Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.106297
Revised: April 15, 2025
Accepted: July 30, 2025
Published online: September 15, 2025
Processing time: 178 Days and 0 Hours
Entecavir (ETV) and tenofovir fumarate (TDF) are recommended first-line agents for the treatment of chronic hepatitis B virus (HBV) infection. However, the effect of these 2 antiviral agents on the risk for recurrence of HBV-associated hepatocellular carcinoma (HCC) after radical hepatectomy remains controversial.
To compare the effect of TDF vs ETV on the risk for HCC recurrence after radical surgery for HBV-related HCC.
Data from consecutive patients, who received TDF or ETV after radical hepatec
The median follow-up for 100 patients [median age, 61 years; 84 male (84%)] who underwent radical resection for HBV-related HCC - Barcelona Clinic Liver Cancer stage 0 [n = 16 (16%)], stage A [n = 61 (61%)] - was 29 months (range, 12-60 months); the median tumor size was 3.0 cm (range, 2.1-4.3 cm). Sixty-eight (68%) patients exhibited HBV-DNA levels > 1000 IU/mL. Twenty-two (22%) patients tested positive for hepatitis B e antigen, in whom the HCC recurrence rate was 59.1% (13/22). After PSM, HCC recurrence rates in the ETV and TDF groups after hepatectomy were 66% (n = 33) and 42% (n = 21), respectively (P = 0.016), and cumulative recurrence rates at 1, 3, and 5 years were 26%, 58%, and 66%, and 18%, 38%, and 42%, respectively (P = 0.045).
TDF treatment is associated with a lower risk for HCC-related outcomes than that for ETV in patients with HBV-associated HCC after curative therapy.
Core Tip: This study compared the recurrence rate of hepatocellular carcinoma (HCC) after radical resection of HCC in patients treated with entecavir (ETV) or tenofovir fumarate (TDF) after propensity score matching. Results revealed that HCC recurrence was observed in 33 (66.0%) patients in the ETV group and 21 (42.0%) in the TDF group, and the risk for HCC recurrence was lower in the TDF group than in the ETV group (P = 0.016). The cumulative recurrence rates of HCC at 1, 3, and 5 years in the ETV and TDF groups were 26.0%, 58.0%, and 66.0%, and 18.0%, 38.0%, and 42.0%, respectively (P = 0.045).
- Citation: Liu HM, Zhang X, Huang HY, Sun JM, Tong QD. Comparative impact of antiviral therapies on postoperative recurrence risk in patients with hepatitis B virus-related hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(9): 106297
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/106297.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.106297
Hepatocellular carcinoma (HCC) is the fourth most common cancer worldwide and the second most common cause of cancer-related mortality[1]. Chronic hepatitis B virus (HBV) infection is among the leading causes of HCC worldwide, with HBV infecting > 296 million individuals globally, infecting 1.5 million each year, and killing > 820000. In China, approximately 100 million individuals are infected with HBV and 20% develop chronic infections[2]. Chronic infection with HBV can lead to liver failure or HCC, for which hepatic resection is a potentially curative option[3]. The five-year recurrence rate after radical HCC resection is > 70%, which hampers long-term outcomes[4].
In HBV-associated HCC, active viral replication is associated with recurrence after hepatectomy. Antiviral therapy effectively suppresses viral reactivation and may reduce risk[4]. Tenofovir disoproxil fumarate (TDF) is a nucleotide analog prodrug that inhibits HBV polymerase and demonstrates strong anti-HBV activity. Its efficacy and safety were sustained for > 7 years in pivotal phase III trials, with no resistance observed. Additionally, TDF treatment for 5 years has been linked to the regression of liver fibrosis and cirrhosis. Entecavir (ETV), a guanosine nucleoside analog that also inhibits HBV polymerase, is a first-line treatment for chronic HBV that is both effective and well-tolerated[5-7].
