 and CHI3 L1 are inversely correlated with pancreatic cancer prognosis, suggesting their potential value in early screening[18,19].
CA-19-9, a carbohydrate antigen, is a mucin-type glycoprotein secreted by pancreatic acinar cells and biliary epithelial cells[20]. In this study, the level of CA-19-9 in the healthy group was significantly lower than that in the pancreatic cancer group. The CA-19-9 Level in the stage I pancreatic cancer group was significantly lower than that in the stage II pancreatic cancer group, and the data showed statistically significant differences (P < 0.05). CA-19-9 was positively correlated with pancreatic cancer, stage I, and stage II pancreatic cancer. Analysis suggests that under normal conditions, the content of CA-19-9 in human blood is low. However, when pancreatic tissue becomes cancerous, pancreatic cancer cells synthesize and secrete large amounts of CA-19-9, leading to a significant increase in CA-19-9 Levels in the blood.
Studies indicate that CA-19-9 Levels correlate with tumor aggressiveness, size, and metastasis, rising progressively with disease progression. However, CA-19-9 is not specific to pancreatic cancer, as conditions like cholangitis and pancreatitis can also elevate its levels, albeit with distinct patterns of elevation and duration. Thus, CA-19-9 serves as a valuable adjunct for diagnosis, disease monitoring, and treatment evaluation but cannot independently confirm pancreatic cancer.
CEA, a glycoprotein initially expressed in fetal gut and certain tumor cells, is a broad-spectrum tumor marker used in the diagnosis and monitoring of multiple cancers[21]. In this study, CEA levels were significantly lower in the healthy group compared to the pancreatic cancer group, with stage I patients showing lower levels than stage II patients (P < 0.05). CEA was positively correlated with pancreatic cancer across all stages. Although nonspecific for pancreatic cancer, elevated CEA levels are common in patients and may increase with tumor progression. However, factors like smoking and inflammatory diseases can also influence CEA levels. Thus, while CEA elevation supports pancreatic cancer diagnosis, it cannot independently confirm the disease.
sICAM-1, the soluble form of ICAM-1, belongs to the immunoglobulin superfamily and is involved in immune cell adhesion, migration, and signaling. Its levels are closely linked to tumorigenesis, progression, and invasion[22,23]. In this study, sICAM-1 Levels were significantly lower in the healthy group compared to the pancreatic cancer group, with stage I patients showing lower levels than stage II patients (P < 0.05). sICAM-1 correlated positively with pancreatic cancer at all stages. This may reflect tumor microenvironment-driven inflammation, where cytokines (e.g., tumor necrosis factor α, interleukin-1β) induce sICAM-1 expression and proteolytic shedding, leading to elevated systemic levels. Additionally, sICAM-1 may facilitate immune evasion and tumor invasion by modulating interactions between tumor and immune cells, highlighting its potential in early diagnosis and therapeutic monitoring.
CHI3 L1, a secretory glycoprotein of the 18-glycosylhydrolase family, is produced by macrophages, dendritic cells, chondrocytes, and tumor cells. It regulates processes like cell proliferation, migration, anti-inflammatory responses, and tissue remodeling[24]. In this study, CHI3 L1 Levels were significantly lower in the healthy group compared to the pancreatic cancer group, with stage I patients showing lower levels than stage II patients (P < 0.05). CHI3 L1 correlated positively with pancreatic cancer at all stages. It promotes tumor progression by modulating extracellular matrix metabolism, suppressing immune cell activity, and activating pathways like phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinase to enhance tumor survival and proliferation. Thus, CHI3 L1 holds promise as both a diagnostic/prognostic biomarker and a therapeutic target.
Multivariate logistic regression analysis confirmed that serum CA-19-9, CEA, sICAM-1, and CHI3 L1 are independent risk factors for pancreatic cancer. ROC curve analysis revealed the following AUC values for individual markers: Pancreatic cancer (0.750, 0.724, 0.585, 0.562), stage I (0.766, 0.752, 0.622, 0.572), and stage II (0.783, 0.758, 0.626, 0.671). Combined use of these markers achieved higher diagnostic performance, with AUCs of 0.782, 0.824, and 0.862 for pancreatic cancer, stage I, and stage II, respectively (P < 0.05), underscoring their clinical utility. However, this study has limitations: (1) Its retrospective design and limited sample size may introduce selection bias, necessitating future prospective, large-scale studies; and (2) Dynamic monitoring of these biomarkers was not performed.
