Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106846
Revised: March 27, 2025
Accepted: April 27, 2025
Published online: June 15, 2025
Processing time: 97 Days and 4.5 Hours
Peritoneal metastasis occurs in about 20% of patients with colorectal cancer (CRC) and is associated with a 5-year survival rate of only 6%. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy reportedly improves survival in selected patients. Achieving complete cytoreduction, indicated by a low completeness of cytoreduction (CCR) score, is a key factor in extending survival. Here, we present a case in which multimodal therapy yielded long-term survival in a patient, even though she had a CCR score of 3.
A 61-year-old female with CRC and extensive peritoneal metastases presented with abdominal distention. Cytoreductive surgery was not completed due to the extent of the disease (CCR score: 3). The patient underwent palliative omen
Aggressive multimodal treatment may yield long-term survival and quality of life improvement in patients with advanced disease, even with high CCR scores.
Core Tip: Long-term survival of patients with a completeness of cytoreduction score of 3 is rarely observed; nonetheless, a favorable outcome was attained in this case by using tailored, multimodal treatment. Despite a substantial residual tumor burden, the 61-year-old female patient survived at least 6 years, suggesting the benefit of aggressive, multimodal treatment even in advanced disease. Despite the challenges in preoperatively identifying patients suited for cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy, administering the most effective therapy that the patient can tolerate is likely to produce positive outcomes.
- Citation: Suh JW, Jo JW, Park DG. Long-term survival of a patient with colorectal cancer with peritoneal carcinomatosis and low completeness of cytoreduction score: A case report. World J Gastrointest Oncol 2025; 17(6): 106846
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106846.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106846
The peritoneum is the second most prevalent metastatic site in colorectal cancer (CRC), occurring in 19%-23% of cases, following the liver, which accounts for 60%-74% of cases[1-3]. Up to 5%-8% of such peritoneal metastases (PMs) are identified synchronously at the initial diagnosis, and up to 5% are discovered metachronously during surveillance[4-7]. In a previous population-based study, the 5-year cumulative survival rate for PM identified during follow-up was 6%[8,9]. The prognosis for PM is 16.3 months, even with optimal systemic chemotherapy, which is inferior to that of liver-only metastases (19.1 months) and lung-only metastases (24.6 months)[6,8,10]. It is associated with a diminished quality of life due to symptoms including abdominal pain, distention caused by malignant ascites, cachexia, and bowel obstruction[6,11]. Multimodal treatment, including cytoreductive surgery (CRS) to remove PMs followed by hyperthermic intraperitoneal chemotherapy (HIPEC) to eliminate residual tumor cells, reportedly extends survival in selected patients with CRC and PM by up to 41 months[8]. CRS and HIPEC significantly improve outcomes, with 1-year and 5-year survival rates as high as 72% and 32%, respectively, in CRC[12-14]. This survival advantage largely depends on achieving an R0 resection, which indicates complete cytoreduction. The completeness of cytoreduction (CCR) score (ranging from 0 to 3) and the extent of peritoneal disease, quantified using the peritoneal cancer index (PCI, ranging from 0 to 39), are recognized as the most reliable predictors of survival[15,16]. To achieve complete cytoreduction, extensive resections of the visceral and parietal peritoneum are sometimes required, contributing to morbidity and mortality rates of up to 42% and 4%, respectively[12,14].
This report concerns a patient who, despite substantial residual disease (CCR score: 3), survived long without recurrence after HIPEC and systemic chemotherapy. Additionally, we conducted a comprehensive review of the latest relevant literature.
A 61-year-old female patient visited the local obstetrics and gynecology hospital due to abdominal distension and discomfort.
The abdominal distension and discomfort had developed 2 weeks before the patient’s visit.
The patient had no history of cancer treatment aside from treatment for rheumatoid arthritis.
There was no personal or family history of cancer treatment in the patient.
A large amount of ascites was noted during the original physical examination.
Upon a visit to a local clinic, the patient’s cancer antigen 125 (CA125) concentration was elevated (391 U/mL, normal range: 0-55.0 U/mL), leading to a referral to a tertiary university hospital. There, a tumor marker test confirmed the increased CA125 concentration (500 U/mL), but the carcinoembryonic antigen and CA19-9 concentrations were within normal limits at 1.4 ng/mL (normal range: 0-7.0 ng/mL) and 0.4 U/mL (normal range: 0-37.0 U/mL), respectively.