Studies have shown that antiviral therapy in patients with HBV-associated HCC yielded a reduction in HCC recurrence after R0 hepatectomy and significantly improved overall survival (OS)[8,9]. In a study by Choi et al[10], the use of TDF therapy resulted in a substantial reduction in the risk for HCC recurrence and an increase in OS compared with ETV. However, based on findings reported by Kao et al[11], it can be inferred that TDF and ETV exhibit similar health benefits in terms of recurrence-free survival (RFS) and OS in patients with HCC. Nevertheless, the choice of antiviral agent(s), such as TDF and ETV, in patients with HBV-related HCC undergoing curative therapy remains controversial. Among the available nucleoside analogs, ETV and TDF are agents both used in first-line regimens due to their high efficacy and low resistance rates. However, comparisons between TDF and ETV have been controversial for decades. A recent meta-analysis by Gok Sargin et al[12] indicated that TDF was superior to ETV in suppressing HBV viral load while maintaining a similar safety profile. However, the effect of TDF and ETV on the risk for HCC recurrence after radical surgery in patients with HBV-related HCC remains unclear.
Propensity score matching (PSM) analysis is commonly used in retrospective studies because it adjusts for various baseline parameters, simulating results similar to those of randomized studies[13]. As such, this study compared the effects of TDF and ETV on the risk for HCC recurrence after radical surgery in patients with HBV-related HCC, with the aim of guiding clinical choices for postoperative antiviral therapy.
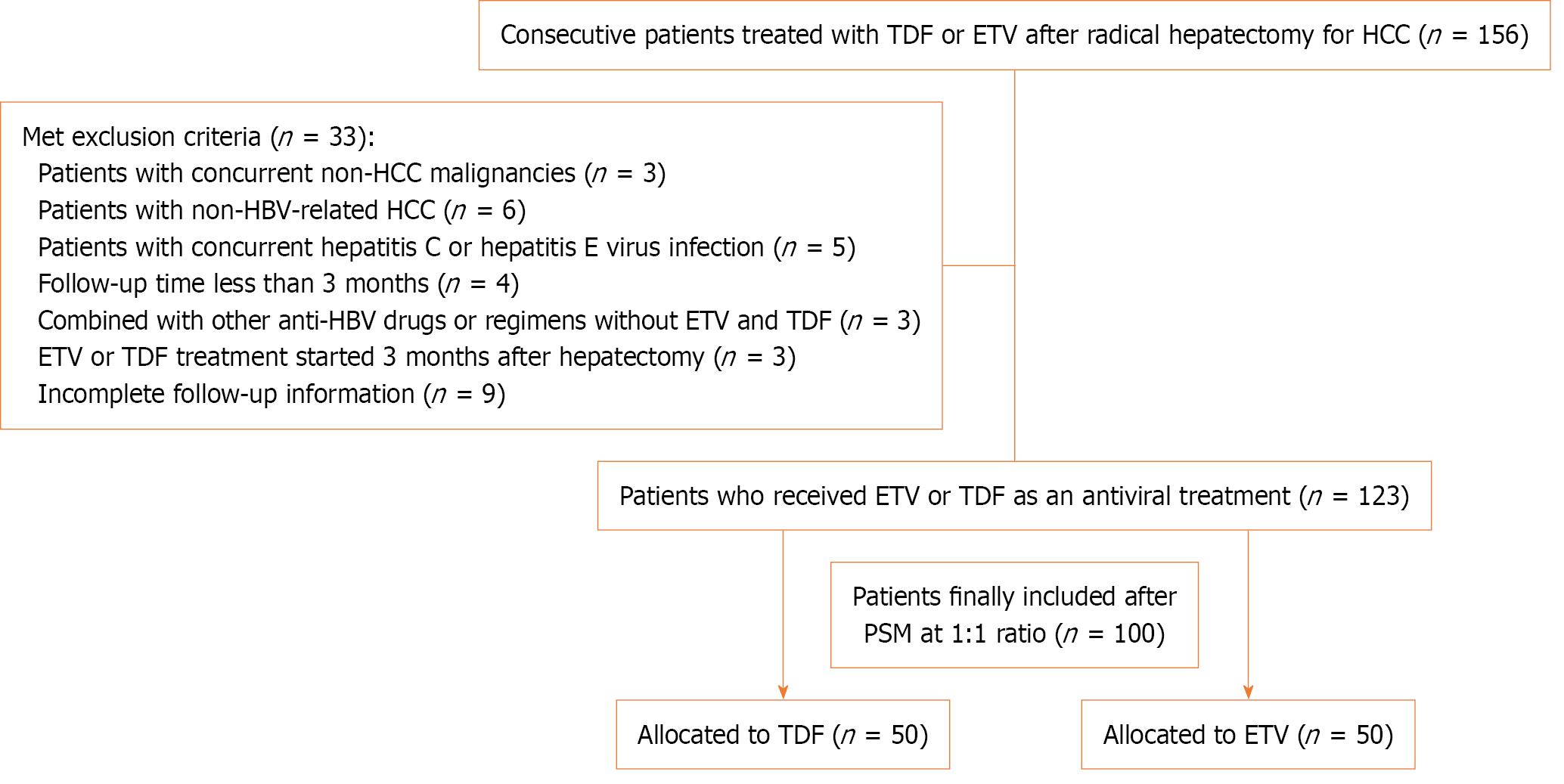
Data from 156 consecutive patients, who were admitted to the Second Hospital of Longyan and treated with TDF or ETV after radical hepatectomy for HCC between December 2018 and December 2023, were retrospectively reviewed. All clinical data were obtained from the electronic database of the hospital.
All patients were diagnosed with HBV-associated HCC and were receiving ETV or TDF at the time of hepatectomy or started medication within 3 months of surgery. Serum HBV DNA levels were measured using real-time polymerase chain reaction at the time of hepatectomy or 1 month before surgery (linear dynamic detection range, 15 to 1 × 108 IU/mL. The diagnostic kits were obtained from Da An Gene Co., Ltd. (Guangzhou, China).