This study demonstrated that the combined detection of CA19-9, CEA, sICAM-1 and CHI3 L1 can significantly improve the accuracy and sensitivity of the diagnosis of pancreatic cancer and its different stages. Among them, the combined detection of these markers has higher diagnostic value for early-stage pancreatic cancer, which provides a new idea and method for the early screening and diagnosis of pancreatic cancer. However, further large-scale, multi-center studies are needed to validate these findings and explore the potential clinical application value of this combined detection method.
| 1. | Bukys T, Kurlinkus B, Sileikis A, Vitkus D. The Prospect of Improving Pancreatic Cancer Diagnostic Capabilities by Implementing Blood Biomarkers: A Study of Evaluating Properties of a Single IL-8 and in Conjunction with CA19-9, CEA, and CEACAM6. Biomedicines. 2024;12:2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Gyimesi G, Keczer B, Rein P, Horváth M, Szűcs Á, Marjai T, Szijártó A, Hritz I. Diagnostic performance of intracystic carcinoembryonic antigen (CEA) versus glucose in differentiation of mucinous and non-mucinous pancreatic cysts. Pathol Oncol Res. 2024;30:1611881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Yablecovitch D, Nadler M, Ben-Horin S, Picard O, Yavzori M, Fudim E, Duchan MT, Sakhnini E, Lang A, Lahav M, Saker T, Neuman S, Selinger L, Freitz B, Dvir R, Raitses-Gurevich M, Golan T, Levy I, Laish I. Serum matrix metalloproteinase-7, Syndecan-1, and CA 19-9 as a biomarker panel for diagnosis of pancreatic ductal adenocarcinoma. Cancer Med. 2024;13:e70144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Yao J, Yan Y, Ruan M, Lin H, Li D, Zeng Z. Drug-induced Autoimmune-like Hepatitis With Pathological Features of Giant Cell Hepatitis. J Clin Exp Hepatol. 2025;15:102498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ahmadipour M, Bhattacharya A, Sarafbidabad M, Syuhada Sazali E, Krishna Ghoshal S, Satgunam M, Singh R, Rezaei Ardani M, Missaoui N, Kahri H, Pal U, Ling Pang A. CA19-9 and CEA biosensors in pancreatic cancer. Clin Chim Acta. 2024;554:117788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 6. | Stoop TF, Doppenberg D, Katz MHG, Tzeng CD, Wei AC, Zureikat AH, Groot Koerkamp B, Besselink MG; Trans-Atlantic Pancreatic Surgery (TAPS) Consortium. ASO Author Reflections: The Value of Serum CEA for Prognostication at Staging and Response Evaluation in Patients with Localized Pancreatic Adenocarcinoma and Nonelevated CA19-9. Ann Surg Oncol. 2024;31:1842-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Doppenberg D, Stoop TF, van Dieren S, Katz MHG, Janssen QP, Nasar N, Prakash LR, Theijse RT, Tzeng CD, Wei AC, Zureikat AH, Groot Koerkamp B, Besselink MG; Trans-Atlantic Pancreatic Surgery (TAPS) Consortium. Serum CEA as a Prognostic Marker for Overall Survival in Patients with Localized Pancreatic Adenocarcinoma and Non-Elevated CA19-9 Levels Treated with FOLFIRINOX as Initial Treatment: A TAPS Consortium Study. Ann Surg Oncol. 2024;31:1919-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | McConnell A, Stoneman T, Hewlett S. Extraordinarily high serum CA 19-9 in setting of pancreatic necrosis and underlying pancreatic adenocarcinoma: a case report. J Surg Case Rep. 2023;2023:rjad550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Lo Presti E, Cupaioli F, Scimeca D, Unti E, Di Martino V, Daidone R, Amata M, Scibetta N, Soucie E, Meraviglia S, Iovanna J, Dusetti N, De Gaetano A, Merelli I, Di Mitri R. The pancreatic tumor microenvironment of treatment-naïve patients causes a functional shift in γδ T cells, impairing their anti-tumoral defense. Oncoimmunology. 2025;14:2466301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Irajizad E, Kenney A, Tang T, Vykoukal J, Wu R, Murage E, Dennison JB, Sans M, Long JP, Loftus M, Chabot JA, Kluger MD, Kastrinos F, Brais L, Babic A, Jajoo K, Lee LS, Clancy TE, Ng K, Bullock A, Genkinger JM, Maitra A, Do KA, Yu B, Wolpin BM, Hanash S, Fahrmann JF. A blood-based metabolomic signature predictive of risk for pancreatic cancer. Cell Rep Med. 2023;4:101194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Janga LSN, Sambe HG, Yasir M, Man RK, Gogikar A, Nanda A, Mohammed L. Holistic Understanding of the Role of Carbohydrate Antigen 19-9 in Pancreatic Cancer Screening, Early Diagnosis, and Prognosis: A Systematic Review. Cureus. 