INTRODUCTION
Duodenal cancer, a rare yet clinically distinct malignancy of the gastrointestinal tract, accounts for less than 1% of all gastrointestinal neoplasms and 30%-45% of small intestinal cancers[1]. Duodenal malignant tumors mainly include duodenal adenocarcinoma, stromal tumors, and neuroendocrine tumors. This review only discusses duodenal adenocarcinoma. With an exceptionally low incidence of < 0.5 cases per 100000 individuals, it represents approximately 0.3%-1.0% of gastrointestinal malignancies globally[2]. Recent molecular profiling advancements have revolutionized biomarker discovery in this field. Extreme Gradient Boost modeling identified three-tiered molecular signatures: Alcohol dehydrogenase 1C, defensin alpha 5, carbamoyl phosphate synthetase 1, secreted phosphoprotein 1, deleted in malignant brain tumors 1, VCAN antisense RNA 1, and apolipoprotein B (cancer vs adenoma); LOC399753, apolipoprotein A4, microRNA 548x, and alcohol dehydrogenase 1C (adenoma subtypes); and small nucleolar RNA C/D box 123, CEA cell adhesion molecule 6, small nucleolar RNA C/D box 78, annexin A10, serine peptidase inhibitor Kazal type 1, and carbamoyl phosphate synthetase 1 (normal vs adenoma)[3]. These signatures provide critical insights into early detection and risk stratification.
The gut microbiota plays a pivotal role in modulating the progression and treatment of duodenal malignancies, with emerging research highlighting its influence on tumor biology, therapeutic efficacy, and patient outcomes[4]. Duodenal malignancies are associated with gut microbiota dysbiosis, characterized by a decline in beneficial bacteria (e.g., Bifidobacterium, Faecalibacterium) and enrichment of pathobionts like Escherichia coli and Bilophila wadsworthia[5]. These shifts correlate with elevated serum triglycerides and liver dysfunction, exacerbating inflammation and tumor progression. Dysbiosis impairs immune surveillance, potentially diminishing responses to immunotherapy[6]. Conversely, Bifidobacterium enrichment enhances programmed cell death protein 1 (PD-1) inhibitor efficacy in preclinical models. Specific species (e.g., Bacteroidetes depletion, Proteobacteria enrichment) correlate with disease severity and treatment response[7]. The gut microbiota’s dual role as a driver of tumor progression and a modulator of therapeutic response underscores its potential as a therapeutic target.
Prognostic biomarkers have also emerged as clinical tools. Caudal-type homeobox transcription factor 2 (CDX2), a transcription factor critical in intestinal development, is highly expressed in gastrointestinal malignancies. Its utility in duodenal cancer lies in distinguishing primary tumors from metastases and predicting therapeutic responses. CDX2 demonstrates a 78.7% positivity rate (48/61) in duodenal carcinoma, with CDX2-positive cases showing significantly improved survival compared to CDX2-negative counterparts [hazard ratio (HR): 0.42, 95% confidence interval (CI): 0.24-0.73], establishing it as an independent prognostic determinant[8]. Similarly, cell-free DNA (cfDNA) levels stratify survival outcomes: Patients with high plasma cfDNA (> 9288 copies/mL) exhibit markedly reduced 1-year (58.7% vs 95.2%) and 5-year (17.6% vs 64.6%) survival rates compared to low-cfDNA cohorts, confirming its role as a robust independent predictor of overall survival (OS)[9]. Circulating tumor DNA, a subset of cfDNA, enables non-invasive tumor profiling and real-time monitoring of treatment response.
Prognosis is multifactorial, influenced by tumor type (adenocarcinoma vs neuroendocrine), location (periampullary vs extra-ampullary), metastatic burden, resectability status, and diagnostic timing[10]. Lymph node involvement dramatically impacts outcomes, with 5-year survival rates plummeting from 65% (node-negative) to 21% (node-positive)[11]. A retrospective analysis of 201 surgically treated patients (1999-2014) revealed striking survival disparities: Median survival of 57 months postcurative resection vs 7 months after palliative surgery (P < 0.001)[12]. Established negative prognostic factors include lymphovascular invasion (HR: 2.18, 95%CI: 1.18-4.03) and perineural infiltration (HR: 2.21; P = 0.002)[13]. Over the past decade, multidisciplinary advances - encompassing endoscopic innovations, minimally invasive surgery, molecularly targeted therapies, and immunotherapy - have reshaped the therapeutic landscape.