Patients with concomitant non-HCC malignancies (n = 3), non-HBV-related HCC (n = 6), hepatitis C or E virus infection (n = 5), follow-up < 3 months (n = 4), combination with other anti-HBV drugs or regimens without ETV and TDF (n = 3), ETV or TDF treatment started 3 months after hepatectomy (n = 3), and incomplete follow-up information (n = 9) were excluded. The remaining 123 patients were matched PSM, and 100 consecutive patients who received TDF or ETV after radical hepatectomy for HBV-related HCC were ultimately included in the analysis, with 50 patients each in the TDF and ETV groups.
PSM was used to minimize the impact of confounding factors between the TDF and ETV groups. The caliper width was set at 0.05, and a 1:1 matching ratio was applied using nearest-neighbor matching based on propensity scores. Conse
This study was designed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and approved by the Ethics Committee of the Second Hospital of Longyan. Given the retrospective design of the study and the use of anonymized patient data, requirements for informed consent were waived.
Data entry was performed by dedicated personnel in accordance with uniform standards, thus ensuring the integrity and homogeneity of the data. Detailed demographic information was collected for all patients, including age and sex, as well as clinicopathological features, including HBV infection status, liver function, and tumor-related parameters. Preopera
The primary outcome was the HCC recurrence rate.
Recurrence criteria: According to imaging examination data (ultrasound, contrast-enhanced CT, MRI, contrast-enhanced ultrasound, positive electron emission tomography/CT), new lesions found in and outside the liver[12].
Recurrence rate = number of patients who relapsed/total number of patients in each group × 100.
The secondary outcomes were HCC recurrence rates in patients positive for hepatitis B e antigen (HBeAg), and a comparison of 1, 3, and 5-year cumulative HCC recurrence rates between the ETV and TDF groups.
Follow-up commenced on the date of the initial tumor diagnosis and ended on the date of the last follow-up or recurrence. The usual follow-up regimen was the same for the patients treated with ETV or TDF. It included four-stage ambulatory CT, biochemical tests of liver function, alpha-fetoprotein levels every 3 months for the first 2 years after hepatectomy, and enhanced MRI with gadoxetic acid, when ambulatory CT did not confirm HCC recurrence during follow-up.