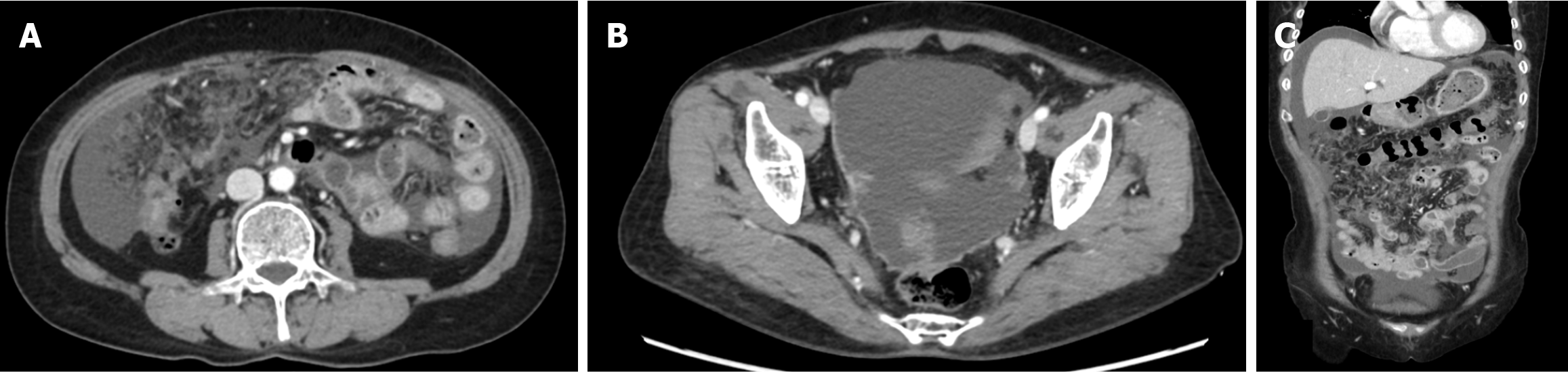
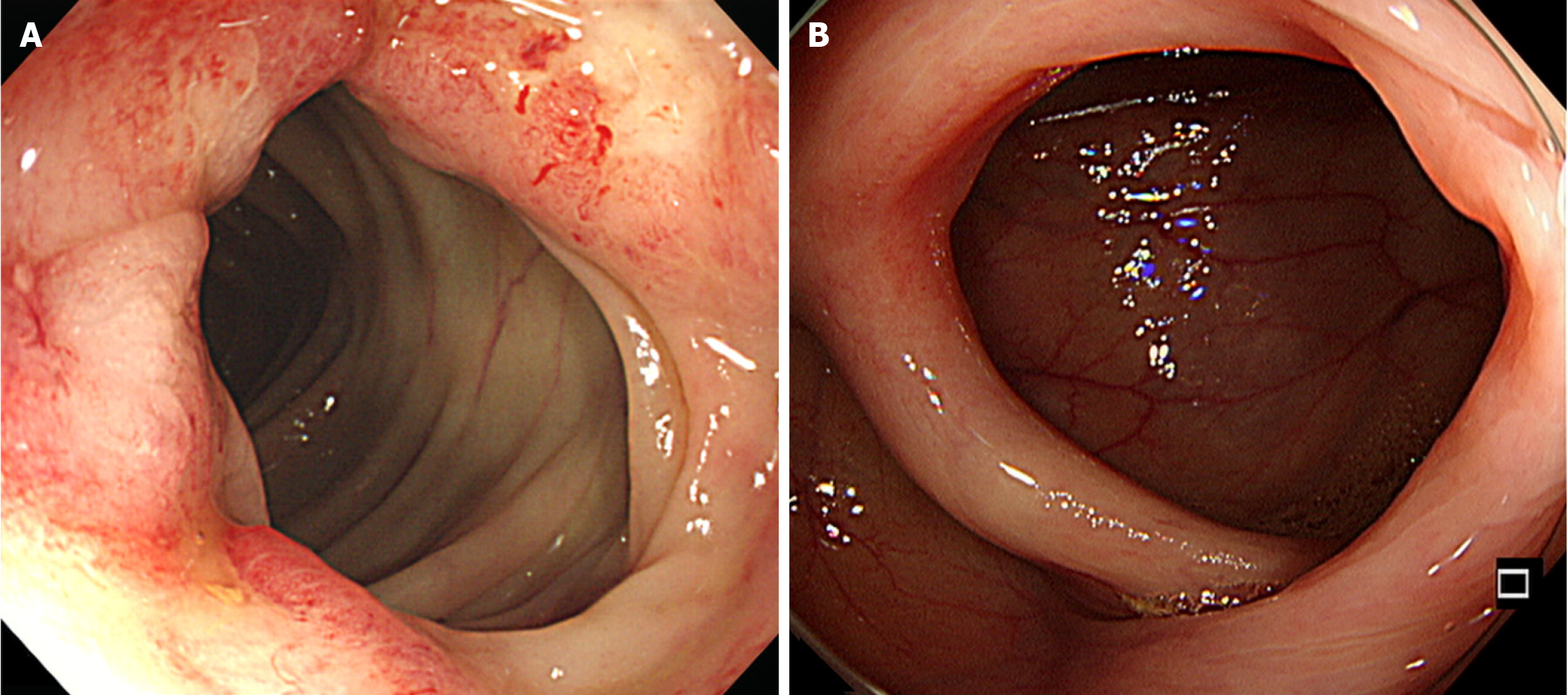
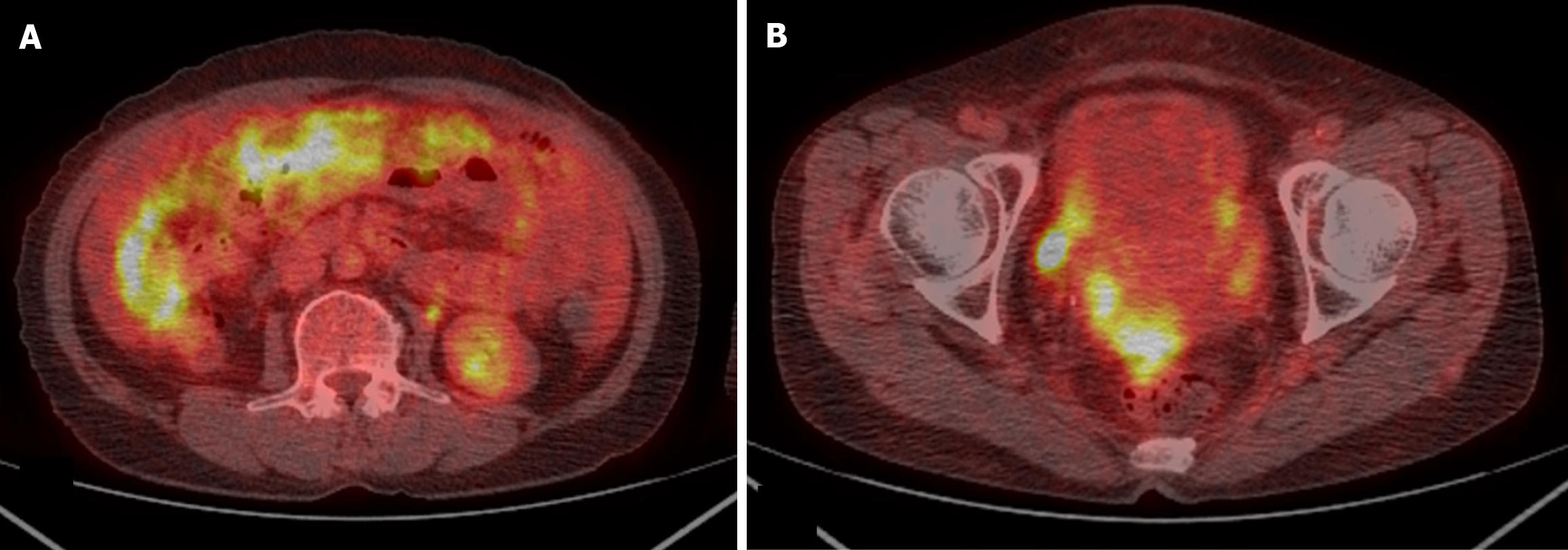
Abdominal-pelvic (AP) computed tomography (CT) revealed moderate to large volumes of diffuse ascites; smudged, nodular omentum in the abdomen and pelvic cavity; and focal abutting or invasion of the anterior wall of the mid-rectum in the cul-de-sac area (Figure 1). An ulcerofungating mass (4 cm in diameter) was observed 45 cm from the anal verge during colonoscopy (Figure 2A), and the histological results confirmed moderately differentiated adenocarcinoma. Positron emission tomography (PET)-CT revealed a substantially elevated metabolic uptake in the abdomen, suggestive of peritoneal seeding (Figure 3).