ENDOSCOPIC AND SURGICAL RESECTION
Endoscopic resection (ER) has become a cornerstone in the management of Tis/T1-stage duodenal tumors, accounting for approximately two-thirds of all resection procedures. This approach is favored due to its oncological efficacy, reduced operative trauma, and lower morbidity compared to major surgery[14]. A cohort study (2004-2017) reported that ER was utilized in 66% of Tis/T1-stage cases and was associated with significantly improved OS (HR: 0.70, 95%CI: 0.52-0.95; P = 0.02) and disease-specific survival (HR: 0.32, 95%CI: 0.17-0.60, P < 0.001) compared to surgical resection. Additionally, ER demonstrated a lower cumulative mortality rate due to postoperative infectious diseases (P = 0.03)[15]. A multicenter study demonstrated ER feasibility for lesions ≤ 3 cm with well-differentiated histology (G1/G2), achieving R0 resection rates of 92% compared to 81% for larger/complex lesions. However, lesions involving the ampulla or with lymphovascular invasion remain contraindications due to higher recurrence risks[16]. A meta-analysis of 1284 patients (2010-2023) revealed 89.2% 5-year overall survival. ER patients reported faster return to work (median, 7 days vs 42 days) and fewer chronic pain episodes (4% vs 22%)[17].
For more advanced tumors, surgical resection remains the primary treatment, with the goal of achieving complete tumor removal (R0 resection). The two main surgical approaches are segmental duodenal resection and pancreaticoduodenectomy (Whipple procedure)[18]. The choice between these techniques depends on factors such as tumor location, size, stage, metastasis, and patient health. A recent multicenter retrospective study (2012-2023) found that radical surgery was associated with favorable OS, with no significant difference between extra-ampullary and peri-ampullary duodenal adenocarcinoma. Minimally invasive surgery has also gained prominence, offering advantages such as reduced intraoperative blood loss, fewer transfusions, and shorter hospital stays[19].
Segmental duodenal resection is increasingly employed for primary duodenal tumors. A retrospective study (2007-2013) reported a median operative time of 191 minutes and a median blood loss of 675 mL, with a 30-day morbidity rate of 82% (78% Clavien grade ≤ 2). Resected specimens included adenocarcinoma, gastrointestinal stromal tumor, adenoma, and lymphoma, with a median hospital stay of 14 days[20]. A meta-analysis of 37 observational studies (1728 patients) revealed a 30-day postoperative mortality rate of 3.2% and a median 5-year OS of 46.4%. Surgical resection significantly improved prognosis compared to palliative therapy (odds ratio: 15.76, P < 0.001), with lymph node metastasis, poor tumor differentiation, perineural invasion, and lymphovascular invasion identified as independent predictors of decreased OS[21]. Lymph node dissection is generally recommended for tumors extending beyond the submucosa, although it may be omitted for intra-mucosal lesions. Lymph node metastasis is an independent prognostic factor, categorizing lymph node stations into Np (upstream) and Nd (other) based on lymphatic flow. Dissection of Np stations is associated with acceptable survival, while metastasis to Nd stations correlates with poor outcomes[22]. For tumors deeper than the submucosa, pancreaticoduodenectomy is the standard procedure, whereas local resection without lymph node dissection may be considered for intra-mucosal cancers. Postoperative follow-up with imaging is recommended to detect distant metastasis and local recurrence[23].