2023;15:e44382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Nikiforova MN, Wald AI, Spagnolo DM, Melan MA, Grupillo M, Lai YT, Brand RE, O'Broin-Lennon AM, McGrath K, Park WG, Pfau PR, Polanco PM, Kubiliun N, DeWitt J, Easler JJ, Dam A, Mok SR, Wallace MB, Kumbhari V, Boone BA, Marsh W, Thakkar S, Fairley KJ, Afghani E, Bhat Y, Ramrakhiani S, Nasr J, Skef W, Thiruvengadam NR, Khalid A, Fasanella K, Chennat J, Das R, Singh H, Sarkaria S, Slivka A, Gabbert C, Sawas T, Tielleman T, Vanderveldt HD, Tavakkoli A, Smith LM, Smith K, Bell PD, Hruban RH, Paniccia A, Zureikat A, Lee KK, Ongchin M, Zeh H, Minter R, He J, Nikiforov YE, Singhi AD. A Combined DNA/RNA-based Next-Generation Sequencing Platform to Improve the Classification of Pancreatic Cysts and Early Detection of Pancreatic Cancer Arising From Pancreatic Cysts. Ann Surg. 2023;278:e789-e797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Yoo J, Lee JM, Joo I, Lee DH, Yoon JH, Yu MH, Jang JY, Lee SH. Post-neoadjuvant treatment pancreatic cancer resectability and outcome prediction using CT, (18)F-FDG PET/MRI and CA 19-9. Cancer Imaging. 2023;23:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 14. | Rosiek V, Bocian-Jastrzębska A, Kos-Kudła B. Selected Serum Biomarkers (Leptin, Chromogranin A, CA19-9, CEA) in Patients with Pancreatic Neuroendocrine Neoplasm and Associations with Metabolic Syndrome. Cancers (Basel). 2023;15:2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Zhao Y, Nie YH, Zhao YG, Zhang CF, Zhang ZH. [Diagnostic values of endoscopic ultrasound-guided fine-needle aspiration cytology combined with histopa- thology for pancreatic space occupying lesions]. Shiyong Yixue Zazhi. 2023;39:899-903. [DOI] [Full Text] |
| 16. | Zhao B, Zhao B, Chen F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022;34:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Hartlapp I, Valta-Seufzer D, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens UM, Waldschmidt D, Pelzer U, Fuchs M, Kullmann F, Boeck S, Ettrich TJ, Held S, Keller R, Anger F, Germer CT, Stang A, Kimmel B, Heinemann V, Kunzmann V; German Pancreatic Cancer Group (AIO-PAK) and NEOLAP investigators. Corrigendum to "Prognostic and predictive value of CA 19-9 inlocally advanced pancreatic cancer treated with multiagent induction chemotherapy: results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113)": [ESMO Open 7 (2024) 100552]. ESMO Open. 2024;9:103705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Ermiah E, Eddfair M, Abdulrahman O, Elfagieh M, Jebriel A, Al-Sharif M, Assidi M, Buhmeida A. Prognostic value of serum CEA and CA19-9 levels in pancreatic ductal adenocarcinoma. Mol Clin Oncol. 2022;17:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 19. | Hata T, Chiba K, Mizuma M, Masuda K, Ohtsuka H, Nakagawa K, Morikawa T, Hayashi H, Motoi F, Unno M. Levels of tumor markers CEA/CA 19-9 in serum and peritoneal lavage predict postoperative recurrence in patients with pancreatic cancer. Ann Gastroenterol Surg. 2022;6:862-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Hall C, McLaren M, Mosse C. A pancreatic mass and extreme elevation of CA 19-9: a benign masquerade of cholangiocarcinoma. J Surg Case Rep. 2022;2022:rjac018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Yan XT, Qian Y. Prognostic Value of Pancreatic Cancer B7-H4 and Related Serum tumor markers. Zhongguo Mianyixue Zazhi. 2022;38:201-205, 210. [DOI] [Full Text] |
| 22. | Raza SS, Khan H, Hajibandeh S, Hajibandeh S, Bartlett D, Chatzizacharias N, Roberts K, Marudanayagam R, Sutcliffe RP. Can preoperative Carbohydrate Antigen 19-9 predict metastatic pancreatic cancer? Results of a systematic review and meta-analysis. HPB (Oxford). 2024;26:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Stuhr LK, Madsen K, Johansen AZ, Chen IM, Hansen CP, Jensen LH, Hansen TF, Kløve-Mogensen K, Nielsen KR, Johansen JS. Combining sCD163 with CA 19-9 Increases the Predictiveness of Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2023;15:897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Boyd LNC, Ali M, Comandatore A, Garajova I, Kam L, Puik JR, Fraga Rodrigues SM, Meijer LL, Le Large TYS, Besselink MG, Morelli L, Frampton A, van Laarhoven HWM, Giovannetti E, Kazemier G. Prediction Model for Early-Stage Pancreatic Cancer Using Routinely Measured Blood Biomarkers. JAMA Netw Open. 2023;6:e2331197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/