The team, comprising a colorectal surgeon, gastroenterologist, pathologist, and radiologist, convened to evaluate the disease.
The multidisciplinary team made a diagnosis of malignant ascites resulting from peritoneal seeding of the colorectal malignancy.
Given the extremely low risk of metastases outside the abdominal cavity, the team decided to proceed with CRS and HIPEC. Intraoperative examinations revealed extensive PMs throughout the mesentery of the small bowel, sigmoid mesocolon, and parietal peritoneum, with a PCI of 39, rendering a complete cytoreduction unattainable. The planned CRS was abandoned, and palliative omentectomy was performed to alleviate the patient’s abdominal discomfort caused by the mass effect and ascites. Thereafter, we performed HIPEC using mitomycin C (30 mg + 10 mg) for 90 minutes. Early postoperative intraperitoneal chemotherapy (EPIC) was planned for control of residual tumors, and intraperitoneal chemotherapy was conducted on postoperative days 1 to 4 by using 5-fluorouracil. Molecular pathology testing revealed no mutations in K-ras, N-ras, or BRAF. On postoperative day 14, the patient was discharged without marked discomfort.
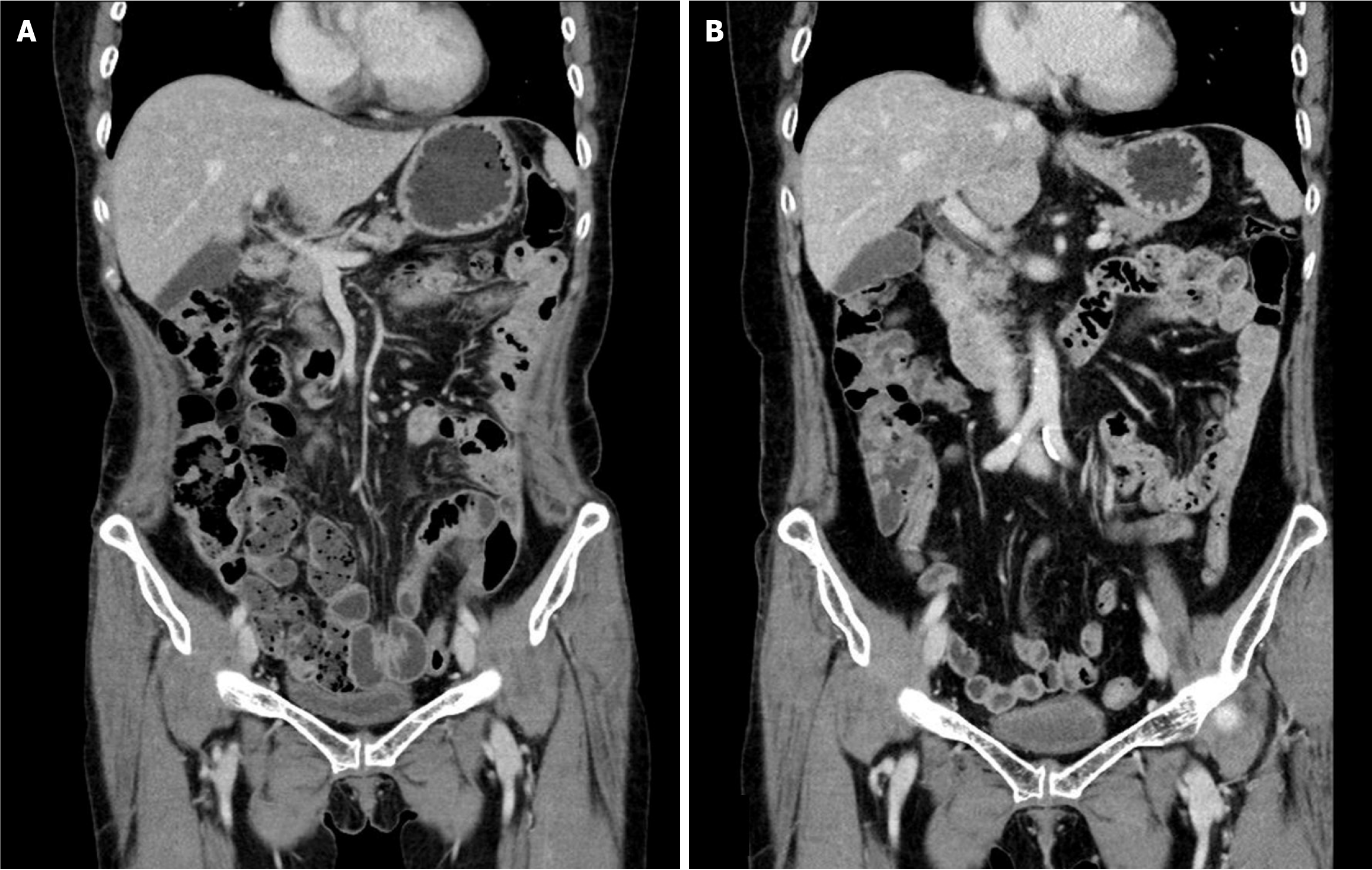
Intraperitoneal chemotherapy with 5-fluorouracil was administered on postoperative days 37 to 40. Palliative chemotherapy (FOLFIRI + bevacizumab) was scheduled for postoperative days 68 and 81 but was not administered due to thrombocytopenia and neutropenia, respectively. On postoperative day 103, in consideration of the patient’s overall state, the chemotherapy regimen was altered to oral capecitabine (1300 mg, twice a day). The treatment was conducted in a cycle of 2 weeks of medication followed by 1 week of cessation. AP-CT and tumor marker tests were conducted at three-cycle intervals to evaluate the patient’s response to chemotherapy. Before the 5th chemotherapy cycle, neutropenia occurred, necessitating dose reduction; in addition, hand-foot syndrome developed during the 14th cycle. In the 16th chemotherapy cycle, a second dose reduction was implemented due to a decline in the patient’s condition and exacerbation of hand-foot syndrome. Following the completion of 19 cycles of chemotherapy, the patient’s overall condition deteriorated, and her hand-foot syndrome worsened. Chemotherapy was discontinued after no signs of recurrence were observed upon evaluation, including via CT (Figure 4A).