CHEMOTHERAPY AND RADIATION THERAPY
Chemotherapy plays a pivotal role in the management of duodenal cancer, serving as both adjuvant therapy following curative resection and palliative treatment for advanced or metastatic disease. In advanced stages, chemotherapy remains the standard of care, with fluoropyrimidine-based regimens combined with platinum derivatives (e.g., FOLFOX, CAPEOX, FOLFOXIRI) being the most used first-line treatments. For salvage therapy, the FOLFIRI regimen is frequently employed[24]. Fluorouracil-based chemotherapy, often combined with platinum analogs or radiation therapy, is widely utilized due to the histological similarities between duodenal adenocarcinoma and colorectal cancer. However, a systematic review and meta-analysis found no significant survival benefit for adjuvant chemotherapy after curative resection, highlighting the need for further research to evaluate the efficacy of multimodal (neo) adjuvant therapies[25]. For unresectable or recurrent duodenal cancer, systemic chemotherapy with fluoropyrimidine and oxaliplatin is weakly recommended, as it has shown modest improvements in OS and progression-free survival in advanced disease[26].
A meta-analysis of 15 retrospective cohorts (6672 patients) demonstrated that adjuvant chemotherapy was associated with a significant OS benefit (HR: 0.60, 95%CI: 0.53-0.68), particularly in stage III tumors (HR: 0.55, 95%CI: 0.48-0.64). Patients with stage II disease also derived benefit (HR: 0.83, 95%CI: 0.69-0.98). Additionally, adjuvant chemotherapy was linked to improved relapse-free survival (HR: 0.65, 95%CI: 0.50-0.85), with the magnitude of benefit varying by disease stage. Similar OS benefits were observed in both duodenal (HR: 0.67, 95%CI: 0.56-0.81) and jejunal/ileal adenocarcinomas (HR: 0.70, 95%CI: 0.62-0.80)[15]. Fluoropyrimidine-oxaliplatin regimens have consistently outperformed other combinations in terms of survival and response rates, making them a preferred choice. While cisplatin-based therapies have shown limited survival benefits in some studies, they may be more effective in duodenal adenocarcinomas due to their anatomical proximity to the pancreas and stomach[27].
Neoadjuvant chemoradiotherapy has been explored in locally advanced duodenal cancer, with some studies reporting improved resectability and potential survival benefits. Adjuvant radiation therapy is occasionally used to reduce the risk of local recurrence, particularly in cases where the tumor involves critical structures such as the pancreas or major blood vessels[28]. Ongoing clinical trials, such as the international Phase 3 Global BALLAD trial, are comparing observation alone, fluoropyrimidine monotherapy, and FOLFOX in patients with stage I-III small intestinal adenocarcinoma, with disease-free survival as the primary endpoint[29]. Similarly, the Japanese J-BALLAD trial, initiated in May 2017, is evaluating the efficacy of postoperative CAPOX (capecitabine and oxaliplatin) vs observation in patients with curatively resected small bowel adenocarcinoma[30]. Future research should focus on tailoring therapies to molecular tumor characteristics, such as the use of (neo) adjuvant immunotherapy for patients with localized mismatch repair-deficient (dMMR) small intestinal adenocarcinomas.
TARGETED THERAPIES
Duodenal cancer, a rare and aggressive gastrointestinal malignancy, has seen significant advancements in treatment with the emergence of targeted therapies and immunotherapy. These approaches offer new hope by specifically targeting molecular pathways and genetic alterations driving tumor growth. Below, we summarize key targeted therapies and related research findings in duodenal cancer. The erb-b2 receptor tyrosine kinase 2 (ERBB2) gene has been identified as a potential therapeutic target in small bowel adenocarcinoma, including duodenal cancer. ERBB2 amplification drives tumor growth via hyperactivation of downstream pathways like phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase[31]. HER2 blockade enhances efficacy by preventing heterodimerization. An antibody-drug conjugate with a topoisomerase I inhibitor payload. In HER2-positive gastrointestinal cancers, trastuzumab deruxtecan (T-DXd) achieved a 43% objective response rate in a Phase 2 trial (DESTINY-PanTumor02), suggesting potential for duodenal cancer[32]. Notably, cases of complete pathologic response have been reported in metastatic ERBB2-amplified duodenal cancer following neoadjuvant treatment with trastuzumab (an ERBB2 inhibitor) combined with FOLFOX chemotherapy[33]. This highlights the potential of ERBB2-targeted therapies in selected patients. The epidermal growth factor receptor (EGFR) is another common target in cancer therapy. EGFR inhibitors block signaling pathways that promote cancer cell proliferation and survival. Preclinical models and early-phase clinical trials have shown promising results for EGFR-targeted therapies in duodenal cancer, particularly in tumors with EGFR overexpression or activating mutations[34]. NCT05113251 is evaluating T-DXd in HER2-positive gastrointestinal cancers, including duodenal tumors. NCT04526470 is testing copanlisib (PI3K inhibitor) + nivolumab in advanced gastrointestinal malignancies.