Data processing was performed using SPSS version 26.0 (IBM Corporation, Armonk, NY, United States). Continuous data are expressed as median [interquartile range (IQR)] and compared using the Mann-Whitney U test. PSM was used for matching; the matching ratio was 1:1, and the caliper width was 0.05. Categorical variables are expressed as number (percentage) and compared using the χ2 or Fisher's exact tests as appropriate. All statistical tests were two-sided and differences with P < 0.05 were considered to be statistically significant.
One hundred patients treated for HBV-associated HCC were identified; a flow-diagram illustrating the patient screening process is presented in Figure 1. The median follow-up period was 29 months (IQR 12-60 months). At baseline (time to curative treatment for HCC), the median age of the patients was 61 years, and 84 (84%) were male. The median HCC was 3.0 cm (IQR 2.1-4.3 cm), 16 (16%) patients had Barcelona Clinic Liver Cancer (BCLC) stage 0, and 61 (61%) had BCLC stage A. Sixty-eight patients (68%) exhibited HBV DNA levels > 1000 IU/mL, and 22 (22.0%) patients tested positive for HBeAg. Cirrhosis was detected in 58 (58.0%) patients. MVI and capsular infiltration were observed in 39 (39.0%) and 51 patients (51.0%), respectively. Fifteen (15.0%) patients exhibited satellite nodules, with poorly differentiated tumors observed in 48 (48.0%) patients.
The incidence of portal vein tumor thrombus in the TDF group was similar to that in the ETV group (26.0% vs 24.0%), as was alanine aminotransferase level > 40 U/L (48.0% vs 46.0%), the incidence of satellite nodules (14.0% vs 16.0%), HBV-DNA > 1000 IU/mL (66.0% vs 70.0%), and aspartate aminotransferase level > 40 U/L (58.0% vs 62.0%).
Compared with the ETV group, the Eastern Cooperative Oncology Group performance status (i.e., ECOG-PS) score 0-1 in the TDF group (60.0% vs 56.0%), Child-Pugh classification A (44.0% vs 46.0%), BCLC stage A (60.0.0% vs 62.0%) were similar. The baseline characteristics of the 2 groups were comparable after PSM matching (Table 1).
| Characteristics | Overall (n = 100) | ETV group (n = 50) | TDF group (n = 50) | P value |