Following the conclusion of chemotherapy, AP-CT, chest CT, and tumor marker tests were conducted every 6 months, whereas esophagogastroduodenoscopy and colonoscopy were performed annually for the first 2 years. Subsequently, all follow-up examinations were conducted at annual intervals. The complete removal of the primary tumor was confirmed via follow-up colonoscopy (Figure 2B). No sign of recurrence was observed up to 6 years post-surgery (Figure 4B), and routine follow-up is planned for up to 10 years.
A prior systematic review and meta-analysis of CRS and HIPEC revealed that the extent of PM and the completeness of resection were the sole significant prognostic factors; the risk of mortality among patients with a CCR score of 0/1 was 1.61-fold that of patients with a CCR score of 2 or higher[16]. In a previous study at our center, overall survival (OS) was influenced by the PCI, CCR score, and postoperative chemotherapy. Although PCI and postoperative chemotherapy were revealed as significant predictors of OS, the CCR score was not[17]. In a meta-analysis, patients who received postoperative chemotherapy had increased OS and disease-free survival rates relative to those who underwent surgery alone (hazard ratio = 0.81, P = 0.00001 and hazard ratio = 0.82, P = 0.003, respectively)[18]. Although preoperative chemotherapy was not significantly associated with an improvement in OS[18], it may decrease the peritoneal tumor burden, enhance the likelihood of achieving a CCR score of 0 and minimize the extent of surgical intervention, which may, in turn, lead to a lower incidence of postoperative complications[19,20]. Preemptive intervention for hematogenous micrometastases may diminish the likelihood of extraperitoneal recurrence after surgery[3]. Patients with tumors deemed unresectable during laparoscopy before preoperative chemotherapy are highly unlikely to be eligible for radical surgery (CCR score = 0) after preoperative chemotherapy[21], as they may experience an increased incidence of surgical complications[22,23]. Those who are initially deemed operable may be disqualified because of toxicity or a poor response[24]. In 2017, our clinic prioritized surgery when surgical resection was feasible, thereafter administering postoperative chemotherapy, including targeted therapy. Palliative omentectomy with HIPEC, followed by FOLFIRI and bevacizumab, was prioritized; however, oral capecitabine was eventually administered in this case, due to the patient’s condition. After verifying the absence of tumor recurrence and metastasis following two dose reductions owing to chemotherapy side effects, the treatment was concluded.