The PI3K/AKT/mTOR pathway, frequently dysregulated in cancer, promotes cell survival, proliferation, and metabolism. Inhibitors targeting this pathway have shown promise in preclinical studies and are currently being evaluated in clinical trials for duodenal cancer. These agents may offer therapeutic benefits, particularly in tumors with activating mutations in PI3K or AKT[35]. PIK3CA mutations constitutively activate PI3Kα, driving uncontrolled proliferation. mTOR overactivation promotes metabolic reprogramming and therapy resistance[36]. NCT04632992 tests alpelisib + trastuzumab in PIK3CA-mutant gastrointestinal cancers. NCT04145263 evaluates capivasertib + paclitaxel in advanced solid tumors.
Vascular endothelial growth factor (VEGF) plays a critical role in angiogenesis, the process by which tumors develop new blood vessels to sustain their growth. VEGF inhibitors, such as bevacizumab, disrupt this process, effectively starving the tumor of nutrients. Bevacizumab, when combined with chemotherapy, has demonstrated improved survival outcomes in patients with advanced duodenal cancer[37]. Molecular profiling of tumors is essential for identifying actionable genetic mutations and protein expressions that drive cancer growth. Techniques such as next-generation sequencing (NGS) enable the detection of specific alterations, such as ERBB2 amplification, EGFR mutations, or microsatellite instability (MSI), which can guide the selection of targeted therapies. For example, tumors with high MSI (MSI-H) may benefit from immune checkpoint inhibitors, while ERBB2-amplified tumors may respond to HER2-targeted agents[38]. Despite the promise of targeted therapies, many remain in early-phase clinical trials, and their efficacy in duodenal cancer requires further validation. However, preclinical models and preliminary clinical data suggest significant potential, particularly when combined with chemotherapy or immunotherapy. The integration of molecular profiling and precision medicine is expected to further enhance the effectiveness of these therapies, enabling personalized treatment strategies tailored to individual patients’ molecular profiles.
IMMUNOTHERAPY
Immunotherapy has emerged as a transformative treatment strategy for duodenal cancer[39]. PD-1/programmed death-ligand 1 (PD-L1) blockade restores T cell-mediated tumor killing by blocking inhibitory signals. cytotoxic T-lymphocyte associated protein 4 inhibition enhances T cell priming but has limited activity as monotherapy[40]. These molecular characteristics enhance tumor immunogenicity, making them more responsive to immune checkpoint inhibitors. Below, we summarize key advances and ongoing research in this field. A landmark study reported three cases of locally advanced MSI-H duodenal adenocarcinoma treated with neoadjuvant pembrolizumab (a programmed cell death protein 1 inhibitor). United States Food and Drug Administration-approved for MSI-H/dMMR duodenal cancer (KEYNOTE-158: 39% objective response rate). After two cycles of therapy, all patients exhibited partial metabolic and endoscopic responses. Following 4-6 cycles of treatment, duodenopancreatectomy revealed complete pathological responses in all cases, suggesting that neoadjuvant immunotherapy could enable organ-sparing surgery in patients achieving complete clinical remission[41]. Similarly, the combination of nivolumab (PD-1 inhibitor) and ipilimumab (cytotoxic T lymphocyte antigen-4 inhibitor) has demonstrated remarkable efficacy in MSI-H/dMMR rectal cancer, with complete pathological responses observed in clinical trials. Phase 2 data show 50% 1-year survival in refractory cases by nivolumab + ipilimumab. This dual checkpoint blockade strategy holds promise for application in duodenal cancer, particularly in locally advanced or metastatic settings[42]. The tumor immune microenvironment plays a critical role in shaping therapeutic responses. A recent study analyzing immune cell infiltration in duodenal cancers found that high densities of tumor-infiltrating lymphocytes, particularly CD8+ cytotoxic T cells, and M1-polarized macrophages were associated with reduced recurrence rates and improved survival. Conversely, elevated levels of immunosuppressive cells (e.g., regulatory T cells, M2 macrophages) correlated with poorer outcomes. Tumor mutation burden and PD-L1 expression have potential in personalized treatment strategies. These findings suggest that immune-related biomarkers could enhance prognostic stratification and guide immunotherapy selection[39].