| Age (years), median (interquartile range) | 61 (52-69) | 60 (52-67) | 62 (54-69) | 0.308 |
| Gender (male) | 84 (84.0) | 42 (84.0) | 42 (84.0) | 1.000 |
| HBeAg | 22 (22.0) | 10 (20.0) | 12 (24.0) | 0.751 |
| HBV-DNA (> 103 IU/mL) | 68 (68.0) | 35 (70.0) | 33 (66.0) | 0.236 |
| Liver cirrhosis | 58 (58.0) | 28 (56.0) | 30 (60.0) | 0.635 |
| Tumor size (cm) | 3.0 (2.1, 4.3) | 3.0 (2.2, 4.3) | 2.9 (2.1, 3.8) | 0.381 |
| MVI | 39 (39.0) | 19 (38.0) | 20 (40.0) | 0.942 |
| Capsular invasion | 51 (51.0) | 25 (50.0) | 26 (52.0) | 0.576 |
| Satellite nodules | 15 (15.0) | 8 (16.0) | 7 (14.0) | 0.263 |
| Tumor differentiation | 0.759 | |||
| Well | 3 (3.0) | 2 (4.0) | 1 (2.0) | |
| Moderate | 49 (49.0) | 25 (50.0) | 24 (48.0) | |
| Poorly | 48 (48.0) | 23 (46.0) | 25 (50.0) | |
| PVTT | 25 (25.0) | 12 (24.0) | 13 (26.0) | 0.246 |
| AFP (> 400 ng/mL) | 51 (51.0) | 26 (52.0) | 25 (50.0) | 0.803 |
| TBIL (> 28 μmol/L) | 3 (3.0) | 2 (4.0) | 1 (2.0) | 0.237 |
| ALT (> 40 U/L) | 47 (47.0) | 23 (46.0) | 24 (48.0) | 0.345 |
| AST (> 40 U/L) | 60 (60.0) | 31 (62.0) | 29 (58.0) | 0.549 |
| ALB (> 40 g/L) | 69 (69.0) | 34 (68.0) | 35 (70.0) | 0.296 |
| Follow-up duration, months | 29 (12-60) | 28 (12-47) | 31 (12-60) | 0.677 |
| BCLC stage | 0.755 | |||
| 0 | 16 (16.0) | 7 (14.0) | 9 (18.0) | |
| A | 61 (61.0) | 31 (62.0) | 30 (60.0) | |
| B | 23 (23.0) | 12 (24.0) | 11 (22.0) | |
| Child-Pugh score | 0.726 | |||
| A | 45 (45.0) | 23 (46.0) | 22 (44.0) | |
| B | 55 (55.0) | 27 (54.0) | 28 (56) | |
| ECOG score | 0.271 | |||
| 0-1 | 58 (58.0) | 28 (56.0) | 30 (60.0) | |
| 2 | 42 (42.0) | 22 (44.0) | 20 (40.0) |
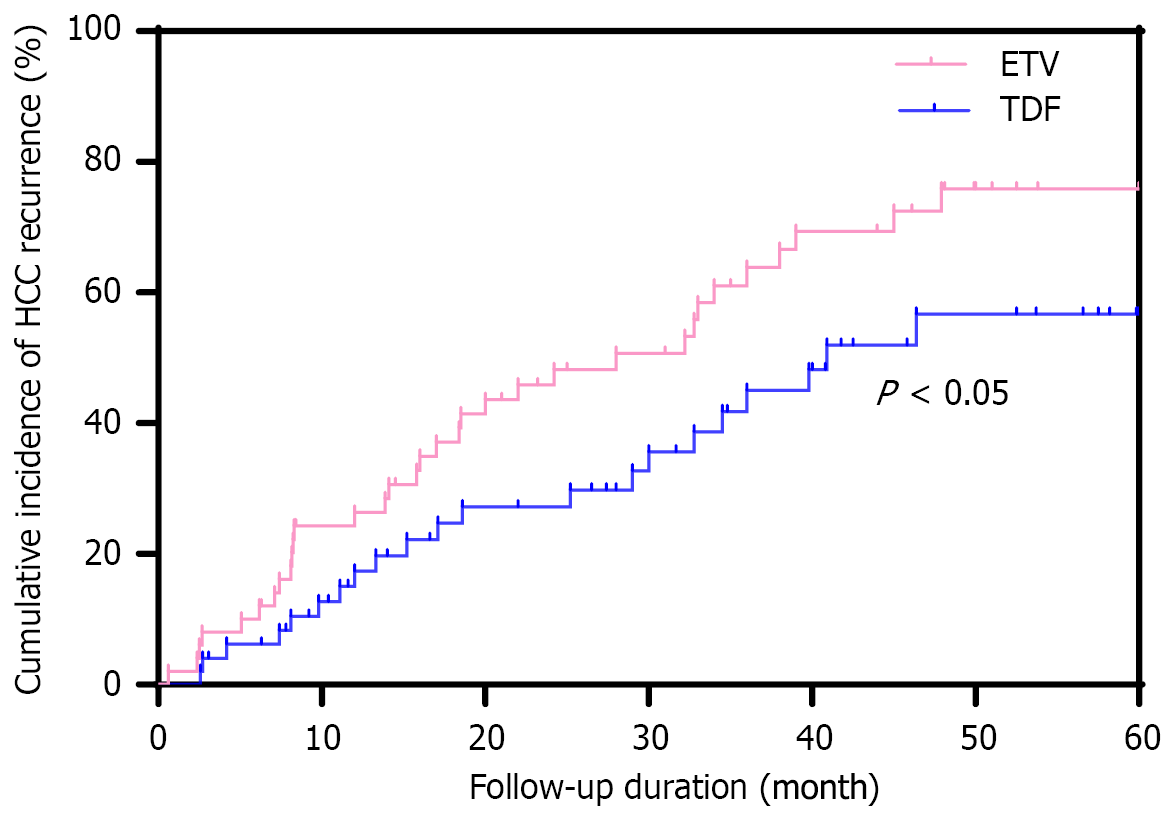
At the end of the last follow-up, HCC recurrence was observed in 33 (66.0%) and 21 (42.0%) patients in the ETV and TDF groups, respectively, with a lower risk for HCC recurrence in the TDF group than that in the ETV group (P = 0.016). The HCC recurrence rate in 22 HBeAg e-positive patients was 59.1% (13/22). Cumulative recurrence rates at 1, 3, and 5 years for the ETV and TDF groups were 26%, 58%, and 66%, and 18%, 38%, and 42%, respectively (P = 0.045). The cumulative incidence of HCC recurrence in both groups is reported in Figure 2.