For patients with CRC and PM, CT is the predominant imaging modality for the evaluation of tumor size and extent, with a reported sensitivity of 75%, specificity of 92%, positive predictive value of 90%, and negative predictive value of 79% for 64-row CT[25]. The reported average sensitivity is 89% for lesions ≥ 0.5 cm, whereas it was merely 43% for those measuring < 0.5 cm[26]. Additionally, CT-based assessment of factors determining complete resection of the neoplasm remains challenging, as the detection rate for tiny, localized lesions, such as diffuse peritoneal, small-intestinal, and mesenteric involvement, is low. To compensate for the low detection rate of CT in these regions, magnetic resonance imaging and PET-CT examinations are conducted, but they remain sufficient. In this case, although the smudged, nodular omentum upon CT and the significantly elevated metabolic uptake in the relevant area upon PET-CT were suggestive of peritoneal carcinomatosis, the diffuse peritoneal, small-intestinal, and mesenteric involvement could not be anticipated preoperatively.
In this case, the patient received one supplementary intraperitoneal chemotherapy regimen following HIPEC and EPIC, as most of the disease remained. A recent study of EPIC vs HIPEC indicated that EPIC was a risk factor for significant surgical complications, whereas survival rates were comparable between EPIC and HIPEC[27]. A study of HIPEC plus EPIC vs HIPEC alone indicated that adding EPIC did not yield a substantial survival benefit, but that the rate of major complications increased[28]. However, that study was conducted exclusively on individuals with CCR scores of 0 or 1, and no investigations on patients with a CCR score of 3 have been reported. In the case of patients with a CCR score of 3, suppressing disease progression was the top priority in our hospital at the time of the study. To reduce the decline in quality of life due to ascites in a patient with a large volume of residual cancer, we performed an additional round of intraperitoneal chemotherapy to HIPEC plus EPIC, judging that the maximum feasible intraperitoneal chemotherapy was possible considering the patient’s condition.
This study was limited by its nature as a single-case report, necessitating further validation through multi-institutional or longitudinal studies. Furthermore, this case report lacks specific clinical markers and data to assess the long-term effects of treatment, especially considering incomplete cytoreduction. Future efforts may involve the accumulation of cases of long-term survival achieved through active multimodal treatment despite incomplete cytoreduction, allowing for an analysis of characteristics associated with prolonged survival. Insights gained from such analyses would inform the optimization of future treatment strategies for similar high-risk patients.
Long-term survival in patients with CCR scores of 3 is rarely documented. Nonetheless, maximal, customized combination therapy has yielded successful results among individuals with substantial degrees of remaining malignancy. Despite the challenges in preoperatively identifying patients suited for CRS plus HIPEC, administering the most effective therapy the patient can tolerate will likely produce positive outcomes.