The emerging role of gut microbiota transplantation (FMT) in enhancing immunotherapy efficacy represents a paradigm shift in oncology[43]. Specific bacteria, such as Parabacteroides distasonis, enhance dendritic cell maturation and promote CD8+ T cell recruitment into tumors. Bifidobacterium and Akkermansia muciniphila stimulate natural killer cell cytotoxicity via short-chain fatty acids production, which enhances interferon-γ secretion and tumor cell lysis[44]. FMT from immune checkpoint inhibitor-responsive donors restored F. prausnitzii and B. longum in colorectal cancer patients, reversing primary resistance to PD-1 inhibitors[45]. This was linked to increased mucosal CD8+ T cell infiltration and reduced interleukin 17-driven inflammation. FMT emerges as a potent adjunct to immunotherapy, leveraging microbial ecosystems to overcome resistance and amplify anti-tumor immunity[46].
Despite these advances, challenges remain. Only 5%-10% of duodenal adenocarcinomas exhibit MSI-H/dMMR status, limiting the applicability of checkpoint inhibitors to a small subset of patients[39]. Microsatellite stable (MSS) tumors exhibit low CD8+ T cell infiltration (≤ 5% of the tumor microenvironment) and high stromal content (≥ 50%). While PD-1 inhibitors benefit MSI-H patients, MSS tumors require innovative strategies like chimeric antigen receptor-modified T cells or dual checkpoint blockade. Additionally, the optimal timing and duration of immunotherapy—neoadjuvant, adjuvant, or palliative - require further investigation. Ongoing multicenter trials, such as the NEOPEMBRO trial (NCT04868604), are evaluating pembrolizumab in the neoadjuvant setting for MSI-H gastrointestinal cancers, including duodenal adenocarcinoma. Preliminary results are expected to clarify the role of immunotherapy in organ preservation and survival improvement.