Tumor resection is the only effective treatment for HCC, and R0 resection is essential for long-term survival; however, long-term survival after liver resection is unsatisfactory because > 70% of tumors recur within the first 5 years[14]. Previous studies have shown that chronic HBV infection is a major risk factor for the development of HCC, and there is a close relationship between the two[15]. Chronic inflammation and cytokine release largely promote hepatocyte proliferation and malignant transformation[16]. In addition, HBV DNA integration may lead to host genome instability and insertion mutations in various cancer-related genes, thereby increasing the risk for HCC[17]. Antiviral therapy has demonstrated a positive effect in slowing the progression of HCC, especially in patients with a high HBV load, and the risk for HCC recurrence after surgical resection is significantly increased[18]. In this study, 33 patients (66.0%) in the ETV group and 21 (42.0%) in the TDF group developed HBV-related HCC recurrence, although the risk for recurrence was significantly lower in the TDF group than that in the ETV group (P = 0.016). This result is consistent with previous findings, suggesting that TDF may be superior to ETV for preventing HCC recurrence[19].
Two previous studies have compared ETV with TDF in preventing HCC recurrence; however, the results have been inconclusive[20]. One study involving 726 patients who underwent radiofrequency ablation (RFA) or excision exhibited no significant difference in HCC recurrence, whereas another study, which included 1695 patients with early stage HCC, reported that TDF has an advantage in preventing HCC recurrence. In the present study, we included all patients with HBV-related HCC and compared the effects of ETV or TDF antiviral therapy after hepatectomy. To better understand the differences in the effects of these 2 antiviral agents on the recurrence rate of HCC, we adjusted for clinical variables other than antiviral regimen using PSM. Results revealed that the recurrence rate with TDF treatment was significantly lower than that for ETV treatment (42.0% vs 66.0%, P = 0.016), whereas the cumulative recurrence rates of HCC at 1, 3, and 5 years were 26.0%, 58.0%, and 66.0% in the ETV group, and 18.0%, 38.0%, and 42.0% in the TDF group, respectively (P = 0.045). These results support the superiority of TDF in preventing HCC recurrence.
In conclusion, TDF therapy appears to result in a lower rate of HCC recurrence than ETV therapy in patients with HBV-associated HCC who experience curative postoperative recurrence. These findings may provide important guidance for the development of antiviral treatment regimens for HCC at this specific stage.