| 1. | Larsen SG, Goscinski MA, Dueland S, Steigen SE, Hofsli E, Torgunrud A, Lund-Iversen M, Dagenborg VJ, Flatmark K, Sorbye H. Impact of KRAS, BRAF and microsatellite instability status after cytoreductive surgery and HIPEC in a national cohort of colorectal peritoneal metastasis patients. Br J Cancer. 2022;126:726-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Thomassen I, van Gestel YR, Lemmens VE, de Hingh IH. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum. 2013;56:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, Wijsman JH, Slooter GD, Rutten HJ, Creemers GJ, Lemmens VE. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Hassan S, Malcomson L, Soh YJ, Wilson MS, Clouston H, O'Dwyer ST, Kochhar R, Aziz O. Patterns and Timing of Recurrence following CRS and HIPEC in Colorectal Cancer Peritoneal Metastasis. Eur J Surg Oncol. 2023;49:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 5. | Lurvink RJ, Bakkers C, Rijken A, van Erning FN, Nienhuijs SW, Burger JW, Creemers GJ, Verhoef C, Lemmens VE, De Hingh IH. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur J Surg Oncol. 2021;47:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, Saltz L, Punt CJA, Koopman M, Tournigand C, Tebbutt NC, Diaz-Rubio E, Souglakos J, Falcone A, Chibaudel B, Heinemann V, Moen J, De Gramont A, Sargent DJ, Grothey A; Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 517] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 7. | Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Rufián-Peña S, Casado-Adam Á, Cosano-Álvarez A, Briceño-Delgado J. Colorectal peritoneal metastases: Optimal management review. World J Gastroenterol. 2019;25:3484-3502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, Juzyna B, de Forges H, Paineau J, Glehen O; UNICANCER-GI Group and BIG Renape Group. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 577] [Article Influence: 115.4] [Reference Citation Analysis (2)] |
| 9. | Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 367] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 10. | Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 11. | Lambert LA, Wiseman J. Palliative Management of Peritoneal Metastases. Ann Surg Oncol. 2018;25:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 12. | Oemrawsingh A, de Boer NL, Brandt-Kerkhof ARM, Verhoef C, Burger JWA, Madsen EVE. Short-term complications in elderly patients undergoing CRS and HIPEC: A single center's initial experience. Eur J Surg Oncol. 2019;45:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 813] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 14. | Levine EA, Stewart JH 4th, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 15. | Tonello M, Baratti D, Sammartino P, Di Giorgio A, Robella M, Sassaroli C, Framarini M, Valle M, Macrì A, Graziosi L, Fugazzola P, Lippolis PV, Gelmini R, Biacchi D, Kasamura S, Deraco M, Cenzi C, Del Bianco P, Vaira M, Sommariva A. External validation of COMPASS and BIOSCOPE prognostic scores in colorectal peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol. 2023;49:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hallam S, Tyler R, Price M, Beggs A, Youssef H. Meta-analysis of prognostic factors for patients with colorectal peritoneal metastasis undergoing cytoreductive surgery and heated intraperitoneal chemotherapy. BJS Open. 2019;3:585-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Jo MH, Suh JW, Yun JS, Namgung H, Park DG. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer: 2-year follow-up results at a single institution in Korea. Ann Surg Treat Res. 2016;91:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Tonello M, Cenzi C, Pizzolato E, Fiscon R, Del Bianco P, Pilati P, Sommariva A. Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis. Cancers (Basel). 2024;16:1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Passot G, Vaudoyer D, Cotte E, You B, Isaac S, Noël Gilly F, Mohamed F, Glehen O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E, Glehen O. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21:2608-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Hompes D, Aalbers A, Boot H, van Velthuysen ML, Vogel W, Prevoo W, van Tinteren H, Verwaal V. A prospective pilot study to assess neoadjuvant chemotherapy for unresectable peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16:O264-O272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Glockzin G, Zeman F, Croner RS, Königsrainer A, Pelz J, Ströhlein MA, Rau B, Arnold D, Koller M, Schlitt HJ, Piso P. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients With Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin Colorectal Cancer. 2018;17:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Simkens GA, Rovers KP, van Oudheusden TR, Nienhuijs SW, Rutten HJ, de Hingh IH. Major influence of postoperative complications on costs of cytoreductive surgery and HIPEC in patients with colorectal peritoneal metastases. Medicine (Baltimore). 2018;97:e0042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Bushati M, Rovers KP, Sommariva A, Sugarbaker PH, Morris DL, Yonemura Y, Quadros CA, Somashekhar SP, Ceelen W, Dubé P, Li Y, Verwaal VJ, Glehen O, Piso P, Spiliotis J, Teo MCC, González-Moreno S, Cashin PH, Lehmann K, Deraco M, Moran B, de Hingh IHJT. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur J Surg Oncol. 2018;44:1942-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Xia W, Geng Y, Hu W. Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer. Cancers (Basel). 2023;15:5641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Marin D, Catalano C, Baski M, Di Martino M, Geiger D, Di Giorgio A, Sibio S, Passariello R. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: correlation with histopathological findings. Abdom Imaging. 2010;35:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 27. | Jeong MH, Kang SJ, Park SY, Kwak SG, Seo AN, Park S, Park JS, Kim HJ, Choi GS. Comparison of EPIC Versus HIPEC in the Treatment of Colorectal Peritoneal Metastases and Appendix Tumors Using Inverse Probability of Treatment Weighting. Ann Surg Oncol. 2024;31:7111-7121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Lam JY, McConnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/