DISCUSSION
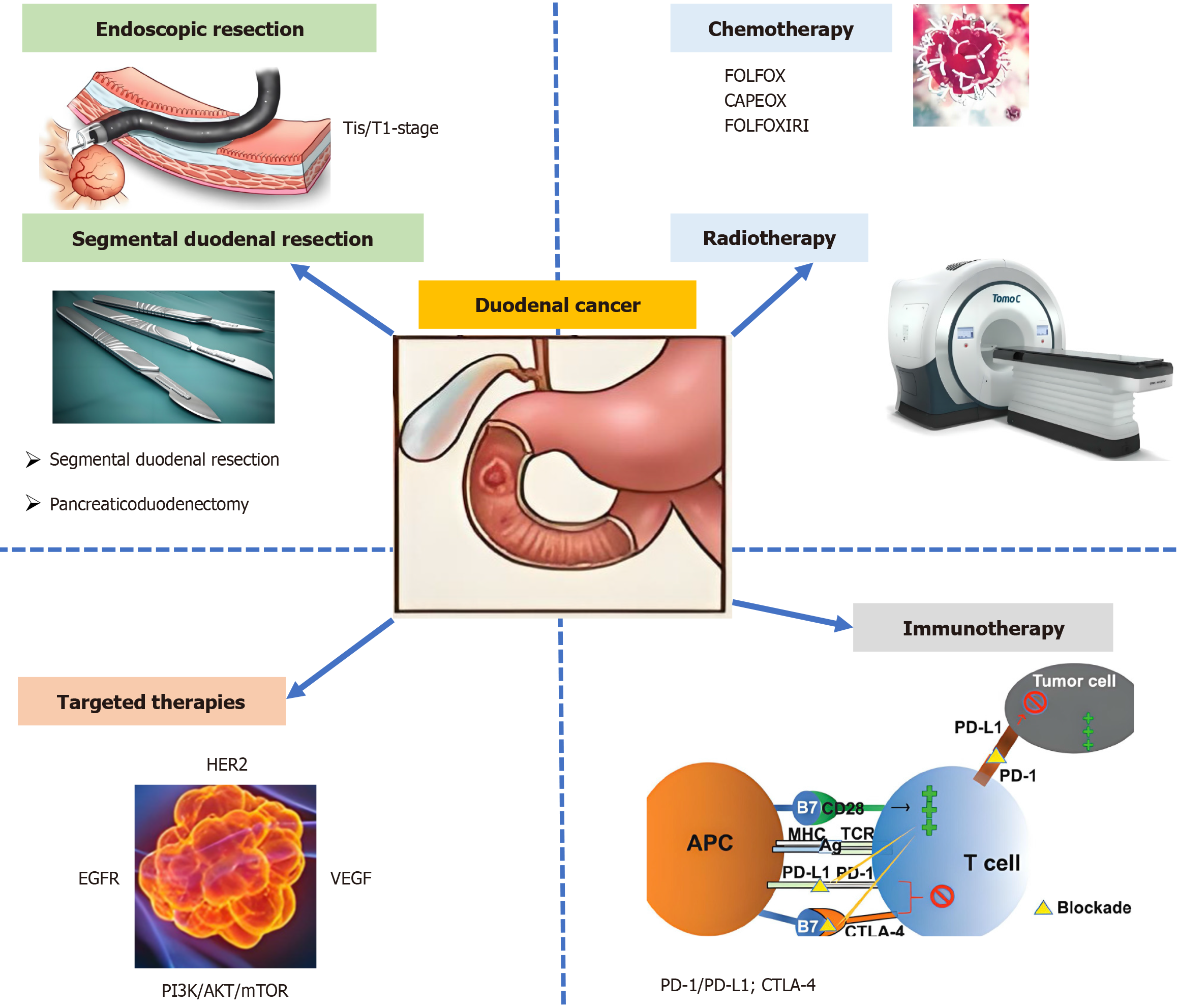
This review synthesized current evidence on the management of duodenal cancer, with emphasis on evolving surgical paradigms, systemic therapies, and emerging precision medicine approaches. Several surgical strategies are available for duodenal adenocarcinoma, including ER, partial (wedge) resection, segmental resection, and pancreaticoduodenectomy. For early-stage tumors, endoscopic and segmental resections are effective, while pancreaticoduodenectomy is preferred for advanced cases. Lymph node dissection is recommended for tumors beyond the submucosa, and careful follow-up is essential to monitor for recurrence. Chemotherapy and radiation therapy are integral to the treatment of duodenal cancer (Figure 1). Adjuvant chemotherapy has been shown to improve OS and relapse-free survival, particularly in stage III tumors, with fluoropyrimidine-oxaliplatin being the preferred regimen. Neoadjuvant and adjuvant radiation therapy may also provide benefits in locally advanced cases. Ongoing clinical trials and molecular profiling efforts are expected to further refine these strategies and improve patient outcomes
Figure 1 Progress in the treatment of duodenal cancer.