Results of this study revealed that after radical surgery for HBV-related HCC, the rate of HCC recurrence was signifi
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4362] [Article Influence: 545.3] [Reference Citation Analysis (6)] |
| 2. | Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, Inoue M. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011;21:401-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Dang Z, Lu P, Qian Y, Lin K, Pan Z, Lau WY, Zhou W. Impact of pathological response after preoperative transcatheter arterial chemoembolization (TACE) on incidences of microvascular invasion and early tumor recurrence in hepatocellular carcinoma: a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr. 2022;11:386-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Sun YH, Du Y, Shen JR, Ai DY, Huang XY, Diao SH, Lin SB, Zhang R, Yuan L, Yang YP, He LL, Qin XJ, Zhou JG, Chen C. A modified lung ultrasound score to evaluate short-term clinical outcomes of bronchopulmonary dysplasia. BMC Pulm Med. 2022;22:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Lu H, Zheng C, Xiong B, Xia X. TACE versus TACE + entecavir versus TACE + tenofovir in the treatment of HBV associated hepatocellular carcinoma. BMC Cancer. 2023;23:235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Teng YX, Li MJ, Xiang BD, Zhong JH. Tenofovir may be superior to entecavir for preventing hepatocellular carcinoma and mortality in individuals chronically infected with HBV: a meta-analysis. Gut. 2020;69:1900-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1410] [Article Influence: 108.5] [Reference Citation Analysis (1)] |
| 8. | Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015;261:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Hui VW, Chan SL, Wong VW, Liang LY, Yip TC, Lai JC, Yuen BW, Luk HW, Tse YK, Lee HW, Chan HL, Wong GL. Increasing antiviral treatment uptake improves survival in patients with HBV-related HCC. JHEP Rep. 2020;2:100152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Choi J, Jo C, Lim YS. Tenofovir Versus Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma After Surgical Resection. Hepatology. 2021;73:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Kao WY, Tan EC, Lee HL, Huang YH, Huo TI, Chang CC, Chiou JF, Hou MC, Wu JC, Su CW. Entecavir versus tenofovir on prognosis of hepatitis B virus-related hepatocellular carcinoma after curative hepatectomy. Aliment Pharmacol Ther. 2023;57:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Gok Sargin Z, Celik U, Dusunceli I, Ustundag Y. Comparison of the side effects of antivirals in chronic hepatitis B patients: a single-center experience. Acta Gastroenterol Belg. 2022;85:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Prognostic Impact of MAFLD Following Surgical Resection of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Nationwide Cohort Study. Cancers (Basel). 2022;14:5002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Zhang YL, Nie CH, Chen F, Zhou TY, Zhou GH, Zhu TY, Chen SQ, Chen XH, Wang HL, Wang BQ, Yu ZN, Jing L, He ZM, Sun JH. Adjuvant Transarterial Chemoembolization for Barcelona Clinic Liver Cancer Stage A Hepatocellular Carcinoma After Hepatectomy. Front Oncol. 2020;10:1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Ji J, Zhang Z, Peng Q, Hao L, Guo Y, Xue Y, Liu Y, Li C, Shi X. The Effects of Qinghao-Kushen and Its Active Compounds on the Biological Characteristics of Liver Cancer Cells. Evid Based Complement Alternat Med. 2022;2022:8763510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Zheng Y, Ming P, Zhu C, Si Y, Xu S, Chen A, Wang J, Zhang B. Hepatitis B virus X protein-induced SH2 domain-containing 5 (SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction. J Biol Chem. 2019;294:4815-4827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Huang MB, Gao Z, Xia M, Zhao X, Fan X, Lin S, Zhang L, Huang L, Wei A, Zhou H, Wu JY, Roth WW, Bond VC, Leng J. Improved Aitongxiao prescription (I-ATXP) induces apoptosis, cell cycle arrest and blocks exosomes release in hepatocellular carcinoma (HCC) cells. Int J Physiol Pathophysiol Pharmacol. 2022;14:90-113. [PubMed] |
| 18. | Tang K, Cheng H, Wang H, Guo Y. Meta-analysis of the occurrence of hepatocellular carcinoma after the treatment of entecavir and tenofovir for chronic hepatitis B. Medicine (Baltimore). 2023;102:e32894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Huang Y, Chen L, Huang R, Zhu C, Shang J, Qian Y, Lian J, Liu L, Jiang J, Liu C, Gui H, Xie Q. Tenofovir is superior to entecavir in reducing HCC for patients with HBV-related compensated cirrhosis at high HCC risk scores. Ther Adv Chronic Dis. 2022;13:20406223221102791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Kim BK, Park SY, Tak WY, Park JY, Kim DY, Ahn SH, Sinn DH, Kim SU. The efficacies of entecavir and tenofovir in terms of enhancing prognosis after curative treatment of hepatitis B virus-related hepatocellular carcinoma. Eur J Intern Med. 2021;89:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/