AKT: Protein kinase B; APC: Antigen-presenting cell; CTLA-4: Cytotoxic T-lymphocyte antigen 4; EGFR: Epidermal growth factor receptor; MHC: Major histocompatibility complex; mTOR: Mammalian target of rapamycin; PD-L1: Programmed death-ligand 1; PI3K: Phosphatidylinositol-3-kinase; TCR: T cell receptor; VEGF: Vascular endothelial growth factor.
Targeted therapies represent a promising approach to treating duodenal cancer by addressing specific molecular pathways and genetic alterations. Key targets include ERBB2, EGFR, VEGF, and the PI3K/AKT/mTOR and RAS-RAF-MEK pathways. Molecular profiling and advanced technologies like NGS are critical for identifying actionable targets and developing personalized treatment plans. While further research is needed to fully establish their role, targeted therapies are poised to revolutionize the management of this rare and aggressive malignancy. The management of duodenal cancer is poised for transformative advances through the integration of multimodal therapies and innovative research strategies. Building on current evidence, future efforts should prioritize the following directions to optimize patient outcomes.
Synergistic combination therapies
Pairing immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1) with agents targeting VEGF (e.g., bevacizumab) or EGFR (e.g., cetuximab) may overcome immunosuppressive microenvironments and enhance antitumor responses. Preclinical data suggest VEGF inhibition normalizes tumor vasculature, improving T-cell infiltration, while EGFR targeting may modulate PD-L1 expression. Leverage radiotherapy’s potential to induce immunogenic cell death and systemic “abscopal effects” by combining it with immunotherapy. Clinical trials should explore sequencing (neoadjuvant vs concurrent) and dosing strategies to maximize synergy.
Multiomics and biomarker discovery
Integrate genomics, proteomics, metabolomics, and microbiomics to identify composite biomarkers predictive of treatment response. For example, tumor mutation burden combined with gut microbiome signatures may refine immunotherapy eligibility beyond MSI-H/dMMR status. Establish global platforms for sharing multiomics data, paired with clinical outcomes, to accelerate biomarker validation. Initiatives like the AACR GENIE registry could serve as models.
Resistance mechanisms and tumor microenvironment
Decipher stromal-immune interactions (e.g., cancer-associated fibroblast-mediated immunosuppression) using spatial transcriptomics. Target tumor microenvironment components with stromal-modifying agents (e.g., transforming growth factor beta inhibitors) to enhance drug delivery. The integration of surgical, systemic, and precision-guided strategies demands a collaborative, data-driven approach. By prioritizing biomarker discovery, adaptive trial designs, and mechanistic studies on resistance, the field can transform duodenal cancer into a manageable chronic condition, ultimately improving survival and quality of life.
CONCLUSION
Duodenal cancer management has advanced significantly through surgical refinements (endoscopic/minimally invasive techniques for early-stage, Whipple procedures for advanced cases) and systemic therapies. Chemotherapy (e.g., FOLFOX) and neoadjuvant chemoradiotherapy improve survival and resectability. Targeted therapies (ERBB2/EGFR inhibitors) and immunotherapy (pembrolizumab for MSI-H/dMMR tumors) show transformative potential in molecularly defined subgroups, supported by molecular profiling (NGS and cfDNA). Critical gaps remain in adjuvant therapy validation and clinical trial scalability due to disease rarity. Future priorities include: (1) Precision oncology: Multiomics-guided biomarker discovery; (2) Global collaboration: Multicenter trials to accelerate evidence; and (3) Survivorship: Standardized long-term monitoring protocols. Multidisciplinary integration of surgical innovation, molecular targeting, and immunotherapy promises improved outcomes, necessitating sustained research to optimize survival and quality of life.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C, Grade C, Grade C
Novelty: Grade B, Grade B, Grade C, Grade C, Grade C
Creativity or Innovation: Grade B, Grade B, Grade C, Grade C, Grade C
Scientific Significance: Grade B, Grade B, Grade C, Grade C, Grade D
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Liao HM; Wu LL; Yang CH S-Editor: Wei YF L-Editor: Filipodia P-Editor: Zhang L